Abstract
Background
Corn silk contains proteins, vitamins, carbohydrates, Ca, K, Mg and Na salts, fixed and volatile oils, steroids such as sitosterol and stigmasterol, alkaloids, saponins, tannins, and flavonoids. Base on folk remedies, corn silk has been used as an oral antidiabetic agent in China for decades. However, the hypoglycemic activity of it has not yet been understood in terms of modern pharmacological concepts. The purpose of this study is to investigate the effects of corn silk on glycaemic metabolism.
Methods
Alloxan and adrenalin induced hyperglycemic mice were used in the study. The effects of corn silk on blood glucose, glycohemoglobin (HbA1c), insulin secretion, damaged pancreatic β-cells, hepatic glycogen and gluconeogenesis in hyperglycemic mice were studied respectively.
Results
After the mice were orally administered with corn silk extract, the blood glucose and the HbA1c were significantly decreased in alloxan-induced hyperglycemic mice (p < 0.05, p < 0.01, respectively), while the level of insulin secretionn was markedly elevated in alloxa-induced hyperglycemic mice (p < 0.05). The alloxan-damaged pancreatic β-cells of the mice were partly recovered gradually after the mice were administered with corn silk extract 15 days later. Also, the body weight of the alloxan-induced hyperglycemic mice was increased gradually. However, ascension of blood glucose induced by adrenalin and gluconeogenesis induced by L-alanine were not inhibited by corn silk extract treatment (p > 0.05). Although corn silk extract increased the level of hepatic glycogen in the alloxan-induced hyperglycemic mice, there was no significant difference between them and that of the control group(p > 0.05).
Conclusion
Corn silk extract markedly reduced hyperglycemia in alloxan-induced diabetic mice. The action of corn silk extract on glycaemic metabolism is not via increasing glycogen and inhibiting gluconeogenesis but through increasing insulin level as well as recovering the injured β-cells. The results suggest that corn silk extract may be used as a hypoglycemic food or medicine for hyperglycemic people in terms of this modern pharmacological study.
Background
Corn silk (ZeamaysL.) refers to the stigmas from the female flowers of maize. Fresh corn silk resembles soft silk threads 10-20 cm long that are either light green or yellow-brown in color. Corn silk contains proteins, vitamins, carbohydrates, Ca, K, Mg and Na salts, fixed and volatile oils, steroids such as sitosterol and stigmasterol, alkaloids, saponins, tannins, and flavonoids [1-14]. There have been many reports on the biological activities of corn silk constituents. Methanol extracts of corn silk showed an antioxidative activity on the level of lipid peroxidation [15]. Volatiles from corn silk inhibited the growth of Aspergillus flavus, indicating that it has an antifungal activity [16]. In addition, extract of corn silk inhibited TNF and LPS-induced cell adhesion, but not cytotoxic activity or TNF production [17].
Corn silk has been used in many parts of the world for the treatment of edema as well as for cystitis, gout, kidney stones nephritis and prostatitis [13,18]. Base on folk remedies, corn silk has been used as an oral antidiabetic agent in China for decades. However, in spite of its widespread use, the mechanisms underlying hypoglycemic activity of corn silk was not yet understood. Therefore, the purpose of this study was to investigate the effects of corn silk on glycaemic metabolism. The effects of corn silk on blood glucose, HbA1c, insulin secretion, damaged pancreatic β-cells, hepatic glycogen and gluconeogenesis in hyperglycemic mice were studied respectively.
Materials and methods
Animals
Kunming strain mice weighing 20-22 g, Grade II, Certificate SCXK (Lu) 20080006, were purchased from the Experimental Animal Center, Shandong University, China. The mice were maintained at room temperature under alternating natural light/dark photoperiod, and had access to standard laboratory food and fresh water ad libitum. This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals. Care was taken to minimize discomfort, distress, and pain to the animals.
Chemicals
Alloxan and adrenaline were analytical grade. Alloxan was purchased from Sigma Co., Ltd and adrenaline was purchased from Tianjin Amino acid Co., Ltd. China. Xiaoke pills were purchased from Jilin Liuhe Pharmaceutic Factory, China. Xiaoke pill is a kind of Chinese medicine used in the treatment of diabetes. It is composed of glibenclamide and several traditional Chinese herbs, including Radix Puerariae, Radix Rehmannia, Radix Astragali, Radix Trichosanthis, Corn Stigma, Fructus Schisandrae and Rhizoma Dioscoreae.
Corn silk extract (CSE) preparation
Corn silk was obtained from local market. Sample was produced by the way introduced by Velazquez [1]. Briefly, corn silk was dried at room temperature (24.2 ± 1.0°C) and an aqueous extraction was performed by adding 100 ml boiling water to 10 g corn silk, filtering after 20 min and then lyophilizing.
Experimental design
Blood samples from alloxan-induced hyperglycemic mice
One hundred mice were fasted for 12 h and then injected (iv) with alloxan (75 mg/kg) dissolved in sterile saline [19]. Forty-eight hours later, blood samples were collected from the tail veins of the mice. The blood glucose was analyzed with a Glucometer-4 (Bayer). Sixty hyperglycemic mice (the blood glucose level greater than 11.1 mmol/L) were selected and randomly divided into 6 groups. From then on, the 6 groups of mice were administered orally saline (control), Xiaoke Pill and 0.5, 1.0, 2.0 or 4.0 g/kg body wt. of corn silk extract (CSE) dissolved in the same amount of saline (experimental). The body weights of the mice were measured on the 0th day, 5th day, 10th day, 15th day and the 20th day. At the same time, after fasting the mice for 12 h on the 20th day, blood samples were obtained from the tail veins to determine the blood glucose levels. On the 45th day, blood samples were collected from the orbital veins to measure the HbA1c with the HbA1c Apparatus (Variantα, Bio-Rad Laboratories) and insulin with an enzyme-linked immunosorbant assay (ELISA) kit (Biosource, Europe) respectively [20]. Then, the mice were sacrificed. The pancreas was dissected out and placed in 10% buffered formalin and the liver was dissected out for the measurement of hepatic glycogen. Estimation of the damaged pancreatic β-cells
The pancreatic tissues were embedded in paraffin blocks after formalin fixation
Paraffin sections were cut at 4- μm thickness and were deparaffinized in xylene twice for 5 min and then were rehydrated with the graded ethanol. The sections were examined after hematoxylin and eosin (H&E) staining [21].
Estimation of hepatic glycogen
The liver was homogenized in ice-cold 0.6 M HClO4. The mixture was immediately centrifuged at 3000 g for 10 min at 4°C to obtain the supernatant. Free glucose in the tissue was measured with the glucose oxidase method. Amyloglucosidase solution (10 U/ml) in 0.2 M sodium acetate buffer (pH 4.8) was then mixed and incubated in the mixture at 40°C for 2 h. After incubation, pH of the mixture was adjusted to 7 and subjected to determination of total glucose. Free glucose was subtracted from total glucose to obtain glycogen content. The glycogen was expressed as mg/g wet tissue [22].
Estimation of gluconeogenesis
Thirty normal mice were selected and allocated equally into 3 groups: Xiaoke Pill-treated group, CSE-treated group and saline group used as the control group. From then on, these 3 groups of mice were administered orally with Xiaoke Pill, CSE (4.0 g/kg) and saline respectively. At the end of the experimental period (15 days later), animals were fasted 12 h. After administration 1 h later, the mice were injected (s.c.) with L-alanine. The blood samples from the tail vein of the mice were collected at the 0th min and 60th min to determine blood glucose level.
Blood samples from adrenaline -induced hyperglycemic mice
Sixty healthy mice were allocated equally into 6 groups. Then these 6 groups of mice were also orally administered with saline, Xiaoke Pill and CSE (0.5, 1.0, 20. and 4.0 g/kg), respectively. On the 14th day, they were fasted over night. After administration 1 h later, animals were injected (sc) with adrenaline. Blood samples from the tail vein of the mice were collected at the 0th min and 60th min to determine blood glucose level just as above.
Statistical analysis
All data were analyzed by a one-way analysis of variance, and the differences between means were established by Duncan's multiple-range test [23]. The data represents means and standard deviations. The significant level of 5% (p <0.05) was used as the minimum acceptable probability for the difference between the means.
Results and Discussion
Alloxan and streptozotocin are the most prominent diabetogenic chemicals in diabetes research. Both are toxic glucose analogues that preferentially accumulate in pancreatic beta cells via the GLUT2 glucose transporter [24-28]. Streptozotocin is split into its glucose and methylnitro-sourea moiety. Owing to its alkylating properties, the latter modifies biological macromolecules, fragments DNA and destroys the beta cells, causing a state of insulin-dependent diabetes (type 1 diabetes mellitus) [27,28]. On the other hand, alloxan has two distinct pathological effects: it selectively inhibits glucose-induced insulin secretion through specific inhibition of glucokinase, the glucose sensor of the beta cell, and it causes a state of insulin-dependent diabetes by selective necrosis of beta cells in type 1 and type 2 diabetes mellitus [24-26]. So alloxan is the agent of choice for induction of diabetic experimental animals in this study.
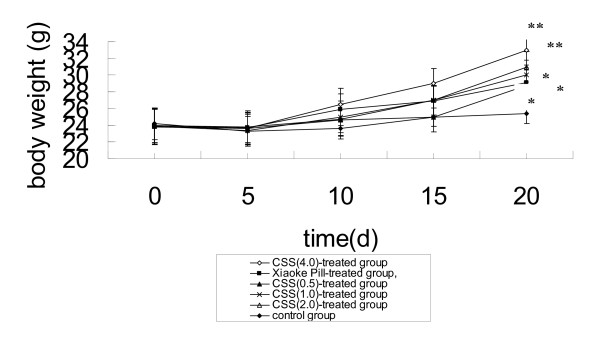
The body weights of the hyperglycemic mice induced by alloxan are presented in Fig. 1. Contrasted with the control group, the body weights of mice in CSE-treated group were increased gradually 20 days later (p < 0.05, p < 0.01). The body weights of mice in Xiaoke Pill-treated group were also increased. The body weights of hyperglycemic mice induced by alloxan were increased when they were administrated with CSE. It indicates that corn silk could act as supplement nutrients to mice.
Figure 1.
Effects of CSE on body weights of hyperglycemic mice. Values are means ± SEM, n = 10. *Different from control group P < 0.05; **different from control group, P < 0.01.
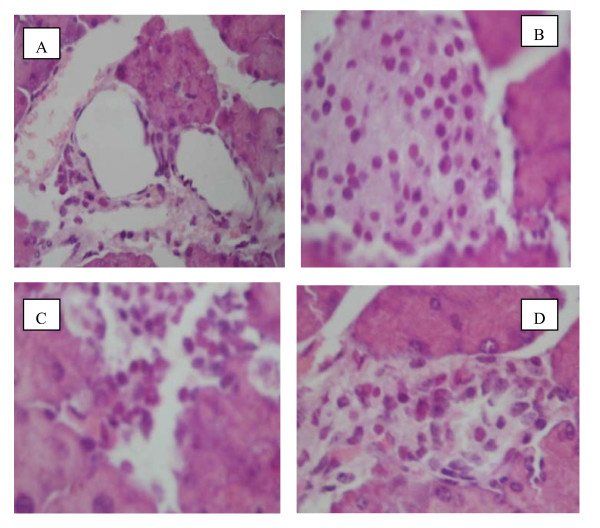
The results of blood glucose from hyperglycemic mice induced by alloxan are presented in Table 1. The levels of blood glucose were decreased after administration of CSE(2.0 and 4.0) and the Xiaoke Pill (p < 0.05). However, low doses CSE-treated groups (0.5 and 1.0 g/kg) did not reach the same result. The mechanisms of the hypoglycaemic effects of CSE have been also studied in this paper. As shown in Table 2, the levels of serum insulin were elevated after administration of 4.0 g/kg body wt CSE (9.8 ± 0.5 μU/mL p < 0.05) and the Xiaoke Pill (8.6 ± 0.7 μU/mL). However, the same results did not occur in the saline treated group(3.8 ± 0.4 μU/mL) throughout the total duration of the study. It is possible that CSE could aid in releasing of insulin from the surviving β-cells, as well from the recovered β-cells by CSE. The β-cells of the mice fed with CSE (4.0 g/kg) were partially recovered (Fig. 2). β-Cells death and alteration of islet cell population were prominent in the diabetic mice (Fig. 2A). In contrast, the cell damage was not observed in the islet cells of the CSE-fed mice in spite of the alloxan treatment (Fig. 2B).
Table 1.
Effect of CSE on blood glucose levels in alloxan-hyperglycemic mice
| Different groups | Blood glucose (mmol/L) |
|---|---|
| Control group | 21.2 ± 2.1b |
| Xiaoke Pill-treated | 13.4 ± 3.0a |
| CSE(4.0)-treated | 11.5 ± 2.1a |
| CSE(2.0)-treated | 15.6 ± 3.0A |
| CSE(1.0)-treated | 18.9 ± 2.8b |
| CSE(0.5)-treated | 19.9 ± 2.7b |
Values are shown as means ± SEM, n = 10. The different letters in the same column indicate a statistical difference (p < 0.05)
Table 2.
Effect of CSE on serum insulin level in alloxan-induced diabetic mice
| Different groups | Serum insulin (μU/mL) |
|---|---|
| Saline-treated | 3.8 ± 1.4a |
| Xiaoke Pill-treated | 8.6 ± 0.7b |
| CSE (4.0)-treated | 9.8 ± 0.5b |
Values are shown as means ± SEM, n = 10. The different letters in the same column indicate a statistical difference. CSE-treated groups were compared with diabetic group, p < 0.05.
Figure 2.
Islet cell death and replication represented by hematoxylin--eosin. The islet cells of diabetic mice of alloxan-treatment (A) showed extensive cell lysis, representing loss of plasma membrane with condensed nuclei and dissolved cytoplasm in wide intercellular spaces. In contrast, the islet cells of CSE(4.0)-fed mice (B) and Xiaoke Pill-treated (D) mice were partly recovered. The islet cells of CSE(2.0)-fed mice was C.
HbA1c is a more useful parameter in diabetes. CSE(4.0 g/kg) could decrease the concentration of HbA1c in plasma of alloxan- induced hyperglycemic group 45 days later (p < 0.01), as shown in Table 3. However the same result did not occur in other groups.
Table 3.
Effect of CSE on HbA1c from hyperglycemic mice induced by alloxan (%)
| Different groups | Results of HbA1c |
|---|---|
| Control group | 11.8 ± 0.23b |
| Xiaoke Pill-treated | 9.9 ± 0.30b |
| CSE(4.0)-treated | 6.9 ± 0.28a |
| CSE(2.0)-treated | 9.0 ± 0.26b |
| CSE(1.0)-treated | 10.8 ± 0.33b |
| CSE(0.5)-treated | 11.0 ± 0.22b |
Values are shown as means ± SEM, n = 10. The different letters in the same column indicate a statistical difference (p < 0.01)
Adrenaline activates glycogenolysis and glyconeogenesis to elevate serum glucose level [29] and its effect is relatively rapid [30]. The results of blood glucose from hyperglycemic mice induced by adrenaline are presented in Table 4. It showed that ascension of blood glucose induced by adrenaline was not inhibited (p > 0.05) after mice were administered orally with CSE for 15 days. It indicates that the mechanisms of the hypoglycaemic effects of CSE might via increasing insulin level as well as recovering the injured β-cells but not through inhibiting gluconeogenesis and glycogenolysis. It is consistent with the results presented in Table 1, 3, 5 and 6.
Table 4.
Effect of CSE on blood glucose levels in adrenaline -hyperglycemic mice
| Different groups | Blood glucose (mmol/L) at 0th min |
Blood glucose (mmol/L) at 60th min |
|---|---|---|
| Control group | 5.7 ± 2.6 | 15.1 ± 1.0 |
| Xiaoke Pill-treated | 5.6 ± 2.2 | 12.3 ± 0.6* |
| CSE(4.0)-treated | 5.8 ± 3.1 | 12.6 ± 1.5* |
| CSE(2.0)-treated | 5.6 ± 2.6 | 14.0 ± 1.2* |
| CSE(1.0)-treated | 5.7 ± 2.3 | 13.9 ± 1.6* |
| CSE(0.5) -treated | 5.7 ± 3.3 | 15.0 ± 3.2* |
Values are shown as means ± SEM, n = 10. * p > 0.05 vs. Control group
Table 5.
Effect of CSE on hepatic glycogen
| Different groups | Hepatic glycogen (mg/g tissue) |
|---|---|
| Saline-treated | 14.2 ± 3.4 |
| Xiaoke Pill-treated | 16.8 ± 0.5* |
| CSE (4.0)-treated | 17.0 ± 4.2* |
Values are shown as means ± SEM, n = 10. * p > 0.05 vs. Saline - treated group.
Table 6.
Effect of CSE on Gluconeogenesis
| Different groups | Blood glucose (mg/mL) at 0th min |
Blood glucose (m/mL) at 60th min |
|---|---|---|
| Xiaoke Pill-treated | 92.5 ± 15.5 | 99.8 ± 11.9* |
| CSE (4.0)-treated | 92.1 ± 17.9 | 102.6 ± 9.0* |
| Saline-treated | 91.6 ± 14.7 | 100.0 ± 12.2 |
Values are shown as means ± SEM, n = 10. * p > 0.05 vs. Saline - treated group.
Glycogen storage in the liver is another way to maintain blood glucose concentration in mammals. Decreased hepatic glucose production is induced by glycogen synthesis and the treatment effects of CSE were further confirmed by the assay of evaluating glycogen storage in the liver. CSE treatment increased the level of hepatic glycogen. The glycogen levels were 17.0 ± 4.2 mg/g tissue in CSE treated mice. Concentrations of hepatic glycogen were lower in saline-treated mice (14.2 ± 3.4 mg/g) than those in CSE treated mice(Table 5). However, there was no significant difference between them (p > 0.05).
As shown in Table 6, At the 60th min, the level of blood glucose of the mice in the CSE group was increased from 92.1 ± 17.9 mg/mL to 102.6 ± 9.0 mg/mL at the 60th min, after the mice were injected (s.c.) with L-alanine. At the same time, the level of blood glucose of the mice in the control group was increased after the mice were injected (s.c.) with L-alanine (from 91.6 ± 14.7 mg/mL to 100.0 ± 12.2 mg/mL). The result was shown in Table. 6 (p > 0.05). Gluconeogenesis is one of the key metabolic pathways in the liver. It is an important mechanism for maintaining blood glucose within a normal range. The result shown in Table. 6 indicates that the mechanisms of the hypoglycaemic effects of CSE were not by inhibiting gluconeogenesis.
Conclusion
Our results showed that CSE treatment markedly reduced hyperglycemia in alloxan -induced diabetic mices. The action of CSE on glycaemic metabolism is not via increasing glycogen and inhibiting gluconeogenesis but through increasing insulin level as well as recovering the injured β-cells.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors were involved in the design of this study; and performed laboratory analyses and statistics. JG drafted the manuscript along with the other authors. All authors read and approved the final manuscript.
Contributor Information
Jianyou Guo, Email: guojianyou@126.com.
Tongjun Liu, Email: liutongjun2003@yahoo.com.cn.
Linna Han, Email: wenhelp@126.com.
Yongmei Liu, Email: yongmei_l@126.com.
Acknowledgements
This work was supported by projects for Young Scientist from Institute of Psychology, Chinese Academy of Sciences (08CX043004), National Natural Science Foundation of China (30800301),China Postdoctoral Science Foundation (20090450546) and Shandong Province Higher Educational Science and Technology Program (J08LH62).
References
- Velazquez DVO, Xavier HS, Batista JEM, de Castro-Chavas C. Zea mays L. extracts modify glomerular function and potassium urinary excretion in conscious rats. Phytomedicine. 2005;12:363–369. doi: 10.1016/j.phymed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Namba T, Xu H, Kadota S, Hattori M, Takahashi T, Kojima Y. Inhibition of Ig E formation in mice by glycoproteins from corn silk. Phytother Res. 1993;7:227–230. doi: 10.1002/ptr.2650070303. [DOI] [Google Scholar]
- Tang L, Ding X, You L, Gu W, Yu F. Bio-activesubstances from corn silk. Wuxi Qinggong Daxue Xuebao. 1995;14:319–324. in Chinese. [Google Scholar]
- Abdel-Wahab SM, El-Tanbouly ND, Kassem HA, Mohamed EA. Phytochemical and biological study of corn silk (styles and stigmas of Zea mays L.) Bull Fac Pharm. 2002;40:93–102. [Google Scholar]
- Li Q, Tian Z, Jia T, Xu Q. Determination of-sitosterol in corn silk by HPLC-ELSD. Zhongcaoyao. 2005;36:1883–1884. in Chinese. [Google Scholar]
- Ren S, Din X. Determination of organic acids in corn silk with GC-MS. Wuxi Qinggong Daxue Xuebao. 2003;22:89–91. in Chinese. [Google Scholar]
- Guevara P, Perez-Amador MC. Flavones in corn silks and resistance to insect attacks. Phyton. 2000;69:151–156. [Google Scholar]
- Ren S, Ding X. Study on determination methods of flavonoids from corn silk. Shipin Kexue. 2004;25:139–142. in Chinese. [Google Scholar]
- Cantelo WW, Jacobson M. Corn silk volatiles attract many pest species of moths. J Environ Sci Health Part A. 1979;14:695–707. doi: 10.1080/10934527909374907. [DOI] [Google Scholar]
- Flath RA, Forrey RR, John JO, Chan BG. Volatile components of corn silk (Zea mays L.): Possible Heliothis zea (Boddie)attractants. J Agric Food Chem. 1978;26:1290–1293. doi: 10.1021/jf60220a012. [DOI] [Google Scholar]
- Buttery RG, Ling LC, Teranishi R. Volatile of corn tassels: Possible corn ear worm attractant. J Agric Food Chem. 1980;28:771–774. doi: 10.1021/jf60230a020. [DOI] [Google Scholar]
- Zeringue HJ. Identification and effects of maize silk volatiles on cultures of Aspergillus flavus. J Agric Food Chem. 2000;48:921–925. doi: 10.1021/jf990061k. [DOI] [PubMed] [Google Scholar]
- Newal CA, Anderson LA, Phillipson JD. In: Herbal Medicine: a Guide for Health-care Professionals. Newal CA, editor. London, Pharmaceutical Press; 1996. p. 90. [Google Scholar]
- Fleming T. In: PDR® for Herbal Medicines™. Second. Fleming T, editor. New Jersey, Medical Economics Company; 2000. pp. 224–225. [Google Scholar]
- Maksimovic ZA, Kovacevic N. Preliminary assay on the antioxidative activity of Maydis stigma extracts. Fitoterapia. 2003;74:144–147. doi: 10.1016/S0367-326X(02)00311-8. [DOI] [PubMed] [Google Scholar]
- Ziringue HJ. Identification and effects of maize silk volatiles on cultures of Aspergillus flavus. J Agric Food Chem. 2000;48:921–925. doi: 10.1021/jf990061k. [DOI] [PubMed] [Google Scholar]
- Habtemariam S. Extracts of corn silk inhibits the tumor necrosis factor-alpha- and bacterial lipopolysaccharide-induced cell adhesion and ICAM-1 expression. Planta Med. 1998;64:314–318. doi: 10.1055/s-2006-957441. [DOI] [PubMed] [Google Scholar]
- Grases F, March JG, Ramis M, Costa-Bauza A. The influence of Zea mays on urinary risk factors for kidney stones in rats. Phytother Res. 1993;7:146–149. doi: 10.1002/ptr.2650070210. [DOI] [Google Scholar]
- You Y, Lin Z. Antioxidant effect of Ganoderma polysaccharide peptide. Acta Pharm Sinica. 2003;38:85–88. [PubMed] [Google Scholar]
- Anderson L, Dinesen B, Jorgenson P, Poulsen F, Roder M. Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem. 1993;38:578. [PubMed] [Google Scholar]
- Zhou G, Han C. The co-effect of vanadium and fermented mushroom of Coprinus comatus on glycaemic metabolism. Biol Trace Elem Res. 2008;124:20–27. doi: 10.1007/s12011-008-8118-7. [DOI] [PubMed] [Google Scholar]
- Keppler D, Decker K. In: Glycogen. Methods of Enzymatic Analysis. Bergmeyer HU, editor. New York, Academic Press; 1974. pp. 11–17. [Google Scholar]
- Duncan DB. Multiple range tests for correlated and heteroscedastic means. Biometrics. 1957;13:164–76. doi: 10.2307/2527799. [DOI] [Google Scholar]
- Weaver DC, Barry CD, McDaniel ML, Marshall GR, Lacy PE. Molecular requirements for recognition at glucoreceptor for insulin release. Mol Pharmacol. 1979;16:361–368. [PubMed] [Google Scholar]
- Gorus FK, Malaisse WJ, Pipeleers DG. Selective uptake of alloxan by pancreatic B-cells. Biochem J. 1982;208:513–515. doi: 10.1042/bj2080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner M, Tiedge M, Guldbakke B, Munday R, Lenzen S. Importance of the GLUT2 glucose transporter for pancreatic beta cell toxicity of alloxan. Diabetologia. 2002;45:1542–1549. doi: 10.1007/s00125-002-0955-x. [DOI] [PubMed] [Google Scholar]
- Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia. 2000;43:1528–1533. doi: 10.1007/s001250051564. [DOI] [PubMed] [Google Scholar]
- Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43:1326–1333. doi: 10.2337/diabetes.43.11.1326. [DOI] [PubMed] [Google Scholar]
- Cherrington AD, Fuchs H, Stevenson RW, Williams PE, Alberti KG, Steiner KE. Effect of epinephrine on glycogenolysis and gluconeogenesis in conscious overnight-fasted dogs. Am J Physiol. 1984;247:E137–144. doi: 10.1152/ajpendo.1984.247.2.E137. [DOI] [PubMed] [Google Scholar]
- Issekutz B Jr, Allen M. Effect of catecholamines and methylprednisolone on carbohydrate metabolism of dogs. Metabolism. 1972;21:48–59. doi: 10.1016/0026-0495(72)90019-4. [DOI] [PubMed] [Google Scholar]