Abstract
Background:
There is limited evidence to support the efficacy of current pharmaceutical agents in reducing the risk of hip fracture in older postmenopausal women with established osteoporosis.
Objective:
To clarify the efficacy of risedronate in reducing the risk of hip fracture in elderly postmenopausal women aged ≥ 70 years with established osteoporosis, i.e., those with bone mineral density-defined osteoporosis and a prevalent vertebral fracture.
Methods:
Post hoc analysis of the Hip Intervention Program (HIP) study, a randomized controlled trial comparing risedronate with placebo for reducing the risk of hip fracture in elderly women. Women aged 70 to 100 years with established osteoporosis (baseline femoral neck T-score ≤ −2.5 and ≥1 prior vertebral fracture) were included. The main outcome measure was 3-year hip fracture incidence in the risedronate and placebo groups.
Results:
A total of 1656 women met the inclusion criteria. After 3 years, hip fracture had occurred in 3.8% of risedronate-treated patients and 7.4% of placebo-treated patients (relative risk 0.54; 95% confidence interval 0.32–0.91; P = 0.019).
Conclusion:
Risedronate significantly reduced the risk of hip fracture in women aged up to 100 years with established osteoporosis.
Keywords: osteoporosis, postmenopausal, hip fracture, risedronate
Introduction
Non-vertebral fractures are the most frequent fractures in patients who present with a clinical fracture at an emergency unit.1 Of these, hip fractures are the most devastating because they lead to serious disability, an increased risk of mortality, and a high socioeconomic cost.2 Only two studies have studied prevention of hip fracture as a primary end point: the Hip Intervention Program (HIP);3 and, more recently, the Health Outcomes and Reduced Incidence with Zoledronic acid ONce yearly Pivotal Fracture Trial (HORIZON-PFT).4 Risedronate reduced the risk of hip fractures by 30%3 and zoledronic acid by 41%.4 In a subgroup analysis of the HIP study, risedronate had no effect on hip fracture in women aged ≥80 years, but 58% of these patients were selected exclusively on the basis of them having ≥1 fall risk3 and only 16% had documented bone mineral density (BMD) or vertebral fracture status, indicating that selection of patients occurred mainly on the basis of fall risks alone. In a post hoc analysis of the HIP and Vertebral Efficacy with Risedronate Therapy (VERT) studies in women aged ≥80 years with osteoporosis or at least one prevalent vertebral fracture, risedronate reduced the risk of vertebral fractures,5 indicating that fracture prevention with risedronate is possible in the very elderly with bone-related fracture risks.
The incidence of hip fractures in women increases exponentially with age and is related to bone- and fall-related risks,6 but 66% of hip fractures in women occur before the age of 85 years.7 We studied the effect of risedronate on hip fractures in women older than 70 years and aged up to 100 years with well-documented bone-related fracture risks, and tested the hypothesis of hip fracture prevention with risedronate in an available group of patients, which included the very elderly, with established osteoporosis from the HIP study.
Methods
Study included in the analysis
The present analysis focused on a subgroup of the intention-to-treat (ITT) population from the HIP study comprising women aged 70 to 100 years with National Health and Nutrition Examination Survey (NHANES) III defined baseline femoral neck T-score of ≤ −2.5 and at least one prior vertebral fracture consistent with the World Health Organization/International Osteoporosis Foundation criteria for established postmenopausal osteoporosis.8,9 As in the original analysis, time-to-first fracture methodology was used to estimate the 3-year hip fracture incidence and observed treatment efficacy in women randomized to 2.5 mg or 5.0 mg risedronate, or to placebo. Hip fracture incidence in the placebo group in our analysis and in the overall ITT population was compared in order to further delineate the efficacy of risedronate in the subgroup of women eligible for inclusion in our study.
The HIP study3 was a 3-year, double-blind, placebo-controlled, randomized study conducted between November 1993 and April 1998 at 183 study centers in Europe, North America, New Zealand, and Australia. Ambulatory postmenopausal women were enrolled in two groups: (1) women aged 70 to 79 years on the basis of low BMD; and (2) women aged ≥80 years on the basis of low BMD (16% of the group population) or the presence of ≥1 non-skeletal, fall-related risk factors (58% of the group population) which, at the time, were documented to predispose the patient to osteoporosis-related hip fracture (see Table 1).3,10,11 These risk factors included difficulty standing from a sitting position; poor tandem gait; fall-related injury during the previous year; psychomotor score of ≤5 on the Clifton Modified Gibson Spiral Maze Test;11 current smoking or smoking during the previous 5 years; maternal history of hip fracture; previous hip fracture; and/or hip-axis length of ≥11.1 cm.
Table 1.
Criteria for enrollment in the HIP study3
| Age 70–79 years | Age ≥ 80 years |
|---|---|
| Femoral neck T-scorea ≤ −4 | Femoral neck T-scorea ≤ −4 |
| or | or |
| Femoral neck T-scorea ≤ −3 plus ≥1 clinical risk factor for hip fractureb | Femoral-neck T-scorea ≤ −3 plus hip-axis length ≥11.1 cm |
| or | |
| ≥1 non-skeletal risk factor for hip fractureb |
Bone mineral density at the femoral neck more than 4 or 3, as applicable, standard deviations below the mean peak value in young adults. For purposes of enrollment, femoral neck T-scores were calculated according to the manufacturer’s reference data base for the densitometer. The femoral-neck T-scores at baseline were later recalculated according to reference data from nhAnes III.10
Difficultystanding from a sitting position; poor tandem gait; fall-related injury during the previous year; psychomotor score of ≤5 on the Clifton ModifiedGibson Spiral Maze Test;11 current smoking or smoking during the previous 5 years; maternal history of hip fracture; previous hip fracture; and/or hip-axis length of ≥11.1 cm.
Women were randomized to daily treatment with 2.5 mg or 5.0 mg risedronate, or an identical-appearing placebo. All women received 1000 mg elemental calcium daily, and those with baseline serum 25-hydroxyvitamin D < 40 nmol/L were given vitamin D ≤ 500 IU daily.
All women underwent physical examination at the beginning and end of the study. Information about adverse events was collected at regular intervals during the study. The study design, patients enrolled, and methods have been described in detail previously.3
Measurement of efficacy
The primary end point of the HIP study was the incidence of radiographically confirmed hip fractures. Baseline vertebral fracture status was determined by examination of spinal radiographs according to published methods.12
Statistical analysis
The HIP study ITT population comprised 9331 women who had received ≥1 dose of either risedronate or placebo. Kaplan-Meier survival (time-to-first fracture) estimates were used to calculate the incidence of hip fracture, and the log-rank test was used to assess the significance of differences between the treatment groups. Proportional-hazards analysis was used to estimate the relative risk (with the 95% confidence interval [CI]) of hip fracture in the risedronate group compared with the placebo group. All tests were two-sided.
Results
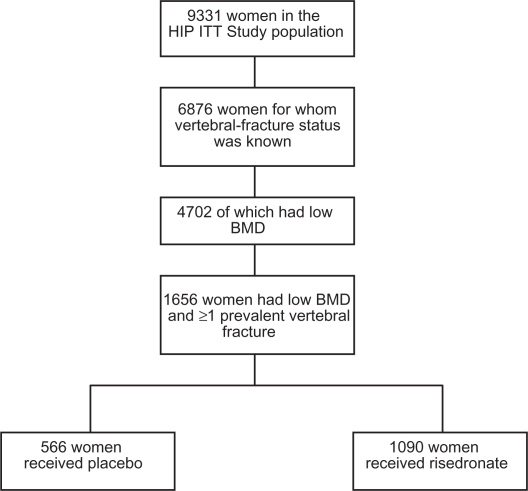
Out of 9331 women enrolled in the original study, 6876 had available and evaluable vertebral fracture status of which 4702 had low BMD. A total of 1656 women had low BMD and at least one prevalent vertebral fracture and were, therefore, eligible for analysis. Of these, 1090 received risedronate and 566 received placebo (see Figure 1). Baseline characteristics of the placebo and treatment groups were similar, and were comparable to the baseline characteristics of the original ITT population (Table 2).
Figure 1.
Study population.
Abbreviations: BMD, bone mineral density; ITT, intention to treat.
Table 2.
Demographic and baseline characteristics
|
Women with established osteoporosisa |
||
|---|---|---|
| Placebo n = 566 | Risedronate n = 1090 | |
| Age, years | ||
| Mean ±s D | 77 ± 5 | 77 ± 5 |
| ≥80 years, n (%) | 122 (22) | 250 (23) |
| Time since menopause, years, mean ± SD | 31 ± 9 | 31 ± 9 |
| Height, cm, mean ± SD | 155.1 ± 7.2 | 154.6 ± 7.0 |
| Weight, kg, mean ± SD | 58.8 ± 10.6 | 58.1 ± 10.4 |
Femoral neck T-score NHANES III ≤ −2.5 and ≥ 1 prevalent vertebral fractures.
Abbreviation: SD, standard deviation.
Hip fracture occurred in 7.4% of women receiving placebo in our study compared with 3.9% of women receiving placebo in the ITT population of the HIP study, confirming that women with established osteoporosis are at higher risk of fracture than are those of the same age who do not have established osteoporosis.
In women with established osteoporosis, the incidence of hip fracture in those receiving risedronate was 3.8% compared with 7.4% in women receiving placebo. Compared with placebo, risedronate 2.5 mg or 5.0 mg once daily reduced the risk of hip fracture by 46% (relative risk [RR] 0.54; 95% CI 0.32–0.91; P = 0.019) in women aged 70–100 years with established osteoporosis (Table 3).3 This was a numerical larger risk reduction than that found in the overall ITT population from the HIP study (RR 0.7; 95% CI 0.6–0.9; P = 0.02) (Table 3).
Table 3.
Cumulative hip fracture incidence (Kaplan-Meier) 0–3 years and hip fracture efficacy of risedronate
|
ITT population from the HIP study3 |
Women with established osteoporosisa |
|||
|---|---|---|---|---|
| Placebo n = 3134 | Risedronate n = 6197 | Placebo n = 566 | Risedronate n = 1090 | |
| Hip fracture incidence, % | 3.9 | 2.8 | 7.4 | 3.8 |
| Hip fracture efficacy | ||||
| RR (95% CI) | – | 0.7 (0.6–0.9) | – | 0.54 (0.32–0.91) |
| P-value | 0.02 | 0.019 | ||
Femoral neck T-score NHANES III ≤ −2.5 and ≥ 1 prevalent vertebral fractures.
Abbreviations: ITT, intention to treat; CI, confidence interval; RR, relative risk.
In an analysis of the women included in our study, the proportion of women who had any adverse event, who had a serious adverse event, or who withdrew because of an adverse event was similar regardless of treatment group. The incidence of death among women in our study was similar regardless of treatment group.
Discussion
Our analysis represents the first and only evidence to suggest that an oral bisphosphonate is effective in reducing the risk of hip fractures in elderly women aged up to 100 years. The HIP study demonstrated that, compared with placebo, risedronate significantly reduced the risk of hip fracture in the overall ITT population by 30% (RR 0.7; 95% CI 0.6–0.9; P = 0.02).3 In women aged 70–79 years with low BMD, risedronate reduced the risk of hip fracture by 40% (RR 0.6; 95% CI 0.4–0.9; P = 0.009), and in those with low BMD and a prevalent vertebral fracture by 60% (RR 0.4; 95% CI 0.2–0.8; P = 0.003). In contrast, active treatment did not significantly reduce the incidence of hip fracture compared with placebo among women aged ≥80 years (RR 0.8; 95% CI 0.6–1.2; P = 0.35). However, most (58%) of the women aged ≥80 years were recruited solely according to the presence of at least one non-skeletal risk factor, and only a minority (16%) were recruited on the basis of a T-score ≤ −4 at the femoral neck (manufacturer’s reference database for the densitometer). The present study demonstrates that risedronate is efficacious in older postmenopausal women with established osteoporosis, reducing the risk of hip fracture by 46% compared with placebo. This significant reduction in fracture risk occurred in a high-risk group of patients, with a placebo fracture incidence of 7.4% (almost double that of patients receiving placebo in the overall ITT population [3.9%]).
The HORIZON-PFT is the only other study to demonstrate the efficacy treatment with a bisphosphonate (zoledronic acid infused once yearly) in reducing the risk of hip fracture as a primary end point in older (aged 65–89 years, mean 73) post-menopausal women. Over 3-years, hip fracture incidence was reduced from 2.5% in the placebo group to 1.4% with annual infusions of zoledronic acid (RR 0.59; 95% CI 0.42–0.83; P = 0.002).4
It has also been documented that drug treatment aimed at improving the bone component of fracture risk is effective when patients have well-defined bone-related risk. For example, in the Fracture Intervention Trial (FIT-1), in women aged 55 to 81 years with low BMD and at least one vertebral fracture, alendronate was shown to significantly reduce the risk of hip fracture, which was captured as a secondary end point in the prospective study.13 However, in the FIT-2 trial, in patients with low BMD and without baseline vertebral fracture, alendronate was not shown to reduce the risk of hip fractures.14 In the Treatment of Peripheral Osteoporosis (TROPOS) study, strontium ranelate did not significantly reduce the incidence of hip fracture in the overall ITT population at 3 years (RR 0.85; 95% CI 0.61–1.19; P = 0.333).15 In a post hoc analysis, strontium ranelate was shown to significantly reduce the risk of hip fractures at 3-years compared with placebo in a high fracture-risk subgroup of women aged ≥74 years with a T-score of ≤ −3.0 (≤−2.4 NHANES III) (RR 0.64; 95% CI 0.41–1.00; P = 0.046).15 A study to demonstrate the efficacy of ibandronate included women aged 55–80 years.16 Ibandronate was not shown to reduce non-vertebral fractures in the overall study population but did demonstrate fracture risk reduction in a post hoc analysis in a high-risk group of patients with low BMD.16 Hip fractures were not assessed separately. Taken together, these findings support the hypothesis that drug treatment which focuses on the bone component of fracture risk is more effective if bone-related risks are present and documented.
In addition to addressing the bone component of fracture risk, it is worth noting that most hip fractures result from a fall or stumble,17 and an older individual’s increased fracture risk reflects not only their reduced bone strength, but also an increased propensity to fall and a loss of protective reflexes.1,18 Some authors have, therefore, suggested that the focus of fracture prevention should be shifted from the treatment of osteoporosis to the prevention of falls.19 In the USA, the Surgeon General guideline on fracture prevention advises an integrated approach to prevent fractures, including lifestyle advice, fall prevention advice, and drug treatment to prevent fractures when appropriate.20 However, this should be in addition to, rather than instead of, osteoporosis treatment.21 The hypothesis that a combined bone- and fall-related approach reduces fracture risk more than when only bone-directed drug treatment is given, still needs to be documented. Although there are some data demonstrating the efficacy of focused or multifactorial fall-prevention programs on fall risk,22 none have demonstrated an effect on fracture prevention.23
Our study provides evidence for the efficacy of an oral bisphosphonate in a group of older postmenopausal women at highest risk of fracture. However, there are several limitations to be considered. As 98% of women in the HIP study were white, our results do not necessarily apply to older postmenopausal women with severe osteoporosis in other racial groups. Our study also shares the limitations of all subgroup analyses, especially when performed post hoc, including diminished power to detect real differences, increase in the variance around the mean estimate, and increasing statistical likelihood of a false finding.24 These limitations are, however, mitigated by the fact that hip fracture prevention was the primary end point in the HIP study and the inclusion of a large, clearly defined subgroup of the ITT population in our analysis.
The findings of this analysis show that risedronate is effective in reducing the risk of hip fracture in elderly women aged up to 100 years with established osteoporosis. It is also acknowledged, particularly in the elderly, that falls are associated with an increased fracture risk. However, it remains to be shown that the addition of fall prevention measures to bone-directed therapy will have further benefit for fracture prevention.
Acknowledgments
The authors received editorial/writing support in the preparation of this manuscript from The Alliance for Better Bone Health (Procter and Gamble Pharmaceuticals and sanofi-aventis US, Inc.). Betty Thompson, PhD, from Excerpta Medica provided editorial and writing assistance. The authors were fully responsible for all content and editorial decisions and received no other financial support or other form of compensation related to the development of the paper.
Footnotes
Disclosures
TM: has received financial support to attend conferences and for research from the following companies: Merck, Procter & Gamble, Roche, Novartis, Shire, Servier, Strackan; he has also sat in Advisory Board meetings for the above mentioned companies.
MM: has received research grants and/or consulting fees from Amgen, Lilly, Merck, Novartis, Procter & Gamble and sanofi-aventis.
PG: has received research grants and/or consulting fees from Amgen, Lilly, Merck, Roche, Servier, Novartis, Procter & Gamble, sanofi-aventis, Wyeth, Schering-Plough and Abbott.
Declaration of funding sources
The study was funded by Procter and Gamble Pharmaceuticals and sanofi-aventis. All authors had access to the data necessary for this analysis.
References
- 1.van Helden S, van Geel AC, Geusens PP, Kessels A, Nieuwenhuijzen Kruseman AC, Brink PR. Bone and fall-related fracture risks in women and men with a recent clinical fracture. J Bone Joint Surg Am. 2008;90(2):241–248. doi: 10.2106/JBJS.G.00150. [DOI] [PubMed] [Google Scholar]
- 2.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367(9527):2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 3.McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women Hip Intervention Program Study Group. N Engl J Med. 2001;344(5):333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 4.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 5.Boonen S, McClung MR, Eastell R, El-Hajj Fuleihan G, Barton IP, Delmas P. Safety and efficacy of risedronate in reducing fracture risk in osteoporotic women aged 80 and older: implications for the use of antiresorptive agents in the old and oldest old. J Am Geriatr Soc. 2004;52(11):1832–1839. doi: 10.1111/j.1532-5415.2004.52506.x. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 7.Chang KP, Center JR, Nguyen TV, Eisman JA. Incidence of hip and other osteoporotic fractures in elderly men and women: Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 2004;19(4):532–536. doi: 10.1359/JBMR.040109. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization WHO Technical Report Series 843. Assessment of fracture risk and its application to screening for post-menopausal osteoporosis. Report of a WHO Study Group. Available from: http://whqlibdoc.who.int/trs/WHO_TRS_843.pdf Accessed July 21, 2009. [PubMed]
- 9.Kanis JA, Glüer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11(3):192–202. doi: 10.1007/s001980050281. [DOI] [PubMed] [Google Scholar]
- 10.Looker AC, Johnston CC, Jr, Wahner HW, et al. Prevalence of low femoral bone density in older US women from NHANES III. J Bone Miner Res. 1995;10(5):796–802. doi: 10.1002/jbmr.5650100517. [DOI] [PubMed] [Google Scholar]
- 11.Pattie AH, Gilleard CJ. Manual of the Clifton Assessment Procedures for the Elderly (CAPE) London: Hodder and Stoughton Educational; 1979. [Google Scholar]
- 12.Melton LJ, 3rd, Lane AW, Cooper C, Eastell R, O’Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3(3):113–119. doi: 10.1007/BF01623271. [DOI] [PubMed] [Google Scholar]
- 13.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348(9041):1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 15.Reginster JY, Seeman E, De Vernejoul MC, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90(5):2816–2822. doi: 10.1210/jc.2004-1774. [DOI] [PubMed] [Google Scholar]
- 16.Chesnut CH, III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization WHO Technical Report Series 921. Prevention and management of osteoporosis. Report of a WHO Scientific Group. Geneva: 2003 [PubMed] [Google Scholar]
- 18.Parker M, Johansen A. Hip fracture. BMJ. 2006;333(7557):27–30. doi: 10.1136/bmj.333.7557.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Järvinen TL, Sievänen S, Khan KM, Heinonen A, Kannus P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ. 2008;336(7636):124–126. doi: 10.1136/bmj.39428.470752.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services . Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of The Surgeon General; 2004. [Google Scholar]
- 21.British Orthopaedic Association, British Geriatrics Society . The Care of Patients with Fragility Fracture. London: BOA; 2007. [Google Scholar]
- 22.Gates S, Fisher JD, Cooke MW, Carter YH, Lamb SE. Multifactorial assessment and targeted intevention for preventing falls and injuries among older people in community and emergency care settings: a systematic review and meta-analysis. BMJ. 2008;336(7636):130–133. doi: 10.1136/bmj.39412.525243.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JT, Morton SC, Rubenstein LZ, et al. Interventions for the prevention of falls in older adults: systematic review and meta-analysis of randomised clinical trials. BMJ. 2004;328(7441):680. doi: 10.1136/bmj.328.7441.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sleight P. Debate: Subgroup analyses in clinical trials: fun to look at – but don’t believe them! Curr Control Trials Cardiovasc Med. 2000;1(1):25–27. doi: 10.1186/cvm-1-1-025. [DOI] [PMC free article] [PubMed] [Google Scholar]