Abstract
Munc18-1 binds to syntaxin-1A via two distinct sites referred to as the “closed” conformation and N terminus binding. The latter has been shown to stimulate soluble N-ethylmaleimide-sensitive factor attachment protein receptor-mediated exocytosis, whereas the former is believed to be inhibitory or dispensable. To precisely define the contributions of each binding mode, we have engineered Munc18-1/-2 double knockdown neurosecretory cells and show that not only syntaxin-1A and -1B but also syntaxin-2 and -3 are significantly reduced as a result of Munc18-1 and -2 knockdown. Syntaxin-1 was mislocalized and the regulated secretion was abolished. We next examined the abilities of Munc18-1 mutants to rescue the defective phenotypes. Mutation (K46E/E59K) of Munc18-1 that selectively prevents binding to closed syntaxin-1 was unable to restore syntaxin-1 expression, localization, or secretion. In contrast, mutations (F115E/E132A) of Munc18-1 that selectively impair binding to the syntaxin-1 N terminus could still rescue the defective phenotypes. Our results indicate that Munc18-1 and -2 act in concert to support the expression of a broad range of syntaxins and to deliver syntaxin-1 to the plasma membrane. Our studies also indicate that the binding to the closed conformation of syntaxin is essential for Munc18-1 stimulatory action, whereas the binding to syntaxin N terminus plays a more limited role in neurosecretory cells.
INTRODUCTION
The Sec1-Munc18 (SM) proteins are essential proteins that regulate soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) machineries through the interaction with specific syntaxins and modulation of the SNARE complex formation. In mammals there are three Munc18 isoforms: -1, -2, and -3 (also called Munc18a, b, and c). Munc18-1 is expressed primarily in neurons and neuroendocrine cells (Hata et al., 1993; Pevsner et al., 1994; Garcia et al., 1994); Munc18-2 is expressed widely except in the brain; and Munc18-3 is expressed ubiquitously (Hata and Südhof, 1995; Katagiri et al., 1995; Tellam et al., 1995; Halachmi and Lev, 1996; Riento et al., 1996). Munc18-1 and -2 can bind to syntaxin-1A, -1B, -2, and -3, whereas Munc18-3 binds to syntaxin-2 and -4 (Tellam et al., 1995, 1997; Hata and Südhof, 1995; Halachmi and Lev, 1996; Riento et al., 1998, 2000; Tamori et al., 1998; Kauppi et al., 2002; Latham et al., 2006; Hu et al., 2007).
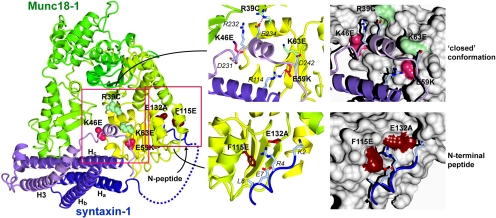
Recent studies have revealed that the interaction between Munc18 and syntaxin is complex; Munc18 can bind to a short N-terminal peptide of syntaxin (Bracher and Weissenhorn, 2002; Hu et al., 2007; Rickman et al., 2007; Burkhardt et al., 2008), in addition to binding to syntaxin in the previously recognized “closed” conformation that incorporates both the central Habc domain and the H3 SNARE helix (Misura et al., 2000) (see Figure 4). Although the physiological significance of these distinct modes of binding remains to be elucidated, the newly described interaction of Munc18-1 with the N terminus of syntaxin was shown to allow Munc18-1 to bind to the SNARE complex (Zilly et al., 2006; Dulubova et al., 2007, Rickman et al., 2007; Shen et al., 2007; Rodkey et al., 2008).
Figure 4.
Structure of the Munc18-1/syntaxin-1 complex (Burkhardt et al., 2008) highlighting mutations used in this study. Munc18-1 is shown colored yellow to green (N to C terminus) and syntaxin-1 is colored dark blue to light blue. The disordered loop connecting the N-terminal syntaxin-1 peptide and the Habc domain is indicated with a dashed line. Mutations in Munc18-1 predicted to inhibit closed conformation binding that are defective in syntaxin-1 binding, trafficking and secretion are indicated in salmon (K46E and E59K), those predicted to inhibit closed conformation binding but have no effect on syntaxin-1 binding, trafficking and secretion are indicated in light green (R39C and K63E), and those predicted to inhibit binding to the N-terminal peptide of syntaxin-1 but have only small effects on binding, trafficking and secretion are indicated in dark red (F115E and E132A). Right panels show blow-ups of regions of interest in ribbon and surface representations, with contacting syntaxin side-chains labeled. Figure generated with CCP4MG (Potterton et al., 2004).
The essential function of neuronal Munc18-1 and its orthologues in exocytosis from neurons and neuroendocrine cells has been supported through the analysis of null mutants. In these mutants, the synaptic neurotransmitter release was completely blocked in mice (Verhage et al., 2000) or reduced by ∼85% in Caenorhabditis elegans and Drosophila (Hosono et al., 1992; Harrison et al., 1994). Dense-core vesicle exocytosis from Munc18-1–deficient chromaffin cells in mice was also severely affected. However, a low albeit significant level of exocytosis remained unaffected in these Munc18-1 null neurosecretory cells (∼10–15% of the wild type) (Voets et al., 2001), suggesting the possible contribution of another isoform of Munc18 in these cells. Knockout of Munc18-3 leads to embryonic lethality (Kanda et al., 2005; Oh et al., 2005). The analysis of heterozygous Munc18-3-knockout mice uncovered impaired insulin sensitivity in an insulin tolerance test as well as defects in skeletal muscle insulin-stimulated glucose transporter (GLUT)4 translocation. Glucose-stimulated insulin secretion from islets isolated from the heterozygous mice was also significantly compromised indicative of a critical role for Munc18-3 in GLUT4 translocation in skeletal muscles and insulin secretion from pancreatic β cells (Oh et al., 2005). Unlike Munc18-1 and -3, the physiological function of Munc18-2 by using knockouts has not yet been addressed, but it has been suggested that Munc18-2 plays regulatory roles in exocytosis in platelets (Houng et al., 2003; Schraw et al., 2003) and in mast cells (Tadokoro et al., 2007) as well as in apical membrane trafficking in epithelial cells (Riento et al., 2000).
Although there is compelling evidence that Munc18-1 plays a critical role in neurotransmitter exocytosis from synaptic vesicles in neurons and from dense-core vesicles in neuroendocrine cells, the precise modality of Munc18-1 contribution to exocytosis is still poorly understood. At least three important functions of Munc18-1 have been proposed: 1) molecular chaperone of syntaxin-1 for proper localization of syntaxin-1 (Rowe et al., 1999; 2001; Medine et al., 2007; Arunachalam et al., 2008; McEwen and Kaplan, 2008); 2) priming/stimulation of the SNARE-mediated membrane fusion (Shen et al., 2007, Tareste et al., 2008; Rodkey et al., 2008; Südhof and Rothman, 2009); and 3) docking of large dense-core vesicles to the plasma membrane (Voets et al., 2001; Toonen et al., 2006).
The hypothesis that Munc18-1 plays a role in syntaxin-1 trafficking originated from transfection experiments in heterologous cells; when exogenous syntaxin1 was transfected into fibroblasts, it remained stuck in the Golgi and/or the endoplasmic reticulum (ER). However, when Munc18-1 was cotransfected, syntaxin-1 was appropriately distributed to the plasma membrane, suggesting a direct role of Munc18-1 in syntaxin-1 transport and delivery to the plasma membrane (Rowe et al., 1999, 2001; Rickman et al., 2007; Medine et al., 2007). Munc18-1 was hypothesized to prevent the formation of inappropriate, ectopic SNARE complexes (Medine et al., 2007). This hypothesis was recently supported by loss-of-function studies of endogenous Munc18-1 in PC12 cells (Arunachalam et al., 2008) and of Unc-18 in C. elegans neurons (McEwen and Kaplan, 2008). In Munc18-1 knockdown PC12 cells, syntaxin-1, but not the other target membrane (t)-SNARE, synaptosomal-associated protein (SNAP)-25, is mislocalized and concentrated in the perinuclear region. Syntaxin-1 mislocalization was rescued upon reexpression of wild-type Munc18-1 (Arunachalam et al., 2008). Anterograde transport of syntaxin homologue (Unc-64) was severely disrupted in unc-18 mutants in C. elegans (McEwen and Kaplan, 2008). In addition, the expression level of syntaxin-1 strikingly decreased in Munc18-1-deficicient neurons and neuroendocrine cells (Verhage et al., 2000; Voets et al., 2001; Toonen et al., 2005). Similarly, knockout of Munc18-3 resulted in a strong reduction in syntaxin-4 expression but not of SNAP-23 or synaptobrevin-2 (Kanda et al., 2005). Thus, one of the functions of Munc18 seems to be to stabilize syntaxin-1 and -4 and to specifically localize these t-SNARE proteins to the plasma membrane. Munc18's chaperone function seems to involve binding to the closed conformation of syntaxin because in the presence of Munc18-1, “open” conformation of syntaxin-1, but not N-terminally truncated syntaxin-1, was shown to be misplaced even in the presence of Munc18-1 in fibroblast cells (Medine et al., 2007). We also showed that syntaxin-1 is properly rescued at the plasma membrane with the Munc18-1(F115E/E132A) mutant, which abolishes binding to the N terminus of syntaxin-1, in Munc18-1 single knockdown cells (Malintan et al., 2009).
Munc18-1 as a priming factor/stimulator of SNARE-mediated membrane fusion/exocytosis has recently become a central hypothesis for the function of this protein (Shen et al., 2007; Südhof and Rothman, 2009). This exciting new hypothesis originated from liposome fusion assays in vitro in which Munc18-1 can enhance SNARE-pin–mediated liposome fusion (Shen et al., 2007) and the fusion between large vesicles and giant membranes (Tareste et al., 2008). Importantly, this enhancement was abolished when the N terminus of syntaxin-1 was deleted or mutated, suggesting that the interaction between N terminus of syntaxin-1 and Munc18-1 is critical for this effect. The residues in the hydrophobic pocket of Munc18-3 that physically bind to syntaxin-4 N-terminal peptide have been defined by crystallography (Hu et al., 2007) and a similar interaction was described for the interaction between Munc18-1 and syntaxin-1 N terminus (Burkhardt et al., 2008). The rescue experiments using unc-18 mutants in C. elegans suggest a critical function for the interaction between Unc18 and the N terminus of syntaxin homologue (Unc-64), whereas Unc18's binding to closed syntaxin seems dispensable (Johnson et al., 2009). However, it is unclear whether the Unc-18 mutant tested really disrupted binding to the closed conformation of Unc-64. In addition, our recent study showed that mutations abrogating the interaction between Munc18-1 and syntaxin-1 N terminus have a limited impact on Ca2+-dependent exocytosis in rescuing Munc18-1 knockdown PC12 cells (Malintan et al., 2009).
To analyze the role for Munc18-1 in neuroendocrine PC12 cells, we previously generated stable Munc18-1 knockdown cells by using RNA interference and found that syntaxin-1 was severely mislocalized. Interestingly, in our Munc18-1 knockdown cells, Munc18-2 seemed to be up-regulated (Arunachalam et al., 2008). We hypothesized that Munc18-2 supports the remaining secretion in the Munc18-1-deficient chromaffin cells and PC12 cells. To study the function of Munc18-1/2 as a whole, we have now generated stable Munc18-1/-2 double knockdown PC12 cells. In these cells, we show here that the expression of not only syntaxin-1 (A and B) but also of syntaxin-2 and -3 is significantly reduced. Also, syntaxin-1 is severely mislocalized and the regulated secretion is completely abolished in these double knockdown cells. These unique cell lines allow us to dissect in detail the structure/function relationship of Munc18-1 in the context of mammalian neurosecretory cells. Through the rescue of these cells with a series of Munc18-1 mutants, we demonstrate the essential function of Munc18-1's binding to the closed conformation of syntaxin-1 whereas Munc18-1's binding to the N terminus syntaxin-1 is not as critical as previously suggested. We also show a striking correlation among Munc18-1's abilities to up-regulate syntaxin-1 expression, to localize syntaxin-1 to the plasma membrane, and to restore dense-core vesicle secretion.
MATERIALS AND METHODS
General Materials
Parental pLKO plasmid for lentivirus-mediated knockdown was purchased from Sigma Chemical (Oakville, ON, Canada). pCMV8.74 and pMD2G to generate recombinant lentivirus were kind gifts from Dr. Tomoyuki Mashimo (University of Texas Southwestern Medical Center at Dallas, Dallas, TX). We obtained monoclonal antibodies against syntaxin-1A and -1B (clone HPC-1) (Barnstable et al., 1985) from Sigma Chemical; Munc18-1 from BD Biosciences (Mississauga, ON, Canada); SNAP-25 (clone SMI 81) from Covance Research Products (Princeton, NJ); and polyclonal antibodies against syntaxin-2 and -3 from Synaptic Systems (Gottingen, Germany). Rabbit anti-Munc18-2 antibody (Riento et al., 1998) and anti-VCP/p97 antibody (Sugita and Südhof, 2000) were kind gifts from Dr. Vesa Olkkonen (National Public Health Institute, Helsinki, Finland) and Dr. Thomas Südhof (University of Texas Southwestern Medical Center at Dallas), respectively.
Construction of Munc18-2 Knockdown Plasmids
Because our stable Munc18-1 knockdown (KD43, KD39) and control (C1, C14) cells were already puromycin resistant (Arunachalam et al., 2008), we replaced the puro gene in the parental knockdown plasmid pLKO-puro with a neo gene, generating pLKO-neo. This plasmid allowed us to isolate stable Munc18-2 knockdown cells with G418 (0.7 mg/ml). To knockdown the rat Munc18-2 gene, we targeted the 21-nucleotide sequence of GGGCATCACCATTGTGGAAGA (residues 165–185) in rat Munc18-2. We used CTCGAG as a linker sequence. Fifty-eight base-pair oligonucleotides containing sense and antisense of the target sequences were annealed and subcloned into the AgeI-EcoRI sites of pLKO-neo, generating the Munc18-2 knockdown plasmid (pLKO-Munc18-2-1). Inserted sequences were verified by sequencing.
Isolation of Stable Munc18-2 Knockdown and Munc18-1/-2 Double Knockdown PC12 Cells
Munc18-1 knockdown and control cells were maintained in DMEM (Invitrogen, Carlsbad, CA) containing 5% calf serum, 5% horse serum (both from HyClone Laboratories, Logan, UT), penicillin (100 U/ml)/streptomycin (0.1 mg/ml) (Sigma Chemical) (Wang et al., 2004, 2005; Fujita et al., 2007; Li et al., 2007; Arunachalam et al., 2008), and, in some cases, 250 ng/ml amphotericin B (Sigma Chemical). We generated recombinant lentivirus by cotransfecting each of pLKO-neo (control, 9 μg) or pLKO-rMunc18-2-1 (9 μg) with two other plasmids (pMD2G, 3 μg; pCMV-dR8.74, 4.8 μg) into human embryonic kidney (HEK)-293FT (Invitrogen, CA) cells by using 40 μl of polyethylenimine (1.2 mg/ml; pH 7.2). These recombinant lentiviruses were applied to the control (C1 and C14) and the Munc18-1 knockdown cells (KD43 and KD39), and the infected host cells were isolated with G418 (0.7 mg/ml). For each recombinant virus, a pool of heterogeneous cells that had survived in G418-containing medium over the period of 1 mo was subjected to immunoblot analysis using anti-Munc18-1 and -2 antibodies. We found that the recombinant virus encoding pLKO-Munc18-2-1 effectively silenced Munc18-2. From the pool of heterologous cells, we have further isolated several independent clones in which the silencing of Munc18-2 is particularly strong. These isolated cells were grown, frozen and kept in a liquid nitrogen tank until use. Control clones were similarly isolated from a heterologous pool of C1 cells infected with recombinant lentiviruses generated from pLKO-neo plasmid.
Lentivirus-mediated Munc18-1 Expression Constructs
We have generated the lentivirus-mediated expression constructs of various Munc18-1 mutants so that these proteins stably express in the Munc18-1/-2 double knockdown cells. The parental expression plasmid was developed by modifying pLKO-puro. First, the puro gene was replaced by a blast gene so that we could select the infected cells with blasticidin. Second, the cytomegalovirus (CMV) promoter followed by multiple cloning site from pCMV5 was subcloned into the AgeI/EcoRI site just downstream of U6 promoter of pLKO-blast, generating pLCMV-blast. Third, cDNA sequence for emerald green fluorescent protein (EmGFP) was sucloned into BamHI site, generating pLCMV-EmGFP-blast. In our previous article (Arunachalam et al., 2008), we introduced six silent nucleotide mutations (SNMs) (GTCCGTGCACAGCCTGATC, underlines indicate SNM) within the target sequence in the Munc18-1 gene to protect the mRNA transcripts transcribed from the Munc18-1 expression plasmid [=pCMV-Munc18-1(SNM)] from being degraded by the anti-Munc18-1 RNA interference machineries already induced within the Munc18-1 knockdown cells. The Munc18-1(SNM) gene digested from pCMV-Munc18-1(SNM) was subcloned into EcoRI/XbaI site of pLCMV-EmGFP-blast plasmid. This Munc18-1 expression plasmid was cotransfected with pCMV8.74 and pMD2G into HEK-293FT cells to generate recombinant lentiviruses that express Munc18-1 fused with EmGFP. The Munc18-1/-2 double knockdown cells that were infected with lentiviruses expressing rescue proteins were selected with blasticidin (5 μg/ml). In some cases, the cells that express Munc18-1 proteins fused with GFP relatively strongly were further enriched using fluorescence activated cell sorting (FACS) facility in our institution.
[3H]Norepinephrine (NE) Release Assays from PC12 Cells
PC12 cells were plated in 24-well plates; 3–4 d after plating, the cells were labeled with 0.5 μCi of [3H]NE in the presence of 0.5 mM of ascorbic acid for 12–16 h. The labeled PC12 cells were incubated with the fresh complete DMEM for 1–5 h to remove unincorporated [3H]NE. The cells were washed once with physiological saline solution (PSS) containing 145 mM NaCl, 5.6 mM KCl, 2.2 mM CaCl2, 0.5 mM MgCl2, 5.6 mM glucose, and 15 mM HEPES, pH 7.4, and NE secretion was stimulated with 200 μl of PSS or high K+-PSS (containing 81 mM NaCl and 70 mM KCl). Secretion was terminated after a 15-min incubation at 37°C by chilling to 0°C, and samples were centrifuged at 4°C for 3 min. Supernatants were removed, and the pellets were solubilized in 0.1% Triton X-100 for liquid scintillation counting.
Cell Preparation for Confocal Immunofluorescence Microscopy
Sterilized circular glass coverslips (0.25 mm in width, 1.8 cm in diameter) were placed in 2.2-cm wells within 12-well cell culture plates. The coverslips were then coated for 1 h with poly-d-lysine (0.1 mg/ml) at room temperature. Cells were allowed to adhere to the cover slips overnight and then differentiated on the cover slips for 3–4 d in DMEM that contained 100 ng/ml nerve growth factor (NGF) (Sigma Chemical), 1% horse serum, 1% calf serum, and penicillin/streptomycin. The cells were washed with phosphate-buffered saline (PBS), fixed for 15 min with PBS containing 4% paraformaldehyde, and permeabilized with PBS containing 0.2% Triton X-100 and 0.3% bovine serum albumin (BSA) for 5 min. Nonspecific sites were blocked for 1 h at room temperature in PBS containing 0.3% BSA. Primary antibodies against syntaxin-1 (HPC-1 diluted 1:1000). After three washes in blocking buffer, red-x-conjugated anti-mouse antibodies (diluted 1:1000) (Jackson ImmunoResearch Laboratories, West Grove, PA) were diluted in blocking buffer and applied for 1 h at room temperature. Samples were washed again three times in blocking buffer and mounted in Fluoromount-G reagent (Southern Biotechnology, Birmingham, AL). Immunofluorescence staining was recorded with a laser confocal scanning microscope (LSM 510; Carl Zeiss, Jena, Germany) with an oil immersion objective lens (63×).
Yeast Two-Hybrid Assays
Full-length wild-type Munc18-1 with SNM (see above) or indicated mutant Munc18-1(SNM) was subcloned into SmaI-PstI site of a bait vector, pLexN. A cytoplasmic domain (residues 1–261) of rat syntaxin-1A and residues 129–451 of rat mint-1, which contains a Munc18-1 interacting domain (residues 226–314) (Okamoto and Südhof, 1997), were subcloned into EcoRI-BglII site of a prey vector, pVP16-3 (Okamoto and Südhof, 1997). Yeast strain L40 (Vojtek et al., 1993) was transfected with bait and prey vectors by using the lithium acetate method (Schiestl and Gietz, 1989). Transformants were plated on selection plates lacking uracil, tryptophan, and leucine. After 2 d of incubation at 30°C, colonies were inoculated into supplemented minimal medium lacking uracil, tryptophan, and leucine and placed in a shaking incubator at 30°C for 2 d.
β-Galactosidase assays were performed as follows. Yeast cells were chilled on ice and harvested by centrifugation (2000 rpm for 5 min). The collected yeast cells were resuspended in 250 μl of breaking buffer (100 mM Tris-Cl, pH 8.0, 1 mM dithiothreitol [DTT], and 20% glycerol). Next, glass beads (0.45–0.5 mm; Sigma Chemical) were added to the yeast suspension to a level just below the meniscus of the liquid, together with 12.5 μl of phenylmethylsulfonyl fluoride stock solution (40 mM in 100% isopropanol stored at −20°C). The mixture was then vortexed six times at top speed in 15-s bursts, with chilling on ice between bursts. After that, another 250 μl of breaking buffer was added, mixed well, and the liquid extract was withdrawn and transferred to new tubes. The extracted liquid was later on clarified by centrifuging for 15 min in a microfuge. To perform the assay, 80 μl of the extract was added to 720 μl of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 2.7 ml/l β-mercaptoethanol, pH 7.0). The mixture was then incubated in a water bath at 28°C for 5 min. The reaction was initiated by adding 0.16 ml of stock solution (4 mg/ml o-nitrophenyl-β-d-galactoside in Z buffer; −20°C), and the reaction mixture was incubated at 28°C. The reaction was precisely terminated at the end of 7-min incubation by addition of 0.4 ml of 1 M Na2CO3 stock solution in distilled water, and the optical density of the reaction mixture was measured at 420 nm by using a spectrophotometer. At the same time, the protein concentration in the extract was measured using Bradford dye-binding assay. A standard curve was prepared using serial dilutions of BSA dissolved in breaking buffer. Ten microliters of the extract was added to 1 ml of the Bradford reagent (Bio-Rad Laboratories, Hercules, CA), and the blue color formed was measured at 595 nm by using a spectrophotometer. The specific activity of β-galactosidase in the extract was calculated according to the following formula: (OD420 × 1.36)/[0.0045 × protein concentration [mg/ml] × extract volume [0.08 ml] × 7 min], where OD420 is the optical density of the product o-nitrophenol at 420 nm. The factor 1.36 corrects for the reaction volume, and the factor 0.0045 is the optical density of a 1 nmol/ml solution of o-nitrophenol. The unit of β-galactosidase–specific activity is therefore expressed as nanomoles per minute per milligram of protein.
Isothermal Titration Calorimetry (ITC)
The syntaxin-1A (residues 2–243) and Munc18-1 (wild type [WT] and F115E or E132A mutant) were prepared as described in Malintan et al. (2009). The proteins were further purified for the ITC experiment by gel filtration into 20 mM sodium phosphate buffer, pH 7.5, 150 mM NaCl, and 1 mM DTT (ITC buffer). Isothermal titration calorimetry was done at 298 K by using a MicroCal iTC200 (GE Healthcare, Chalfont St. Giles, Buckinghamshire, United Kingdom), with 13 × 3.1 μl injections of 30 μM syntaxin-1A(2-243) into 3 μM Munc18-1 proteins (WT and F115E or E132A mutant). ITC data were analyzed using Origin software (OriginLab, Northampton, MA) to obtain the thermodynamic parameters, stoichiometry N, equilibrium association constant Ka (= Kd−1) and the binding enthalpy ΔH. The Gibbs free energy ΔG and entropy ΔS of the interactions were determined from the equations ΔG = −RTln(Ka) and ΔG = ΔH − TΔS. The thermodynamic parameters for the syntaxin1A-Munc18-1 interaction were expressed as mean ± SEM, calculated from six independent Munc18-1-WT experiments and three independent mutant experiments.
hPLAP Secretion Assay from PC12 Cells
PC12 cells at 70–80% confluence in 10-cm dishes were cotransfected with 3 μg of a reporter plasmid, pCMV-neuropeptide Y(NPY)-hPLAP (Li et al., 2007; Fujita et al., 2007; Arunachalam et al., 2008), and 10 μg of empty pCMV5 (for control) or pCMV-Munc18-1(SNM) (for rescue) by using electroporation. After 48 h, the cells were harvested and replated in 24-well plates. Six or 7 d after electroporation, the plated cells were washed once with PSS, and NPY-hPLAP secretion was stimulated with 200 μl of PSS or high K+-PSS. Secretion was terminated after a 25-min incubation at 37°C by chilling to 0°C, and samples were centrifuged at 4°C for 3 min. Supernatants were removed, and the pellets were solubilized in 200 μl of PSS containing 0.1% Triton X-100. The amounts of NPY-hPLAP secreted into the medium and retained in the cells were measured by the Phospha-Light Reporter Gene Assay System (Applied Biosystems, Foster City, CA). We treated the samples at 65°C for 30 min to inactivate nonplacental alkaline phosphatases and assayed an aliquot (10 μl) for placental alkaline phosphatase activity with the kit. The total volume of the assay was 120 μl. After 5–10 min, chemiluminescence was quantified by FB12 luminometer (Berthold Detection Systems, Zylux Corporation, Oak Ridge, TN).
RESULTS
Diminished Regulated Secretion in Stable Munc18-1 and -2 Double Knockdown Cells
In contrast to Munc18-1–deficient neurons in which neurotransmitter release is abolished (Verhage et al., 2000), dense-core vesicle secretion from Munc18-1–deficient adrenal chromaffin cells is reduced by 85–90% (Voets et al., 2001). In Munc18-1 knockdown PC12 cells, secretion is reduced by 45–70% (Arunachalam et al., 2008). The partial secretion defects can be explained by the presence of Munc18 isoforms. Whereas Munc18-1 is the sole isoform expressed in neurons, both Munc18-1 and Munc18-2 are expressed in PC12 cells and adrenal medullary cells (Figure 1A). In addition, up-regulation of Munc18-2 was found in our Munc18-1 knockdown PC12 cells (Arunachalam et al., 2008). These findings led us to a hypothesis that Munc18-2 supports the remaining secretion in the Munc18-1–deficient chromaffin cells and PC12 cells (Voets et al., 2001; Arunachalam et al., 2008). We therefore aimed to establish PC12 cell lines in which Munc18-1 and/or Munc18-2 are stably silenced to determine the precise functions of Munc18-1 and -2.
Figure 1.
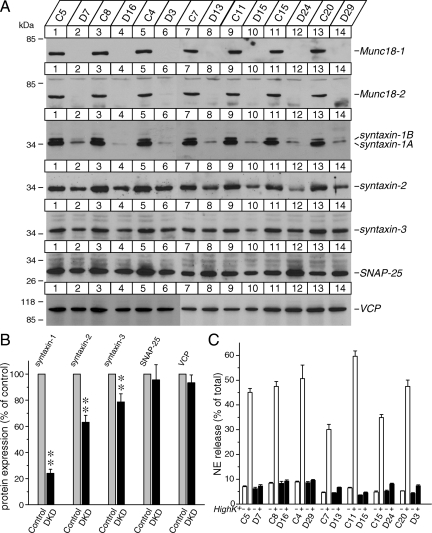
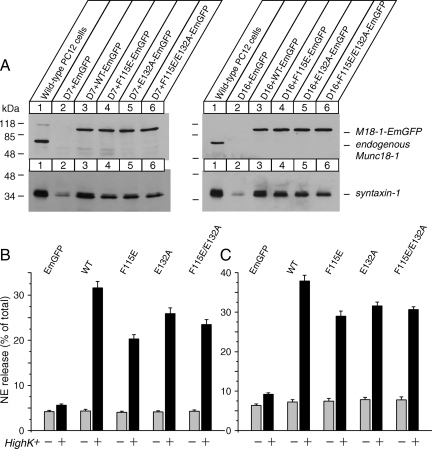
Down-regulation of both Munc18-1 and Munc18-2 results in dramatic reductions in syntaxin-1 expression and NE secretion. (A) Expression of Munc18-2 in rat adrenal glands and wild-type PC12 cells. Twenty micrograms of total homogenates from the rat adrenal glands and wild-type PC12 cells was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting using anti-Munc18-2 antibodies. The signal was detected with enhanced chemiluminescence detection system. A number on the left indicates the position of a molecular weight marker. (B) Control (C1) and Munc18-1 knockdown (KD43) clones [isolated in Arunachalam et al. (2008)] were infected with lentiviruses generated from pLKO-neo (control) or pLKO-Munc18-2 knockdown plasmid. The infected cells were selected with neomycin (0.8 mg/ml). Thirty micrograms of total homogenates from heterologous pools of the surviving cells was analyzed by SDS-PAGE and immunoblotting using anti-Munc18-1 and -2 and syntaxin-1, -2, and -3 antibodies. (C) NE release was stimulated by 70 mM KCl for 15 min from heterologous pools of control, Munc18-1 single knockdown, Munc18-2 single knockdown, and Munc18-1/-2 double knockdown cells. Error bars indicate SEM (n = 15).
To generate stable Munc18-2 knockdown PC12 clones, we used the lentivirus-mediated infection system (see Materials and Methods). The recombinant lentiviruses to knockdown Munc18-2 were applied to two lines of the control (C1 and C14) and of the Munc18-1 knockdown cells (KD43 and KD39) (Arunachalam et al., 2008), and the infected host cells were isolated with G418. For each recombinant virus, a pool of heterogeneous cells that had survived in G418-containing medium over the period of 1 mo was subjected to immunoblot analysis using anti-Munc18-1 and -2 antibodies. We found that the recombinant virus encoding pLKO-Munc18-2 effectively silenced Munc18-2 in a heterologous pool of the cells (Figure 1B).
We then analyzed the secretory phenotype of the heterologous pool of the knockdown cells with a biochemical [3H]NE release assay. Secretion was triggered with 15-min incubation in PSS or high K+-PSS. High K+-PSS depolarizes the membrane potential of PC12 cells to induce the opening of voltage-sensitive Ca2+ channels, thereby evoking Ca2+-dependent exocytosis. Using this assay, we found that Munc18-1 knockdown resulted in a reduction of Ca2+-dependent neurotransmitter release by ∼65%, whereas Munc18-2 knockdown resulted in an inhibition by ∼20%. Importantly, the secretion from Munc18-1/-2 double knockdown cells was strikingly inhibited (by ∼90%) (Figure 1C). From the pool of heterologous cells, we have further isolated several independent clones in which the silencing of Munc18-2 is particularly strong along with control clones (Figure 2A). In these clonal double knockdown cells in which both Munc18-1 and -2 are strongly silenced, we found that high K+-mediated secretion is almost abolished (Figure 2C).
Figure 2.
Munc18-1/-2 double knockdown clones exhibit significant reductions in the expression of all the plasma membrane syntaxins (syntaxin-1, -2, and -3) and abolish regulated NE secretion. (A) Immunoblot analysis of isolated multiple clones in which both Munc18-1 and -2 are strongly down-regulated. Thirty micrograms of total homogenates from the Munc18-1/-2 double knockdown (D) and control (C) clones was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and immunoblotting using anti-Munc18-1 and -2; syntaxin-1, -2, and -3; SNAP-25; and VCP/p97 (as a loading control) antibodies. Signals were detected with enhanced chemiluminescence detection system. A number on the left indicates the position of a molecular weight marker. (B) Quantification of changes in protein expression of syntaxin-1, -2, and -3; SNAP-25; and VCP between control and Munc18-1/-2 double knockdown clones. Images of these proteins on the film were quantified using ImageJ. **p < 0.01), statistically significant difference. (C) NE release was stimulated by 70 mM KCl for 15 min. Seven pairs of control and double knockdown clones were examined. Error bars indicate SEM (n = 6–12).
Striking Reduction in Syntaxin-1 Expression and Significant Reductions in Syntaxin-2 and -3 Expression in Munc18-1/-2 Double-Knockdown PC12 Cells
We previously found a consistent, but relatively limited (∼15%) reduction in the expression of syntaxin-1 (A and B) in Munc18-1 knockdown cells (Arunachalam et al., 2008). Reduced expression of syntaxin-1 is more severe (∼50–70%) in Munc18-1–deficient neurons (Verhage et al., 2000). We hypothesize that the limited reduction in syntaxin-1 expression in our Munc18-1 knockdown cells was probably due to the presence of compensatory up-regulated Munc18-2 (Arunachalam et al., 2008). Therefore, we examined the expression levels of syntaxin-1 in heterologous pools of the control, Munc18-1 knockdown, Munc18-2 knockdown and Munc18-1/-2 double knockdown cells using immunoblot analysis (Figure 1B). As found previously, the expression level of syntaxin-1(A and B) was reduced by ∼15% in Munc18-1 knockdown cells, whereas it was unchanged in Munc18-2 knockdown cells. However, it was dramatically reduced (∼75%) in Munc18-1/-2 double-knockdown cells (Figure 1B).
In PC12 cells as well as adrenal chromaffin cells and neurons, other isoforms of syntaxin are present such as syntaxin-2 and -3. However, it was unknown whether the expression of these isoforms is also affected by the knockdown of Munc18-1 and/or -2. We found that syntaxin-2 expression was reduced by ∼50% in the Munc18-1/-2 double-knockdown cells whereas syntaxin-3 expression seemed to be almost unchanged (Figure 1B). These results strongly suggest that Munc18-1 and -2 act in concert to control the expression levels of not only syntaxin-1 but also of syntaxin-2.
Although changes in syntaxin-3 were not clearly detected in the heterologous pool of Munc18-1/-2 double knockdown cells, this may be due to the modest reductions in syntaxin-3 being overshadowed by the presence of incomplete Munc18-2 knockdown cells within the heterologous pool or that syntaxin-3 expression is not regulated by Munc18-1 and -2. To verify this, we have thus further examined the expression of syntaxin-1,-2, and -3 in isolated clones of strong Munc18-1/-2 double knockdown cells (Figure 2A). Quantification of the immunoblot signal from the paired control and double knockdown clones cells by using ImageJ software (National Institutes of Health, Bethesda, MD) showed significant reductions in all the isoforms (syntaxin-1: t6 = 17.6, p < 0.01; syntaxin-2: t6 = 7.21, p < 0.01; and syntaxin-3: t6 = 3.97, p < 0.01), although the level of reductions is different among the isoforms (Figure 2B). We also examined the other t-SNARE protein, SNAP-25 (Sollner et al., 1993), as well as a ubiquitous trafficking protein VCP/p97 (Peters et al., 1990). No changes were found in the expression of these proteins (SNAP-25: t6 = 0.69, n.s.; VCP: t6 = 1.28, n.s.) (Figure 2B). We conclude that Munc18-1 and -2 are critical in maintaining the expression levels of plasma membrane isoforms of syntaxin including synataxin-1(A/B), -2, and -3 but not the other t-SNARE, SNAP-25.
Severe Mislocalization of Syntaxin-1 in Munc18-1/-2 Double-Knockdown PC12 Cells
We showed previously that Munc18-1 is critical for plasma membrane localization of syntaxin-1 and attributed this mislocalization of syntaxin-1 to the loss-of-function of Munc18-1 (Arunachalam et al., 2008). However, this mislocalization might have been artificially caused by the action of up-regulated Munc18-2 in the absence of Munc18-1. Therefore, we have examined whether mislocalization of syntaxin-1 persisted after Munc18-2 was removed in addition to Munc18-1. To better visualize mislocalized syntaxin-1 in control and Munc18-1/-2 double knockdown PC12 cells, we differentiated PC12 cells by using NGF. The cell bodies of differentiated PC12 cells enlarge and become flatter, which allow us to better examine the localization of syntaxin-1. These cells were infected with EmGFP to visualize the whole cell shape. We found syntaxin-1 to be present at the plasma membrane in control cells (Figure 3A), whereas syntaxin-1 was accumulated at the perinuclear region in Munc18-1/-2 double knockdown (DKD) cells (Figure 3B). In contrast, SNAP-25 was present at the plasma membrane in the knockdown cells (Figure 3C). The selective defect in syntaxin-1 transport found in Munc18-1/-2 double knockdown cells prove that the mislocalization of syntaxin-1 in Munc18-1 knockdown cells observed in our previous study (Arunachalam et al., 2008) cannot be assigned to the compensatory up-regulation of Munc18-2. The degree of mislocalization of syntaxin-1 seemed to be more severe in the Munc18-1/-2 double knockdown cells compared with the Munc18-1 single knockdown cells (Arunachalam et al., 2008). However, quantitative comparison is technically difficult because the expression level of syntaxin-1 is different between Munc18-1 single knockdown cells and the double knockdown cells. We also aimed to examine the subcellular localization of syntaxin-2 and -3 in the double knockdown cells using commercially available anti-syntaxin-2 and -3 antibodies (SySy Systems, Goettingen, Germany) but found that these antibodies were not suitable for immunofluorescence detection due to the high level of background. Nonetheless, our results suggest that mislocalization of syntaxin-1 is clearly attributable to the combined loss-of-function of Munc18-1 and -2.
Figure 3.
Confocal immunofluorescence microscopy reveals dramatic mislocalization of syntaxin-1, but not SNAP-25, in Munc18-1/-2 double knockdown cells. Confocal images of NGF-differentiated control PC12 cells (A) and Munc18-1/-2 double knockdown (DKD, D7) cells (B and C) that were infected with EmGFP-expressing lentiviruses (middle) and stained with anti-syntaxin-1 (left, A and B) or SNAP-25 (left, C) monoclonal antibodies followed by Alexa 488-conjugated goat anti-mouse antibodies. Right panels are merged pictures. Bar, 10 μm.
Generation of a Munc18-1 Mutant (K46E/E59K) That Abolishes the Binding to the Closed Conformation of Syntaxin-1A
In previous sections, we generated stable Munc18-1/-2 double-knockdown cells and found that the expression of syntaxin-1/-2/-3 is reduced, syntaxin-1 is severely mislocalized and that the regulated secretion is abolished in these cells. Nonetheless, the double knockdown cells are healthy and their growth and division rate are comparable with that of control cells. Thus, these unique cell lines are ideal models to analyze the structure/function relationship of Munc18-1 in detail through rescue experiments.
We first examined the functional significance of the binding of Munc18-1 to the closed conformation of syntaxins involving the syntaxin Habc domain and H3 SNARE helix. The crystal structure of Munc18-1 bound to syntaxin-1 has been solved allowing for identification of key Munc18-1 residues governing this interaction (Misura et al., 2000; Burkhardt et al., 2008) (Figure 4). Some of the key residues specifically contacting closed syntaxin lie in domain 1 (largely formed by residues 38-71) and domain 3a (residues 271-280 and 331-338) of Munc18-1. By mutating these residues, we aimed to generate Munc18-1 mutants that specifically abolish the binding to the closed conformation of syntaxin-1 but not to the N terminus of syntaxin-1. We therefore generated three point mutants in domain 1 of Munc18-1: K46E, E59K, and K63E. Lys46 of Munc18-1 specifically contacts residues Asp231 and Arg232 in the H3 helix of syntaxin-1, Glu59 of Munc18-1 forms a buried salt-bridge with Arg114 within the H3 domain of syntaxin-1, and Lys63 packs against the syntaxin H3 helix. We also generated the R39C mutant, which is believed to at least partially lose binding to the closed conformation of syntaxin-1 (Johnson et al., 2009) (Figure 4). Arg39 forms an exposed salt-bridge with Glu234 within the syntaxin H3 domain.
To examine the binding between Munc18-1 mutants and the cytoplasmic domain of syntaxin-1, we performed the yeast two-hybrid assay (Hata and Südhof, 1995). This assay is an unbiased method that can examine many interactions simultaneously and provides more in vivo like environment for binding interaction compared with the biochemical binding experiments. We also examined the interaction between mint-1 (another Munc18-interacting protein) (Okamoto and Südhof, 1997) and the Munc18-1 mutants. Our rationale using mint-1 as reference was that if the interaction with syntaxin-1, but not with mint-1, is disrupted, we can conclude that this mutant is properly folded and specifically loses its ability to bind to syntaxin-1.
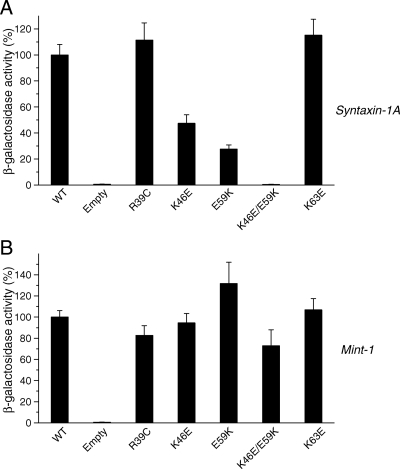
As expected, we found that K46E and E59K mutations reduced Munc18-1 binding to syntaxin-1 but not to mint-1, whereas K63E had no effect on binding to either syntaxin-1 or mint-1 (Figure 5, A and B). We then generated a double mutant, K46E/E59K, which showed abolishment of binding to syntaxin-1 (Figure 5A), while leaving that of mint-1 unaffected (Figure 5B). These results strongly suggest that K46E/E59K selectively loses the ability to bind to syntaxin-1. The Munc18-1 K46E/E59K double mutant should still be able to bind to the N terminus syntaxin-1 and thus to the SNARE complex because the mutated residues are distinct from the surface of Munc18-1 required for binding to the N-terminal peptide of syntaxin-1 (Figure 4). As shown below, the mutations in the residues of this surface of Munc18-1 required for binding to the N terminus resulted in reductions in the binding to mint-1 (see Figure 8). These results suggest that the nature of K46E/E59K mutation is distinct from that of the residues involved in syntaxin-1 N terminus binding.
Figure 5.
Mutations in domain 1 of Munc18-1 result in dramatic reductions in the binding to syntaxin-1 but not to mint-1. Mutations were introduced in the residues in domain 1 of Munc18-1 that are involved in the binding to the ‘closed’ conformation of syntaxin-1 (Misura et al., 2000). The binding between these Munc18-1 mutants and syntaxin-1A or mint-1 was analyzed with yeast two-hybrid assays. In each assay, β-galactosidase activities of the transformed yeast clones were quantified (see Materials and Methods) and normalized so that the activity of the yeast clones transformed with the wild-type Munc18-1 was set to 100%. Error bars indicate SEM (n = 12–14 for binding to syntaxin-1; n = 8–12 for binding to mint-1). Note that K46E/E59K mutation in Munc18-1 abolished the binding to syntaxin-1 but not to mint-1.
Figure 8.
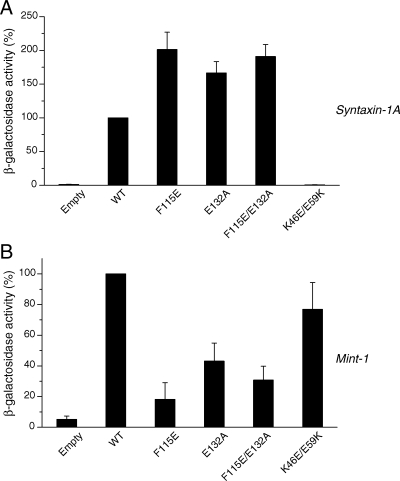
Mutations in the hydrophobic pocket of Munc18-1 result in reductions in the binding to mint-1, but not to syntaxin-1A. Mutations (F115E, E132A, and F115E/E132A) were introduced in the hydrophobic pocket of Munc18-1 that is involved in the binding to N-terminus of syntaxin-1. The binding of these mutants to syntaxin-1A (A) and mint-1 (B) was analyzed by yeast-two hybrid assays. In each assay, β-galactosidase activities of the yeast clones were quantified and normalized so that the activity of the clones transformed with the wild-type Munc18-1 was set to 100%. Error bars indicate SEM (n = 9).
Unlike K46E and E59K, R39C mutant showed a similar level of binding to both syntaxin-1 and mint-1. Our results strongly suggest that R39C mutation does not significantly affect the binding properties of Munc18-1.
K46E/E59K Mutant Was Unable to Restore Syntaxin-1 Expression and Localization in Munc18-1/-2 Double Knockdown Cells
We then tested the ability of the wild-type and K46E/E59K mutant of Munc18-1 to rescue various phenotypes in Munc18-1/-2 double knockdown cells including reduced syntaxin-1 expression, syntaxin-1 mislocalization, and secretion defects. We expressed these proteins fused with EmGFP (Munc18-1-EmGFP) by using the lentivirus system in the Munc18-1/-2 double-knockdown PC12 cells (D7 and D16). The expression plasmid contains the blasticidin resistance gene to enable us to select infected cells. The recombinant virus was applied to D7 and D16 cells, and the infected cells were isolated using blasticidin. Using FACS, we further enriched green fluorescent protein (GFP)-positive cells. For the wild-type Munc18-1-EmGFP, we separated two populations of cells in which the GFP level differs (high and low, Figure 6A). The expression level of Munc18-1-EmGFP proteins was verified by immunoblot analysis using anti-Munc18-1 antibody.
Figure 6.
Stable reexpression of wild-type Munc18-1, but not K46E/E59K mutant, restores syntaxin-1 expression and NE-secretion in Munc18-1/-2 double knockdown clones (D7 and D16). (A) Munc18-1/-2 double knockdown clones (D7 and D16) were infected with lentiviruses that express EmGFP, wild-type Munc18-1-EmGFP or mutant (K46E/E59K) Munc18-1-EmGFP, and the infected cells were selected with blasticidin. The surviving cells were further enriched by a FACS using the GFP signal. For the wild-type Munc18-1-EmGFP, we separated two populations of cells in which the GFP level differs (high vs. low). Thirty micrograms of total homogenates from heterologous pools of the enriched cells was analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotting using anti-Munc18-1 and syntaxin-1 antibodies. Signals were detected with enhanced chemiluminescence detection system. A number on the left indicates the position of a molecular weight marker. (B and C) NE release was stimulated by 70 mM KCl for 15 min in the rescued cells (B for D7 clones; C for D16 clones). Error bars indicate SEM (n = 15).
In D7 cells, differences in the expression level (high vs. low) of the wild-type Munc18-1-EmGFP within the FACS-sorted cells disappeared during the recovery and growth period, whereas the differences persisted in D16 cells (Figure 6A). We also found that K46E/E59K mutant fused with EmGFP express at a similar level to the endogenous Munc18-1 and the exogenous wild-type Munc18-1 fused with EmGFP (Figure 6A). Importantly, the exogenous expression of wild-type Munc18-1-EmGFP, but not K46E/E59K-EmGFP, resulted in a dramatic recovery of syntaxin-1 expression (Figure 6A).
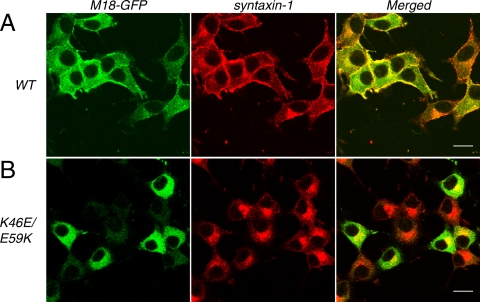
We then examined whether wild-type and K46E/E59K mutant are able to rescue syntaxin-1 plasma membrane localization in the double knockdown cells (Figure 7). We found that the plasma membrane localization of syntaxin-1 was recovered by the reexpression of the wild-type Munc18-1-EmGFP, whereas syntaxin-1 remained in the perinuclear region of the cells upon expression of Munc18-1 K46E/E59K-EmGFP mutant (Figure 7, A and B). These results indicate that K46E/E59K loses the ability to support the expression and plasma membrane localization of syntaxin-1, demonstrating the essential role for these domain 1 residues critical for binding to the closed conformation of syntaxin-1. We also found intriguing differences in the localization between the exogenously expressed wild-type and K46E/E59K Munc18-1-EmGFP. The wild-type Munc18-1-EmGFP showed a strong enrichment at the plasma membrane region, whereas K46E/E59K Munc18-1-EmGFP showed a broad cytoplasmic distribution (Figure 7B). Considering that Munc18-1 is a cytosolic protein, the enrichment of the wild-type Munc18-1-EmGFP is probably due to the interaction with syntaxin-1 that was correctly trafficked to the plasma membrane.
Figure 7.
Rescue of syntaxin-1 localization upon reintroduction of the wild-type Munc18-1-EmGFP, but not of K46E/E59K Munc18-1-EmGFP, into Munc18-1/-2 double knockdown clones. Confocal images of D7 cells infected with lentiviruses that express either wild-type Munc18-1-EmGFP (left, top) or K46E/E59K Munc18-1-EmGFP (left, bottom) and stained with anti-syntaxin-1 antibodies followed by red-x-conjugated anti-mouse antibodies (middle). Right panels are merged pictures. Bar, 10 μm.
K46E/E59K Mutant Was Unable to Restore Dense-Core Vesicle Secretion in Munc18-1/-2 Double Knockdown Cells
We then examined whether wild-type and K46E/E59K mutant of Munc18-1 can restore regulated secretion of Munc18-1/-2 double knockdown cells. We found robust recovery of secretion with the wild-type Munc18-1-EmGFP. In D16 cells, different (high vs. low) levels of the wild-type Munc18-1-EmGFP rescued secretion defects similarly, indicating that their rescue effects were saturating (Figure 6, B and C). In contrast, K46E/E59K-EmGFP did not rescue secretion defects, almost similar to EmGFP alone (Figure 6, B and C). These results show that Munc18-1's binding to the closed conformation of syntaxin-1 is crucial for its secretory functions in addition to its chaperone functions for syntaxins. Because K46E/E59K is unable to restore syntaxin-1 expression and plasma membrane localization, we suggest that the inability of K46E/E59K mutant to restore secretion can be attributable at least in part to reduced expression and mislocalization of syntaxin.
F115E, E132A, and F115E/E132A Munc18-1 Mutants Show Reduced Binding to mint-1, but Not to Syntaxin-1A
We next examined functional roles for Munc18-1's binding to syntaxin-1's N terminus. The residues in Munc18-3 that are involved in binding to the N terminus of syntaxin-4 have been identified through crystallography (Hu et al., 2007), as have the analogous residues of Munc18-1 for binding to the syntaxin-1 N terminus (Burkhardt et al., 2008; Malintan et al., 2009). These residues are located in a relatively hydrophobic pocket of Munc18-1 (Figure 4). We used the mutants of Munc18-1 in which the critical residues for binding to the N-terminal syntaxin-1 were mutated (Malintan et al., 2009). These were F115E, E132A, and F115E/E132A (double mutation), which dramatically reduced the ability of Munc18-1 to interact with syntaxin-1 within the SNARE complex but had only a modest effect on binding to the free syntaxin-1 protein (Malintan et al., 2009).
We first characterized binding characteristics of these mutants to syntaxin-1 and mint-1 using yeast two-hybrid assays (Figure 8). We found that all the mutants clearly retain binding to syntaxin-1 (Figure 8A). This is in line with our previous ITC results (Malintan et al., 2009) and with other studies using truncated syntaxin-1 lacking the N-terminal peptide (Burkhardt et al., 2008), which showed that the dominant contribution to binding of free syntaxin-1 comes from the association of syntaxin-1 Habc and H3 domains, not the N-terminal peptide. In fact, Burkhardt et al. (2008) could not detect the measurable affinity between syntaxin-1A N-terminal peptide alone (residues 1-20) and Munc18-1 by using ITC (see table 1 in Burkhardt et al., 2008). As a consequence, Malintan et al. (2009) measured the affinity between a cytoplasmic domain of syntaxin-1A (residues 2–243) and Munc18-1 wild-type or F115E/E132A mutant using ITC and found that the dissociation constant of the syntaxin-1A binding affinity to the F115E/E132A mutant was not significantly changed compared with that of the wild-type. However, the binding enthalpy (ΔH) was significantly altered by the mutations, with −19.98 ± 0.88 kCal/mol for the wild type and −14.17 ± 0.17 kCal/mol for the F115E/E132A (Malintan et al., 2009). Here, we performed additional ITC experiments that examine the affinity between a cytoplasmic domain of syntaxin-1A and F115E and E132A single mutants (Supplemental Figure 1). Similar to F115E/E132A mutant, we found significant reductions in the binding enthalpy (ΔH) for each mutant (−20.6 ± 1.05 kCal/mol for the wild-type, −14.3 ± 2.01 kCal/mol for F115E, and −15.5 ± 1.40 for F115E for E132A; p < 0.05, n = 6), whereas the dissociation constant (Kd) was not significantly changed for these mutants (Table 1). Thermodynamically the binding of Munc18-1 mutants was characterized by a subsequently less unfavorable entropic contribution (TΔS in Table 1). A possible explanation for the decreased enthalpic and increased entropic contributions to binding is that the overall binding surface area is reduced when the syntaxin-1A N-terminal peptide is unable to bind, but there is a smaller entropic penalty incurred by the folding of the unstructured syntaxin-1A N-terminal region upon binding. Together, these results indicate that mutations in the Munc18-1 hydrophobic pocket, predicted to mediate binding to the syntaxin-1A N-terminal peptide, affect the thermodynamics and kinetic properties of association without causing a major change in the equilibrium binding affinity. However, it is important to point out that, in the context of the full SNARE complex, the mutant Munc18-1 had almost abrogated binding as expected when the N-peptide makes a relatively greater contribution to the binding interface (Malintan et al., 2009).
Table 1.
Thermodynamic parameters for the binding of syntaxin-1A (Sx1a, residues 2-243) to Munc18-1 determined by ITC
| Proteins interaction | ΔH (kCal mol−1) | TΔS (kCal mol−1) | ΔG (kCal mol−1) | Kd (nM) | n |
|---|---|---|---|---|---|
| Munc18-1-WT:Sx1a2-243 | −20.6 ± 1.05 | −10.1 ± 1.08 | −10.5 ± 0.10 | 22.8 ± 3.94 | 1.04 ± 0.02 |
| Munc18-1-F115E:Sx1a2-243 | −14.3 ± 2.01* | −3.86 ± 2.01* | −10.4 ± 0.04 | 23.1 ± 1.42 | 0.96 ± 0.03 |
| Munc18-1-E132A:Sx1a2-243 | −15.5 ± 1.40* | −5.31 ± 1.41* | −10.1 ± 0.03* | 35.8 ± 1.20 | 1.05 ± 0.02 |
Values are expressed as means ± SEM (Munc18-1-WT, n = 6; Munc18-1-F115E or Munc18-1-E132A, n = 3). All experiments were performed at 298 K.
Significant at *p < 0.05.
Unexpectedly, we found that all of the mutants (F115E, E132A, and F115E/E132A) exhibited significant reductions in binding to mint-1 in yeast two-hybrid assays (Figure 8B). These results imply that the residues/domains of Munc18-1 for binding to the N terminus syntaxin-1 and mint-1 might overlap. This also suggests the intriguing possibility that competitive binding of mint-1 may be able to regulate association of Munc18-1 with the SNARE complex, where interaction with the syntaxin-1 N-terminal peptide is critical (Burkhardt et al., 2008; Malintan et al., 2009).
F115E, E132A, and F115E/E132A Munc18-1 Mutants Largely Rescue Phenotypes of Munc18-1/-2 Double Knockdown Cells
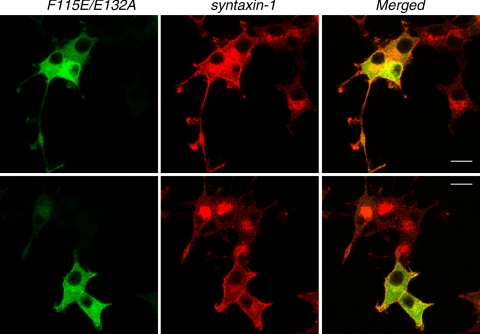
We then infected Munc18-1/-2 double knockdown cells with lentivirus that express these mutant Munc18-1 proteins fused with EmGFP (Figure 9A). Unlike the K46E/E59K mutant, we found that these mutants were capable of restoring the expression level of syntaxin-1, although not as efficiently as the wild-type protein (∼70–80% of the wild type) (Figure 9A). We then examined whether the F115E/E132A mutant could rescue syntaxin-1 mislocalization. We found that the cells that express F115E/E132A-EmGFP show stronger plasma membrane localization of syntaxin-1 compared with the neighboring cells that did not express F115E/E132A-EmGFP (Figure 10). However, we noted that the plasma membrane localization of syntaxin-1 in these cells was not as prominent as in the cells that express the wild-type Munc18-1-EmGFP. We often observed the existence of perinuclear localization of syntaxin-1 in the presence of its apparent plasma membrane localization (Figure 10, top).
Figure 9.
Stable reexpression of mutants in the hydrophobic pocket of Munc18-1 largely restores syntaxin-1 expression and NE-secretion in the double knockdown clones (D7 and D16). (A) Munc18-1/-2 double knockdown clones (D7, D16) were infected with lentiviruses that express EmGFP, wild-type Munc18-1-EmGFP, or mutant (F115E, E132A. F115E/E132A) Munc18-1-EmGFPs and the infected cells were isolated with blasticidin. Thirty micrograms of total homogenates from heterologous pools of the surviving cells was analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotting using anti-Munc18-1 and syntaxin-1 antibodies. Signals were detected with enhanced chemiluminescence detection system. A number on the left indicates the position of a molecular weight marker. (B and C) NE release was stimulated by 70 mM KCl for 15 min in the rescued cells (B for D7 clones; C for D16 clones). Error bars indicate SEM (n = 8 for D7 and n = 12 for D16).
Figure 10.
Rescue of syntaxin-1 localization upon reintroduction of F115E/E132A mutant Munc18-1-EmGFP into Munc18-1/-2 double knockdown clones. Confocal images of D7 cells that were infected with lentiviruses that express F115E/E132A Munc18-1-EmGFP (left) and stained with anti-syntaxin1 antibodies followed by red-x-conjugated anti-mouse antibodies (middle). Right panels are merged pictures. The existence of perinuclear localization of syntaxin-1 in the presence of its apparent plasma membrane localization was apparent in top panels but not in bottom panels. Bar, 10 μm.
We then examined whether the mutants in the hydrophobic pocket of Munc18-1 could restore regulated secretion of Munc18-1/-2 double knockdown cells. The secretion was significantly recovered by these mutant Munc18-1-EmGFPs but not by EmGFP alone (Figure 9, B and C). Thus, Munc18-1's binding to the N terminus of syntaxin-1 is largely dispensable for its secretory functions.
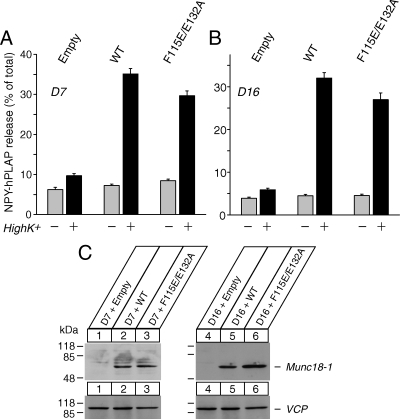
We also confirmed the abilities of F115E/E132A mutant to rescue secretion by using transient transfection. In this case, the wild-type and mutant Munc18-1 were expressed without EmGFP (Figure 11). Because transfection rate was low (usually 10–50% range also depending on the expression construct), we used cotransfected peptide (neuropeptide Y fused with a soluble marker of human placental alkaline phosphatase [NPY-hPLAP]) as a marker of transfection. Secretion assays were performed to measure secretion of NPY-hPLAP (Fujita et al., 2007; Li et al., 2007; Arunachalam et al., 2008; Malintan et al., 2009). We found that transient expression of F115E/E132A mutant Munc18-1 can rescue secretion of NPY-hPLPAP to a large extent compared with the wild-type Munc18-1 (Figure 11), further strengthening our conclusion.
Figure 11.
Defects in peptide secretion are rescued upon tranfection-mediated reintroduction of the wild-type and F115/E132 mutant, of Munc18-1. (A and B) Secretion of NPY-hPLAP from the double knockdown clones (D7 in A; D16 in B) that were transfected with the control plasmid or the Munc18-1 expression plasmid was stimulated by 70 mM KCl for 25 min. Error bar indicates SEM (n = 15 for D7; n = 18 for D16). (C) The expression of transfected Munc18-1 proteins in D7 and D16 clones. Thirty micrograms of total homogenates from D7 and D16 clones that were transiently transfected with Munc18-1 expressing plasmids was analyzed by SDS-polyacrylamide gel electrophoresis and immunoblotting using anti-Munc18-1 and anti-VCP (loading control) antibodies. Signals were detected with enhanced chemiluminescence detection system. A number on the left indicates the position of a molecular weight marker.
DISCUSSION
In this study, we have generated Munc18-2 single knockdown PC12 cells and Munc18-1/-2 double-knockdown cells in attempt to dissect the relative contribution of these two isoforms to the expression levels of various syntaxin isoforms present in neurosecretory cells. Although Munc18-2 single knockdown did not affect the expression of syntaxin-1, the double knockdown of Munc18-1 and -2 caused drastic (∼75%) reductions in syntaxin-1 expression accompanied by a dramatic mislocalization of this protein (Figs. 1–3). In addition, the double-knockdown of Munc18-1 and -2 significantly reduced the expression of both syntaxin-2 and -3. These results clearly indicate that Munc18-1 and -2 have overlapping function in the maintenance and trafficking of plasma membrane syntaxins.
Whether the function of Munc18-1 (and -2) in the transport of syntaxin-1 to the plasma membrane and the maintenance of the expression of syntaxins is independent or interrelated remains to be elucidated. The results from our rescue experiments using four Munc18-1 mutants (K46E/E59K, F115E, E132A, and F115E/E132A) showed that the abilities of the mutants to rescue syntaxin-1 localization and to restore the syntaxin-1 expression are well correlated (Figures 6, 7, 9, and 10). Thus, we suggest that these two functions of Munc18 might be interrelated. We speculate that the reduction in syntaxins' amounts in the absence of Munc18-1 and -2 may be a consequence of inappropriate localization of these proteins. Syntaxins that are trapped in the Golgi complex might be more susceptible to protein degradation. It is known that the SNARE interactions are not selective (Yang et al., 1999) and, without Munc18-1, plasma membrane syntaxins form ectopic SNARE complexes (Medine et al., 2007). We suggest that a crucial function of Munc18 is to prevent plasma membrane syntaxins from forming the ectopic SNARE complex at the Golgi region and to allow them to exit from the Golgi complex and be transported to the plasma membrane.
Crystal structures identified the residues in Munc18-1 that are critical for binding to the closed conformation of syntaxin-1, whereby the H3 SNARE helix and Habc domains of syntaxin-1 are tightly packed (Misura et al., 2000; Burkhardt et al., 2008). We found that Lys46 and Glu59 of Munc18-1 are particularly important for syntaxin binding and that the double mutation of these residues results in the complete loss of binding to syntaxin-1A but not mint-1 (Figure 5). This mutant failed to rescue the expression, localization of syntaxin-1 or secretion defects, indicating the essential role for the binding between Munc18-1 and the closed conformation of syntaxin-1.
It was recently reported that a mutant (E59K) of Munc18-1, which is similar to our mutant (K46E/E59K), exhibited significantly reduced ability to rescue exocytosis defects in Munc18-1 deficient neurons compared with the wild type (Deák et al., 2009). The authors showed that this mutation does not affect the binary interaction between Munc18-1 and syntaxin-1 by using ITC. However, this residue (Glu59) was shown to directly interact with Arg114 in the Habc domain of syntaxin-1 (Misura et al., 2000). In addition, a previous mutagenesis study showed that E59K mutation in Munc18-2 loses the binding to syntaxins 1–4 (Kauppi et al., 2002). Results from our yeast two-hybrid assays also support that E59K significantly reduces the binary binding to syntaxin-1 but not to mint-1 (Figure 5). Thus, we suggest that the reduced rescue activity of E59K in Munc18-1 deficient neurons (Deák et al., 2009) can be interpreted at least in part by the loss of binary binding with syntaxin-1. It is unknown whether E59K expression can restore the expression of syntaxin-1 in Munc18-1-deficient neurons. Based on our results that K46E/E59K loses the ability to rescue syntaxin-1 expression and localization in Munc18-1/-2 double knockdown cells (Figures 6A and 7), we predict that E59K mutant would not be able to rescue syntaxin-1 expression.
The Arg39 residue is also part of the residues in domain 1 of Munc18-1 that are critical for binding to the closed syntaxin-1 (Misura et al., 2000). As a consequence, R39C has been presumed to be a mutant that lacks binding to the closed conformation of syntaxin-1. Nonetheless, this mutant can support secretion defects of Munc18-1–deficient chromaffin cells (Gulyás-Kovács et al., 2007). Similarly, R50C mutant of Rop functions as gain-of-function mutant in Drosophila (Harrison et al., 1994), and R39C mutant can rescue unc-18 mutant in C. elegans (Johnson et al., 2009). Our yeast two-hybrid analysis suggests that R39C mutation does not significantly reduce the binding to Munc18-1 or mint-1 (Figure 5). Therefore, we suggest that the use of R39C as a Munc18-1 mutant that abolishes binding to the closed conformation of syntaxin-1 is misleading.
We have used three mutants (F115E, E132A, and F115E/E132A) that have been shown to lose binding to the N terminus of syntaxin-1 (Malintan et al., 2009) based on the information from the crystal structure (Hu et al., 2007; Burkhardt et al., 2008). We showed previously that these mutants lost the binding to the syntaxin-1 containing SNARE complex but had only a small effect on binding to free-syntaxin-1 (Malintan et al., 2009). Our yeast two-hybrid assays further confirmed that mutation of the N-terminal peptide binding surface does not significantly affect the binary binding to syntaxin-1, although interestingly they do significantly affect the binding to mint-1 (Figure 8). The F115E/E132A mutants rescued the phenotypes of Munc18-1/-2 knockdown at levels of 70–80% of the wild type. Thus, the interaction between the N terminus of syntaxin-1 and the hydrophobic pocket of Munc18-1 plays only a limited role in syntaxin-1 trafficking and dense-core vesicle secretion in PC12 cells.
Does Munc18-1 binding to the syntaxin-1 N terminus contribute to SNARE-mediated neurotransmitter release? The ability of Munc18-1 to directly stimulate SNARE-mediated vesicle fusion was demonstrated through liposome fusion assays and the fusion assays between large vesicles and giant membranes (Shen et al., 2007; Tareste et al., 2008). In regulated neurosecretion, SNARE-mediated fusion is stimulated or triggered by Ca2+, whereas Munc18-1-dependent stimulation of liposome fusion does not require the presence of Ca2+ (Shen et al., 2007). Thus, it remains to be determined whether Munc18-1 interaction with syntaxin-1 N terminus is truly able to stimulate vesicle fusion in regulated Ca2+-dependant exocytosis. Recent experiments that showed the ability of syntaxin-1 N-terminal peptide to inhibit synaptic release are major steps forward to support the function of syntaxin-1 N terminus in exocytosis (Khvotchev et al., 2007). Our results that the F115E, E132A, and F115E/E132A mutants still do not fully rescue the secretory phenotypes of Munc18 depletion may also support the role of Munc18-1 binding to syntaxin-1 N terminus in neurosecretion. However, the F115E, E132A or F115E/E132A mutants do not rescue syntaxin-1 expression or localization as efficiently as the wild type (Figures 9A and 10). Thus, the incomplete rescue of secretion we observe with these mutants may be attributable to the incomplete rescue of syntaxin-1 expression and localization by these mutants. Although the removal of N terminus of syntaxin-1 that interacts with the hydrophobic pocket of Munc18-1 only slightly affects the binary interaction with Munc18-1, it greatly reduces the ability of Munc18-1 to prevent syntaxin-1 from forming the SNARE complex in vitro (Burkhardt et al., 2008). To prove the priming/fusion activity of Munc18-1, it will be essential to identify Munc18-1 mutants that lose secretory functions yet maintain the chaperone functions for syntaxins. More work is necessary to assess the contribution of interaction between Munc18-1 and N-terminal syntaxin-1 to neuronal exocytosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. T. Mashimo, V. Olkkonen and T. Südhof for providing reagents for this study. We thank members of Sugita laboratory for critically reading the manuscript. This research was supported by the Canada Research Chair program, Heart and Stroke Foundation (grant NA6217), Natural Sciences and Engineering Research Council of Canada (grant 456042), the Canadian Institute of Health Research (grants MOP-57825 and MOP-64465), a grant from the National Health and Medical Research Council of Australia (to B.M.C. and F.A.M.) and a fellowship to F.A.M. from the National Health and Medical Research Council of Australia.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-08-0712) on October 7, 2009.
REFERENCES
- Arunachalam L., et al. Munc18-1 is critical for plasma membrane localization of syntaxin1 but not of SNAP-25 in PC12 cells. Mol. Biol. Cell. 2008;19:722–734. doi: 10.1091/mbc.E07-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Hofstein R., Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Bracher A., Weissenhorn W. Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. EMBO J. 2002;21:6114–6124. doi: 10.1093/emboj/cdf608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt P., Hattendorf D. A., Weis W. I., Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F., Xu Y., Chang W. P., Dulubova I., Khvotchev M., Liu X., Südhof T. C., Rizo J. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J. Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I., Khvotchev M., Liu S., Huryeva I., Südhof T. C., Rizo J. Munc18-1 binds directly to the neuronal SNARE complex. Proc. Natl. Acad. Sci. USA. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Xu A., Xie L., Arunachalam L., Chou T. C., Jiang T., Chiew S. K., Kourtesis J., Wang L., Gaisano H. Y., Sugita S. Ca2+-dependent activator for secretion 1 is critical for constitutive and regulated exocytosis, but not for loading of transmitters into dense-core vesicles. J. Biol. Chem. 2007;282:21392–21403. doi: 10.1074/jbc.M703699200. [DOI] [PubMed] [Google Scholar]
- Garcia E. P., Gatti E., Butler M., Burton J., De Camilli P. A rat brain Sec1 homologue related to Rop and UNC18 interacts with syntaxin. Proc. Natl. Acad. Sci. USA. 1994;91:2003–2007. doi: 10.1073/pnas.91.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás-Kovács A., de Wit H., Milosevic I., Kochubey O., Toonen R., Klingauf J., Verhage M., Sørensen J. B. Munc18-1, sequential interactions with the fusion machinery stimulate vesicle docking and priming. J. Neurosci. 2007;27:8676–8686. doi: 10.1523/JNEUROSCI.0658-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halachmi N., Lev Z. The Sec1 family: a novel family of proteins involved in synaptic transmission and general secretion. J. Neurochem. 1996;66:889–897. doi: 10.1046/j.1471-4159.1996.66030889.x. [DOI] [PubMed] [Google Scholar]
- Harrison S. D., Broadie K., van de Goor J., Rubin G. M. Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron. 1994;13:555–566. doi: 10.1016/0896-6273(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Hata Y., Südhof T. C. A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic. J. Biol. Chem. 1995;270:13022–13028. doi: 10.1074/jbc.270.22.13022. [DOI] [PubMed] [Google Scholar]
- Hata Y., Slaughter C. A., Südhof T. C. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- Hosono R., Hekimi S., Kamiya Y., Sassa T., Murakami S., Nishiwaki K., Miwa J., Taketo A., Kodaira K. I. The unc-18 gene encodes a novel protein affecting the kinetics of acetylcholine metabolism in the nematode Caenorhabditis elegans. J. Neurochem. 1992;58:1517–1525. doi: 10.1111/j.1471-4159.1992.tb11373.x. [DOI] [PubMed] [Google Scholar]
- Houng A., Polgar J., Reed G. L. Munc18-syntaxin complexes and exocytosis in human platelets. J. Biol. Chem. 2003;278:19627–19633. doi: 10.1074/jbc.M212465200. [DOI] [PubMed] [Google Scholar]
- Hu S. H., Latham C. F., Gee C. L., James D. E., Martin J. L. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc. Natl. Acad. Sci. USA. 2007;104:8773–8778. doi: 10.1073/pnas.0701124104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Ferdek P., Lian L. Y., Barclay J. W., Burgoyne R. D., Morgan A. Binding of UNC-18 to the N-terminus of syntaxin is essential for neurotransmission in Caenorhabditis elegans. Biochem. J. 2009;418:73–80. doi: 10.1042/BJ20081956. [DOI] [PubMed] [Google Scholar]
- Kanda H., Tamori Y., Shinoda H., Yoshikawa M., Sakaue M., Udagawa J., Otani H., Tashiro F., Miyazaki J., Kasuga M. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J. Clin. Invest. 2005;115:291–301. doi: 10.1172/JCI22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H., et al. A novel isoform of syntaxin-binding protein homologous to yeast Sec1 expressed ubiquitously in mammalian cells. J. Biol. Chem. 1995;270:4963–4966. doi: 10.1074/jbc.270.10.4963. [DOI] [PubMed] [Google Scholar]
- Kauppi M., Wohlfahrt G., Olkkonen V. M. Analysis of the Munc18b-syntaxin binding interface. Use of a mutant Munc18b to dissect the functions of syntaxins 2 and 3. J. Biol. Chem. 2002;277:43973–43979. doi: 10.1074/jbc.M208315200. [DOI] [PubMed] [Google Scholar]
- Khvotchev M., Dulubova I., Sun J., Dai H., Rizo J., Südhof T. C. Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J. Neurosci. 2007;27:12147–12155. doi: 10.1523/JNEUROSCI.3655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham C. F., et al. Molecular dissection of the Munc18c/syntaxin4 interaction: implications for regulation of membrane trafficking. Traffic. 2006;7:1408–1419. doi: 10.1111/j.1600-0854.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- Li G., et al. RalA and RalB function as the critical GTP sensors for GTP-dependent exocytosis. J. Neurosci. 2007;27:190–202. doi: 10.1523/JNEUROSCI.2537-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malintan N. T., Nguyen T. H., Han L., Latham C. F., Osborne S. L., Wen P. J., Lim S. J., Sugita S., Collins B. M., Meunier F. A. Abrogating Munc18-1-SNARE complex interaction has limited impact on exocytosis in PC12 cells. J. Biol. Chem. 2009;284:21637–21646. doi: 10.1074/jbc.M109.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J. M., Kaplan J. M. UNC-18 promotes both the anterograde trafficking and synaptic function of syntaxin. Mol. Biol. Cell. 2008;19:3836–3846. doi: 10.1091/mbc.E08-02-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medine C. N., Rickman C., Chamberlain L. H., Duncan R. R. Munc18-1 prevents the formation of ectopic SNARE complexes in living cells. J. Cell Sci. 2007;120:4407–4415. doi: 10.1242/jcs.020230. [DOI] [PubMed] [Google Scholar]
- Misura K. M., Scheller R. H., Weis W. I. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- Oh E., Spurlin B. A., Pessin J. E., Thurmond D. C. Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes. 2005;54:638–647. doi: 10.2337/diabetes.54.3.638. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Südhof T. C. Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J. Biol. Chem. 1997;272:31459–31464. doi: 10.1074/jbc.272.50.31459. [DOI] [PubMed] [Google Scholar]
- Peters J. M., Walsh M. J., Franke W. W. An abundant and ubiquitous homo-oligomeric ring-shaped ATPase particle related to the putative vesicle fusion proteins Sec18p and NSF. EMBO J. 1990;9:1757–1767. doi: 10.1002/j.1460-2075.1990.tb08300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner J., Hsu S. C., Scheller R. H. n-Sec 1, a neural-specific syntaxin-binding protein. Proc. Natl. Acad. Sci. USA. 1994;91:1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potterton L., McNicholas S., Krissinel E., Gruber J., Cowtan K., Emsley P., Murshudov G.N., Cohen S., Perrakis A., Noble M. Developments in the CCP4 molecular-graphics project. Acta. Crystallogr. D. Biol. Crystallogr. 2004;60:2288–2294. doi: 10.1107/S0907444904023716. [DOI] [PubMed] [Google Scholar]
- Rickman C., Medine C. N., Bergmann A., Duncan R. R. Functionally and spatially distinct modes of munc18-syntaxin 1 interaction. J. Biol. Chem. 2007;282:12097–12103. doi: 10.1074/jbc.M700227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K., Galli T., Jansson S., Ehnholm C., Lehtonen E., Olkkonen V. M. Interaction of Munc-18-2 with syntaxin 3 controls the association of apical SNAREs in epithelial cells. J. Cell Sci. 1998;111:2681–2688. doi: 10.1242/jcs.111.17.2681. [DOI] [PubMed] [Google Scholar]
- Riento K., Jantti J., Jansson S., Hielm S., Lehtonen E., Ehnholm C., Keranen S., Olkkonen V. M. A sec1-related vesicle-transport protein that is expressed predominantly in epithelial cells. Eur. J. Biochem. 1996;239:638–646. doi: 10.1111/j.1432-1033.1996.0638u.x. [DOI] [PubMed] [Google Scholar]
- Riento K., Kauppi M., Keranen S., Olkkonen V. M. Munc18-2, a functional partner of syntaxin 3, controls apical membrane trafficking in epithelial cells. J. Biol. Chem. 2000;275:13476–13483. doi: 10.1074/jbc.275.18.13476. [DOI] [PubMed] [Google Scholar]
- Rodkey T. L., Liu S., Barry M., McNew J. A. Munc18a scaffolds SNARE assembly to promote membrane fusion. Mol. Biol. Cell. 2008;19:5422–5434. doi: 10.1091/mbc.E08-05-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J., Corradi N., Malosio M. L., Taverna E., Halban P., Meldolesi J., Rosa P. Blockade of membrane transport and disassembly of the Golgi complex by expression of syntaxin 1A in neurosecretion-incompetent cells: prevention by rbSEC1. J. Cell Sci. 1999;112:1865–1877. doi: 10.1242/jcs.112.12.1865. [DOI] [PubMed] [Google Scholar]
- Rowe J., Calegari F., Taverna E., Longhi R., Rosa P. Syntaxin 1A is delivered to the apical and basolateral domains of epithelial cells: the role of munc-18 proteins. J. Cell Sci. 2001;114:3323–3332. doi: 10.1242/jcs.114.18.3323. [DOI] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- Schraw T. D., Lemons P. P., Dean W. L., Whiteheart S. W. A role for Sec1/Munc18 proteins in platelet exocytosis. Biochem. J. 2003;374:207–217. doi: 10.1042/BJ20030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Tareste D. C., Paumet F., Rothman J. E., Melia T. J. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Sollner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Rothman J. E. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S., Südhof T. C. Specificity of Ca2+-dependent protein interactions mediated by the C2A domains of synaptotagmins. Biochemistry. 2000;39:2940–2949. doi: 10.1021/bi9920984. [DOI] [PubMed] [Google Scholar]
- Tadokoro S., Kurimoto T., Nakanishi M., Hirashima N. Munc18-2 regulates exocytotic membrane fusion positively interacting with syntaxin-3 in RBL-2H3 cells. Mol. Immunol. 2007;44:3427–3433. doi: 10.1016/j.molimm.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Tamori Y., Kawanishi M., Niki T., Shinoda H., Araki S., Okazawa H., Kasuga M. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3–L1 adipocytes. J. Biol. Chem. 1998;273:19740–19746. doi: 10.1074/jbc.273.31.19740. [DOI] [PubMed] [Google Scholar]
- Tareste D., Shen J., Melia T. J., Rothman J. E. SNAREpin/Munc18 promotes adhesion and fusion of large vesicles to giant membranes. Proc. Natl. Acad. Sci. USA. 2008;105:2380–2385. doi: 10.1073/pnas.0712125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam J. T., McIntosh S., James D. E. Molecular identification of two novel Munc-18 isoforms expressed in non-neuronal tissues. J. Biol. Chem. 1995;270:5857–5863. doi: 10.1074/jbc.270.11.5857. [DOI] [PubMed] [Google Scholar]
- Tellam J. T., Macaulay S. L., McIntosh S., Hewish D. R., Ward C. W., James D. E. Characterization of Munc-18c and syntaxin-4 in 3T3–L1 adipocytes: putative role in insulin-dependent movement of GLUT-4. J. Biol. Chem. 1997;272:6179–6186. doi: 10.1074/jbc.272.10.6179. [DOI] [PubMed] [Google Scholar]
- Toonen R. F., de Vries K. J., Zalm R., Südhof T. C., Verhage M. Munc18-1 stabilizes syntaxin 1, but is not essential for syntaxin 1 targeting and SNARE complex formation. J. Neurochem. 2005;93:1393–1400. doi: 10.1111/j.1471-4159.2005.03128.x. [DOI] [PubMed] [Google Scholar]
- Toonen R. F., Kochubey O., de Wit H., Gulyas-Kovacs A., Konijnenburg B., Sørensen J. B., Klingauf J., Verhage M. Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J. 2006;25:3725–3737. doi: 10.1038/sj.emboj.7601256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M., et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Voets T., Toonen R. F., Brian E. C., de Wit H., Moser T., Rettig J., Südhof T. C., Neher E., Verhage M. Munc18-1 promotes large dense-core vesicle docking. Neuron. 2001;31:581–591. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- Vojtek A. B., Hollenberg S. M., Cooper J. A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Wang L., Li G., Sugita S. RalA-exocyst interaction mediates GTP-dependent exocytosis. J. Biol. Chem. 2004;279:19875–19881. doi: 10.1074/jbc.M400522200. [DOI] [PubMed] [Google Scholar]
- Wang L., Li G., Sugita S. A central kinase domain of type I phosphatidylinositol phosphate kinases is sufficient to prime exocytosis: isoform specificity and its underlying mechanism. J. Biol. Chem. 2005;280:16522–16527. doi: 10.1074/jbc.M413263200. [DOI] [PubMed] [Google Scholar]
- Yang B., Gonzalez L., Jr., Prekeris R., Steegmaier M., Advani R. J., Scheller R. H. SNARE interactions are not selective. Implications for membrane fusion specificity. J. Biol. Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- Zilly F. E., Sorensen J. B., Jahn R., Lang T. Munc18-bound syntaxin readily forms SNARE complexes with synaptobrevin in native plasma membranes. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.