Abstract
Context
Generalized social phobia (GSP) is characterized by fear/avoidance of social situations. Previous studies have examined the neural responses in GSP to one class of social stimuli, facial expressions. However, studies have not examined the neural response in GSP to another equally important class of social stimuli, the communication of praise or criticism.
Objective
To examine the neural response to receipt of praise or criticism in GSP; specifically, to determine whether patients with GSP show an increased response to the receipt of both praise and criticism and whether self-relevance modulates this relationship.
Design
Case-control study.
Setting
Government clinical research institute.
Participants
Unmedicated individuals with GSP (n=17) and age-, IQ-, and sex-matched healthy comparison individuals (n=17).
Main Outcome Measure
Blood oxygenation level–dependent signal, as measured via functional magnetic resonance imaging. During functional magnetic resonance imaging scans, individuals read positive (eg, You are beautiful), negative (eg, You are ugly), and neutral (eg, You are human) comments that could be either about the self or about somebody else (eg, He is beautiful).
Results
Hypothesized significant group×valence×referent interactions were observed within regions of the medial prefrontal cortex and bilateral amygdala. In these regions, the patients with GSP showed significantly increased blood oxygenation level–dependent responses, relative to comparison individuals, to negative comments (criticism) referring to themselves. However, in contrast, there were no significant group differences with respect to negative comments referring to others or neutral or positive comments referring to self or others.
Conclusions
These results implicate the medial prefrontal cortex, involved in the representation of the self, together with the amygdala, in the pathophysiology of GSP. Further, findings demonstrate a meaningful effect of psychological context on neural-circuitry hyperactivity in GSP.
Generalized social phobia (GSP) is characterized by fear/avoidance of social situations and fear of being judged negatively by others. It is the most common anxiety disorder in the general population, with the lifetime prevalence estimated at 13.3%,1,2 and it is associated with a high risk for depression, alcohol and drug abuse, and suicide.1,3
Unsurprisingly, given the disorder's core features, laboratory-based studies with GSP have typically involved the presentation of social stimuli, particularly facial expression.4-13 The behavioral and physiological literature emphasize GSP-related difficulties specifically in the processing of negative valence expressions. However, imaging studies find more generalized perturbation in facial expression processing. Thus, functional magnetic resonance imaging (fMRI) studies have indicated that GSP involves increased activity in several regions, including the amygdala and anterior cingulate, to various facial expressions, including harsh,11 angry,4,5,8,9 fearful,4,5 disgusted,6 happy,8 and neutral5,10 expressions. That is, GSP appears to involve increased responsiveness to social stimuli in emotion-relevant brain regions regardless of valence.
There are complexities, however, inherent in the use of facial stimuli. In particular, attention modulates the response to facial expressions both in healthy individuals14-16 and patients.17,18 Moreover, abnormalities in a region critically implicated in GSP, the amygdala, influence attention to emotional-expression stimuli.19,20 Indeed, patients with GSP have been observed to show anomalous attentional responses21,22 to facial stimuli. Finally, based on the nature of hypersensitivities in GSP, all face types, even those expressing neutral or positive emotion, have the potential to convey threatening information to patients with GSP. Therefore, the degree to which current findings in GSP implicate valence-specific perturbations remains unclear.
The goal of the current study was to examine response of patients with GSP to another class of social signal: receiving language-based praise or criticism. Such stimuli can be subtly, but precisely, manipulated to probe for specific hypersensitivities in GSP by manipulating target variables while keeping other parameters constant. Thus, it is possible through simple adjective substitutions (eg, ugly, beautiful) to alter dramatically a sentence's valence and potential threat relevance in GSP. Similarly, by manipulating only subjective personal pronouns (eg, you, she), it is possible to precisely manipulate whether a sentence is self-referential or not. Studies with healthy populations examining self-referential–type processing have demonstrated an effect of self-processing as well as mentalizing about other people's mental states on engagement of the medial prefrontal cortex (MPFC).23-29 Previous work on information-processing biases suggests that GSP involves an abnormal tendency to retrospectively ruminate30,31 and to appraise the self negatively, based on either self-generated or external cues.31 Therefore, given prior data implicating the MPFC in self-referential reasoning,23-28 work on information-processing biases indirectly implicates the MPFC in the pathophysiology of GSP. Moreover, emerging evidence more directly implicates MPFC hyperactivity in pediatric anxiety disorders, specifically during self-referential tasks.17 However, to our knowledge, self-referential processing has not been investigated in patients with GSP.
In short, we used a novel verbal comment–based paradigm to implement a 2 (referential target: self or other) ×3 (valence: negative, neutral, and positive) ×2 (group: GSP, healthy comparison [HC]) design. This enabled us to address the following 2 principal questions: First, do patients with GSP show an increased response to the comments regardless of the comments’ valence? Second, do patients with GSP show an increased response to the comments regardless of the comments’ self-relevance? The core descriptor of GSP involves fear of being evaluated negatively by others, and prior work demonstrates increased sensitivity to negative social feedback, specifically, on conditioning tasks.32 Accordingly, we hypothesize that GSP involves hyperresponsiveness, specifically, to self-referential criticism. If so, patients with GSP will show, specifically to self-referential criticism, increased responses within the MPFC and possibly emotion-relevant regions, such as the amygdala. However, the current study uses a novel paradigm, and prior work demonstrates a link between praise and negatively experienced embarrassment, as well as an increased propensity for embarrassment in GSP. Therefore, it also is possible that patients with GSP show atypically increased responses to self-referential praise. If so, one would expect patients with GSP to show increased responses within the MPFC and possibly emotion-relevant regions, such as the amygdala, to both self-referential criticism and praise, as opposed to criticism in particular. This study tests these contrasting predictions.
METHODS
SUBJECTS
This study included 17 patients with GSP and 17 HC individuals, group matched on age, sex, and IQ (Table 1). Subjects were recruited from advertisements approved by the National Institute of Mental Health institutional review board.
Table 1.
Subject Characteristics
| Mean (SE) |
|||
|---|---|---|---|
| Patients With GSP (n=17) | Healthy Subjects (n=17) | P Value | |
| Age, y | 35.1 (2.47) | 29.7 (2.28) | .12 |
| Sex, No. | .49 | ||
| F | 6 | 9 | |
| M | 11 | 8 | |
| Race, No. | |||
| White | 14 | 13 | |
| African American | 2 | 3 | |
| Asian | 1 | 1 | |
| IQ | 115.6 (2.80) | 120.4 (2.55) | .22 |
| LSAS-SR | 61.4 (5.09) | 18.9 (3.17) | <.001 |
| BAI | 7.4 (1.77) | 2.9 (0.88) | <.05 |
| IDS | 10.1 (1.73) | 4.2 (0.95) | <.01 |
Abbreviations: BAI, Beck Anxiety Inventory; GSP, generalized social phobia; IDS, Inventory of Depressive Symptomatology; LSAS-SR, Liebowitz Social Anxiety Scale self-report.
Subjects with GSP had to meet criteria for GSP according to the DSM-IV (1994) criteria based on the Structural Clinical Interview for DSM-IV Axis I disorders33 and a confirmatory clinical interview by a board-certified psychiatrist (D.S.P.). No patient with GSP had another Axis I diagnosis; all were currently medication-free. Healthy comparisons were excluded if they had a history of any psychiatric illness. All subjects were in good physical health, as confirmed by a complete physical examination, and provided written informed consent. Patients with GSP reported significantly greater depression, social anxiety, and general anxiety than the HCs (Table 1).
BEHAVIORAL TASK
Subjects viewed comments that varied according to referential target such that the comment could either be about themselves (eg, You're a genius) or about somebody else (eg, She's a genius). In addition, the comments could be negative (eg, You're an idiot; She's an idiot), neutral (eg, You're a human; She's a human), or positive (You're a genius; She's a genius). Thirty-two negative, 32 positive, and 32 neutral comments, matched on number of letters and words, were used in the study. Moreover, care was taken to ensure that the framing of the comments was consistent across the 3 valences (eg, You are sexy looking; You are ugly looking; You are average looking). Prior to scanning, subjects were told that they would view different comments and that the comments could either be about themselves or somebody else. They were told to think about somebody whose opinion they really care about saying the comments. For each comment, regardless of referent target or valence, subjects were simply required to press a button with their left hand when they had read the comment. The task involved no feedback. Each comment was presented for 2500 milliseconds with a 500-millisecond interstimulus interval and was presented in a fully randomized order within each run, such that the time at which any specific comment occurred was random throughout the experiment. In addition, for each experimental run, 34 trial-length fixation points were presented between the stimuli (4 at the beginning of the run, 4 at the end of the run, and 26 randomized throughout the run). These stimuli provided an implicit baseline against which all other events could be contrasted. Moreover, the inclusion of such “null events,” which occurred randomly throughout the run, further ensured that the timing of each experimental stimulus event occurred randomly. This excluded the possibility that timing-related factors differentially influenced neural responses to one or another event class.
In addition to 34 null events, each run included 8 negative comments about the self, 8 negative comments about a male other, 8 negative comments about a female other, 8 neutral comments about the self, 8 neutral comments about a male other, 8 neutral comments about a female other, 8 positive comments about the self, 8 positive comments about a male other, and 8 positive comments about a female other, resulting in a total of 72 comments per run. Thus, the 72 comments and 34 null events combined to produce runs of 106 stimuli. Subjects completed 4 randomly presented runs.
Following EPI acquisition, subjects rated each individual comment on a 7-point Likert scale, according to how the comments made them feel, where 1=extremely unhappy, 4=neither unhappy nor happy, and 7=extremely happy.
fMRI PARAMETERS
Whole-brain blood oxygen level–dependent (BOLD) fMRI data were acquired using a 1.5-T GE MRI scanner (GE Medical Systems, Milwaukee, Wisconsin). Following saggital localization, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence (matrix=64×64 mm, repetition time=3000 milliseconds, echo time=30 milliseconds, field of view=240 mm [3.75×3.75×4-mm voxels]). Images were acquired in 31 contiguous 4-mm axial slices per brain volume, with each run lasting 5 minutes 18 seconds. In the same session, a high-resolution T1-weighed anatomical image was acquired to aid with spatial normalization (3-dimensional spoiled gradient recalled acquisition in a steady state, repetition time=8.1 milliseconds, echo time=3.2 milliseconds, flip angle=20°, field of view=240 mm, 124 axial slices, thickness=1.0 mm, 256×256 acquisition matrix).
Data were analyzed within the framework of the general linear model using Analysis of Functional Neuroimages (AFNI).34 Both individual and group-level analyses were conducted. The first 4 volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. Motion correction was performed by registering all volumes in the EPI data set to a volume collected close to acquisition of the high-resolution anatomical data set.
The EPI data sets for each subject were spatially smoothed (isotropic 6-mm kernel) to reduce variability among individuals and generate group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each point by the mean signal intensity of that voxel for each run and multiplying the result by 100, producing regression coefficients representing the percentage of signal change. Regressors for 6 comment categories (self negative, self neutral, self positive, other [he/she] negative, other [he/she] neutral, other [he/she] positive) were created by convolving the train of stimulus events with a γ-variate hemodynamic response function. Linear regression modeling was performed using these regressors plus regressors for a first-order baseline drift function. This produced, for each voxel and each regressor, a β coefficient and its associated t statistic.
Voxel-wise group analyses involved transforming single-subject β coefficients into the standard coordinate space of Talairach and Tournoux.35 Subsequently, a 2 (group: GSP, HC) ×2 (referential target: self, other) ×3 (valence: negative, neutral, positive) analysis of variance (ANOVA) was performed to produce statistical maps of the main effect of group and valence and group×valence interaction (P<.005). In addition, and in accordance with our hypotheses, we applied an anatomically defined amygdala mask to tests of the group×referential target×valence interaction (at P<.05). To correct for multiple comparisons for the whole-brain analysis at P<.005, we performed a spatial clustering operation using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) with 1000 Monte Carlo simulations taking into account the entire EPI matrix. This procedure yielded a minimum cluster size of 6 voxels (337.5 mm3) with a mapwise false-positive probability of P<.05, corrected for multiple comparisons.
After observing hypothesized group differences, post hoc analyses were performed to facilitate interpretations. For these analyses, the average percentage of signal change was measured across all voxels within each region of interest (ROI) generated from the functional mask, and data were analyzed using appropriate follow-up tests within SPSS (SPSS Inc, Chicago, Illinois).
FUNCTIONAL CONNECTIVITY
We conducted a psychophysiological interaction analysis (http://afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html) to examine connectivity between the amygdala and MPFC in the 3-way group×referential target×valence interaction. Each individual subject's time series was converted to common Talairach space according to his or her structural data set. The first eigenvariate time series was extracted across all voxels within each of the amygdala ROIs generated from the 3-way group×referential target×valence interaction mask. The BOLD signal at the seed region was deconvolved using an assumed form of γ impulse response function implemented in AFNI before creating the interaction term. To examine activation specifically related to each of the individual 6 events, the average signal across the 5 other events was used as a covariate in the correlation analysis. The proportion of the variation in the signal that could be explained by the interaction between the seed and each of the 6 event types was determined by squaring the resulting correlation coefficient associated with the interaction regressor. Correlation coefficients were converted to a gaussian variable using a Fisher transformation formula to reduce the skew and normalize the sampling distribution. Subsequently, a 2 (group: GSP, HC) ×2 (referential target: self, other) ×3 (valence: negative, neutral, positive) ANOVA was performed to produce a statistical map of the 3-way group×referential target×valence interaction (P<.005). After observing hypothesized group×referential target×valence interaction in the MPFC, post hoc analyses were performed to facilitate interpretation of these differences. For these post hoc analyses, the average percentage of signal change was measured across all voxels within the MPFC ROI generated from the functional mask, and the data were analyzed using appropriate follow-up tests within SPSS.
RESULTS
EPI DATA
Blood oxygenation level–dependent response data were analyzed by a 2 (group: GSP, HC) ×2 (referential target: self, other) ×3 (valence: negative, neutral, positive) ANOVA. First, the main interaction with respect to our predictions (group×referential target×valence) is described, providing a test of our a priori hypothesis. Next, we briefly consider the secondary interaction with respect to our predictions (group×referential target).
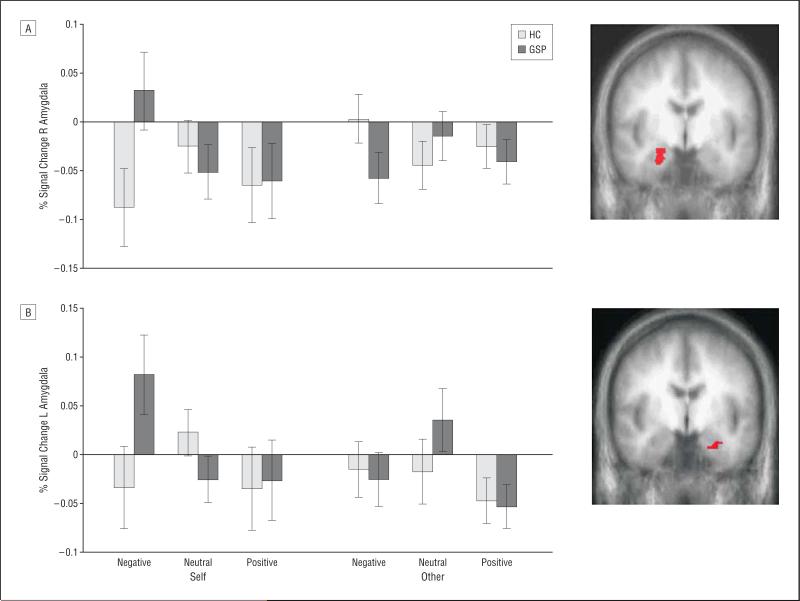
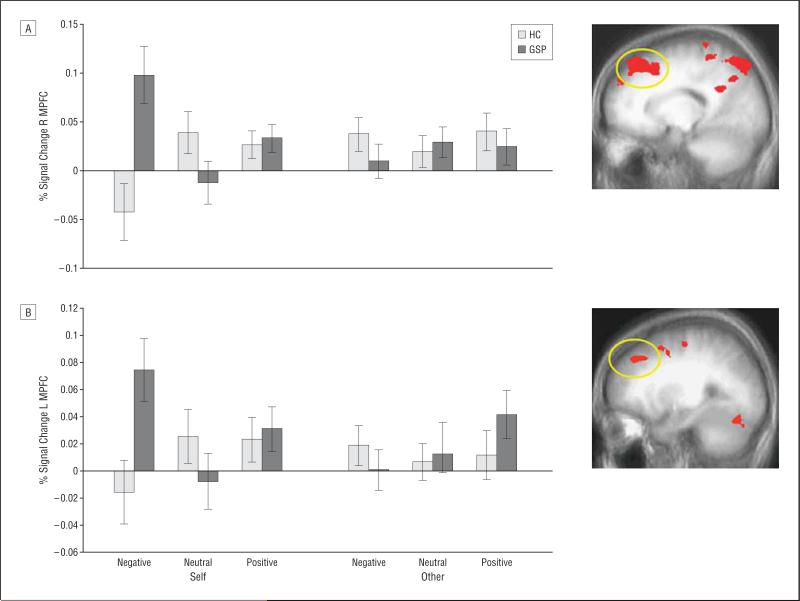
Our principal interest was to determine whether patients with GSP show atypically increased BOLD responses to self-referential criticism within the MPFC and amygdala. Consistent with this hypothesis, the 3-way group×referential target×valence interaction was significant, both in the MPFC and bilateral amygdala (amygdala significant at P<.05, uncorrected for multiple comparisons) (Table 2)(Figure 1 and Figure 2). In line with predictions, patients with GSP showed significantly greater BOLD responses in all regions to negative comments about the self (self negative) relative to the HCs (range, P < .01-.001 for the MPFC regions; P < .05, for the right amygdala; P=.056, for the left amygdala). However, the 2 groups did not differ significantly in the BOLD response for any of the regions for any of the other comments categories (self neutral; self positive; other negative; other neutral; other positive) (Table 2). Importantly, the significant group difference in response to negative comments about the self was not due to deactivations in the HCs; BOLD responses to negative and neutral comments about the self did not differ significantly in that group (F=1.61 and F=1.67; P=.22 and P=.21 for the left and right amygdala, respectively, and F=2.14 and F=3.94; P=.16 and P=.06 for the left and right MPFC, respectively) (Figure 1 and Figure 2).
Table 2.
Significant Areas of Activation for the Group×Referential Target×Valence Interactiona
| Region | BA | mm3 | x | y | z | F Value |
|---|---|---|---|---|---|---|
| R medial prefrontal cortex | 9 | 380 | 18 | 49 | 33 | 8.18 |
| R medial prefrontal cortex | 8 | 16 828 | 16 | 35 | 48 | 12.53 |
| L medial prefrontal cortex | 8/9 | 822 | –26 | 35 | 38 | 9.78 |
| R middle frontal gyrus | 6 | 1212 | 27 | –9 | 61 | 10.18 |
| L anterior cingulate gyrus | 24 | 749 | –13 | 3 | 44 | 8.49 |
| L amygdalab | 147 | –23 | –3 | –22 | 3.32 | |
| R amygdalab | 471 | 24 | –2 | –22 | 3.71 | |
| L precentral gyrus | 4 | 638 | –17 | –23 | 54 | 9.89 |
| L postcentral gyrus | 3 | 727 | –41 | –23 | 50 | 9.99 |
| R precuneus | 7 | 13 113 | 9 | –51 | 54 | 12.99 |
| L precuneus | 7 | 911 | –9 | –51 | 53 | 11.50 |
Abbreviations: BA, Brodmann area; L, left; R, right.
All activations are effects observed in whole-brain analyses significant at P<.005 corrected for multiple comparisons (significant at P<.05).
Significant at P<.05 uncorrected for multiple comparisons.
Figure 1.
Interactions of group×referential target×valence in the amygdala. Bold oxygen level–dependent responses within the right (R) amygdala (A) (x, y, z=24, –2, –22) and left (L) amygdala (B) (x, y, z=–23, –3, –22) to negative, neutral, and positive comments about the self or somebody else for the 2 groups. HC indicates healthy comparison; GSP, generalized social phobia.
Figure 2.
Interactions of group×referential target×valence in the medial prefrontal cortex (MPFC). Bold oxygen level–dependent responses within the right (R) MPFC (A) (x, y, z=16, 35, 48) and left (L) MPFC (B) (x, y, z=–26, 35, 38) to negative, neutral, and positive comments about the self or somebody else for the 2 groups. HC indicates healthy comparison; GSF, generalized social phobia.
Independent of statement valence, the group×referential target interaction identified 3 regions (Table 3). The 2 groups did not differ significantly in their responses to comments about others. However, in line with predictions, the patients with GSP showed significantly greater BOLD responses to comments about the self relative to the HCs in the MPFC/Brodmann area (BA) 10 (F=13.01; P<.005; x, y, z=–27, 76, 13). There was also a significant group×referential target interaction in the right parahippocampal gyrus/amygdala; the patients with GSP again showed significantly greater BOLD responses to comments about the self relative to the HCs (F=9.38; P<.005). These 2-way interaction effects, observed in both regions, primarily reflected increased activation to self-referential negative comments, as reflected in the 3-way group×referential target×valence interaction (see earlier).
Table 3.
Significant Main Effect and Interaction Areas of Activation for Group and Referential Target and Valencea
| Region | BA | mm3 | x | y | z | F Value |
|---|---|---|---|---|---|---|
| Moderated by referential target: self > other | ||||||
| L inferior frontal gyrus | 45 | 3904 | –41 | 22 | 17 | 26.10 |
| R middle frontal gyrus | 45/46 | 1033 | 50 | 25 | 23 | 16.54 |
| L medial prefrontal cortexb | 9 | 321 | –20 | 36 | 25 | 10.72 |
| L middle frontal gyrus | 6 | 752 | –35 | –3 | 47 | 24.64 |
| L middle temporal gyrus | 22/41 | 9186 | –44 | –43 | 9 | 33.41 |
| R superior temporal gyrus | 22 | 2993 | 45 | –21 | –5 | 22.04 |
| R superior temporal gyrus | 21/38 | 456 | 49 | 2 | –10 | 19.11 |
| L supramarginal gyrus | 588 | –60 | –57 | 33 | 20.31 | |
| L lingual gyrus | 19 | 614 | –28 | –56 | –1 | 21.81 |
| R lingual gyrus | 19 | 439 | 28 | –73 | –9 | 14.27 |
| Moderated by valence | ||||||
| Negative = neutral > positive | ||||||
| L inferior frontal gyrus | 47 | 4184 | –42 | 15 | 24 | 11.93 |
| L uncus/amygdala | 444 | –28 | –6 | –28 | 8.20 | |
| L superior temporal gyrus | 22 | 435 | –50 | –1 | –3 | 9.70 |
| Negative > neutral > positive | ||||||
| L declive | 975 | –37 | –66 | –19 | 9.00 | |
| L superior temporal gyrus | 21/22 | 3079 | –43 | –32 | –1 | 11.44 |
| Group×referential target interaction | ||||||
| L medial prefrontal cortex | 10 | 2529 | –27 | 76 | 13 | 25.46 |
| R parahippocampal gyrus/amygdalab | 175 | 44 | 38 | 41 | 13.95 | |
| R parahippocampal gyrus | 535 | 19 | –21 | –12 | 20.55 | |
| Group×valence interaction | ||||||
| L inferior frontal gyrus | 47 | 388 | –22 | 28 | 0 | 7.67 |
| L middle frontal gyrus | 6 | 461 | –30 | 5 | 41 | 7.67 |
| L cingulate gyrus | 31 | 1765 | –20 | –50 | 28 | 11.97 |
| L parahippocampal gyrus | 466 | –25 | –27 | –8 | 7.38 | |
| R declive | 504 | 40 | –68 | –17 | 8.68 | |
| L thalamus | 2289 | –11 | –29 | 5 | 9.28 | |
| R supramarginal gyrus | 39 | 504 | 37 | –47 | 26 | 10.58 |
| R precuneus | 31 | 487 | 18 | –48 | 30 | 9.80 |
| R middle occipital gyrus | 19 | 1242 | 42 | –77 | 4 | 10.40 |
| Referential target×valence interaction | ||||||
| R middle frontal gyrus | 6 | 1559 | 16 | –2 | 57 | 7.89 |
| L middle frontal gyrus | 10 | 574 | –32 | 41 | 23 | 8.69 |
| R medial prefrontal cortex | 10 | 432 | 25 | 48 | 23 | 7.08 |
| L precentral gyrus | 6 | 1517 | –56 | 4 | 14 | 10.53 |
| R postcentral gyrus | 5 | 855 | 14 | –40 | 59 | 7.79 |
| L superior temporal gyrus | 40 | 807 | –54 | –48 | 21 | 9.39 |
| R inferior parietal lobule | 40 | 548 | 65 | –35 | 36 | 8.78 |
Abbreviations: See Table 2.
All activations are effects observed in whole-brain analyses significant at P<.005 corrected for multiple comparisons (significant at P<.05).
Significant at P<.005 uncorrected for multiple comparisons.
PSYCHOPHYSIOLOGICAL INTERACTION FUNCTIONAL CONNECTIVITY ANALYSIS
The results from the interactions suggest that amygdala-MPFC relationships are important in task-related group-specific differences. If true, one would expect group differences in amygdala-MPFC connectivity specifically on trials involving self-referential negative comments. We therefore used psychophysiological interaction to measure amygdala-MPFC functional connectivity, using the amygdala ROIs from the 3-way group×referential target×valence interaction.
The results from the 2 (group: GSP, HC) ×2 (referential target: self, other) ×3 (valence: negative, neutral, positive) ANOVA involving the left amygdala identified a region within the MPFC (F=7.71; P<.005; x, y, z=17, 29, 33) proximal to those identified by our main ANOVA, where there was a significant 3-way group×referential target×valence interaction. In line with predictions, the strength of amygdala-MPFC connectivity was significantly greater for the GSP group relative to the HC group to negative comments about the self (self negative) (F=4.91; P<.05). However, the 2 groups did not differ significantly in the amygdala-MPFC connectivity for any of the other comments categories (F=0.32-4.00; P=.57-.053). There was no significant 3-way group×referential target×valence interaction for right amygdala–MPFC connectivity at P<.005.
EPI–BEHAVIORAL MEASURES CORRELATIONAL ANALYSIS
Using correlational analysis, we examined whether there was a significant relationship between level of symptoms in GSP as indexed by the Beck Anxiety Inventory, Liebowitz Social Anxiety Scale, or Inventory of Depressive Symptomatology and amygdala or MPFC activation to negative or positive, relative to neutral, comments about the self or others. Following correction for multiple comparisons, there was no significant correlation involving any of the regions for any of the comments categories or scales (Pearson r range=±0.013 to 0.571).
BEHAVIORAL DATA
Ratings and reaction time (RT) data collected after scanning were analyzed by separate 2 (group: GSP, HC) ×2 (referential target: self, other) ×3 (valence: negative, neutral, positive) ANOVAs. For ratings, there was a significant main effect of valence (F=289.24; P<.001); subjects rated the negative comments as significantly more unpleasant (F=213.28; P<.001) and the positive comments as significantly more pleasant (F=217.03; P<.001) relative to the neutral comments (mean [SE], negative, 2.37 [0.09]; positive, 5.42 [0.09]; neutral, 4.21 [0.06]) (Table 4). There was also a significant main effect of referential target (F=10.79; P<.005); subjects rated the comments about themselves more positively than the comments about others (mean [SE], self, 4.04 [0.04]; other, 3.95 [0.03]). There was a significant valence×group interaction (F=4.42; P<.05) and a trend toward the GSP group rating the negative comments as significantly more unpleasant (F=3.11; P<.05, 1-tailed) than the HC group. The 2 groups did not differ significantly in their ratings of neutral comments. There was also a significant referential target×valence interaction (F=59.07; P<.001); subjects rated positive and neutral comments about the self significantly more pleasant (F=65.17 and F=4.31; P<.001 and P<.05, respectively) and negative comments significantly more unpleasant (F=50.85; P<.001) than comments about others (Table 4). We also had group×referential target×valence interaction effects. Patients with GSP rated self-referential negative comments as significantly more unpleasant than the HCs (F=4.35; P<.05; mean [SE], GSP, 1.77 [0.10]; HC, 2.16 [0.16]). However, the 2 groups did not differ significantly in their ratings of neutral or positive comments about the self or negative, neutral, or positive comments about others.
Table 4.
Ratings and RTs for the 6 Comment Categories
| Mean (SE) |
||||
|---|---|---|---|---|
| Ratings |
RTs |
|||
| Comment Category | GSP | HC | GSP | HC |
| Self negative | 1.77 (0.10) | 2.16 (0.16) | 1079.14 (84.58) | 1165.91 (50.56) |
| Self neutral | 4.34 (0.13) | 4.15 (0.05) | 1102.29 (92.63) | 1092.73 (45.10) |
| Self positive | 6.19 (0.10) | 5.66 (0.17) | 1115.67 (75.21) | 1118.79 (44.31) |
| Other negative | 2.66 (0.19) | 2.89 (0.13) | 1056.11 (82.79) | 1100.10 (51.19) |
| Other neutral | 4.25 (0.11) | 4.09 (0.05) | 1100.61 (95.93) | 1071.04 (44.31) |
| Other positive | 5.08 (0.18) | 4.74 (0.14) | 1073.15 (89.45) | 1085.15 (54.15) |
Abbreviations: GSP, generalized social phobia; HC, healthy comparison; RTs, reaction times.
For RTs, there was a significant main effect of referential target (F=5.25; P<.05); RTs to comments about the self were significantly higher than RTs to comments about others (mean [SE], self, 1112.42 [45.87] milliseconds; other, 1081.03 [49.62] milliseconds). There were no other significant main effects, and there were no significant interactions (F= 0.09-2.02; P=.93-.65) (Table 4). Finally, no correlations emerged between any RT index and neural response within those regions of the frontal cortex identified in other analyses (Pearson r range, ±0.001 to 0.444; P=.99-.009, not significant after correction for multiple comparisons).
COMMENT
The current study addressed 2 questions on the nature of BOLD responses to self- or other referential comments of a critical, neutral, or complimentary nature. First, does GSP-related hyperresponsiveness to social stimuli occur for any valence language or is it specific to negative comments? Second, is it generalized or is it for self-referential language only? Our data indicated that GSP-related hyperresponsiveness occurs specifically to negative comments, particularly negative self-referential comments.
Previous work implicates emotion-relevant hyperresponsiveness in GSP for one class of social stimuli, facial expressions.4-6,8,9,11,36 Specifically, research shows that GSP involves greater responding than in healthy subjects to a variety of facial expressions, including negative (harsh, angry, fearful, or disgusted),4-6,8,9,11 positive,8 and neutral5,10 expressions. However, little work has directly examined the neural response to other classes of social stimuli in GSP. Because neutral or positive valence faces might convey threat to patients with GSP, alternative stimulus classes may allow more refined explication of valence effects. The current data demonstrate such effects. Specifically, herein we extend the previous work using faces by showing emotion-relevant increased responses in GSP to another class of social stimuli, language-based praise and criticism. We also extend the previous work by showing that hyperresponsiveness occurs specifically to self-referential criticism rather than to broader classes of stimuli.
Prior work shows that self-directed praise or criticism can increase embarrassment. Given that embarrassment is a concern in GSP, one might expect patients with GSP to show greater BOLD responses than healthy subjects to self-directed praise as well as criticism. However, this was not seen. For all regions identified by group×referential target×valence interactions, patients with GSP showed significantly increased BOLD responses, relative to HCs, only to self-directed criticism. Interestingly, valence ratings provided by the 2 groups also demonstrated more negative ratings in GSP only for self-directed criticism. Arousal ratings collected subsequent to the study from 15 additional healthy individuals who did not participate in the fMRI study showed no significant difference between the arousal ratings for the positive and negative comments. These data suggest that our results are unlikely to be affected by differential levels of baseline arousal for positive and negative comments.
Previous work has reported increased amygdala response in patients with GSP to facial expressions.4,5,11 In the current study, we found similarly increased amygdala BOLD response in the patients with GSP relative to the HCs to self-referential criticism, another class of social stimuli. However, we also observed strong selective differential responses in GSP to self-referential criticism within the MPFC (BA 8, BA 9). Such MPFC differences may reflect primary amygdala-activation differences, with reverberating influences on the MPFC further emphasizing the critical role of the amygdala in GSP. Alternatively, given that prior work implicates the MPFC in self-representations,23-29 the MPFC may modulate amygdala engagement to initiate and maintain aspects of GSP. Thus, GSP-related dysfunction may, at least partly, reflect negative attitude toward the self, particularly in response to social stimuli, as instantiated in the MPFC.
In this regard, there are data to suggest that a subdivide might be made between the ventral and dorsal regions of the MPFC, with the ventral MPFC particularly associated with self-referential/relevant processing24,26-28,37 and the dorsal MPFC associated more with mentalizing about other people's mental states.37-40 In the current study, patients with GSP, relative to HCs, showed significantly elevated responses to self-referential criticism in more dorsal regions of the MPFC (x, y, z=18, 49, 33; 16, 35, 48; and –26, 35, 38). This may suggest enhanced fostering of representation of the other individuals’ mental states, particularly when patients with GSP manifest concern about others’ views of the patient. However, future work is clearly needed to further investigate this issue to determine the role and importance of MPFC functioning in GSP. Future work also should consider the other factors that might account for the differences observed herein. For example, monitoring of physiological parameters, such as carbon dioxide or heart rate during EPI acquisition, might reveal the degree to which the BOLD differences observed herein reflected differences in peripheral physiology. Nevertheless, prior studies suggest that such physiological differences are unlikely to account for the current results. That is, prior research finds only weak associations between individual differences in social anxiety and peripheral physiology,41-43 with no evidence of such differences on a task such as the one used herein. Regardless, this issue has not received systematic investigation across different emotive conditions within a balanced factorial design.
It is worth briefly considering cognitive models of GSP. These emphasize the role of cognitive processes in the maintenance of the disorder.30,31 For example, the Clark and Wells30 model identifies 4 processes that contribute to the maintenance of this anxiety: self-schemata, self-focused attention, in-situation safety behaviors, and anticipatory and postevent processing. The fourth maintaining factor, postevent processing, appears of most relevance to the current data. Postevent processing refers to the tendency for individuals with social phobia to engage in a detailed review or “post mortem” of events following a social interaction. This conceptualization is similar to the Rapee and Heimberg31 suggestion that social anxiety is generated and maintained by retrospective rumination. Work has shown that patients with GSP maintain negative appraisals of task performance over time (in contrast to healthy individuals who show increased positivity over their performance with time44). Retrospective rumination is thought to be initiated by information elicited from external and internal cues during the social event itself.31 In the current study, the patients with GSP showed significantly heightened amygdala and MPFC responses to negative “social” self-referential appraisals. In short, it is possible that the amygdala and MPFC mediate postevent processing/retrospective rumination, maintaining a negative self-referential evaluation in response to cues (in the current case, externally generated explicit cues).
In summary, we found that the neural response in GSP to social comments was increased specifically to self-referential comments, and in particular self-referential comments that were critical. The regions implicated in this increased neural response included regions of the MPFC and the amygdala. Given that MPFC regions are involved in representations of the self, it might be suggested that these regions, together with the amygdala, play a primary role in the development and maintenance of GSP and that the pathology in the disorder at least partly reflects a negative attitude toward the self, particularly in response to social stimuli—that in GSP what engages the mind is others’ criticism. This highly context-dependent response in GSP helps constrain existing models of the disorder and may thus guide future therapeutic formulations in the treatment of the disorder.
Funding/Support
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Mental Health.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Kessler RC. The impairments caused by social phobia in the general population: implications for intervention. Acta Psychiatr Scand Suppl. 2003;(417):19–27. doi: 10.1034/j.1600-0447.108.s417.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Magee WJ, Eaton WW, Wittchen HU, McGonagle KA, Kessler RC. Agoraphobia, simple phobia, and social phobia in the National Comorbidity Survey. Arch Gen Psychiatry. 1996;53(2):159–168. doi: 10.1001/archpsyc.1996.01830020077009. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000;12(suppl 1):69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney D, Blair RJR, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. doi: 10.1176/appi.ajp.2008.07071060. [published online ahead of print May 15, 2008] doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 6.Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry. 2005;57(9):975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Straube T, Mentzel HJ, Glauer M, Miltner WH. Brain activation to phobia-related words in phobic subjects. Neurosci Lett. 2004;372(3):204–208. doi: 10.1016/j.neulet.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 8.Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52(3):163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- 9.Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004;56(12):921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9(6):1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- 11.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Res. 2007;154(1):93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res. 2006;148(1):55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ. The impact of processing load on emotion. Neuroimage. 2007;34(3):1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Pine DS, Klein RG, Mannuzza S, Moulton JL, III, Lissek S, Guardino M, Woldehawariat G. Face-emotion processing in offspring at risk for panic disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(7):664–672. doi: 10.1097/01.chi.0000162580.92029.f4. [DOI] [PubMed] [Google Scholar]
- 19.Adolphs R, Tranel D, Young AW, Calder AJ, Phelps EA, Anderson AK, Lee GP, Damasio AR. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37(10):1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 20.Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, Solak E, Abeygunawardane AI. Attention to the eyes and fear-recognition deficits in child psychopathy. Br J Psychiatry. 2006;189:280–281. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- 21.Horley K, Williams LM, Gonsalvez C, Gordon E. Social phobics do not see eye to eye: a visual scanpath study of emotional expression processing. J Anxiety Disord. 2003;17(1):33–44. doi: 10.1016/s0887-6185(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 22.Horley K, Williams LMLM, Gonsalvez CC, Gordon EE. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Res. 2004;127(12):43–53. doi: 10.1016/j.psychres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(pt 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 24.Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 25.Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H. In search of the emotional self: an FMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160(11):1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- 26.Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci. 2006;18(9):1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- 28.Seger CA, Stone M, Keenan JP. Cortical activations during judgments about the self and an other person. Neuropsychologia. 2004;42(9):1168–1177. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social Phobia: Diagnosis, Assessment, and Treatment. Guilford Press; New York, NY: 1995. pp. 69–93. [Google Scholar]
- 31.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther. 1997;35(8):741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 32.Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS, Grillon C. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am J Psychiatry. 2008;165(1):124–132. doi: 10.1176/appi.ajp.2007.06091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 34.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 35.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; Stuttgart, Germany: 1988. [Google Scholar]
- 36.Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2005;62(7):799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57(2):109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- 39.Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ’theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 40.Brunet E, Sarfati Y, Hardy-Bayle MC, Decety JA. PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11(2):157–166. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- 41.Papp LA, Gorman JM, Liebowitz MR, Fyer AJ, Cohen B, Klein DF. Epinephrine infusions in patients with social phobia. Am J Psychiatry. 1988;145(6):733–736. doi: 10.1176/ajp.145.6.733. [DOI] [PubMed] [Google Scholar]
- 42.Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biol Psychiatry. 2000;47(2):85–95. doi: 10.1016/s0006-3223(99)00222-x. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann SG, Moscovitch DA, Kim HJ. Autonomic correlates of social anxiety and embarrassment in shy and non-shy individuals. Int J Psychophysiol. 2006;61(2):134–142. doi: 10.1016/j.ijpsycho.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Abbott MJ, Rapee RM. Post-event rumination and negative self-appraisal in social phobia before and after treatment. J Abnorm Psychol. 2004;113(1):136–144. doi: 10.1037/0021-843X.113.1.136. [DOI] [PubMed] [Google Scholar]