Abstract
Purpose
To measure the hypermethylation of four genes in primary tumors and paired plasma samples to determine the feasibility of gene promoter hypermethylation markers for detecting breast cancer in the plasma.
Materials and Methods
DNA was extracted from the tumor tissues and peripheral blood plasma of 34 patients with invasive breast cancer, and the samples examined for aberrant hypermethylation in cyclin D2, retinoic acid receptor β (RARβ), twist and high in normal-1 (HIN-1) genes using methylation-specific PCR (MSP), and the results correlated with the clinicopathological parameters.
Results
Promoter hypermethylation was detected at high frequency in the primary tumors for cyclin D2 (53%), RARβ (56%), twist (41%) and HIN-1 (77%). Thirty-three of the 34 (97%) primary tumors displayed promoter hypermethylation in at least one of the genes examined. The corresponding plasma samples showed hyperme thylation of the same genes, although at lower frequencies (6% for cyclin D2, 16% for RARβ, 36% for twist, and 54% for HIN-1). Overall, 22 of the 33 (67%) primary tumors with hypermethylation of at least one of the four genes also had abnormally hypermethylated DNA in their matched plasma samples. No significant relationship was recognized between any of the clinical or pathological parameters (tumor size, axillary lymph node metastasis, stage, or Ki-67 labeling index) with the frequency of hypermethylated DNA in the primary tumor or plasma.
Conclusion
The detection of aberrant promoter hypermethylation of cancer-related genes in the plasma may be a useful tool for the detection of breast cancer.
Keywords: Methylation, Plasma, Breast neoplasms
INTRODUCTION
Breast cancer is the most common malignancy in women in Korea and Western countries, accounting for 16.8% of all cancers in Korean female patients (1). The chance of being cured is greatest for those individuals whose primary tumor or tumor relapse are detected at an early stage, which permits curative surgery. Standard techniques for the detection and monitoring of breast cancer rely on palpation or radiological images, but many tumors escape these detection methods until they reach a relatively advanced stage. Therefore, it is important that new methods be developed to provide more sensitive approaches to the detection of new breast cancers or their recurrences.
One possible approach to the detection of breast cancer detection is via analysis of the circulating DNA from cancer cells. Nanogram quantities of DNA circulating in the blood are present in healthy individuals, while cancer patients have an average of 219 ng DNA/ml plasma (10~1,200 ng/ml plasma) (2). In seeking more specific markers, several studies have shown that it is possible to identify microsatellite alterations in the plasma and serum DNA of patients with head and neck (3) and small cell lung carcinomas (4). Additionally, p53 and ras gene mutations have been detected in the plasma and serum of patients with colorectal and pancreatic carcinomas (5,6).
Another DNA abnormality detected in the plasma and serum of cancer patients is that of gene promoter hypermethylation. The presence of gene promoter hypermethylation in the serum and plasma DNA has also been demonstrated in patients with cancers of the lung (7), head and neck (8), liver (9), colon (10), stomach (11) and breast (12~15).
In all types of human cancers studied to date, hypermethylation of the CpG islands of genes is associated with transcriptional gene silencing. In the case of breast cancer, genes previously reported to be hypermethylated include several genes involved in DNA repair (BRCA1 and GSTP1), cell cycle regulation (p16INK4A and cyclin D2), cell adhesion (E-cadherin), hormone- and receptor-mediated cell signaling (ER, RARβ, and THRβ) and regulation of cell transcription (HOX5A), as well as other functions (RASSF1A, Twist, and HIN-1) (16).
On the basis of our previous study (16), four genes (cyclin D2, RARβ, twist, and HIN-1) commonly methylated in invasive breast carcinomas were chosen for this study. Cyclin D2 is a member of the D-type cyclin group, consisting of cyclins implicated in cell cycle regulation, differentiation and malignant transformation. In contrast to cyclin D1, it has been suggested that cyclin D2 is involved in a vital tumor suppressor function in normal breast tissue, and that its loss may be related to tumorigenesis (17). RARβ is known as a tumor suppressor gene through its mediation of the growth-inhibitory effects of retinoic acids. The frequency of methylation of RARβ in invasive breast carcinomas was 41%, while the gene was rarely methylated in benign breast tissue (16,18). 'Twist' belongs to the basic-helix-loop-helix family of transcription factors, and has been implicated in lineage-specific cellular differentiation and survival. Little is known about the role of twist in breast cancer development. The high frequency of twist methylation (50~56%) in breast cancer, coupled with no methylation in normal mammary epithelial cells, suggests it plays an important role in carcinogenesis (16,18). Hin-1 is a putative cytokine, recently discovered by SAGE, to be highly expressed in the normal terminal duct-lobular unit (TDLU) and downregulated by promoter hypermethylation in 74% of primary breast tumors (19).
To determine the feasibility and the clinical significance of detecting gene promoter hypermethylation in the plasma of patients with breast cancer, 34 patients with invasive breast cancer were examined, using the sensitive MSP technique, for abnormal promoter hypermethylation in cyclin D2, RARβ, twist and HIN-1 of primary tumors and their paired plasma samples.
MATERIALS AND METHODS
1) Sample collection and DNA extraction
Matched primary tumor specimens and peripheral blood samples were collected from 34 patients diagnosed with breast cancer, between November 2002 and May 2003. Fresh tumor samples were obtained immediately after surgical resection of the breast and stored at -70℃ before DNA extraction. Pathological diagnosis and clinical evaluation disclosed no evidence of metastatic dissemination in any patient. In order to avoid the possible clearance of plasma DNA after removal of the primary tumor, a blood sample was collected from each patient on the day of surgery, prior to the mastectomy or breast-conserving surgery. The sample was later discarded if the histological diagnosis did not conclusively indicate the presence of a malignant lesion. Blood specimens were centrifuged at 3,000 rpm, in EDTA-containing tubes, for 20 minutes at room temperature, and plasma was then stored at either -20 or -70℃ until DNA extraction. Plasma samples from 10 age-matched normal individuals without breast disease were used as controls. Tumor and plasma DNAs were prepared using the QIAamp Tissue Kit (Qiagen, Hilden, Germany) and the QIAamp Blood Kit (Qiagen, Hilden, Germany), respectively, according to a modified protocol of the manufacturer. One ml of plasma was used for DNA extraction, with subsequent sodium bisulfite treatment in both the cancer patients and normal controls. To obtain a higher plasma DNA concentration, one column was used repeatedly until the entire sample had been processed. When spectrophotometrically quantified, 1 ml of plasma yielded an average of 330 ng of DNA (178~630 ng). All samples were collected in accordance with institutional guidelines for protection of human subjects, and the informed consents were obtained from all patients prior to obtaining their blood and tumor samples.
2) Sodium bisulfite treatment of DNA
DNA from the tumor and plasma specimens were treated with sodium bisulfite and analyzed using MSP, as previously described (16). This process converts all unmethylated cytosine to uracil, which is recognized as thymidine by Taq polymerase; this process does not affect methylated cytosines. Total extracted DNA from 1 ml of plasma, and 1 µg of DNA from tumor tissue, was used for this step. DNA, in 50 µl of water, was denatured with 5.5 µl of 2 M sodium hydroxide for 10 min at 37℃. Thirty µl of 10 mM hydroquinone (Sigma-Aldrich, Inc., St. Louis, MO) and 520 µl of 3 M sodium bisulfite (Sigma-Aldrich, Inc., St. Louis, MO) were subsequently added, with mixing. The DNA was overlain with several drops of mineral oil, and the sample incubated at 50℃ for 16 hr. Bisulfite-modified DNA was purified using Microcon® YM-30 centrifugal filter devices (Millipore, Bedford, MA), according to the instructions of the manufacturer. DNA was eluted from the column in 50 µl of distilled water into a 1.5 ml microcentrifuge tube, desulfonated with 5.5 µl of 3 M sodium hydroxide for 10 min at room temperature, and then neutralized with 17 µl of 10 M ammonium acetate. DNA was precipitated with 700 µl of absolute ethanol and 1 µl of glycogen, at -70℃, washed twice with 70% ethanol, air-dried and then resuspended in 20 µl of distilled water. Samples were stored at -20℃ until used. DNA from normal breast tissue was treated in vitro with SssI methyltransferase (New England Biolabs Inc. Beverly, MA), in order to generate completely methylated DNA at all CpGs, which was included in each round of sodium bisulfite treatment as a quality control of the bisulfite conversion process and subsequent methylation reactions.
3) Methylation-specific PCR (MSP)
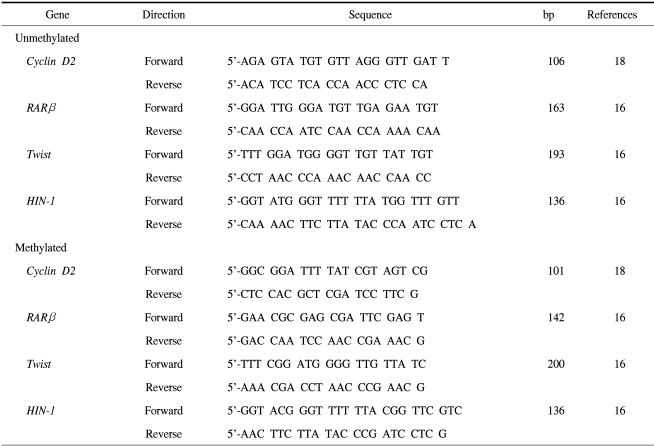
PCR amplification was performed to detect the presence of hypermethylation within the promoter CpG islands of cyclin D2, RARβ, twist and HIN-1 genes, using primers specific for methylated or unmethylated DNA. The primer sequences were based on previous reports, and are listed in Table 1. For these reactions, 1~2 µl of bisulfite-treated DNA was added to bring the reaction volume up to 25 µl, containing 1.25 mM dNTP, 16.6 mM (NH4) 2SO4, 67 mM Tris, pH 8.8, 6.7 mM MgCl2, 10 mM β-mercaptoethanol, 1U RedTaq genomic DNA polymerase (Sigma-Aldrich, Inc., St. Louis, MO) and 300 ng each of the forward and reverse primers specific to the methylated and unmethylated DNA sequences. Methylated and unmethylated primers were tested in separate reactions. Amplification was carried out in a PTC-200 thermal cycler (MJ Research, Inc., Waltham, MA), with a total of 35 and 45 cycles for tumor and plasma DNA, respectively. The conditions were: 95℃ for 5 min, followed by repetitions of 95℃ for 30 sec, 56℃ for 30 sec and 72℃ for 45 sec, with a final extension step of 72℃ for 10 min. In vitro methylated DNA, with SssI methyltransferase, was used as a positive control for the methylated products. As a negative control for each set of PCR, distilled water was used instead of DNA. Ten µl of PCR products were loaded onto a 2% agarose gel mixed, with GelStar® nucleic acid gel stain (Biowhittaker Molecular Applications, Rockland, MA), and visualized under UV illumination. Images were obtained using the Electrophoresis Document and Analysis System (EDAS290, Kodak, Rochester, NY). Samples were scored as methylated DNA when there was clear visible band on the gel when the methylated primers were used (Fig. 1).
Table 1.
Primer sequences used in MSP
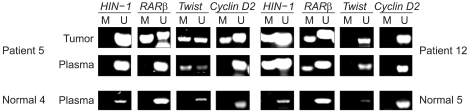
Fig. 1.
Representative MSP results in breast cancer tissues, their paired plasma samples and normal controls. Lanes labeled "M" represent reactions using primers specific for bisulfite-treated DNA products with methylated CpG sites, and lanes labeled "U" represent reactions using primers specific for bisulfite-treated DNA products with unmethylated CpG sites. Some normal controls showed no amplified signals for methylated or unmethylated reactions. In those cases, the MSP was repeated and the absence of a methylated signal, in contrast to the presence of an unmethylated signal, confirmed.
4) Statistical analysis
Fisher's exact and χ2 tests (SPSS 12.0 for windows) were used to analyze the correlation between the gene promoter hypermethylation and clinicopathological features of the breast cancer. Spearman rank correlation was used to find concordant methylation between two different genes. p≤0.05 was considered statistically significant.
RESULTS
1) Patients and tumor characteristics
Thirty-four patients with breast cancer were examined. The clinicopathological features of the patients were obtained from the medical records and pathological reports. Among these 34 patients, there were 30 invasive ductal carcinomas, 1 mucinous carcinoma, 1 medullary carcinoma, 1 papillary carcinoma and 1 micropapillary carcinoma. The mean age of the patients was 46.7 years (range 35~64 years), and that of normal individuals used as controls was 49.6 years (range 33~64 years). The tumor sizes were 2 cm or less in 14 cases (41%), larger than 2 cm, but less than 5 cm, in 19 cases (56%), and larger than 5 cm in one case (3%). Seventeen of the 34 cases (50%) had axillary lymph node metastasis at the time of surgery. High Ki-67 expression (>30% nuclei positive) was observed in 13 of the 34 cases (38%).
2) Gene hypermethylation frequency in primary breast carcinoma
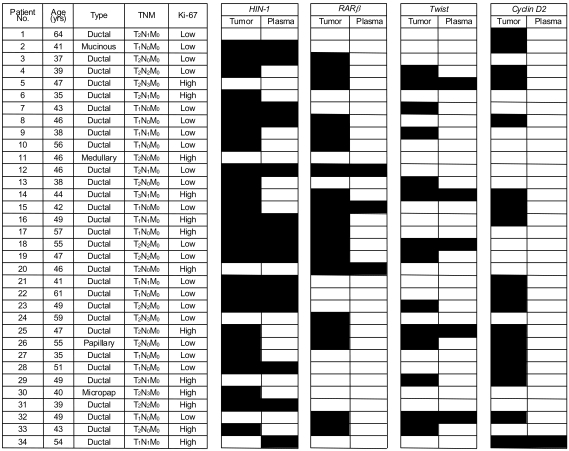
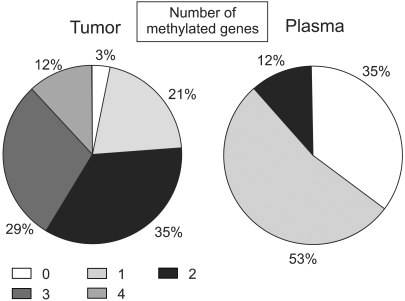
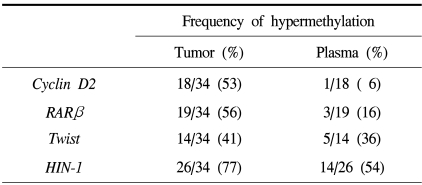
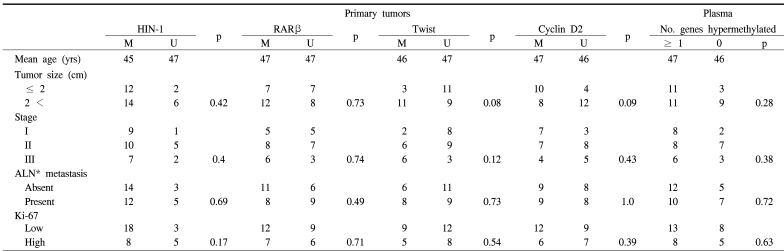
The methylation stati of the cyclin D2, RARβ, twist and HIN-1 gene promoters in 34 invasive breast cancer tissues were analyzed using the MSP technique (Fig. 2). Thirty-three of the 34 (97%) primary breast tumors exhibited abnormal promoter hypermethylation in at least one of the four genes studied (Fig. 3). This frequency was very similar to that (100%) of a previous report, which studied five genes in invasive breast carcinomas (18). Twenty-six (76%) invasive breast carcinomas displayed hypermethylation in two or more genes (Fig. 3). The individual genes were hypermethylated in 53, 56, 41 and 77% of the cyclin D2, RARβ, twist and HIN-1 genes, respectively (Table 2). Significantly concordant methylation was observed for HIN-1 and twist (r=0.41, p=0.04), but methylation among other genes in the primary tumors appeared to occur independently. For each gene, there was no difference between the mean ages of the patients in the groups with the methylated versus the unmethylated genes. The clinicopathological data of the patients was then correlated with the results of MSP. As seen in our previous data (16), the presence of hypermethylation in invasive breast carcinomas was not associated with tumor size, axillary lymph node metastasis, stage or Ki-67 labeling index (Table 3).
Fig. 2.
Clinicopathological data and MSP results of breast cancer patients. Black and white boxes indicate methylated and unmethylated samples, respectively. Criteria for high Ki-67 is nuclear staining in more than 30% of tumor cells.
Fig. 3.
Distribution of patients according to the number of methylated genes in the primary breast tumor and the plasma. The numerals in the box indicate the number of methylated genes.
Table 2.
Frequency of promoter hypermethylation of the cyclin D2, RARβ, twist and HIN-1 genes in tumor tissues and plasma samples of breast cancer patients
Table 3.
Clinical and pathological features of breast cancer patients with promoter hypermethylation in primary tumors and plasma
*axillary lymph node.
3) Promoter hypermethylation in plasma DNA from breast cancer patients
The frequency of hypermethylation for the aforementioned gene promoters was then analyzed in the 34 matched plasma samples from the breast cancer patients to determine the feasibility of MSP analysis for detecting tumor-derived DNA in plasma samples, and the concordance of the methylation profiles between the two specimen types. Promoter hypermethylation in one or more genes was detected in the plasma of 22 (65%) of the breast cancer patients (Fig. 3). In contrast, no hypermethylated DNA was detected in any of the 10 age-matched control plasma samples, or in the plasma from the single patient with a medullary carcinoma that showed no methylation of any of the four genes in the primary tumor.
The relative frequencies of hypermethylation of all four genes were lower in the plasma than in the primary tumors, but these relative frequencies were not uniform for the four genes. Hypermethylation of HIN-1 was detected in the plasma from 14 of the 26 patients (54%) with methylation of the gene in the primary tumor; the other relative frequencies were 6% (1 of 18), 16% (3 of 19) and 36% (5 of 14) for cyclin D2, RARβ and twist, respectively (Table 2). By combining the results for the 4 genes, hypermethylation in the plasma DNA was detected in 22 of the 33 patients (67% sensitivity) with confirmed methylation of the genes in the primary tumor. In the plasma samples, 18 of the 22 patients were found to have one methylated gene, while four patients showed concurrent methylation in two genes. In only one case, a faint band, indicating hypermethylation for HIN-1 gene, was detected in the plasma when it was not detected in the corresponding tumor.
The potential correlations between the presence of hypermethylated genes in the plasma and the clinicopathological parameters of the breast cancers were also studied. A statistical analysis revealed there was no significant difference in the clinicopathological parameters between the subgroups of patients with and without hypermethylated genes in their plasma (Table 3).
DISCUSSION
The finding of tumor-derived DNA in the circulation of cancer patients has inspired further efforts to develop DNA-based assays capable of detecting evidence of cancer using serum or plasma samples. Several previously tested approaches for the detection of cancer-derived DNA in serum or plasma in other types of cancers; however, have shown little promise for the detection of breast cancers. For example, microsatellite shifts (low-level instability) and loss of heterozygosity were detected in the plasma or serum DNAs from patients with head and neck (3) and small cell lung carcinomas (4), but breast cancers rarely exhibited microsatellite instability, with the detection of LOH requiring the majority of the DNA to be tumor-derived (20). Mutant K-ras and p53 DNAs have also been identified in the plasma or serum of patients with colorectal and pancreatic carcinomas (5,6), but K-ras mutations rarely occur in breast cancers, and p53 mutations in only 20~40% (21).
The goal of our study was to determine the feasibility of detecting breast cancers through the measurement of another common class of cancer-specific alterations of DNA, gene promoter hypermethylation, in plasma samples. Although serum and plasma have often been used interchangeably for methylation studies, plasma samples were used in this study because serum may contain higher extra normal DNA due to cell lysis during coagulation (22).
Individual hypermethylation of the cyclin D2, RARβ, twist and HIN-1 genes were found at high frequencies in the tumor samples (41~77%), similar to those previously reported for the same genes in other studies evaluating invasive breast cancers (16,18,23). Furthermore, reasonable complementation was observed among the four genes of the panel for detecting hypermethylation in breast cancer tissues, with almost all (97%) of the invasive cancers used in our study showing methylation of at least one of these markers.
This panel of genes was somewhat less effective at detecting hypermethylated DNA in the peripheral circulation of breast cancer patients; however, an overall sensitivity of 67% was observed for the 33 patients with tumors showing hypermethylation for at least one of the genes compared to two other panels (APC, RASSF1A, DAP-K) (14) and (p16 and CDH1) (15), with 76 and 82% sensitivities, respectively. This can likely be attributed to differences in PCR amplification efficiencies between the primer sets and laboratories, as two panels-(cyclin D2, RARβ, twist and HIN-1) and (APC, RASSF1A, DAP-K)-provided similar diagnostic coverage (97% and 94%, respectively) in the corresponding tumors. It is expected that the detection sensitivity is enhanced by the addition of other genes that are frequently hypermethylated in breast cancers to the panel. Our previous study established no significant differences between two demographically distinct populations or among the different histological breast cancer types with regard to the overall frequency of gene methylation or frequency of methylation for any specific genes, with the exception of the high frequency of BRCA1 methylation observed in mucinous cancers (16). Through MSP analysis, Dulaimi et al. (14) detected methylation in the serum DNA from patients with preinvasive lesions (ductal CIS and lobular CIS). Both of these studies have offered promising suggestion that, if a panel, with a large number of genes specific for breast cancer, was constructed, and the MSP primers or PCR technology optimized, the methylation-based screening of the plasma or serum could cover all races, histological types and stages for the detection of breast cancers. However, it may not be possible to identify hypermethylated genes in the plasma or serum in all patients with hypermethylation of the same gene in the primary tumor, as not all tumors shed DNA into the blood and because; in cancer patients, the tumor DNA fraction varies from 3 to 93% of the total circulating DNA (2). Additionally, circulating DNA fragments have limited stability, and hypermethylation is not a fixed event (24). This would partially explain the differences in the methylation frequencies of the same gene between tumor tissues and plasma, which has been shown in this study as well as other reports (7~11,14,15).
Another issue with using hypermethylated genes as markers for the detection of breast cancer in plasma is that of achieving good specificity. Methylation of our markers was not detected in the normal controls. The sample size of the control group in our study; however, was not large enough to validate 100% specificity. Evron et al. (23) demonstrated low methylation frequency (4%) for RARβ in white blood cells. In our study, methylation of the HIN-1 gene was detected in the plasma of one patient not showing methylation in the same gene in the corresponding tumor tissue. A similar phenomenon was also observed in another group (25). The investigators suggested the presence of other undetected organ malignancies, or irritation by environmental factors, as possible explanations for the positive results. Thus, examination of gene methylation patterns between different normal tissues and the effects of aging, inflammatory diseases of major organs and life styles, such as smoking and air pollution, on the methylation patterns can further validate the specificity of a methylation-based study.
Another potentially important finding of our study was the detection of methylated DNA in the plasma of breast cancer patients, irrespective of tumor stage. This contrasts with the findings of Muller et al. (13) and Hu et al. (15), who reported a strong relationship between the presence of hypermethylated DNA in the serum and clinicopathological features of the underlying breast cancer. Their results appear more convincing due to the presence of tumor-derived DNA in the plasma was related to cellular turn-over, necrosis and apoptosis of tumor cells (2), and also because the presence of tumor-specific hypermethylated genes in the plasma or serum indicates a release of sufficient amounts of tumor DNA into the circulation, which is likely to be correlated with the degree of invasiveness. However, several previous studies, which investigated stomach, lung, liver, and head and neck cancers, also failed to demonstrate any correlation between the detection of promoter hypermethylation in the serum and the tumor stage (7~9,11). The findings of a recently published study support our results, as hypermethylation of APC, RASSF1A and DAP-K were detected by an MSP analysis of the serum DNA from patients with preinvasive breast cancer (ductal CIS and lobular CIS), and from patients with all grades and stages of invasive breast cancer; additionally, positive detection of hypermethylation in the serum was not associated with the tumor stage (14). These apparent discrepancies could also be related to the specific gene markers used, and it would clearly be advantageous for panels of markers to be developed that are both highly sensitive to cancers at all stages, as well as useful for the clinical staging in breast cancer management.
Our study provides encouragement that measurements of gene promoter hypermethylation using sensitive methods, such as MSP, could be used to develop sensitive and relatively specific testing methods for detecting and monitoring breast cancer, although further investigation is needed to confirm the sensitivity and specificity of this and other procedures. The selection of an optimal panel of markers will most likely be critical for increasing the sensitivity of this approach, which will require the location of DNA sequences are commonly methylated in the serum or plasma DNA from cancer patients, as well as in breast tumor tissue.
CONCLUSIONS
The hypermethylation of four genes (cyclin D2, RARβ, twist, and HIN-1) were measured in primary tumors and paired plasma samples of breast cancer patients. Almost all of the primary breast tumors (97%) showed abnormal promoter hypermethylation in at least one of these genes. Hypermethylation in the plasma DNA was detected in 67% of the patients with confirmed methylation of the genes in the primary tumor. Detection of aberrant promoter hypermethylation of cancer-related genes in the plasma could be a useful method for detecting and monitoring breast cancer, although further studies are needed to confirm the sensitivity and specificity of the procedure.
Footnotes
This work was supported in part by grants from the Korea Breast Cancer Foundation and the Yeungnam University Research Grants in 2003.
References
- 1.Shin HR, Jung KW, Won YJ, Park JG 139 KCCR-affiliated Hospitals. 2002 annual report of the Korea central cancer registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103–114. doi: 10.4143/crt.2004.36.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 3.Nawroz H, Koch W, Anker P, Stroun M, Sidransky D. Microsatellite alterations in serum DNA of head and neck cancer patients. Nat Med. 1996;2:1035–1037. doi: 10.1038/nm0996-1035. [DOI] [PubMed] [Google Scholar]
- 4.Chen XQ, Stroun M, Magnenat JL, Nicod LP, Kurt AM, Lyautey J, et al. Microsatellite alterations in plasma DNA of small cell lung cancer patients. Nat Med. 1996;2:1033–1035. doi: 10.1038/nm0996-1033. [DOI] [PubMed] [Google Scholar]
- 5.Hibi K, Robinson CR, Booker S, Wu L, Hamilton SR, Sidransky D, et al. Molecular detection of genetic alterations in serum of colorectal cancer patients. Cancer Res. 1998;58:1405–1407. [PubMed] [Google Scholar]
- 6.Mulcahy HE, Lyautey J, Lederrey C, qi Chen X, Anker P, Alstead EM, et al. A prospective study of K-ras mutations in the plasma of pancreatic cancer patients. Clin Cancer Res. 1998;4:271–275. [PubMed] [Google Scholar]
- 7.Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, Herman JG. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res. 1999;59:67–70. [PubMed] [Google Scholar]
- 8.Sanchez-cespedes M, Esteller M, Wu L, Nawroz-Danish H, Yoo GH, Koch WM, et al. Gene promoter hypermehtylation in tumors and serum of head and neck cancer patients. Cancer Res. 2000;60:892–895. [PubMed] [Google Scholar]
- 9.Wong IH, Lo YM, Zhang J, Liew CT, Ng MH, Wong N, et al. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999;59:71–73. [PubMed] [Google Scholar]
- 10.Grady WM, Rajput A, Lutterbaugh JD, Markowitz SD. Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res. 2001;61:900–902. [PubMed] [Google Scholar]
- 11.Lee TL, Leung WK, Chan MW, Ng EK, Tong JH, Lo KW, et al. Detection of gene promoter hypermethylation in the tumor and serum of patients with gastric carcinoma. Clin Cancer Res. 2002;8:1761–1766. [PubMed] [Google Scholar]
- 12.Silva JM, Dominguez G, Garcia JM, Gonzalez R, Villanueva MJ, Navarro F, et al. Presence of tumor DNA in plasma of breast cancer patients: clinicopathological correlations. Cancer Res. 1999;59:3251–3256. [PubMed] [Google Scholar]
- 13.Muller HM, Widschwendter A, Fiegl H, Ivarsson L, Goebel G, Perkmann E, et al. DNA methylation in serum of breast cancer patients: An independent prognostic marker. Cancer Res. 2003;63:7641–7645. [PubMed] [Google Scholar]
- 14.Dulaimi E, Hillinck J, Ibanez de Caceres I, Al-Saleem T, Cairns P. Tumor suppressor gene promoter hypermethylation in serum of breast cancer patients. Clin Cancer Res. 2004;10:6189–6193. doi: 10.1158/1078-0432.CCR-04-0597. [DOI] [PubMed] [Google Scholar]
- 15.Hu XC, Wong IH, Chow LW. Tumor-derived aberrant methylation in plasma of invasive ductal breast cancer patients: clinical implications. Oncol Rep. 2003;10:1811–1815. [PubMed] [Google Scholar]
- 16.Bae YK, Brown A, Garrett E, Bornman D, Fackler MJ, Sukumar S, et al. Hypermethylation in histologically distinct classes of breast cancer. Clin Cancer Res. 2004;10:5998–6005. doi: 10.1158/1078-0432.CCR-04-0667. [DOI] [PubMed] [Google Scholar]
- 17.Evron E, Umbricht CB, Korz D, Raman V, Loeb DM, Niranjan B, et al. Loss of cyclin D2 expression in the majority of breast cancers is associated with promoter hypermethylation. Cancer Res. 2001;61:2782–2787. [PubMed] [Google Scholar]
- 18.Fackler MJ, McVeigh M, Evron E, Garrett E, Mehrotra J, Polyak K, et al. DNA methylation of RASSF1A, HIN-1, RAR-β, cyclin D2 and twist in in situ and invasive lobular breast carcinoma. Int J Cancer. 2003;107:970–975. doi: 10.1002/ijc.11508. [DOI] [PubMed] [Google Scholar]
- 19.Krop IE, Sgroi D, Porter DA, Lunetta KL, LeVangie R, Seth P, et al. HIN-1, a putative cytokine highly expressed in normal but not cancerous mammary epithelial cells. Proc Natl Acad Sci USA. 2001;98:9796–9801. doi: 10.1073/pnas.171138398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anbazhagan R, Fujii H, Gabrielson E. Microsatellite instability is uncommon in breast cancer. Clin Cancer Res. 1999;5:839–844. [PubMed] [Google Scholar]
- 21.Borresen-Dale AL. TP53 and breast cancer. Hum Mutat. 2003;21:292–300. doi: 10.1002/humu.10174. [DOI] [PubMed] [Google Scholar]
- 22.Cottrell S, Laird RW. Sensitive detection of DNA methylation. Ann N Y Acad Sci. 2003;983:120–130. doi: 10.1111/j.1749-6632.2003.tb05967.x. [DOI] [PubMed] [Google Scholar]
- 23.Evron E, Dooley WC, Umbricht CB, Rosenthal D, Sacchi N, Gabrielson E, et al. Detection of breast cancer cells in ductal lavage fluid by methylation-specific PCR. Lancet. 2001;357:1335–1336. doi: 10.1016/s0140-6736(00)04501-3. [DOI] [PubMed] [Google Scholar]
- 24.Taback B, Hoon DS. Circulating nucleic acids and proteomics of plasma/serum: clinical utility. Ann N Y Acad Sci. 2004;1022:1–8. doi: 10.1196/annals.1318.002. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara K, Fujimoto N, Tabata M, Nishii K, Matsuo K, Hotta K, et al. Identification of epigenetic aberrant promoter methylation in serum DNA is useful for early detection of lung cancer. Clin Cancer Res. 2005;11:1219–1225. [PubMed] [Google Scholar]