Abstract
Functional imaging data were acquired during performance of a reward-contingency task in a unique cohort of adolescents (ages 14–18 years) who were characterized since infancy on measures of temperamental behavioral inhibition. Neural activation was examined in striatal structures (nucleus accumbens, putamen, caudate) with a known role in facilitating response to salient reward-related cues. Adolescents with a history of behavioral inhibition, relative to noninhibited adolescents, showed increased activation in the nucleus accumbens when they believed their selection of an action would affect reward outcome. Neural responses did not differ between the two groups when participants made a prespecified response that they knew would result in reward or when they produced random motor responses that they knew would not be rewarded. These results link inhibited temperament and perturbed neural responses to reward-contingency cues.
About 10 to 15% of healthy infants are highly apprehensive, vigilant, and fearful in the presence of unfamiliar people, objects, and contexts (Kagan, 1994). This temperamental quality, termed behavioral inhibition to the unfamiliar (Kagan, Reznick, Clarke, Snidman, & Garcia-Coll, 1984), reflects increased salience of novel stimuli for this group of infants. Signs of heightened reactivity to novelty can be detected reliably as early as 4 months of age (Calkins, Fox, & Marshall, 1996; Kagan & Snidman, 1991) and tend to persist across childhood. As these children mature, they display social reticence in the presence of peers (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001), are overly stressed in the face of mild challenges (Fox, Henderson, Marshall, Nichols, & Ghera, 2005), and are at an elevated risk for developing anxiety disorders (Biederman et al., 1993; Hirshfeld et al., 1992; Pine, Helfenstein, Bar-Haim, Nelson, & Fox, 2009).
Inhibited children display distinct physiological patterns. Relative to noninhibited children, they tend to have elevated baseline cortisol, higher baseline heart rate, lower heart rate variability, and greater right frontal electroencephalograph asymmetry (Calkins et al., 1996; Fox, 1991; Henderson, Marshall, Fox, & Rubin, 2004; Kagan, Reznick, & Snidman, 1987). These patterns have been attributed to enhanced reactivity of the brain's fear circuitry, particularly the amygdala (Kagan et al., 1987; Kagan, Reznick, & Snidman, 1988; Perez-Edgar et al., 2007; Schwartz, Wright, Shin, Kagan, & Rauch, 2003).
Research on behavioral inhibition has recently widened to include the study of striatal response to appetitive cues (Guyer et al., 2006). This new area of investigation suggests that behavioral inhibition is associated with potentiated responses to an array of motivationally salient stimuli, including both rewards and threats. Two studies using the monetary incentive delay (MID) task (Knutson, Adams, Fong, & Hommer, 2001) support this premise. The MID task is a performance-based paradigm in which participants either obtain a reward or avoid losses according to the speed with which they press a button. A behavioral study of college students selected as high and low in self-reported shyness (Hardin et al., 2006) revealed that the shy students had faster reaction times (RTs) to potential rewards on this task, a result suggesting enhanced reward sensitivity in this group. A functional neuroimaging study tested a group of adolescents who had been assessed for behavioral inhibition in infancy and early childhood while they performed a version of this same MID task (Guyer et al., 2006). Findings revealed greater striatal activation in response to incentives in inhibited relative to noninhibited adolescents, and this was true regardless of whether the incentive was an anticipated gain or an anticipated loss.
Given the nature of the MID task, one cannot tell whether the findings of Hardin et al. (2006) and Guyer et al. (2006) were due to the specific kind of rewarding incentives used (i.e., obtain a gain or avoid a loss) or to the inhibited group experiencing heightened concern about performing poorly on the task. It is also unclear whether such concern about task performance might generalize to simple motor responses that do not involve incentives (see Bar-Haim & Bart, 2006). Prior work in behavioral inhibition suggests that enhanced behavioral and neural responding on the MID task may indeed reflect enhanced sensitivity to circumstances that elicit performance evaluation (McDermott, Perez-Edgar, Henderson, Pine, & Fox, 2009). Nevertheless, this hypothesis remains speculative because of limitations in this task.
To clarify these issues, we relied conceptually on paradigms used previously to engage the striatum in healthy adults, applying motor tasks in which rewards were contingent or noncontingent on participants’ response (Tricomi, Delgado, & Fiez, 2004; Zink, Pagnoni, Martin-Skurski, Chappelow, & Berns, 2004). Our paradigm contained two primary conditions: one in which a prespecified motor response always produced a reward (the noncontingent condition—i.e., reward was not contingent on participants’ selection of an action because the action was prespecified) and another in which participants believed that their selection of a particular motor response had an impact on monetary gain (the contingent condition—i.e., participants believed that reward was contingent on their selection of an action, although in fact rewards were awarded randomly). In a third, control, condition, participants made simple motor responses that were not rewarded (the simple motor condition). Groups of participants who were identified as behaviorally inhibited and as noninhibited underwent functional magnetic resonance imaging (fMRI) while they performed the task. If group differences in striatal activation were driven solely by the presence of a reward, they would be manifested in both the noncontingent and the contingent conditions, but if such differences required a perceived connection between action and outcome, they would be manifested only in the contingent condition. The control condition allowed us to evaluate the possibility that group differences in motor response per se might account for group differences in neural activation patterns.
Thus, this research builds directly on prior fMRI findings in behaviorally inhibited individuals and on previous studies documenting that stimulus salience and perceived action-outcome associations influence striatal response to rewards (Tricomi et al., 2004; Zink, Pagnoni, Chappelow, Martin-Skurski, & Berns, 2006; Zink et al., 2004; Zink, Pagnoni, Martin, Dhamala, & Berns, 2003). Specifically, we tested the hypothesis that variation in early childhood temperament is related to variation in neural activation in three a priori regions of interest (ROIs) within the striatum: caudate, nucleus accumbens, and putamen. Because earlier reports suggested that individual differences in behavioral inhibition may be rooted in variability in amygdalar response to novelty (Perez-Edgar et al., 2007; Schwartz et al., 2003), we also tested for between-group differences in amygdala activation. We predicted that relative to noninhibited adolescents, inhibited adolescents would show greater striatal activation in the contingent condition, and not in the noncontingent condition; this would indicate that temperamental differences in reward processing are specific to motivated action in reward seeking and not to the simple presence of reward or sensitivity to performance evaluation. This prediction is consistent with prior findings implicating perturbed contingency monitoring in behavioral inhibition (McDermott et al., 2009). Furthermore, we predicted that there would be no group differences in the simple motor condition.
METHOD
Participants
At 4 months of age, 433 participants were screened for motor and emotional reactivity to novel visual and auditory stimuli (Calkins et al., 1996; Kagan & Snidman, 1991). Of these 433 infants, 153 with reactivity scores at the high and low extremes were selected for inclusion in a longitudinal study. When the children were 14 and 24 months of age, maternal reports of temperamental social fear (Goldsmith, 1996) and observations of children's reactivity to novel social and nonsocial stimuli (Calkins et al., 1996) were recorded. When the children were 48 months of age, maternal reports of temperamental shyness (Buss & Plomin, 1984) and observations of children's responses to unfamiliar peers (Fox et al., 2001) were recorded. Of these 153 children who were followed longitudinally, 35 were recruited in adolescence to participate in the present study, and 32 (15 females and 17 males) provided usable data (mean age at time of fMRI scan = 16.22 years, range = 14–18 years). Scale scores (one behavioral measure and one maternal-report measure at each age point) were standardized and averaged across the three ages. We selected participants who exhibited sustained inhibition or noninhibition across the different times of observation because this selection strategy ensured that we captured a stable trait, rather than a pattern of behavior that was unusual and was exhibited at only a single testing session. Using the composite scores, we classified 16 participants from the top end of the distribution as behaviorally inhibited and 16 participants from the bottom end of the distribution as noninhibited.
The inhibited group had significantly higher scores on the behavioral-inhibition composite (M = 0.57, SD = 0.63) relative to the noninhibited group (M = –0.72, SD = 0.31), t(30) = 7.42, prep < .99, d = 2.71. The inhibited and noninhibited groups did not differ on age, IQ, or male-to-female ratio (all ps > .15). Furthermore, the groups did not differ on measures of anxiety (State-Trait Anxiety Inventory—Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983; Screen for Child Anxiety Related Emotional Disorders, parent and child versions—Birmaher et al., 1997), depression (The Children's Depression Inventory—Kovacs, 1985), or psychiatric status (Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version—Kaufman et al., 1997) at the time of scanning, all ps > .40.1
Experimental Task
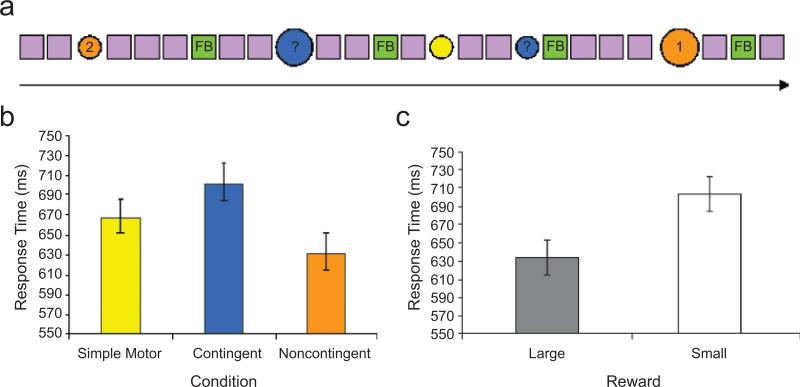
Five stimulus types were used in the experimental task: neutral cues (purple squares), three different experimental cues (circles) that were color-coded to the three experimental conditions (noncontingent, contingent, and simple motor), and cues that provided feedback on performance (green squares). The neutral cues were presented repeatedly, interrupted infrequently with experimental cues that prompted a manual response from the participant (Fig. 1a). Feedback was delivered following the contingent and noncontingent trials, which were separated by a random number of neutral stimuli. The neutral stimuli provided a baseline against which brain activation during presentation of experimental cues was compared (see Tricomi et al., 2004, for a similar approach).
Fig. 1.
Experimental design and task performance. In the reward-contingency task (a), a series of neutral stimuli (purple squares, displayed for 1,000 ms each) was interrupted randomly with infrequent experimental cues (large or small colored circles, displayed for 1,500 ms each). On noncontingent trials, the number “1” or the number “2” was presented in the center of the cue (shown here in orange), and subjects were instructed to press the button (“1” or “2”) that corresponded to the number in the circle. A correct response resulted in a small or large reward (denoted by the circle's size). On contingent trials, a question mark was presented in the center of the cue (shown here in blue), and subjects were instructed to press either button (“1” or “2”). They were led to believe that if their selected response was correct, they would receive a small or large reward (denoted by the circle's size), but in actuality, the reward was predetermined to be awarded on half the trials. On simple motor trials, the cue was always a small colored circle (shown here in yellow), and subjects were instructed to press either button (“1” or “2”). It was made explicit that no reward would be delivered on these trials. Assignment of cue colors to conditions was random across subjects. Feedback (FB; gain or no gain) and cumulative gain were provided (green box) at a variable interval following noncontingent and contingent trials. The graphs show mean response time (with standard errors of the mean) as a function of condition (b) and size of the reward (c).
The three different experimental cues signaling the noncontingent, contingent, and simple motor trials were randomly distributed throughout the task. Each condition was denoted by a uniquely colored circle (orange, blue, or yellow). Color-to-condition mapping was counterbalanced across participants. Each noncontingent cue consisted of a colored (e.g., orange) circle with “1” or “2” printed in its center. Participants were instructed to press the button (“1” or “2”) that corresponded to the number shown in the circle. They were told that these trials would always be followed by a monetary gain if they pressed the correct button. Each contingent cue consisted of a circle of a different color (e.g., blue) with a question mark printed in its center. Participants were instructed to select and press either button (“1” or “2”) and were led to believe that a correct selection would yield a monetary gain and an incorrect selection would result in no reward. In actuality, gains on these trials were determined randomly. After a variable interval (filled with neutral cues) following each noncontingent or contingent trial, feedback (gain or no gain) was provided, along with the cumulative gain for the entire session. Potential gains were of two magnitudes (3 or 6 points, each point equivalent to 10¢), denoted by the size of the experimental cue (small or large). Finally, simple motor trials were signaled by a plain circle of yet another color (e.g., yellow). Again, participants were instructed to press either of the two available buttons (“1” or “2”), but the instructions explicitly indicated that no reward would be distributed on such trials.
Functional neuroimaging data were acquired during task performance using rapid, event-related fMRI techniques. Immediately following the task, after being removed from the scanner, participants completed a series of questionnaires about the task. These questionnaires probed participants’ understanding of the task's parameters and the feelings elicited during the task.
fMRI Data Acquisition
Scanning took place in a General Electric (Waukesha, WI) Signa 3-T magnet. The task stimuli were displayed on a screen at the foot of the scanner bed via back-projection from a head-coil-mounted mirror. Foam padding in the head coil was used to restrict head movement. Participants responded to the stimuli using a handheld two-button response box in their right hand (Cedrus Lumina, San Pedro, CA).
For functional time-series image acquisition, each brain volume contained 30 interleaved 4-mm-thick sagittal slices with an isotropic in-plane voxel dimension of 3.75 mm. We used a T2*-weighted single-shot echo-planar magnetic resonance sequence with a repetition time (TR) of 2,500 ms, echo time (TE) of 23 ms, flip angle of 90°, field of view (FOV) of 24 cm, and matrix size of 64 × 64. One hundred sixty-five volumes were collected per time series.
During the same scanning session, we used a T1-weighted standardized, magnetization-prepared, spoiled gradient-recalled echo sequence to acquire a high-resolution structural image to aid in spatial normalization. The parameters for the structural image were as follows: 124 sagittal slices (1.2 mm thick) with an in-plane resolution of 0.86 mm, TR = 8,100 ms, TE = 32 ms, flip angle = 15°, number of excitations (NEX) = 1, bandwidth = 31.2 kHz, FOV = 24 cm, and matrix size = 256 × 256.
fMRI Data Analysis
For data analysis and image presentation, we used Analysis of Functional and Neural Images (AFNI) software, Version 2.56b (Cox, 1996). Visual inspection of the echo-planar images confirmed good image quality and minimal movement. Subjects with movement greater than 3 mm in any plane were excluded (n = 3). Standard preprocessing of the echo-planar-imaging data included the following steps. Each subject's time series was corrected for slice timing and motion, spatially smoothed to a 4-mm full-width/half-maximum Gaussian kernel, and transformed to percentage signal change from the mean blood-oxygenation-level-dependent (BOLD) activity of the entire time series on a voxel-wise basis.
Events defined from the experimental design and residual-motion parameters were regressed on each subject's processed time series using multiple regression (Neter, Kutner, Machtsheim, & Wasserman, 1996). The statistical regression model used was a gamma variate basis function, set to the onset of each event type, convolved with the hemodynamic response function provided in AFNI. This model included regressors for five event classes corresponding to the two levels of the contingent and noncontingent conditions, as well as the simple motor condition (i.e., large-reward, contingent condition; small-reward, contingent condition; large-reward, noncontingent condition; small-reward, noncontingent condition; and simple motor condition). We also included regressors for six residual-motion parameters: three rotational dimensions (roll, yaw, and pitch) and three translational dimensions (x, y, and z). After convolving the 11 regressors to model the hemodynamic response (Cohen, 1997), we generated whole-brain statistical t maps for each subject to determine the beta value and t statistic for each event type at each voxel. This procedure was followed by group-level, random-effects analyses of individual contrast values.
Given past data (Guyer et al., 2006; Monk et al., 2006; Perez-Edgar et al., 2007; Tricomi et al., 2004) and our a priori hypothesis, we took two approaches in the group-level analyses of the beta coefficients. First, because previous work from our lab (Guyer et al., 2006) showed increased BOLD signal with increased incentive level in regions of the striatum, we sought to determine if contingency of the outcome on the action selected influenced activity in these primary ROIs related to reward-driven behavior. We created standard masks of the right and left side of each region—the nucleus accumbens, ventral and dorsal caudate, and putamen—using anatomical boundaries from the atlas of Talairach and Tournoux (1988). The left and right amygdala were also tested. Between-group differences in these regions were assessed on a voxel-wise basis using the AFNI 3dttest procedure; contrasts of contingent versus noncontingent trials, contingent versus baseline trials, and noncontingent versus baseline trials were used to decompose a significant interaction of condition and group. Within each condition, we collapsed across size of the incentive (large, small) to increase statistical power. The contrast of simple motor versus baseline trials was also examined for group differences in order to assess potential between-group effects due to variations in motor preparation and response. Criteria for statistical significance included thresholds for both height intensity (p < .005) and spatial extent (k > 200 voxels) within ROIs. The AFNI 3dmaskave function was used to compute per-subject average activation of all voxels within each ROI for each contrast. Mean activation values within each ROI were then imported into SPSS for further analyses, and for illustrative purposes.
The second approach involved conducting exploratory whole-brain analyses to examine the degree to which between-group differences occurred more generally throughout the brain. AFNI AlphaSim (1,000 Monte Carlo simulations) was used to correct for multiple comparisons. With this algorithm, significant voxels had to exceed a criterion of p < .001, whole-brain-uncorrected, and had to be in a cluster of at least 1,173 voxels, which corresponded to a whole-brain-corrected value of p < .01. Using this threshold and the AFNI 3dttest procedure, we tested for between-group differences for the same contrasts as in the ROI analyses.
RESULTS
Behavioral Data
RTs were examined in an analysis of variance (ANOVA) with condition (noncontingent, contingent, simple motor) as a within-subjects factor and group (inhibited, noninhibited) as a between-subjects factor. This analysis revealed a main effect of condition, F(2, 60) = 14.19, prep < .99 (Fig. 1b). Follow-up contrasts indicated that mean RTon the noncontingent trials was faster than mean RT on the simple motor trials, t(31) = 2.49, prep < .93, d = 0.89, which in turn was faster than mean RT on the contingent trials, t(31) = 2.90, prep < .96, d = 1.04. Neither the main effect of group nor the Condition × Group interaction approached statistical significance, Fs < 1.
To assess the potential effect of incentive size on RT, we conducted an additional ANOVA with condition (noncontingent, contingent) and incentive size (large, small) as within-subjects factors and group (inhibited, noninhibited) as a between-subjects factor (size of the cue circle did not vary on the simple motor trials, and these trials were therefore not included in this analysis). The results again revealed that the contingent condition elicited slower RTs than did the noncontingent condition, F(1, 30) = 30.13, prep < .99. In addition, large incentives elicited faster RTs than did small incentives (Fig. 1c), F(1, 30) = 4.29, prep < .88. There were no differences in task performance between the inhibited and noninhibited groups, and none of the interactions approached statistical significance (all Fs < 1).
The postscan questionnaires included a series of yes/no questions and a series of ratings on 10-point scales. Responses to these items provided information about how subjects understood the task and how they felt during scanning. No group differences were detected on the following key questions: “I could tell when I was going to win,” “I had a hard time deciding which button to press,” and “I had a strategy to decide which button to choose,” all ts < 1.45. In addition, participants were queried on the meaning of each of the cues, and responses indicated that all participants had a good understanding of what the cues signified.
Functional Imaging Data
ROI Analyses
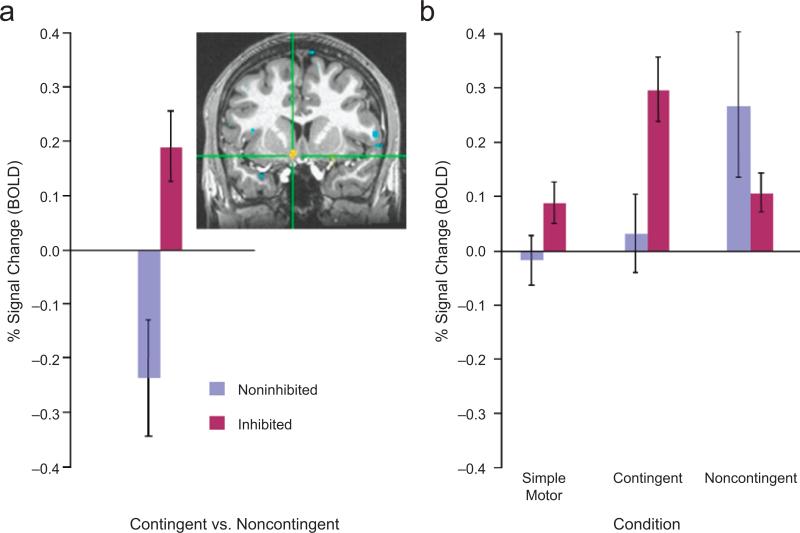
Based on our a priori hypotheses, our main analysis focused on whether the groups differed in change in activation within the three a priori striatal ROIs (nucleus accumbens, caudate, and putamen) during the contingent relative to the noncontingent trials. A significant Group (inhibited, noninhibited) × Condition (noncontingent, contingent) interaction revealed greater activation in the left nucleus accumbens in the inhibited group than in the noninhibited group, t(30) = 3.34, prep < .98, d = 1.22 (Fig. 2a). Post hoc between-group contrasts for activation in each condition relative to the baseline (neutral) condition indicated that the inhibited group showed significantly greater left nucleus accumbens activation than the noninhibited group only when participants believed that the action they selected determined reward outcome (contingent trials), t(30) = 2.74, prep < .95, d = 1.00 (Fig. 2b). The groups did not differ in their activation in the noncontingent condition, t(30) < 1. An additional between-group contrast for the motor-only trials was nonsignificant, t(30) = 1.58, prep < .79, d = 0.58, as were the contrasts related to the other preselected ROIs (Table 1).
Fig. 2.
Results from the region-of-interest analysis of activation in the nucleus accumbens. The graph in (a) shows the average percentage of blood-oxygenation-level-dependent (BOLD) signal change (with standard errors of the mean) in 253 voxels of the nucleus accumbens cluster that surpassed the threshold of p < .05 in the analysis of the Condition (contingent vs. noncontingent) × Group (inhibited vs. noninhibited) interaction. Percentage signal change is shown in separate bars for the inhibited and noninhibited groups. The inset depicts a coronal slice at y = 8 mm, showing in yellow the location of this cluster (maxima of the Talairach coordinates: x = –7, y = 8, z = –9). The graph in (b) presents the mean percentage signal change (with standard errors of the mean) in the three experimental conditions relative to the baseline (neutral) condition, separately for the two groups.
TABLE 1.
Results From the Region-of-Interest Analyses of the Condition (Contingent vs. Noncontingent) × Group (Inhibited vs. Noninhibited) Interaction: Percentage of Blood-Oxygenation-Level-Dependent Signal Change and Statistical Results
| Left hemisphere |
Right hemisphere |
|||||
|---|---|---|---|---|---|---|
| Brain region | % signal change | t(30) | p | % signal change | t(30) | p |
| Nucleus accumbens | 0.41 | 4.10 | .0001 | 0.22 | 1.97 | .08 |
| Caudate (dorsal) | –0.09 | –1.22 | .23 | –0.08 | –1.04 | .31 |
| Caudate (ventral) | 0.08 | 0.83 | .42 | 0.05 | 0.60 | .55 |
| Putamen | –0.03 | –0.58 | .56 | –0.04 | –0.93 | .36 |
| Amygdala | –0.09 | –0.88 | .78 | –0.04 | –0.49 | .63 |
Exploratory Whole-Brain Analyses
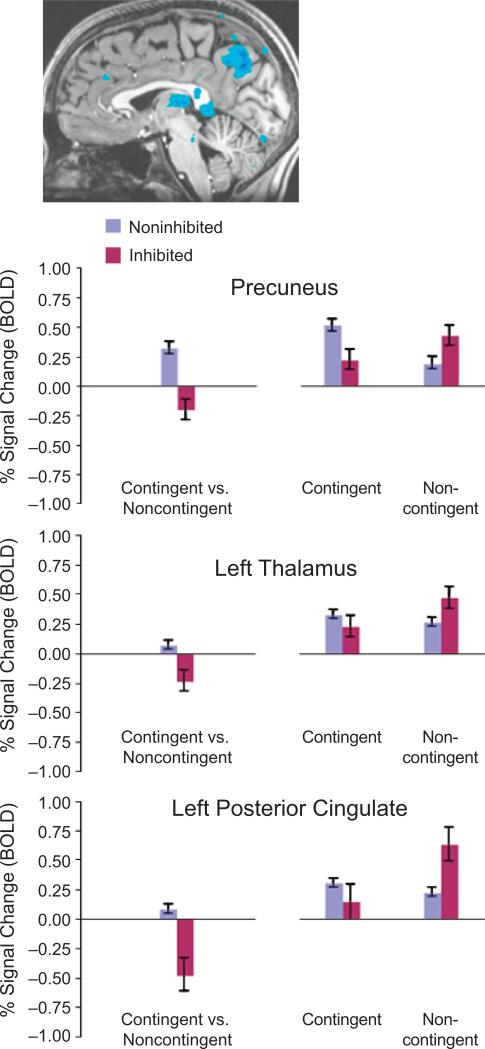
Exploratory whole-brain analyses were conducted for the Condition (noncontingent, contingent) × Group (inhibited, noninhibited) interaction to examine the degree to which between-group differences occurred more generally throughout the brain. The nonstriatal brain activations that emerged from these analyses are reported for completeness only and are not a focus of discussion. Brain areas with activation patterns that survived a Monte Carlo simulation correcting for multiple comparisons are summarized in Table 2 and Figure 3.
TABLE 2.
Results From the Whole-Brain Analyses of the Condition (Contingent vs. Noncontingent) × Group (Inhibited vs. Noninhibited) Interaction: Brain Regions Surviving the Monte Carlo Correction
| Talairach coordinates |
Cluster size (k) | |||||
|---|---|---|---|---|---|---|
| Brain region | x | y | z | t(30) | p | |
| Precuneus | 0 | –69 | 37 | 5,560 | –5.96 | .0001 |
| Left thalamus | –3 | –24 | 9 | 2,085 | –3.87 | .0005 |
| Left posterior cingulate | –1 | –43 | 4 | 7,618 | –3.51 | .0015 |
Note. Talairach coordinates and t and p values refer to the peak voxel in each identified significant cluster (p < .01, minimal cluster size = 1,173 voxels). Cluster size (k) indicates the number of voxels within each significant cluster.
Fig. 3.
Results from the whole-brain analyses. The illustration at the top depicts a sagittal slice showing, in blue, areas of significant activation in the precuneus, left thalamus, and left posterior cingulate, as revealed in the analysis of the Condition (contingent vs. noncontingent) × Group (inhibited vs. noninhibited) interaction. For each region, the graph on the left shows the average percentage of blood-oxygenation-level-dependent (BOLD) signal change in voxels that surpassed a threshold of p < .05 in the interaction analysis, and the graph on the right presents the percentage signal change in each experimental condition relative to the baseline (neutral) condition; results are shown separately for the two groups. Error bars represent standard errors of the mean.
These analyses indicate that relative to the noninhibited group, adolescents who were temperamentally inhibited in early childhood showed lower activation in the precuneus, left thalamus, and left posterior cingulate during contingent trials (i.e., when they believed that their choice of an action would affect reward outcome), and greater activation during noncontingent trials (i.e., when the action was prespecified and rewards were administered on all trials).
DISCUSSION
In this study, adolescents characterized by an enduring pattern of behavioral inhibition (documented at three time points from infancy up to age 4 years) demonstrated enhanced sensitivity of the reward-related neural system, relative to adolescents not classified as behaviorally inhibited. This sensitivity was specific to the experimental condition in which participants believed that their choice of an action determined reward acquisition. Between-group differences in neural activation did not occur in the case of systematically rewarded stimuli or simple motor responses. Taken together, these findings suggest that active agency is needed to bring to light temperament-related individual differences in striatal response. Indeed, as hypothesized, enhanced sensitivity in the inhibited group was manifest as greater nucleus accumbens activation. This group difference emerged even though behavioral performance, as indexed by RT, was comparable in the two groups.
Note that responses to the follow-up debriefing questionnaire (“Describe what each of the three different circles means”) indicated that, as intended, the participants perceived reward on the contingent trials as being affected by their choice of response. Furthermore, responses to questions assessing how subjects experienced the task did not differ between groups. These data suggest that between-group differences in subjective-emotional responses or in reward expectancy, elicited by contingent-reward cues, may not completely explain the observed between-group differences in striatal responding. However, it should be noted that our postscan measures of reward expectancy were unlikely to comprehensively capture psychological processes that occurred immediately upon presentation of the contingent-reward cues. The experimental design allowed us to probe a limited set of behaviors during the task, and future studies should examine between-group differences in the relations between reward expectancy and striatal responding, perhaps by employing alternative designs that probe more comprehensively levels of reward expectancy engaged by presentation of contingent-reward cues.
Regardless of this limitation, the behavioral data showed that the task did engage relevant psychological processes across participants. For example, the differences in RTs to the noncontingent, contingent, and motor cues, in both groups considered together, indicate that these cues were perceived and processed differently, providing further support to the efficacy of the experimental manipulation. Particularly noteworthy is the finding that RTs were longer for contingent cues than for noncontingent cues, which suggests that extra time was needed to execute a selection in the contingent condition.
When combined with prior findings, these new results provide an important framework for understanding the neural correlates of temperamental behavioral inhibition. Although theory and research on behavioral inhibition have typically focused on threat processing and the amygdala (Monk et al., 2006; Pine, 2007; Schwartz et al., 2003), the present study indicates that behaviorally inhibited individuals may also show perturbations in response to positive stimuli and in reward-related neural circuitry. In particular, the nucleus accumbens subserves numerous adaptive and goal-directed behaviors, such as feeding, drinking, sex, and exploration (Kelley, 1999; Meredith & Totterdell, 1999). The present findings suggest that inhibited temperament is related to increased brain activation when active agency is at play in the context of reward processing. This is not surprising considering that inhibited temperament is typically most apparent when active participation is required (Coplan, Rubin, Fox, Calkins, & Stewart, 1994).
Finally, hypersensitivity to contingency-related reward delivery is only one parsimonious, theoretically plausible explanation for these findings. Our findings could also be conceptualized as reflecting exaggerated concerns about performance in the inhibited group, relative to the noninhibited group. A comprehensive evaluation of these alternatives would require more in-depth investigation of relations among perceived action-reward contingency and performance-related concerns in behaviorally inhibited subjects. In this regard, it is noteworthy that levels of anxiety and depression, and psychiatric status, did not differ between the groups in our study.
The exploratory whole-brain analyses revealed between-group differences in activation in additional regions (precuneus, left thalamus, and left posterior cingulate). The activation patterns in these regions, however, were opposite to those for the nucleus accumbens, and possibly reflected additional and complementary processing required by the experimental conditions in our task. Additional work on the functional relatedness of these brain regions to reward processing is needed in order to clarify the full meaning of these preliminary findings.
In conclusion, the present findings extend understanding of behavioral inhibition by more precisely characterizing the neurocognitive processes associated with alterations in responses to reward. Our previous work demonstrated that behavioral inhibition is associated with perturbations in the appetitive-motivational system (Guyer et al., 2006; Hardin et al., 2006). In this study, we found that these perturbations were specific to the condition in which participants believed that choice of self-executed responses determined outcomes, and did not extend to the condition in which outcomes were independent of subjects’ agency. Indeed, a sense of “responsibility,” or self-agency, in a context of uncertainty (probabilistic outcomes) drives the neural system underlying appetitive motivation (i.e., nucleus accumbens) more strongly in temperamentally inhibited than noninhibited adolescents. One important next step will be to determine how the reward system interacts with fear circuitry among subjects characterized by behavioral inhibition and elevated anxiety (Frenkel, Lamy, Algom, & Bar-Haim, 2008). A further goal will be to examine behavioral inhibition and its underlying neural circuitry across development. Finally, a better understanding of factors that contribute to individual differences in reward-related processes may eventually generate insights relevant for therapeutic interventions in anxiety, a diathesis closely related to shyness and behavioral inhibition.
Footnotes
We examined whether the two behavioral-inhibition groups differed in the number of high-motor versus high-negative infants and found that they did not.
REFERENCES
- Bar-Haim Y, Bart O. Motor function and social participation in kindergarten children. Social Development. 2006;15:296–310. [Google Scholar]
- Biederman J, Rosenbaum JF, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, Kagan J. A 3-year follow-up of children with and without behavioral inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32:814–821. doi: 10.1097/00004583-199307000-00016. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Buss AH, Plomin R. Temperament: Early personality traits. Erlbaum; Hillsdale, NJ: 1984. [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, Stewart SL. Being alone, playing alone, and acting alone: Distinguishing among reticence and passive and active solitude in young children. Child Development. 1994;65:129–137. [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Fox NA. If it's not left, it's right: Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Frenkel TI, Lamy D, Algom D, Bar-Haim Y. Individual differences in perceptual sensitivity and response bias in anxiety: Evidence from emotional faces. Cognition & Emotion. 2008;23:688–700. [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the toddler behavior assessment questionnaire. Child Development. 1996;67:218–235. [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Perez-Edgar K, Guyer AE, Pine DS, Fox NA, Ernst M. Reward and punishment sensitivity in shy and non-shy adults: Relations between social and motivated behavior. Personality and Individual Differences. 2006;40:699–711. doi: 10.1016/j.paid.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Marshall PJ, Fox NA, Rubin KH. Psychophysiological and behavioral evidence for varying forms and functions of nonsocial behavior in preschoolers. Child Development. 2004;75:251–263. doi: 10.1111/j.1467-8624.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, et al. Stable behavioral-inhibition and its association with anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Kagan J. Galen's prophecy: Temperament in human nature. Basic Books; New York: 1994. [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral-inhibition to the unfamiliar. Child Development. 1984;55:2212–2225. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral-inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychological Science. 1991;2:40–44. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADSPL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. In: McGinty JF, editor. Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug abuse. Vol. 877. New York Academy of Sciences; New York: 1999. pp. 71–90. Annals of the New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI). Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith G, Totterdell S. Microcircuits in nucleus accumbens’ shell and core involved in cognition and reward. Psychobiology. 1999;27:165–186. [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Neter J, Kutner MH, Machtsheim CJ, Wasserman W. Applied linear statistical models. 4th ed. Irwin; Chicago: 1996. [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. Research review: A neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Helfenstein S, Bar-Haim Y, Nelson E, Fox NA. Challenges in the development of novel therapeutics for the treatment of childhood central nervous system disorders. Neuropsychopharmacology. 2009;34:213–228. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Verlag; Stuttgart, Germany: 1988. [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. NeuroImage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. Journal of Neuroscience. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]