Abstract
Background
Alzheimer's disease (AD) is a progressive neurodegenerative disease that places substantial burdens on those who provide support for family members with declining cognitive and functional abilities. Many AD patients eventually require formal long-term care services because of the absence, exhaustion, or inability of family members to provide care. The costs of long-term care, and especially nursing home care, often deplete private financial resources, placing a substantial burden on state Medicaid programs. Current evidence suggests that pharmacological treatments and caregiver interventions can delay entry into nursing homes and potentially reduce Medicaid costs. However, these cost savings are not being realized because many patients with AD are either not diagnosed or diagnosed at late stages of the disease, and have no access to Medicare-funded caregiver support programs.
Methods and Results
A Monte Carlo cost-benefit analysis, based on estimates of parameters available in the medical literature, suggests that the early identification and treatment of AD have the potential to result in large, positive net social benefits as well as positive net savings for states and the federal government.
Conclusions
These findings indicate that the early diagnosis and treatment of AD are not only socially desirable in terms of increasing economic efficiency, but also fiscally attractive from both state and federal perspectives. These findings also suggest that failure to fund effective caregiver interventions may be fiscally unsound.
Keywords: Alzheimer's disease, Cost-benefit analysis
1. Introduction
With the aging of the United States population, the annual incidence of Alzheimer's disease (AD) is expected to increase from approximately 377,000 in 1995 to one million by 2050 [1]. The rapid increase in AD will have profound implications for the delivery and financing of long-term care (LTC) because the oldest old with AD are the largest consumers of LTC services (especially nursing home care). Although studies estimated a wide range of total annual costs to the United States economy of AD, the most likely estimates are on the order of tens of billions of dollars [2]. Alzheimer's disease has substantial fiscal impacts internationally [3], and in the United States, influences federal and state government costs in both the Medicare and Medicaid programs. Patients with AD incur about 60% higher costs than non-AD patients in the Medicare program [4]. For states, AD patients impose a substantial cost on Medicaid programs through nursing home use. The LTC costs account for 34.6% of Medicaid spending nationally and for 42.9% in Wisconsin [5]. One approach to reducing the cost of LTC is to lower the demand for LTC services by delaying the onset or slowing the progression of AD.
Although the available therapies for AD are less than ideal, accumulating evidence indicates that they may slow the progression of the disease in some patients. In particular, therapies that slow the progression of AD, or support caregivers, have the potential to reduce the risk of nursing home placement [6,7]. A major barrier to implementing these therapies and reducing state and Medicaid LTC costs is the failure of the medical profession to diagnose and treat persons with AD. Studies suggest that between 40% to 80% of persons with dementia are undiagnosed in primary care [8–10] and, as a result, are untreated. The failure to diagnose and treat persons with AD was attributed to the lack of physicians' knowledge about dementing illnesses, the absence of cognitive screening, and the public perception that nothing can be done about the disease [11].
The early diagnosis and treatment of any dementing disorder requires that clinicians be alerted to the presence of potential cognitive problems. The United States Preventive Services Task Force recommends screening only for persons in whom cognitive impairment is already suspected, or for persons who meet certain triggers of suspicion for cognitive impairment [12]. The current recommendations against broader screening ignore the expressed wishes of older adults who, in some studies, overwhelmingly (80%) stated that they would want to know as early as possible that they had AD [13,14]. In general, current recommendations focus on the narrow clinical situation and ignore the growing need for early diagnoses that would allow for patient and caregiver interventions early in the course of the disease.
The present analysis evaluates the costs and benefits of the early identification and treatment of AD patients, using LTC cost data from Wisconsin and data about the potential benefits of pharmacologic and nonpharmacologic therapies. Are the early identification and treatment of AD patients socially desirable? Do the early diagnosis and treatment of AD offer fiscal benefits to states or the federal government? Our analysis answers these questions by predicting the net social benefits and changes in state and federal expenditures for early intervention programs, using Wisconsin as an example.
2. Methods
2.1. Modeling strategy
Our analyses proceed in two steps. First, the net social benefits and net fiscal savings to Wisconsin and the federal government are estimated, assuming early intervention with drug treatment, a program for caregivers, or both of these interventions. Net social benefits algebraically sum the monetized value of impacts of an intervention on all persons, e.g., patients, caregivers, and taxpayers. The fiscal effects are the changes in public expenditures borne by taxpayers. The large variation in AD progression and the uncertainty about a number of parameters call for a stochastic model. Because a large number of uncertainties must be considered and disease progression is irreversible, a Monte Carlo model is used.
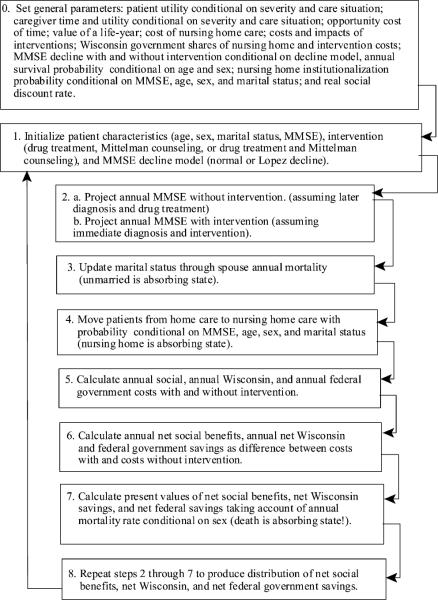
A detailed summary of the modeling strategy for the Monte Carlo trials is shown in Appendix I, and is recapped here. For each set of assumed parameters and interventions, a hypothesized cohort of identical AD patients is followed over the course of their lives. Each patient suffers a random cognitive annual decline, drawn from an appropriate distribution. The cognitive level determines the probability that a patient will be institutionalized in a nursing home, taking into account age, gender, and the presence of a spouse as caregiver. Throughout this process, patients have some probability of surviving until the next year. The experience of the cohort provides a distribution of the present values of net social benefits and fiscal savings from interventions occurring at different stages of the disease, as defined by the Mini-Mental State Examination (MMSE).
Second, the cost of identifying an AD patient is estimated by using the results of an early detection and diagnostic regime from Boustani et al [15]. This analysis incorporates the false-positive rate as well as empirical rates of voluntary participation at various stages of the diagnostic process. The predictions of the benefits of early intervention, and the predicted costs of the diagnostic program, permit an estimate of the overall net social benefits and fiscal savings that would result from the implementation of an early-stage diagnostic and treatment program.
2.2. Monte Carlo parameters
2.2.1. Overview
The Monte Carlo analyses use a number of assumptions related to the calculation of costs and benefits, and are summarized in Appendix II. Many of these assumptions are conditional on cognitive ability, as measured by the MMSE. Although many psychosocial and functional factors influence the risk of institutionalization, the model uses the MMSE because of the data available about the relationship between a given MMSE score and the outcomes on which estimates are based. The analyses classify MMSE scores of 28 to 21 as indicating mild AD, 20 to 11 as indicating moderate AD, and 10 to 1 as indicating severe AD.
Several categories of costs accrue to society during the years of survival of AD patients as a function of their MMSE score: expected nursing home institutionalization, direct costs to caregivers, reductions in the quality of life of patients, and reductions in the quality of life of caregivers. The analyses assume that the primary benefits of drug treatment accrue through reductions in costs resulting from a slower decline according to MMSE score. There may be additional benefits of drug treatment, such as improved patient behavior that reduces caregiver burden [16]. Because our analysis does not account for any effects not related to slowing disease progression, it may underestimate the benefits of drug treatment. As costs and benefits accrue over a number of years, it is necessary to discount them to present values. We do so using midyear discounting with a real discount rate of 3.5%.
2.3. Base-case assumptions
2.3.1. Survival probabilities
The analyses assume that the spouses of patients have sex-specific and age-specific annual survival probabilities, based on the most recent United States life tables [17]. Data from the Cardiovascular Health Study, which followed more than 5000 people over age 65 years for up to 10 years, estimate a hazard ratio for death of 2.1 for AD patients relative to persons with normal cognition [18]. This hazard ratio is applied to the annual survival probabilities for patients, which shortens the expected lifespan. Whereas a 65-year-old with normal cognition would live to about age 81 years on average, a 65-year-old with AD would live on average only to about 76 years. This is a conservative approach, because it is likely that the increased death rate for AD patients is larger for those at more severe stages of the disease, so that uniformly applying the odds ratio results in too many deaths during the mild and moderate stages of the disease, when interventions are most likely to be of benefit. Although one study [19] reported a possible reduction in mortality from donepezil therapy, consistent with the findings of Lopez-Pousa et al [20], we assume that drug treatment does not affect longevity.
2.3.2. Drug costs
The cost of drug treatment is approximately $5 per day or $1,825 per year, and is eligible for Medicare Part D coverage. Mays et al [21] estimated that 15% of participants will be below the doughnut hole where they bear a 25% copayment rate and 24% of program participants will fall in the doughnut hole, where they bear a copayment rate of 100%. As a base-case estimate, we assume that the state will pay the 25% copayment for 15% of the population and 100% for the 24% falling into the doughnut hole or an average of 28% of drug costs. The federal share is 72%. Note that we assume that, to ensure high levels of participation, the state pays this share of drug costs for all AD patients rather than just for those who are Medicaid-eligible.
2.3.3. Institutionalization risk
The estimates of baseline risk of nursing home institutionalization derive from Hauber et al [22]. They estimated a piecewise Cox proportional hazard model of the risk of nursing home institutionalization as a function of MMSE score for the average AD patient, using data collected by the Consortium to Establish a Registry for Alzheimer's Disease. We used their estimated models to produce risks contingent on MMSE score, sex, marital status, and age (older or younger than 72 years). Applications of their models, which were performed piecewise in terms of mild, moderate, and severe AD, required adjustments at the boundaries to maintain a monotonic increase in risk of institutionalization with declining MMSE score. Using this approach, the annual risk of institutionalization is about 1% when the MMSE score falls to 24 points and increases to over 90% when the MMSE score falls to 2 points, averaging across all demographic categories. For single older males and females, the probabilities reach 100% at MMSE scores of around 11. At the beginning of each year in our analysis, surviving AD patients are moved from the community to nursing homes, using the probabilities of Hauber et al [22]. After AD patients are institutionalized in a nursing home, they are assumed to remain there until death.
2.3.4. Caregiver costs
Patients with AD receive some care from family caregivers. Estimates of the time that caregivers provide to AD patients are derived from a study conducted by Bell et al [23]. That study estimated the average time that caregivers spend providing personal-care assistance to patients at home (or in nursing homes) as 15.4 (0.6), 44.5 (1.6), and 70.2 (2.2) hours per week for mild, moderate, and severe AD patients, respectively. In the analyses, estimates of the actual hours spent were randomly selected from uniform distributions, ranging from 50% below to 50% above the estimates of Bell et al [23]. Following common convention, these hours were monetized using the median hourly wage in Wisconsin for 2006 of $14.69.
2.3.5. Nursing home costs
The 2005 private-pay cost for a nursing home day in Wisconsin was $189, or $66,795 per year, and the Medicaid reimbursement amount was $127 per diem, or $46,355 per year [24]. In Wisconsin, on average, 23% of the Medicaid per diem is paid by patients; 40% of the remainder is paid from state funds, and 60% from federal funds. Consequently, on average, 31% of Medicaid nursing home costs are paid from state funds, or 22% of the private-pay cost. The private-pay rate is considered the social cost of nursing homes, with 22% of that amount paid by Wisconsin, and 33% by the federal government.
2.3.6. Quality of life
Changes in the quality of life of both patients and caregivers may also result from slowing the progression of the disease and changing the venue of care. The utility estimates in these analyses reflect how much a person might value the quality of a year of life in a demented versus nondemented state. Neumann et al [25] reported estimates of utilities for both patients and caregivers as a function of AD severity, and in terms of whether care is provided in the community or a nursing home: for patients in community (or nursing home) care, the utilities are 0.68 (0.71), 0.54 (0.48), and 0.37 (0.31) for mild, moderate, and severe AD, respectively. For caregivers of patients in the community (or nursing home), the utilities are 0.86 (0.86), 0.86 (0.88), and 0.86 (0.88) for mild, moderate, and severe AD, respectively. For caregivers, we used the utilities of Neumann et al [25] as base levels, adjusted for the risk of depression. Following Lave et al [26], we assigned a quality of life of 0.59 for caregivers suffering from major depression. The prevalence of depression among caregivers is about 32% [27]. Thus, caregiver utilities are assumed to be the same as the utilities of Neumann et al [25] when depression is absent (68% of the time), and 0.59 when depression is present (32% of the time).
These utilities can be applied to the statistical value of a life-year to obtain monetized, quality-adjusted life-years. The average statistical value of life for the United States population, suggested by a number of meta-analyses of empirical estimates, is about $4 million [28]. At the assumed discount rate of 0.035, this corresponds to the statistical value of a life-year as $187,000. We make the conservative assumption that the statistical value of a life-year is distributed uniformly over a range $93,500 to $187,000.
2.3.7. Rate of cognitive decline
The opportunity to initiate treatment early in AD is a primary benefit of early diagnosis. The analyses predict the impacts of early intervention with AD treatment conditional on age, sex, marital status, and initial MMSE score. An untreated AD patient will typically decline, on average, about 3 to 4 MMSE points per year. Treatment with drugs appears to slow this decline by about 1 to 2 MMSE points per year [29].
Each Monte Carlo trial that assesses drug treatment compares the MMSE path for a person both with and without drug treatment immediately after diagnosis. For each year looking forward, declines in MMSE score are randomly selected from a treatment distribution and a nontreatment distribution. Because there are no lifelong, long-term, randomized controlled trials comparing disease progression in treated and untreated patients, we estimated the effect of treatment using the two models summarized in Appendix III.
The MMSE/Lopez (L) model is based on the results of a multiyear study of 135 matched pairs of patients with probable AD for whom the primary benefit of treatment was to increase the odds (2.5) of running a slow progressive course, defined as a decline of 2 or fewer MMSE points each year [6]. On average, slow progressors had near-zero MMSE point declines (0.1 for the treatment group, and −0.2 for those in the control group). On average, fast progressors in the treatment group lost 4.0 points per year, whereas fast progressors in the control group lost 4.9 points per year. We modeled the annual decline in MMSE score by assuming that slow progressors randomly receive declines from a uniform distribution between −1 and 2 (yielding a mean decline of 0.5), and fast progressors on drug treatment randomly receive declines from a uniform distribution between 3 and 5 (yielding a mean decline of 4). Fast progressors not on drug treatment randomly receive declines from a uniform distribution between 3 and 6.8 (yielding a mean decline of 4.9). Further, the analyses randomly assign 60% of patients on drug treatment to be slow progressors, and 40% of patients not on drug treatment to be slow progressors. Overall, this yields mean declines of 1.9 and 3.1 for treatment and nontreatment, respectively. The 1.2-point difference in mean declines is conservative, in view of other studies involving drug treatment that typically found mean differences between 1.7 and 2.3 MMSE points per year [30–32].
Alternatively, the MMSE/Normal (N) model assumes that declines for those receiving drug treatment are drawn from a normal distribution with a mean of 1.5 and a standard deviation of 1.5 (with negative values set to zero), whereas declines for those not receiving drug treatment are drawn from a normal distribution with a mean of 3.5 and a standard deviation of 1.5 (with negative values set at zero). The truncation in this process yields a mean difference in decline between treated and untreated patients of approximately 1.9 MMSE points.
2.3.8. Caregiver intervention
The early detection of AD also creates the possibility of providing support services to caregivers, such that AD patients remain at home longer. Although a majority of care-givers appear to view delaying placement of loved ones in nursing homes as very important in absolute terms, as well as in terms of reducing mortality risk [33], the strain of providing care, especially for severely affected patients, has the potential to exhaust caregivers and lead to institutionalization. Various support services can be provided to caregivers to help them cope with the burdens of providing care.
We used the results by Mittelman et al [7] of a randomized trial of enhanced counseling and support intervention for spouse caregivers, to estimate the net benefits of caregiver intervention. Over an almost 10-year period, Mittelman et al [7] randomly assigned over 400 spouses of AD patients to receive either the usual care or enhanced counseling and support intervention. The enhanced counseling included 2 individual sessions, 4 family sessions, weekly support group participation, and ad hoc telephone contacts initiated by care-givers. Each counseling session involved, on average, 4.0 hours of professional time, including 0.2 hours for arrangements, 2.0 hours in actual sessions, 1.15 hours in travel time to caregiver homes, and 0.65 hours in peer review of the session. Approximately 45% of caregivers sought telephone counseling per week, with an average counseling session lasting 0.4 hours. Thus, for each participating caregiver on average, there was an initial expenditure of 24 hours of counselor's time, and an additional 9.4 hours per year of participation. Although agreement to participate in support groups (usually in the caregiver's own neighborhood) was a condition for receiving enhanced counseling, after 1 year, only 58% had joined groups, compared with 42% for those in the usual-care group.
Using a Cox proportional hazard model, the impact of enhanced counseling and support services was estimated to be a reduction in the risk of nursing home placement of 0.72, with a 95% confidence interval range of 0.54 to 0.96, or an average delay of nursing home admission by about 1.5 years [7]. In addition, Mittelman et al [7] reported a statistically significant odds ratio of 0.91 for each later year of entry into their sample. For example, those entering in the fifth year of a 10-year program have an odds ratio of 0.61, relative to those entering in the first year.
Our analysis considers the possible replication of the program studied by Mittelman et al [7], using the above assumptions, as summarized in Appendix III. Taking medical and public health social workers (Standard Occupational Code [SOC] 211022, May 2005) in Wisconsin as the employment category, and assuming that benefits comprise 30% of the total compensation (the rate for all civilian employees in June 2006), we assumed that a counselor has an annual salary of $42,290 and benefits of $18,124, for a total of $60,414 per year, or $35.05 per hour. For each AD patient and each year, we applied a random selection from the confidence interval for the odds ratio of the effect of program participation on the risk of institutionalization, adjusted for entry into the program in the fifth year (out of a possible 10 years), to the schedule according to Hauber et al [22] of the risk of nursing home institutionalization as a function of MMSE score.
Mittelman et al [34] reported reductions in caregiver depression of approximately 15.3, 5.7, and 3.8 percentage points for those receiving enhanced counseling at 1, 3, and 5 years, respectively. We applied the 3-year reduction, assuming that the risk of depression for those receiving enhanced counseling is 26.3%, rather than the 32% assumed for those not receiving enhanced counseling.
2.3.9. Induced service use
Those receiving the caregiver intervention possibly made greater use of generally available support services provided through public and private programs. Data on the utilization of these extra-treatment support services were not reported or analyzed by Mittelman et al [7]. Consequently, to predict the marginal utilization of services that likely result from implementation of a program like that of Mittelman et al [7],we used data from the Alzheimer's Disease Project, which involved the provision of case management and community-service reimbursement to a randomized treatment group [35]. In that study, treatment resulted in a 16% increase (from an average base of about 42%) in the fraction of care-givers using any homecare services, as well as a 45-hour per year increase in utilization (from a base of approximately 286 hours per year). Treatment also resulted in an 18-percentage-point increase (from an average base of about 15%) in the fraction of caregivers who used adult daycare services, as well as a 7-day-per-year increase in utilization (from an average base of approximately 166 days per year). Thus, on average, treatment resulted in an increase in homecare services utilization of about 72 hours per year [0.16 (286 hours + 45 hours) + 0.42 (45 hours)], and an increase in adult daycare utilization of about 32 days per year [0.18 (166 days + 7 days) + 0.15 (7 days)]. We monetized these service increments, using national averages of $19 per hour of in-home care and $50 per day of adult daycare, to estimate an incremental annual cost of $2968. We also assume that Wisconsin pays for all marginal services. Because it is unclear how applicable the Alzheimer's Disease Project results are to an intervention like that of Mittelman et al [7], we treated the $2968 as an upper bound. Specifically, in each Monte Carlo trial, a value was randomly drawn from a range of $0 to $2968. This approach may underestimate the social costs of the additional service use that the counseling program entails. However, the assumption that Wisconsin would pay the entire amount most likely overestimates the cost of caregiver intervention to the state.
2.3.10. Counterfactual: those not diagnosed at early stages of disease
The final set of assumptions concerns the counterfactual against which early detection and treatment is compared. Many patients who are not identified at early stages will eventually be diagnosed, and some will be treated with drugs. Based on a retrospective study of patients diagnosed with AD at a memory disorders clinic, average MMSE scores upon presentation were 20.8, 18.8, 16.8, and 15.3 for those referred from screening programs, physicians, family and friends, and other sources, respectively [36]. In a study based on over 12,000 beneficiaries in the 2002 Medicare Current Beneficiary Survey, 24.7% of dementia patients in community settings and 26.3% of dementia patients in long-term care settings received dementia drugs [37]. Drawing on these studies, we assumed that AD patients not detected at early stages of the disease will present for diagnosis at an MMSE score of 19, and have a 25% chance of receiving drug treatment.
3. Results
3.1. Monte Carlo results
Each Monte Carlo analysis produced a similar distribution for net social benefits, net Wisconsin fiscal benefits, and net federal fiscal benefits. This distribution was the basis for predicting mean values, i.e., if a large number of people with a particular set of characteristics were treated, then on average, the reported mean values would result. The means contain some sampling error for any finite number of trials. In the present analyses, 10,000 trials produced 95% confidence intervals of approximately $2000 for estimates of net social and net fiscal benefits.
Table 1 shows the impacts of various interventions for a 70-year-old married woman or man with an MMSE score of 28, 26, or 24 when diagnosed and treated. The first row within each MMSE level shows the net social and fiscal benefits, assuming drug treatment with MMSE/N decline, i.e., those receiving drug treatment experience declines drawn from a normal distribution centered around 1.5, with an standard deviation of 1.5. These impacts are substantially larger than those estimated assuming drug treatment with MMSE/L decline, as shown in the second row. The third row shows the effects of caregiver intervention, assuming MMSE/L decline for untreated patients. The final row for each MMSE level shows the combination of drug treatment and caregiver intervention, assuming MMSE/L decline.
Table 1.
Net benefits of diagnosis and treatment of a 70-year-old married woman (or man, in parentheses) with AD in $1000s
| Present Value of Net Social Benefits | Present Value of Wisconsin Fiscal Benefits | Present Value of Federal Fiscal Benefits | |
|---|---|---|---|
| MMSE = 28 at time of diagnosis | |||
| Drug (MMSE/N) | 172 (147) | 15 (12) | 28 (24) |
| Drug (MMSE/L) | 98 (84) | 6 (5) | 13 (12) |
| Caregiver intervention (MMSE/L) | 10 (7) | 4 (2) | 21 (17) |
| Drug (MMSE/L) and caregiver | 125 (101) | 16 (11) | 34 (27) |
| MMSE = 26 at time of diagnosis | |||
| Drug (MMSE/N) | 149 (129) | 13 (10) | 22 (19) |
| Drug (MMSE/L) | 94 (80) | 5 (4) | 10 (9) |
| Caregiver intervention (MMSE/L) | 11 (9) | 6 (4) | 22 (19) |
| Drug (MMSE/L) and caregiver | 116 (104) | 15 (13) | 31 (28) |
| MMSE = 24 at time of diagnosis | |||
| Drug (MMSE/N) | 122 (106) | 10 (8) | 15 (14) |
| Drug (MMSE/L) | 69 (64) | 4 (3) | 6 (6) |
| Caregiver intervention (MMSE/L) | 15 (11) | 7 (6) | 24 (20) |
| Drug (MMSE/L) and caregiver | 93 (80) | 15 (12) | 29 (25) |
As indicated in Table 1, all cells show positive net social and fiscal benefits. Caregiver intervention offers a much higher ratio of fiscal to social benefits than drug treatment alone. Keeping AD patients at any level of severity out of nursing homes saves the state and federal government money, but caregivers continue to bear time costs. There is a synergistic effect between drug treatment and caregiver intervention: drugs slow the decline in MMSE score, and care-giver intervention reduces the risk of institutionalization for any level of MMSE score.
The net social and fiscal benefits are consistently higher for a woman than for a man. This result is true for different ages and MMSE scores at diagnosis and treatment, and is attributable primarily to the higher expected years of additional life for women. The analysis in Table 1 is for AD patients with spouses at time of screening. Repeating the analysis for unmarried patients yields small reductions in net social benefits (less than $2000 on average) for both men and women.
Fig. 1 shows the distribution of net social benefits for 10,000 trials for a particular Monte Carlo analysis, assuming a drug-treatment effect (MMSE/L) for a 70-year-old married woman with a starting MMSE score of 26. Averaging across trials, the mean net social benefits are $94,000, the mean net Wisconsin fiscal savings are $5000, and the mean net federal fiscal savings are $10,000. As shown, 68.3% of the trials had positive net social benefits, i.e., whereas on average an early intervention is efficient, the net social benefits are negative in about one third of the trials. In many cases, death comes early, before the benefits of treatment-delayed decline can be fully realized. Averaging over trials, the mean age at death is 80.4 years, and the mean number of years spent in a nursing home is reduced by 1.2 years, from 7.6 years to 6.4.
Fig. 1.
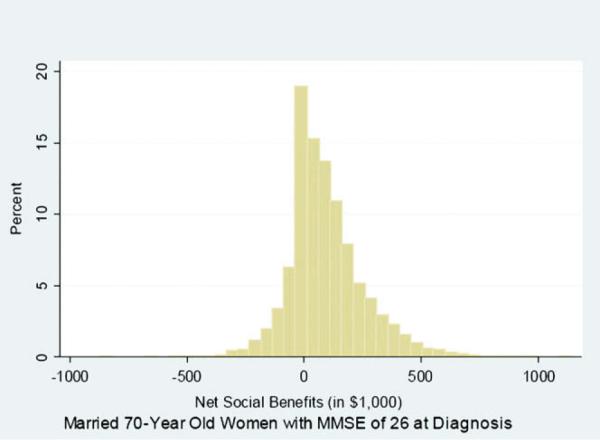
Distribution of 10,000 trials of Monte Carlo analysis, showing net social benefits of diagnosis and treatment of 70-year-old married women with MMSE score of 26 at diagnosis.
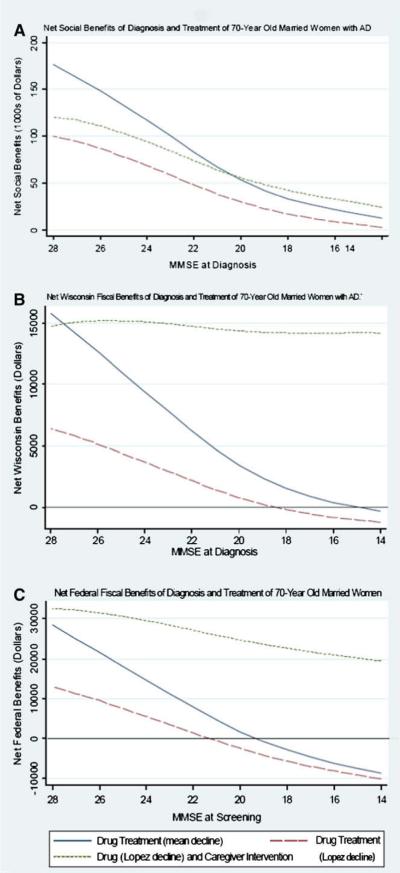
Fig. 2A–C considers the relative benefits of pharmacologic and nonpharmacologic interventions when AD is detected at different disease stages, as defined by MMSE score. Fig. 2A shows the net social benefits, Fig. 2B shows the net Wisconsin fiscal benefits, and Fig. 2C shows the net federal fiscal benefits of interventions after a diagnosis at various levels of MMSE score for a 70-year-old married woman. Assuming either MMSE/N or MMSE/L decline, drug treatment yields declining, but positive, net social benefits as MMSE scores decline from 28 to 14. Adding caregiver intervention to drug treatment (MMSE/L) increases net social benefits at each MMSE score. Repeating the analysis, assuming more effective drug treatment (in terms of MMSE/N decline) combined with caregiver intervention, would dramatically increase the social and fiscal benefits of an intervention (results not shown). The net Wisconsin fiscal benefits become negative at an MMSE score of 18, unless drug and caregiver interventions are combined. This result occurs because the benefit of caregiver intervention goes up with decreasing MMSE score, whereas the benefit of drug therapy declines. As shown in Fig. 2C, the net federal fiscal benefits of combining drug treatment and caregiver intervention yield positive net benefits even when the MMSE score at diagnosis is as low as 14.
Fig. 2.
Net social and fiscal benefits of diagnosis and treatment of 70-year old married women diagnosed at different stages of AD (A–C), as defined by MMSE score.
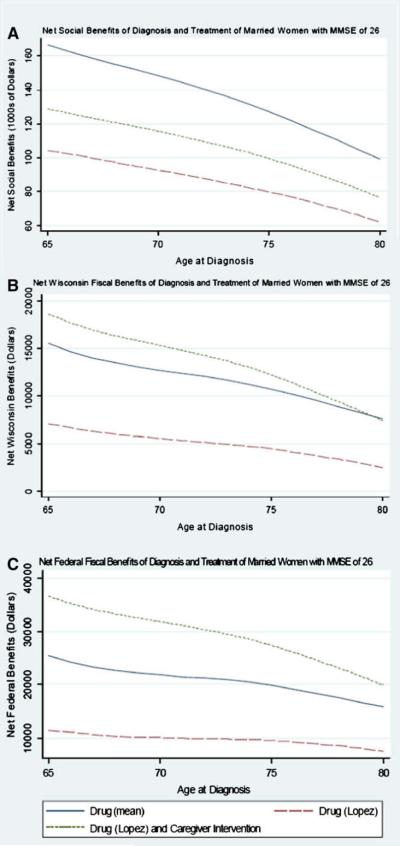
Fig. 3A shows the net social benefits of interventions as a function of age for a married woman with an MMSE score of 26 at diagnosis. As expected, the net social benefits decline with age, but remain positive. Fig. 3B,C shows similar patterns for net fiscal benefits: net Wisconsin and net federal fiscal benefits decline, but remain positive through age 80 years.
Fig. 3.
Net social and fiscal benefits of diagnosis and treatment of married women with MMSE score of 26 (A–C), diagnosed at different ages.
3.2. Early identification and diagnostic evaluation costs
An important question is whether the cost savings generated by early intervention are large enough to offset the costs associated with early identification and diagnostic evaluations. We estimated a cost per diagnosis of dementia of approximately $4000, based on estimated costs and charges as well as negative results and refusals to proceed (48% of those screening positive declined further evaluation) according to Boustani et al [15]. Assuming that 70% of those diagnosed with dementia would be further diagnosed as having AD and would be candidates for intervention, the cost per AD diagnosis was approximately $5700, which we take as an estimate of the social cost of case-finding and diagnostic costs.
Because the dementia population is primarily over 65 years old, it is Medicare-eligible. Assuming that Medicare covered all diagnostic costs, the cost to the federal government per identified AD patient would be $3170. Assuming that Wisconsin covered all other associated costs, the cost to the state per identified AD patient would be $2530.
The cost per diagnosis of this particular protocol is lower than the net fiscal Wisconsin benefits of the combination of drug treatment (MMSE/L) and caregiver intervention shown in Table 1, i.e., if Wisconsin paid all costs of implementing an early identification and caregiver intervention protocol not covered by the federal government, the combined intervention would yield overall savings to the state of approximately $10,000 per diagnosed patient.
4. Discussion
We report the results of a Monte Carlo analysis of the potential benefits of early diagnosis and treatment, using best estimates of the effects of available therapies, both pharmacologic and nonpharmacologic. These analyses suggest that the early recognition and management of persons with AD will generate cost savings. The net benefits were highest when cases were identified at earlier stages, e.g., an MMSE score of 28, and when drug therapy was combined with a caregiver intervention program [7]. We also estimated the state and federal fiscal benefits of early diagnosis and treatment, and as expected, the federal benefits were consistently more substantial than the state benefits. These results indicate that a program implemented at the national level has the potential to generate substantial cost savings to society as a whole, as well as to state and federal governments. Efforts to promote the earlier identification and better management of AD patients seem to hold promise in terms of stemming the future rise in costs associated with an increasing prevalence of AD in an aging population. More effective treatments could be expected to generate even greater cost savings than those reported here.
Our estimates of the benefits of pharmacologic therapy were based on two models: 1) one that assumes a slowing of deterioration through reductions in mean annual decline; and 2) another that uses the findings of Lopez et al [6],in which the major benefit of therapy involved increasing the likelihood that a person would have a slow progressive course. Neither of these models is based on the results of long-term randomized controlled trials (RCTs) evaluating the effects of current drug therapy. There simply are no lifelong, long-term RCTs on which to base assumptions. Because of this, these analyses report the social and fiscal savings that might be realized if available treatments had two different effects on disease course. Our analyses also assume that the use and therefore benefits of drug therapy continue to death. The model of drug benefit by Lopez et al [6] is more conservative, but if treatment is implemented early, it still generates substantial cost savings. If the benefits of future therapies were more robust and reduced the mean MMSE decline to 0.5 rather than 1.5 in the MMSE/N model, then the net social benefits would rise from $149,000 to $406,000 for a 70-year-old married woman diagnosed at an MMSE score of 26. These analyses illustrate the importance of research directed at developing more effective AD-modifying therapies.
We used a model developed by Mittelman et al [7] to estimate the potential costs and benefits of a caregiver intervention. We chose this model because its effectiveness was evaluated in RCTs lasting almost 10 years and the benefits were time-dependent, i.e., they increased with longer participation in the program. Other caregiver interventions were limited by small sample sizes [38] or by interventions lasting less than 1 year [39], and did not show reductions in nursing home use. Net social and fiscal savings were consistently larger when drug treatment was combined with caregiver intervention. These analyses confirm what is already known, i.e., that caregivers are important components of successful dementia-management programs, and should not be ignored. Current Medicare reimbursement policies do not support the development of caregiver interventions similar to the model of Mittelman et al [7].Our analyses suggest that this failure is fiscally unsound.
Our analyses suggest that the net social benefits of interventions are sufficiently large to justify even relatively expensive programs to promote early diagnosis and treatment. In view of the fiscal pressures facing states, the more relevant question is whether an early diagnosis and treatment program can be designed to yield a cost per AD diagnosis sufficiently small to make early diagnosis and treatment, including care-giver intervention, fiscally desirable from the state or federal perspective.
To answer this question, we estimated the results and costs of a dementia-diagnosis protocol, using the findings of Boustani et al [15]. We chose this protocol because we believe it represents a likely high estimate for screening costs and outcomes. The mean MMSE score in that study was 18 at time of diagnosis and 70% of the population was African-American, and as a result, findings from that study may not be generalizable to the larger population. In that study, only 52% of persons screening positive agreed to further evaluation, and of those, 47% were diagnosed with dementia. Our diagnostic cost estimates were also taken from that study, and assumed that neuroimaging and extensive neuropsychological tests were performed in all agreeing to further evaluation. Despite this conservative approach to estimating program costs for early diagnosis, the combined drug/caregiver intervention still generated cost savings. The net fiscal benefits of the combined intervention to Wisconsin were large enough to generate savings of approximately $10,000 per diagnosed patient, even if Wisconsin paid all program costs. However, we also assumed that physicians would act on the results of the diagnostic process to provide drug or caregiver interventions. This may be an overestimate, especially for persons with early AD [40].
Does our analysis suggest that we should implement population-based cognitive screening programs to promote early detection and intervention? We do not think so. We think that scarce resources could be better spent developing more effective disease-altering therapies and financing caregiver interventions that were shown to reduce costs. At present, the benefits of current therapies are marginal, Medicare does not support caregiver interventions, and access to dementia diagnostic services is limited. Until these deficiencies in AD management are resolved, population-based cognitive screening will continue to be controversial.
There are numerous arguments against cognitive screening to promote the early diagnosis and treatment of AD [41]. There is concern that many people will experience fear and anxiety about being labeled with a cognitive disorder such as AD. Studies suggest that this assumption may not be valid [13,42]. The marginal benefits of available therapies are another reason often cited for not screening. However, as illustrated in these analyses, savings do not necessary accrue simply because of pharmacologic treatment. Nonpharmacologic caregiver interventions, like the intervention of Mittelman et al [7], if made available, can offer significant savings to state and federal governments, regardless of the effectiveness of current drugs.
The analyses presented here answer two important public policy questions. First, is the early detection of AD, followed by drug treatment and caregiver support, socially desirable? The estimation of positive net social benefits provides an affirmative answer to this question. Second, from a political economy perspective, do early detection, treatment, and caregiver support offer sufficient fiscal savings to either the federal or state governments, to make these interventions politically viable in a time of fiscal austerity? The analysis also provides an affirmative answer to this question. Potentially large fiscal savings for the federal government should encourage changes in Medicare reimbursement and the present approach to dementia management. Moreover, potential fiscal savings for a state like Wisconsin should encourage the development of state-level programs, even in the absence of a national program. As states devote increasing amounts of their Medicaid dollars to LTC for AD patients, state policymakers are likely to be receptive to the potential for early intervention to reduce these expenditures. These programs could include some form of cognitive screening combined with public and professional education and improved access to dementia diagnostic services, and proven programs of caregiver support.
Over the next 5 to 10 years, emerging therapies may become more effective in slowing the course of the disease and reducing the LTC costs and caregiver burden [43]. Our analyses suggest that improving access to even marginally effective therapies and effective caregiver interventions may be not only good medicine, but also sound fiscal policy. Nevertheless, public policy as well as professional attitudes about AD will need to change from that of neglect to proactive recognition and management, if these savings are to be realized.
Acknowledgments
We thank Elizabeth Drilias and Marc Ratkovic for research assistance, and for financial support we thank the Institute for Clinical and Translational Research, the Community Academic Partnership Program, and the Robert M. La Follette School of Public Affairs (University of Wisconsin-Madison). We thank Dana Mukamel, Aidean Vining, and participants in the 2008 Workshop of the Society for Benefit-Cost Analysis for helpful comments.
Appendix I
Summary of modeling strategy for Monte Carlo trials
Appendix II
Overview of general base-case parameters
| Parameter | Assumed Value | Source |
|---|---|---|
| Annual nursing home cost | Wisconsin Department of Health and Family Services [24] | |
| Private pay, $66,795 | ||
| Medicaid, $46,355 | ||
| Weekly hours of caregiver time at home | Bell et al (2001); assumes variation of ±50% [23] | |
| Mild AD, 15.4 | ||
| Moderate AD, 44.5 | ||
| Severe AD, 70.2 | ||
| Weekly hours of caregiver time for nursing home care | ||
| Mild AD, 0.6 | ||
| Moderate AD, 1.6 | ||
| Severe AD, 2.2 | ||
| Median Wisconsin hourly wage | Center on Wisconsin Strategy (2007) | |
| $14.69 | ||
| Opportunity cost of caregiver time | Opportunity cost of leisure | |
| Median wage | ||
| Patient utility at home | Neumann et al [25] | |
| Mild AD, 0.68 | ||
| Moderate AD: 0.54 | ||
| Severe AD: 0.37 | ||
| Patient utility in nursing home | ||
| Mild AD, 0.71 | ||
| Moderate AD, 0.48 | ||
| Severe AD: 0.31 | ||
| Caregiver utility at home | ||
| Mild AD, 0.86 | ||
| Moderate AD, 0.86 | ||
| Severe AD, 0.86 | ||
| Caregiver utility for patient in nursing home | ||
| Mild AD, 0.86 | ||
| Moderate AD, 0.88 | ||
| Severe AD, 0.88 | ||
| Annual survival probability | Arias [17]; Fitzpatrick et al [18] | |
| Varies by age and sex; applies 2.1 hazard rate for AD patients | ||
| Annual nursing home institutionalization risk | Based on estimated models of Hauber et al [22] | |
| By MMSE, sex, age, and marital status | ||
| Real social discount rate | Boardman et al [28]; upper bound value of life-year consistent with $4 million statistical value of life | |
| 0.035 | ||
| Value of a life-year | ||
| Uniform distribution over $93,500 to $187,000 | ||
| Annual cost of drug treatment | Assuming $5 per day | |
| $1825 | ||
| Wisconsin share of drug costs | Assumes Wisconsin pays out-of-pocket costs under Medicare Part D; Mays et al [21] | |
| 0.28 | ||
| Wisconsin share of nursing home costs | Based on 23% average patient payment, and 40% of remainder paid by state | |
| Medicaid, 0.31 | ||
| Market, 0.22 | ||
| Counterfactual to screening | ||
| Assumed MMSE at presentation, 19 | Barker et al [36] | |
| Gruber-Baldini et al [37] | ||
| Probability of CEI upon diagnosis, 0.25 |
Appendix III
Intervention assumptions
| Drug Intervention | ||
|---|---|---|
| Mean Decline Model (MMSE/N) | Assumption | |
| Source | ||
| Annual MMSE decline without treatment | Normal distribution with a mean of 3.5 and a standard deviation of 1.5, with negative truncation | Consistent with findings by Sabbagh et al [30], Matthews et al [31], and Small et al [32] of mean decline differences across studies of between 1.7 and 2.3 MMSE points per year for untreated compared with treated |
| Annual MMSE decline with treatment | Normal distribution with a mean of 1.5 and a standard deviation of 1.5, with negative truncation | |
| Decline Model of Lopez et al [6] (MMSE/L) | ||
| Probability of being slow progressor without treatment | 0.39 | Lopez et al [6] |
| Annual decline of slow progressors without treatment | Uniform distribution over range of −1 to 2: mean, 0.5 | |
| Annual decline of fast progressors without treatment | Uniform distribution over range of 3 to 6.8: mean, 4.9 | |
| Probability of being slow progressor with treatment | 0.60 | |
| Annual decline of slow progressors without treatment | Uniform distribution over range of −1 to 2: mean, 0.5 | |
| Annual decline of fast progressors without treatment | Uniform distribution over range of 3 to 5: mean, 4.0 |
| Caregiver Intervention | Assumption | Source |
|---|---|---|
| Initial counselor time in hours | 24 | Mittelman et al [7] |
| Annual counselor time in hours | 9.4 | |
| Counselor wage and benefits | $35.05 | SOC Code 211022, May 2005, assuming 30% benefits rate |
| Odds ratio reduction in nursing home risk | Uniform draw from confidence interval of 0.54 to 0.96 | Mittelman et al [7] |
| Base annual risk of caregiver depression | 0.32 | Mittelman et al [34] |
| Annual reduction in caregiver depression risk from counseling | 0.057 | |
| Caregiver utility with depression | 0.59 | Lave et al [26] |
| Annual incremental home service use by counseled caregivers | Uniform draw from range of $0 to $2968 | Upper bound estimated from Newcomer et al (1999); assumes Wisconsin pays entire amount [35] |
References
- [1].Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169–73. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- [2].Bloom BS, de Pouvourville N, Straus WL. Cost of illness of Alzheimer's disease: how useful are current estimates? Gerontologist. 2003;43:158–64. doi: 10.1093/geront/43.2.158. [DOI] [PubMed] [Google Scholar]
- [3].Knapp M, Prince M. Dementia UK: the full report. Alzheimer's Society; London: 2007. [Google Scholar]
- [4].Taylor DH, Jr, Sloan FA. How much do persons with Alzheimer's disease cost Medicare? J Am Geriatr Soc. 2000;48:639–46. doi: 10.1111/j.1532-5415.2000.tb04721.x. [DOI] [PubMed] [Google Scholar]
- [5].Kaiser Family Foundation State health facts. 2006 Available at, www.statehealthfacts.org.
- [6].Lopez OL, Becker JT, Saxton J, Sweet RA, Klunk W, DeKosky ST. Alteration of a clinically meaningful outcome in the natural history of Alzheimer's disease by cholinesterase inhibition. J Am Geriatr Soc. 2005;53:83–7. doi: 10.1111/j.1532-5415.2005.53015.x. [DOI] [PubMed] [Google Scholar]
- [7].Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67:1592–9. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- [8].Valcour VG, Masaki KH, Curb JD, Blanchette PL. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964–8. doi: 10.1001/archinte.160.19.2964. [DOI] [PubMed] [Google Scholar]
- [9].Boise L, Neal MB, Kaye J. Dementia assessment in primary care: results from a study in three managed care systems. J Gerontol Med Sci. 2004;59A:621–6. doi: 10.1093/gerona/59.6.m621. [DOI] [PubMed] [Google Scholar]
- [10].Magsi H, Malloy T. Under-recognition of cognitive impairment. I. Assisted living facilities. J Am Geriatr Soc. 2005;53:295–8. doi: 10.1111/j.1532-5415.2005.53117.x. [DOI] [PubMed] [Google Scholar]
- [11].Boise L, Camicioli R, Morgan DL, Rose JH. Congleton L. Diagnosing dementia: perspectives of primary care physicians. Gerontologist. 1999;39:457–64. doi: 10.1093/geront/39.4.457. [DOI] [PubMed] [Google Scholar]
- [12].United States Preventive Services Task Force . The guide to clinical preventive services. Agency for Healthcare Research and Quality; Rockville, MD: 2005. [Google Scholar]
- [13].Dale W, Hemmerich J, Hill EK, Hougham GW, Sachs GA. What correlates with the intention to be tested for mild cognitive impairment (MCI) in healthy older adults? Alzheimer Dis Assoc Disord. 2008;22:144–52. doi: 10.1097/WAD.0b013e318161103c. [DOI] [PubMed] [Google Scholar]
- [14].Dale W, Hougham GW, Hill EK, Sachs GA. High interest in screening and treatment for mild cognitive impairment in older adults: a pilot study. J Am Geriatr Soc. 2006;54:1388–94. doi: 10.1111/j.1532-5415.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- [15].Boustani M, Callahan CM, Unverzagt FW, Austrom MG, Perkins AJ, Fultz B, et al. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20:572–7. doi: 10.1111/j.1525-1497.2005.0126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Geldmacher DS. Treatment guidelines for Alzheimer's disease: redefining perceptions of primary care. Primary care companion. J Clin Psychiatry. 2007;9:113–21. doi: 10.4088/pcc.v09n0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arias E. United States life tables, 2003. Natl Vital Stat Rep. 2006;54:1–40. [PubMed] [Google Scholar]
- [18].Fitzpatrick AL, Kuller LH, Lopez OL, Kawas CH, Jagust W. Survival following dementia onset: Alzheimer's disease and vascular dementia. J Neurol Sci. 2005;229/230:43–9. doi: 10.1016/j.jns.2004.11.022. [DOI] [PubMed] [Google Scholar]
- [19].Gasper MC, Ott BR, Lapane KL. Is donepezil therapy associated with reduced mortality in nursing home residents with dementia? Am J Geriatr Pharmacother. 2005;3:1–7. doi: 10.1016/j.amjopharm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [20].Lopez-Pousa S, Olmo JG, Franch JV, Estrada AT, Cors OS, Nierga IP, et al. Comparative analysis of mortality in patients with Alzheimer's disease treated with donepezil or galantamine. Age Ageing. 2006;35:365–71. doi: 10.1093/ageing/afj083. [DOI] [PubMed] [Google Scholar]
- [21].Mays J, Brenner M, Neuman T, Cubanski J, Claxton G. Estimates of Medicare beneficiaries—out-of-pocket drug spending in 2006. Kaiser Family Foundation; Menlo Park, CA: 2004. [Google Scholar]
- [22].Hauber AB, Gnanasakthy A, Snyder EH, Bala MV, Richter A, Mauskopf JA. Potential savings in the cost of caring for Alzheimer's disease: treatment with rivastigmine. Pharmacoeconomics. 2000;17:351–60. doi: 10.2165/00019053-200017040-00005. [DOI] [PubMed] [Google Scholar]
- [23].Bell CM, Araki SS, Neumann PJ. The association between caregiver burden and caregiver health-related quality of life in Alzheimer's disease. Alzheimer Dis Assoc Disord. 2001;15:129–36. doi: 10.1097/00002093-200107000-00004. [DOI] [PubMed] [Google Scholar]
- [24].Wisconsin Department of Health and Family Services . Wisconsin nursing homes and residents. Division of Health Care Financing, Bureau of Health Information; Madison, WI: 2006. [Google Scholar]
- [25].Neumann PJ, Hermann RC, Kuntz KM, Araki SS, Duff SB, Leon J, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer's disease. Neurology. 1999;52:1138–45. doi: 10.1212/wnl.52.6.1138. [DOI] [PubMed] [Google Scholar]
- [26].Lave JR, Frank RG, Schulberg HC, Kamlet MS. Cost-effectiveness of treatments for major depression in primary care practice. Arch General Psychiatry. 1998;55:645–51. doi: 10.1001/archpsyc.55.7.645. [DOI] [PubMed] [Google Scholar]
- [27].Covinsky KE, Newcomer R, Fox P, Wood J, Sands L, Dane K, et al. Patient and caregiver characteristics associated with depression in caregivers of patients with dementia. J Gen Intern Med. 2003;18:1006–14. doi: 10.1111/j.1525-1497.2003.30103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boardman AE, Greenberg DH, Vining AR, Weimer DL. Cost-benefit analysis: concepts and practice. 3rd ed. Prentice Hall; Upper Saddle River, NJ: 2006. [Google Scholar]
- [29].Bullock R, Dengiz A. Cognitive performance in patients with Alzheimer's disease receiving cholinesterase inhibitors for up to five years. Int J Clin Pract. 2005;59:817–22. doi: 10.1111/j.1368-5031.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- [30].Sabbagh M, Farlow M, Relkin N, Beach T. Do cholinergic therapies have disease-modifying effects in Alzheimer's disease? Alzheimers Dement. 2006;2:118–25. doi: 10.1016/j.jalz.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [31].Matthews HP, Korbey J, Wilkenson DG, Rowden J. Donepezil in Alzheimer's disease: eighteen months from Southampton Memory Clinic. Int J Geriatr Psychiatry. 2000;15:713–20. doi: 10.1002/1099-1166(200008)15:8<713::aid-gps187>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- [32].Small GW, Kaufer D, Mendiondo MS, Quarg P, Spiegel R. Cognitive performance in Alzheimer's disease patients receiving rivastigmine for up to 5 years. Int J Clin Pract. 2005;59:473–7. doi: 10.1111/j.1368-5031.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- [33].Karlawish JHT, Klocinski JL, Merz J, Clark CC, Asch DA. Caregivers' preferences for the treatment of patients with Alzheimer's disease. Neurology. 2000;55:1008–14. doi: 10.1212/wnl.55.7.1008. [DOI] [PubMed] [Google Scholar]
- [34].Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer's disease. Am J Psychiatry. 2004;161:850–6. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- [35].Newcomer R, Spitalny M, Fox P, Yordi C. Effects of the Medicare Alzheimer's disease demonstration on the use of community-based services. Health Serv Res. 1999;34:645–67. [PMC free article] [PubMed] [Google Scholar]
- [36].Barker WW, Luis C, Harwood D, Loewenstein D, Bravo M, Ownby R, Duara R. The effect of a memory screening program on the early diagnosis of Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19:1–7. doi: 10.1097/01.wad.0000155380.63231.68. [DOI] [PubMed] [Google Scholar]
- [37].Gruber-Baldini AL, Stuart B, Zukerman IH, Simoni-Wastila L, Miller S. Treatment of dementia in community dwelling and institutionalized Medicare beneficiaries. J Am Geriatr Soc. 2007;55:1508–16. doi: 10.1111/j.1532-5415.2007.01387.x. [DOI] [PubMed] [Google Scholar]
- [38].Callahan CM, Boustani MA, Unverzagt FW, Autrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295:2148–57. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- [39].Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized controlled trial. Ann Intern Med. 2006;145:727–38. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Borson S, Scanlan J, Hummel J, Gibbs K, Lessig M, Zuhr E. Implementing routine cognitive screening of older adults in primary care: process and impact of physician behavior. Soc Gen Intern Med. 2007;22:811–7. doi: 10.1007/s11606-007-0202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brayne C, Fox C, Boustani M. Dementia screening in primary care: is it time? JAMA. 2007;298:2409–11. doi: 10.1001/jama.298.20.2409. [DOI] [PubMed] [Google Scholar]
- [42].Carpenter BD, Xiong C, Porensky EK, Lee MM, Brown PJ, Coats M, et al. Reaction to a dementia diagnosis in individuals with Alzheimer's disease and mild cognitive impairment. J Am Geriatr Soc. 2008;56:405–12. doi: 10.1111/j.1532-5415.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- [43].Salloway S, Mintzer J, Weiner MF, Cummings JL. Perspectives: disease-modifying therapies in Alzheimer's disease. Alzheimer's Dement. 2008;4:65–79. doi: 10.1016/j.jalz.2007.10.001. [DOI] [PubMed] [Google Scholar]