Abstract
Aims
The aim was to test the hypothesis that carotid artery plaque expression of lipoprotein-associated phospholipase A2 (Lp-PLA2) predicts cardiac events.
Methods and results
Prospective cohort study of 162 consecutive patients undergoing elective carotid endarterectomy. Lipoprotein-associated phospholipase A2 content was quantified by immunoblotting and lysophosphatidylcholine (lysoPC) by liquid chromatography tandem mass spectrometry. Additional biomolecular profiling by immunoblotting included C-reactive protein, p67phox, and matrix metalloproteinase-2 and -9. Macrophage plaque content was determined by quantitative immunostaining, plaque collagen content by quantitative Sirius red staining. Follow-up for cardiac death and non-fatal acute myocardial infarction was accomplished over a period of 48 ± 14 months. Expression of Lp-PLA2 and lysoPC was higher in carotid plaques of patients with than without cardiac events [median 1.6 (25th, 75th percentile 0.9, 2.5) vs. 0.8 (0.5, 2.0), P = 0.01 and 413 (281, 443) vs. 226 (96, 351) mmol/L, P = 0.03]. Smoking and point increase in carotid Lp-PLA2 expression but no other traditional cardiovascular risk factor, histological or molecular marker remained predictive of cardiac events in the multivariate Cox proportional hazard analyses [HR 3.65 (1.36–9.83), P = 0.01 and HR 1.34 (1.01–1.77), P = 0.039]. Carotid plaque Lp-PLA2 expression above the median constituted a more than three times higher risk for cardiac events [HR 3.39 (1.13–10.17), P = 0.03].
Conclusion
Lipoprotein-associated phospholipase A2 expression in carotid artery plaques is a predictor of long-term cardiac outcome. The current study supports the concept of atherosclerosis as a systemic disease with multi-focal complications and personalized medicine.
Keywords: Arteriosclerosis, Cardiovascular diseases, Plaque, Prognosis
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is a systemic yet segmental process, which by gradual and acute obstruction of the vascular lumen leads to chronic and acute clinical presentations in different vascular territories. Most of the prognostic implications relate to coronary heart disease, as underscored by the fact that even more than half of the patients with peripheral arterial disease (PAD) will eventually die from a cardiac cause.1 Intriguingly, nearly half of the patients presenting with unstable angina have evidence of complex and potentially unstable carotid artery plaques.2 On the basis of these and other observations, the concept of widespread vulnerability in individuals with ASCVD has evolved over the past years.3,4
The oxidative modification hypothesis of atherosclerosis constitutes that low-density lipoprotein (LDL) particles, retained in the subendothelial space, undergo oxidative modification, generating a pathophysiological momentum. Indeed, oxidized LDL particles are metabolized further by enzymes such as lipoprotein-associated phospholipase A2 (Lp-PLA2), yielding fatty acids and lysophospholipids such as lysophosphatidylcholine (lysoPC).5 Lysophosphatidylcholine has been shown to foster oxidative stress, to impair endothelial function and viability, to affect vascular smooth muscle cell (SMC) proliferation and survival, and to contribute to the tissue accumulation of macrophages.6 Even though Lp-PLA2 accumulates in the vascular wall along with the retention of LDL particles, macrophages are the most significant active source for Lp-PLA2 in the vascular wall. In this context, Lp-PLA2 may become a mediator of secondary LDL modifications and oxidative stress in line with the oxidative response to inflammation theory of atherosclerosis.7 Thus, beyond an indicator function, Lp-PLA2 may serve as an active link between oxidative stress and inflammation with important implications for ASCVD.
A number of epidemiological studies demonstrated an association between circulating Lp-PLA2 mass concentrations or activity and cardiovascular events, in particular non-fatal acute myocardial infarction (AMI) and cardiac death.8 In patients with early coronary artery disease (CAD), net production of Lp-PLA2 and lysoPC in the coronary circulation correlates with the extent of structural disease and epicardial endothelial dysfunction, respectively.9 These findings suggest that the vascular production and activity of Lp-PLA2 may give a pathophysiological account for its prognostic implications. Indeed, recent pathoanatomic studies confirmed that Lp-PLA2 plaque expression increases with plaque progression and characterizes complicated and symptomatic plaques.10,11 However, so far no study has investigated the prognostic merit of the expression of Lp-PLA2 in atherosclerotic plaques prospectively, let alone within the concept of ‘the vulnerable patient’.
The present study was therefore designed to test the hypothesis that local expression of Lp-PLA2 in carotid atherosclerotic plaque is predictive of future cardiac events. Within the framework of a longitudinal cohort study, patients underwent carotid endarterectomy (CEA) and molecular plaque profiling, including analysis of plaque levels of Lp-PLA2 and lysoPC, an indicator of its enzymatic activity and were subsequently followed for the occurrence of cardiac death and non-fatal AMI for nearly 5 and a half years.
Methods
Patients
All individuals from the previously characterized cohort of 164 patients who underwent CEA at the Mayo Clinic from February 2002 to July 2005 were considered for the inclusion in this study.11 Two patients were excluded from the study analyses as they were not available for follow-up, leaving a cohort of 162 patients. Follow-up was performed by review of institutional records and phone calls for overall survival and the occurrence of cardiac events, namely AMI (types 1–3 according to the new universal definition12) and cardiac death, and coronary revascularization. For patients who experienced a fatal event, death certificates were reviewed for the underlying cause, and it was deemed cardiac if diagnosed with sudden cardiac death or primarily related to heart failure, cardiac arrhythmia, or AMI. A subsequent, additional set of 41 patients undergoing CEA was recruited for comparative analysis of plaque expression and circulating plasma levels of Lp-PLA2. Approval for the study conduct was given by the Mayo Foundation Institutional Review Board.
Plaque specimens
Carotid plaques were processed in a standardized manner which included transverse dissection at the site of the maximum plaque diameter.11 There was no isolation of any particular plaque area but one cross-sectional half of the entire plaque was always taken for formalin fixation and paraffin embedding and the other half for shock freezing and storage at −80°C.
Western blotting for lipoprotein-associated phospholipase A2, matrix metalloproteinase-2, and nicotinamide dinucleotide phosphate oxidase p67phox
Western blotting was performed as previously described, loading 50 µg per lane and using the following primary antibodies: anti-Lp-PLA2 (monoclonal 4B4, 1:1000, gift from DiaDexus, San Francisco, CA, USA), anti-matrix metalloproteinase (MMP)-2 and anti-MMP-9 (1:7500, Chemicon International), anti-nicotinamide dinucleotide phosphate oxidase p67phox (1:500; Santa Cruz), anti-C-reactive protein (1:1000, Sigma), and anti-β-actin (1:1000, Sigma).11,13 Densitometric signals were analysed using ImageJ software (National Institutes of Health). As we utilized a highly specific monoclonal antibody to Lp-PLA2 similar to the commercial Lp-PLA2 mass serum assay, there is no interference by other PLA2 subtypes.
Liquid chromatography tandem mass spectrometry for lysophosphatidylcholine
Plaque content of lysoPC was measured using liquid chromatography tandem mass spectrometry in 54 randomly selected samples as reported before.11
Immunostaining for macrophage and smooth muscle cells
Macrophages and SMCs were identified by anti-CD68 (1:500, Dako) and anti-α SMC actin (1:1000, Dako) immunostaining, respectively.11,13 Diaminobenzidine (Vector Laboratories) was used as chromogen. Primary antibody isotype immunoglobulins were used as negative controls. Digital images of the stained specimens were taken (SPOT Advanced 3.3, Diagnostic Instruments Inc.) and the percentage of stained area within the entire specimen was quantified by MetaMorph™ Meta Imaging Series 4.6 (Molecular Devices Corporation).
Terminal deoxynucleotidyl transferase end labelling staining for apoptosis
Cells undergoing apoptosis were identified by the Terminal deoxynucleotidyl transferase end labelling (TUNEL) technique applying the ApopTag® In Situ Apoptosis Detection Kit (Intergen).13 Quantification was made by manual count of the number of TUNEL+ cells relative to the total number of cells in the plaque.
Sirius red staining for collagen
The collagen content of carotid plaques was evaluated by Sirius red as outlined before.13 Slides were visualized under both bright field and polarized light microscope, and pictures were taken with identical exposure settings for all sections. The content of collagen, identified by birefringence under polarized light, was quantified as percent of plaque area.
Lipoprotein-associated phospholipase A2 plasma measurement
The Lp-PLA2 levels were measured in plasma aliquots that were obtained at the time of CEA and stored at −70°C using an enzyme-linked immunoassay (PLAC test, DiaDexus, Inc.) as reported before.9,16
Statistics
Continuous non-parametric and parametric data were presented as median (25th, 75th percentile) and mean ± standard deviation (SD), respectively. Categorical data were presented in absolute numbers and percentage. Two group comparisons were made by Mann–Whitney U-test or t-test or for continuous non-parametric and parametric variables or the χ2 test for categorical variables. Regression coefficients were calculated on the basis of Pearson or Spearman product moment for parametric and non-parametric data, respectively. Cox proportional hazard analyses were used for the identification of outcome predictors, both in univariate and multivariate manner, with the results presented as hazard ratio (HR) and 95% confidence interval (95% CI). Kaplan–Meier plot-related group comparisons were made by log-rank test. A P-value less than 0.05 was considered statistical significant for all analysis.
Results
The demographic and clinical profile of the 162 CEA patients is detailed in Table 1. Hypertension and hyperlipidaemia were the most prevalent cardiovascular risk factors in a predominantly aged patient population. Forty-three percent of the patients had known ASCVD elsewhere including 28.6% with combined PAD and CAD (Table 1).
Table 1.
Patient characteristics
| Variable | Total cohort | No cardiac events | Cardiac events |
|---|---|---|---|
| Age (years) | 71.0 (66.0, 76.0) | 71.0 (66.0, 76.0) | 70.0 (62.5, 76.8) |
| Female gender | 58 (35.8) | 53 (37.6) | 5 (23.8) |
| BMI (kg/m2) | 29.0 ± 5.1 | 29.1 ± 4.9 | 28.6 ± 6.4 |
| Laboratory parameters | |||
| Haemoglobin (g/dL) | 13.5 ± 1.6 | 13.6 ± 1.5 | 13.4 ± 1.7 |
| White blood cell count × 109/L | 6.8 (5.7, 8.4) | 6.7 (5.7, 8.4) | 7.4 (6.5, 8.4) |
| Creatinine (mg/dL) | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.2) | 1.2 (1.0, 1.4) |
| Total cholesterol (mg/dL) | 184.0 (153.0, 206.0) | 184.0 (155.5, 205.5) | 190.0 (123.0, 208.0) |
| Low-density lipoprotein (mg/dL) | 106.2 ± 40.2 | 107.0 ± 41.0 | 100.3 ± 34.6 |
| High-density lipoprotein (mg/dL) | 45.5 ± 13.2 | 45.8 ± 13.3 | 42.6 ± 12.0 |
| Triglycerides (mg/dL) | 147.0 (97.8, 210.5) | 149.0 (98.0, 216.0) | 132.0 (95.0, 197.5) |
| Cardiovascular risk factors | |||
| Diabetes mellitus, n (%) | 30 (18.5) | 26 (18.4) | 4 (19.1) |
| Hypertension, n (%) | 140 (86.5) | 119 (84.4) | 21 (100) |
| Hyperlipidaemia, n (%) | 136 (84.0) | 119 (84.4) | 17 (81.0) |
| Current smoking, n (%) | 22 (13.6) | 16 (11.4) | 6 (28.6)* |
| Family history, n (%) | 25 (15.4) | 24 (17.0) | 1 (4.8) |
| Cardiovascular history | |||
| Previous stroke, n (%) | 18 (11.1) | 13 (9.2) | 5 (23.8)* |
| PAD, n (%) | 29 (17.9) | 23 (16.3) | 6 (28.6) |
| AAA, n (%) | 9 (5.6) | 8 (5.7) | 1 (4.8) |
| CAD, n (%) | 67 (41.4) | 56 (39.7) | 11 (52.4) |
| Previous AMI, n (%) | 29 (17.9) | 24 (17.0) | 5 (23.8) |
| Previous CABG, n (%) | 25 (15.4) | 22 (15.6) | 3 (14.3) |
| Previous PCI, n (%) | 25 (15.4) | 21 (14.9) | 4 (19.1) |
| Traditional FRS (%) | 16.3 ± 8.6 | 16.3 ± 8.4 | 16.0 ± 10.6 |
| Medications | |||
| Aspirin, n (%) | 137 (84.6) | 119 (84.4) | 18 (85.7) |
| Clopidogrel, n (%) | 15 (9.3) | 14 (9.9) | 1 (4.8) |
| Coumadin, n (%) | 22 (13.6) | 20 (14.2) | 2 (9.5) |
| LLD/statin– no. (%) | 104 (64.2)/98 (60.5) | 92 (65.2)/86 (61.0) | 12 (57.1)/12 (57.1) |
| ACE-inhibitors/ARB, n (%) | 53 (32.7)/20 (12.3) | 46 (32.6)/18 (12.8) | 7 (33.3)/2 (9.5) |
| Beta-blocker, n (%) | 89 (54.9) | 77 (54.6) | 12 (57.1) |
| CCB/amlodipine, n (%) | 37 (22.8)/17 (10.5) | 35 (24.8)/16 (11.3) | 2 (9.5)/1 (4.8) |
| Nitrates, n (%) | 17 (10.5) | 15 (10.7) | 2 (9.5) |
| Diuretics/HCTZ, n (%) | 55 (34.0)/37 (22.8) | 49 (34.8)/32 (22.7) | 6 (28.6)/5 (23.8) |
| Allopurinol, n (%) | 9 (5.6) | 9 (6.4) | 0 (0.0) |
| Thyroid hormone, n (%) | 12 (7.4) | 9 (6.4) | 3 (14.3) |
| Anti-diabetics/insulin, n (%) | 24 (14.8)/4 (2.5) | 22 (15.6)/3 (2.1) | 2 (9.5)/1 (4.8) |
| Vitamins, n (%) | 52 (32.1) | 48 (34.0) | 4 (19.1) |
Continuous non-parametric and parametric data were presented as median (25th, 75th percentile) and mean ± SD, categorical data as number (%). CEA, carotid endarterectomy; PAD, peripheral arterial disease; AAA, abdominal aortic aneurysm; CAD, coronary artery disease; AMI, acute myocardial infarction; CABG, coronary artery bypass surgery; PCI, percutaneous coronary intervention; FRS, Framingham risk score (traditionally calculated for those without known CAD and diabetes mellitus); LLD, lipid-lowering drugs; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; HCTZ, hydrochlorothiazide.
*P < 0.05.
Twenty-one patients (13%) experienced a cardiac event during a follow-up time of 48 ± 14 months, including 16 non-fatal AMIs and five cardiac deaths. A total of 14 patients (8.6%) died from a non-cardiac cause, including nine patients with cancer.
A clinical history of strokes was more common among patients with future cardiac events, whereas the anatomic extent of carotid artery disease at the time of CEA did not differ between patients with and without a future cardiac event (Tables 1 and 2). There was no significant difference in CAD history between patients with and without coronary events (Table 2). More specifically, 50% of the patients in the non-event group had documented assessment of CAD within 3 months prior or after CEA; of these, 40% had documentation of obstructing CAD by angiography (at least one lesion more than 70%) and 16% had a positive stress test. In the event group, 48% of the patients had documented assessment of CAD around the CEA procedure; of these, 30% had documentation of obstructing CAD by angiography and 10% had a positive stress test. The medication profile was consistent with the disease profile of the study population and did not differ between patients with and without future cardiac events at baseline and during follow-up.
Table 2.
Plaque characteristics
| Variable | Total cohort | No cardiac events | Cardiac events |
|---|---|---|---|
| Clinical presentation | |||
| TIA, stroke, amaurosis fugax within 120 days of CEA, n (%) | 63 (41.4) | 59 (41.8) | 7 (33.3) |
| Time interval from symptoms (days) | 15.0 (7.0, 45.5) | 15.0 (7.0, 42.5) | 17.0 (6.5, 55.0) |
| U/S and MRI findings | |||
| CEA stenosis limited to ICA, n (%) | 36 (22.2) | 31 (22.0) | 5 (23.8) |
| CEA stenosis with ulcerated plaque characteristics, n (%) | 10 (6.2) | 8 (5.7) | 2 (9.5) |
| Moderate to severe contralateral carotid artery disease, n (%) | 78 (48.1) | 68 (48.3) | 10 (47.6) |
| Vertebral artery stenosis, n (%) | 29 (17.9) | 24 (17.0) | 5 (23.8) |
| Histological findings | |||
| Collagen content, % plaque area | 1.0 (0.5, 2.6) | 0.8 (0.5, 2.6) | 2.3 (1.2, 2.4)* |
| Macrophages, % plaque area | 0.6 (0.4, 1.3) | 0.6 (0.4, 1.2) | 0.8 (0.4, 1.5) |
| Smooth muscle cells, % plaque area | 35.2 (22.8, 48.8) | 34.9 (23.7, 48.8) | 39.9 (22.3, 50.9) |
| TUNEL+ cells, % plaque area | 11.6 (6.5, 23) | 10.8 (6.5, 21.9) | 28.1 (12.6, 36.9) |
| Molecular findings | |||
| Lp-PLA2, DMR to β-actin | 0.8 (0.5, 2.1) | 0.8 (0.5, 2.0) | 1.6 (0.9, 2.5)* |
| LysoPC (mmol/L) | 247.7 (105.9, 367.2) | 225.6 (96.1, 351.3) | 413.2 (281.4, 442.7)* |
| p67phox, DMR to β-actin | 1.0 (0.5, 2.1) | 1.0 (0.5, 2.1) | 1.3 (0.5, 2.5) |
| MMP-2, DMR to β-actin | 1.1 (0.7,1.9) | 1.1 (0.7, 1.8) | 1.4 (0.8, 2.5) |
| MMP-9, DMR to β-actin | 0.7 (0.3, 1.4) | 0.7 (0.3, 1.4) | 0.9 (0.4, 3.0) |
| C-reactive protein, DMR to β-actin | 2.3 ± 1.1 | 2.4 ± 1.1 | 1.7 ± 0.6 |
Continuous non-parametric and parametric data were presented as median (25th, 75th percentile) and mean ± SD, categorical data as number (%). CEA, carotid endarterectomy; U/S, ultrasound; MRI, magnetic resonance imaging; ICA, internal carotid artery; TUNEL, Terminal deoxynucleotidyl transferase end labelling; Lp-PLA2, lipoprotein-associated phospholipase A2; DMR, densitometric ratio; lysoPC, lysophosphatidylcholine; MMP, matrix metalloproteinase.
*P < 0.05 for group comparison.
Collagen content was the only histological characteristic that differed among patients who were and were not to experience a future cardiac event (Table 2). Both carotid artery Lp-PLA2 expression and lysoPC content were higher in patients with future cardiac events (Table 2). There was a significant correlation between the expression of Lp-PLA2 and plaque lysoPC content (r = 0.52, P < 0.001). Lipoprotein-associated phospholipase A2 and lysoPC levels correlated significantly with macrophage count (r = 0.422, P < 0.001 and r = 0.514, P = 0.009), MMP-2 expression (r = 0.461, P < 0.001 and r = 0.597, P = 0.02), MMP-9 expression (r = 0.226, P = 0.01 and r = 0.370, P = 0.017), and collagen content (r = 0.823, P < 0.001 and r = 0.335, P = 0.01). Lp-PLA2 expression also correlated with SMC content (r = 0.215, P = 0.03) and lysoPC content with the number of TUNEL+ cells in the carotid plaques (r = 0.829, P = 0.04).
On the basis of Cox proportional hazard analyses, cancer was the only significant predictor of non-cardiac mortality [HR 7.77 (1.59–23.28), P < 0.001]. Smoking and point increase in carotid Lp-PLA2 expression were the only variables that remained predictive of cardiac events in the univariate analyses [HR 3.74 (1.43–9.78), P = 0.01 and HR 1.32 (1.01–1.72), P = 0.04] and in the multivariate Cox proportional hazard analyses [HR 3.65 (1.36–9.83), P = 0.03 and HR 1.34 (1.01–1.77), P = 0.04], when adjusted for those additional variables that differed between patients with and without cardiac events in Tables 1 and 2, including previous stroke and collagen content.
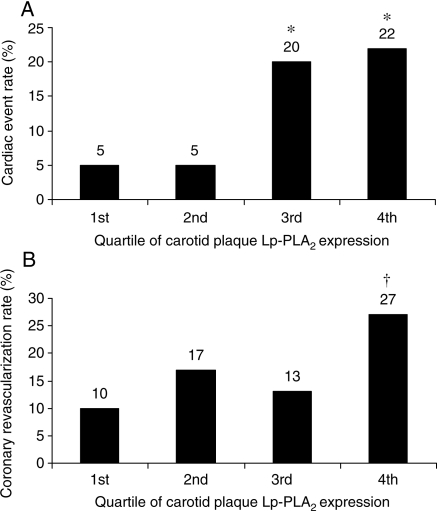
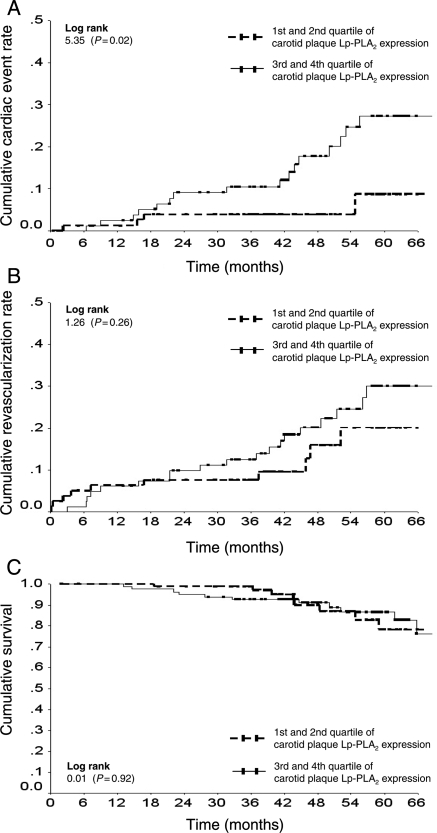
Stratification of long-term cardiac outcome according to quartiles of carotid plaque Lp-PLA2 expression pointed out two risk categories (Figure 1A). Patients with carotid plaque expression of Lp-PLA2 in the two upper quartiles were more than three times more likely to experience a cardiac event [HR 3.39 (1.13–10.17), P = 0.03 in univariate analysis and HR 3.79 (1.24–11.60), P = 0.02 in multivariate analysis with the above outlined co-factors] (Figure 2A). The coronary revascularization rates showed a gradual increase across the quartile spectrum of carotid plaque Lp-PLA2 expression (Figure 1B). Even though the rate of coronary revascularization increased more in the upper than in the lower quartiles of Lp-PLA2 expression, this trend did not reach statistical significance [HR 1.55 (0.72–3.34), P = 0.27] (Figure 2B). Overall cumulative survival did not differ between patients with carotid plaque expression of Lp-PLA2 above and below the median (Figure 2C).
Figure 1.
Incidence of cardiac death and non-fatal acute myocardial infarction (A) and percutaneous coronary intervention and coronary artery bypass surgery (B) during follow-up according to quartiles of lipoprotein-associated phospholipase A2 (Lp-PLA2) expression in carotid artery plaques, *P < 0.05 vs. First and second quartiles, †P < 0.05 vs. first quartile.
Figure 2.
Cumulative incidence of non-fatal acute myocardial infarction and cardiac death over time was higher in patients with lipoprotein-associated phospholipase A2 (Lp-PLA2) expression in carotid artery plaques in the upper two compared with the lower two quartiles (21 vs. 5%, P = 0.02, A). Cumulative coronary revascularization rates over time tended to be higher in the upper than in the lower quartiles (20 vs. 14%, P = 0.31, B). Overall survival did not differ between patients with Lp-PLA2 expression in carotid artery plaques in the upper two quartiles compared with the lower two quartiles (86 vs. 90%, P = 0.46).
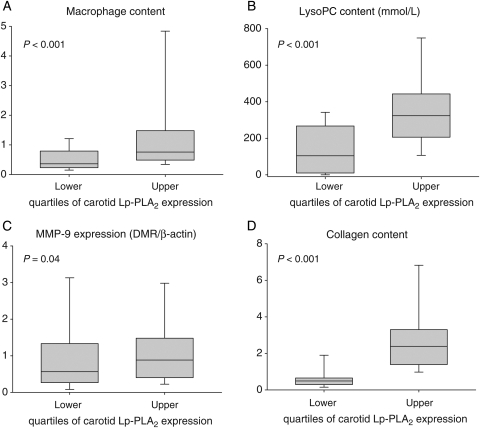
Stratified according to upper and lower quartiles of Lp-PLA2 carotid plaque expression, the plaque parameters that showed significant differences included lysoPC, macrophage content, MMP-9 expression, and collagen content (Figure 3). There was no difference in the prevalence of documented CAD across the quartiles of carotid Lp-PLA2 expression. Also, there was no difference in the medication profile across the quartiles of Lp-PLA2 expression at baseline and during follow-up (data not shown).
Figure 3.
Content of macrophages (A), lysophosphatidylcholine (LysoPC, B), matrix metalloproteinase-9 (MMP-9, C), and collagen (D) was significantly higher in plaques with lipoprotein-associated phospholipase A2 (Lp-PLA2) expression in the upper two quartiles.
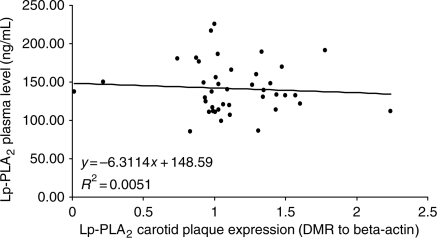
Plasma concentrations Lp-PLA2 were measured at the time of CEA in a separate set of 41 patients and were not found to correlate with carotid artery plaque expression of Lp-PLA2 (Figure 4).
Figure 4.
Lipoprotein-associated phospholipase A2 (Lp-PLA2) plasma mass concentrations plotted over Lp-PLA2 carotid plaque expression in a consecutive series of 41 patients undergoing carotid endarterectomy.
Discussion
The current study demonstrates that carotid artery plaque expression of Lp-PLA2 and lysoPC, the indicator of Lp-PLA2's enzymatic activity, was higher in patients with future cardiac events. The prognostic merit of the carotid plaque profile may relate to its indicative nature for the biological profile of plaques in other vascular territories, including the coronary circulation. Accordingly, the current findings can be viewed in support of the concept that atherosclerosis is a systemic process with multi-focal events and a pathophysiological role Lp-PLA2 within this concept.
The current study shows for the first time that the expression of Lp-PLA2 in carotid artery plaques is a predictor of future cardiac events independent of a number of other well-defined predictors including smoking and previous stroke. Importantly, in the current study, there was no significant difference in the extent of clinical cerebrovascular disease, PAD, and/or CAD between those with and without events and between those with high- and low-Lp-PLA2 levels. These findings are in line with previous studies failing to detect a correlation between systemic Lp-PLA2 levels and the extent of structural ASCVD but still confirming its functional and prognostic merit.14–16 The validity of Lp-PLA2 as a CV biomarker was primarily established in studies involving patients without established ASCVD. In these studies, a higher circulating Lp-PLA2 mass concentration or activity conferred an up to two-fold higher risk for cardiac events such as AMI or cardiac death and ischaemic stroke alike.17–20 Thus, the current study broadens our view by demonstrating that not only systemic biomarkers but also local plaque biomarkers and namely Lp-PLA2 can predict systemic events.
The finding that lysoPC content was also higher in carotid plaques of patients who suffered a future cardiac event suggests that not only the expression but also the activity of Lp-PLA2 is of prognostic significance. Lysophosphatidylcholine is generated by Lp-PLA2's hydrolyzing activity on oxidized lipids such as oxidized LDL and contributes to tissue accumulation of monocytes/macrophages, the main cellular source of Lp-PLA2 in the atherosclerotic plaque.21–23 By virtue of these processes, Lp-PLA2 can be involved in a positive-feedback loop of tissue inflammation. Furthermore, whereas low concentrations of lysoPC are anti-apoptotic, lysoPC evokes apoptosis of endothelial and vascular SMCs at high concentrations.24–26 High levels of Lp-PA2 and its activity may therefore favour the development of ‘vulnerable’ plaques, rich in inflammatory cells and necrotic core. Indeed, the current study confirmed a positive correlation between plaque macrophage count and plaque levels of Lp-PLA2 and lysoPC as well as between lysoPC level and number of apoptotic cells. Furthermore, Lp-PLA2 and lysoPC expression correlated with MMP-2 and MMP-9 expressions, consistent with previous experimental data on the induction of MMP production by lysoPC.27 However, none of these variables, which are often related to unstable plaque remodelling, differed between patients with and without cardiac events. On the contrary, collagen content, usually considered a marker for plaque stability, was higher in patients with future events and correlated with Lp-PLA2 and lysoPC plaque levels. The predominant significance of Lp-PLA2 in this context is underscored by the fact that Lp-PLA2 but not collagen content remained an independent predictor of long-term outcome in multivariate analysis. Possibly, Lp-PLA2 (via lysoPC) leads to an increase in reactive oxygen production, stimulating the transforming growth factor beta-1 pathway and collagen production by myofibroblasts.28 Hence, Lp-PLA2 relates to a number of different biological processes in the atherosclerotic plaque and may be an integrative marker and mediator of plaque biological activity.
The current findings also lend support for the theory that the very process that causes plaque destabilization is not confined to a single vascular bed and that plaque characteristics and stability may be shared among different vascular territories.29 Indeed, patients with acute cardiac events have widespread involvement of the arterial vasculature beyond the coronary circulation.2,4 Vice versa, patients with PAD have a risk of cardiac events that is equivalent to CAD patients. Also, carotid artery plaque as detected by ultrasound is a predictor of AMI in the general population and in those with CV risk factors or disease.30,31 Furthermore, complex features of carotid artery plaques, such as plaque surface irregularity on angiography, are predictive of mainly cardiac mortality.32 In the current study, however, there was no difference in the complexity of anatomic carotid artery disease between those patients with and those patients without cardiac events during follow-up. Neither was there a difference in the history and documented extent of CAD. In addition, the Framingham risk score calculated according to the traditional model in patients without known CAD or diabetes mellitus did not differ between patients with and without events. This is in line with the notion that carotid artery disease, in fact, constitutes a CAD equivalent and all patients in this study cohort are to be viewed at high risk for cardiac events and Framingham risk scores cannot be used for further risk stratification. Assessment of Lp-PLA2 plaque expression, however, helped to re-stratify this otherwise maybe uniformly viewed cohort into an intermediate-risk (1–1.5% per year) and a very high-risk (more than 4% per year) group. Thus, molecular plaque profiling can be of additive value in the risk stratification of patients and even superior to classical anatomic and histological markers of plaque vulnerability.
One might argue that the size of the current study was too small. However, a statistically significant deviation from the null hypothesis was detected in this study with a preset acceptable chance of an alpha (Type I) error of less than 5%. Moreover, on the basis of patient numbers and event rates, the probability of a beta (Type II) error was less than 20% (power 81.6 and 87.8% with and without the Yates correction factor). Hence, chances of committing a Type I or Type II error in this study were within the traditionally set limits for statistical acceptance.
The current study, however, cannot prove a causal role of Lp-PLA2 in the development of adverse cardiovascular events. One option to do so is by prospective studies with Lp-PLA2 inhibitors. Indeed, the IBIS-2 study showed that administration of the Lp-PLA2 inhibitor darapladib halted necrotic core expansion.33 These data are corroborated by the finding that darapladib inhibits Lp-PLA2 activity in carotid plaques as potently as plasma Lp-PLA2 activity and with a notable reduction in pro-apoptotic caspase activities.34 Similarly, in an animal model of accelerated atherosclerosis, inhibition of Lp-PLA2 activity halts necrotic core development and overall plaque formation along with a significant reduction in the expression of pro-atherosclerotic genes and particularly those related to leucocyte activity.35 Hence, available studies so far provide pathomechanistic support along the lines of the current findings but data on the improvement in clinical outcome are still to be obtained.
Another limitation of the current study is that enzymatic activity of Lp-PLA2 was not determined directly; however, the concentration of lysoPC in carotid plaques was measured as a surrogate marker in a subset of patients. Lysophosphatidylcholine is produced from oxidized phosphatidyl choline by the action of Lp-PLA2 and is therefore reflective of its activity taking into consideration though that lysoPC can also be produced by other phospholipases and enzymes such as lecithin cholesterol acyl transferase which degrades non-oxidized phosphatidyl choline to a free fatty acid and lysoPC. In the current study, Lp-PLA2 plasma mass concentration was measured in a subset of patients at the time of CEA and no correlation with the extent of carotid artery plaque expression was found. This finding corresponds to the results of a previous study that did not find a correlation between carotid plaque expression by immunohistochemistry and Lp-PLA2 serum activity.36 In line with these observations, the current study extends previous systemic biomarker studies and provides a more direct link to the involvement of Lp-PLA2 in the pathogenesis and prognosis of vascular disease. Because of the focus on Lp-PLA2-related mechanisms, the molecular plaque profile studied here was limited and might have underestimated the influence of other molecular markers. Future proteomic microarray assays might be needed for more extensive studies of plaque components and culprit candidates. Ideally, future studies should also include simultaneous assessment of multiple vascular sites to further examine the systemic aspects of disease processes and prediction. These studies could further the current findings in support of the concept of the vulnerable patient, the merit of molecular profiling, and the prognostic and pathophysiological role of Lp-PLA2.
In conclusion, Lp-PLA2 expression in carotid artery plaques is a predictor of long-term cardiac outcome. These findings support the concept of systemic vulnerability of ASCVD and a pathophysiological role Lp-PLA2 within this concept.
Funding
This study was supported by NIH grants: K24, HL-69840, R01 HL-63911, DK73608, HL77131, HL085307, and HL092954, the Mayo Stiftung, and by an award from the Mayo Clinic Clinical Immunology and Immunotherapeutic program.
Conflict of interest: none declared.
Acknowledgements
The authors are grateful to Monica L. Olson and Darrell Loeffler for their help in collecting and processing endarterectomy specimens and to Meagan J. Schultz her technical help in processing the lysophosphatidylcholine assay and Ryan Lennon for his statistical support. Finally, we would like to thank DiaDexus for providing the monoclonal anti-Lp-PLA2 antibody. No financial, analytical, or editorial support was received from any commercial company.
References
- 1.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 2.Lombardo A, Biasucci LM, Lanza GA, Coli S, Silvestri P, Cianflone D, Liuzzo G, Burzotta F, Crea F, Maseri A. Inflammation as a possible link between coronary and carotid plaque instability. Circulation. 2004;109:3158–3163. doi: 10.1161/01.CIR.0000130786.28008.56. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Atherosclerosis: disease biology affecting the coronary vasculature. Am J Cardiol. 2006;98:3Q–9Q. doi: 10.1016/j.amjcard.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 5.MacPhee C, Moores K, Boyd H, Dhanak D, Ife R, Leach C, Leake D, Milliner K, Patterson R, Suckling K, Tew D, Hickey D. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338:479–487. [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Y, Zhang P, Zhang L, Osman H, Mohler ER, III, Macphee C, Zalewski A, Postle A, Wilensky RL. Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory responses. Atherosclerosis. 2007;191:54–62. doi: 10.1016/j.atherosclerosis.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Stocker R, Keaney JF., Jr New insights on oxidative stress in the artery wall. J Thromb Haemost. 2005;3:1825–1834. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 8.Lavi S, Lavi R, McConnell JP, Lerman LO, Lerman A. Lipoprotein-associated phospholipase A(2): review of its role as a marker and a potential participant in coronary endothelial dysfunction. Mol Diagn Ther. 2007;11:219–226. doi: 10.1007/BF03256243. [DOI] [PubMed] [Google Scholar]
- 9.Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 10.Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, Virmani R. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 11.Mannheim D, Herrmann J, Versari D, Gossl M, Meyer FB, McConnell JP, Lerman LO, Lerman A. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke. 2008;39:1448–1455. doi: 10.1161/STROKEAHA.107.503193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Versari D, Herrmann J, Gossl M, Mannheim D, Sattler K, Meyer FB, Lerman LO, Lerman A. Dysregulation of the ubiquitin–proteasome system in human carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2132–2139. doi: 10.1161/01.ATV.0000232501.08576.73. [DOI] [PubMed] [Google Scholar]
- 14.Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J. 2005;26:137–144. doi: 10.1093/eurheartj/ehi010. [DOI] [PubMed] [Google Scholar]
- 15.Kardys I, Oei H-HS, van der Meer IM, Hofman A, Breteler MMB, Witteman JCM. Lipoprotein-associated phospholipase A2 and measures of extracoronary atherosclerosis: the Rotterdam study. Arterioscler Thromb Vasc Biol. 2006;26:631–636. doi: 10.1161/01.ATV.0000201289.83256.cf. [DOI] [PubMed] [Google Scholar]
- 16.Yang EH, McConnell JP, Lennon RJ, Barsness GW, Pumper G, Hartman SJ, Rihal CS, Lerman LO, Lerman A. Lipoprotein-associated phospholipase A2 is an independent marker for coronary endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2006;26:106–111. doi: 10.1161/01.ATV.0000191655.87296.ab. [DOI] [PubMed] [Google Scholar]
- 17.Packard CJ, O'Reilly DSJ, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GDO, Docherty G, Burczak JD The West of Scotland Coronary Prevention Study Group. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 18.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the atherosclerosis risk in communities (ARIC) study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 19.Koenig W, Khuseyinova N, Lowel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903–1908. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 20.Oei H-HS, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MMB, Witteman JCM. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam study. Circulation. 2005;111:570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 21.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci USA. 1988;85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, Rice SQ, Tew DG, Karkola K, Yla-Herttuala S. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:2909–2917. doi: 10.1161/01.atv.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 23.Rong JX, Berman JW, Taubman MB, Fisher EA. Lysophosphatidylcholine stimulates monocyte chemoattractant protein-1 gene expression in rat aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2002;22:1617–1623. doi: 10.1161/01.atv.0000035408.93749.71. [DOI] [PubMed] [Google Scholar]
- 24.Chai YC, Howe PH, DiCorleto PE, Chisolm GM. Oxidized low density lipoprotein and lysophosphatidylcholine stimulate cell cycle entry in vascular smooth muscle cells. Evidence for release of fibroblast growth factor-2. J Biol Chem. 1996;271:17791–17797. doi: 10.1074/jbc.271.30.17791. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh CC, Yen MH, Liu HW, Lau YT. Lysophosphatidylcholine induces apoptotic and non-apoptotic death in vascular smooth muscle cells: in comparison with oxidized LDL. Atherosclerosis. 2000;151:481–491. doi: 10.1016/s0021-9150(00)00453-6. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M, Okazaki H, Ogata Y, Takeuchi K, Ikeda U, Shimada K. Lysophosphatidylcholine induces apoptosis in human endothelial cells through a p38-mitogen-activated protein kinase-dependent mechanism. Atherosclerosis. 2002;161:387–394. doi: 10.1016/s0021-9150(01)00674-8. [DOI] [PubMed] [Google Scholar]
- 27.Inoue N, Takeshita S, Gao D, Ishida T, Kawashima S, Akita H, Tawa R, Sakurai H, Yokoyama M. Lysophosphatidylcholine increases the secretion of matrix metalloproteinase 2 through the activation of NADH/NADPH oxidase in cultured aortic endothelial cells. Atherosclerosis. 2001;155:45–52. doi: 10.1016/s0021-9150(00)00530-x. [DOI] [PubMed] [Google Scholar]
- 28.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 29.Hellings WE, Peeters W, Moll FL, Pasterkamp G. From vulnerable plaque to vulnerable patient: the search for biomarkers of plaque destabilization. Trends Cardiovasc Med. 2007;17:162–171. doi: 10.1016/j.tcm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, Wilsgaard T, Lochen ML, Njolstad I, Arnesen E. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromso study. Stroke. 2007;38:2873–2880. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 31.Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der Graaf Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke. 2007;38:1470–1475. doi: 10.1161/STROKEAHA.106.477091. [DOI] [PubMed] [Google Scholar]
- 32.Rothwell PM, Villagra R, Gibson R, Donders RC, Warlow CP. Evidence of a chronic systemic cause of instability of atherosclerotic plaques. Lancet. 2000;355:19–24. doi: 10.1016/s0140-6736(99)04470-0. [DOI] [PubMed] [Google Scholar]
- 33.Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Verheye S, Aschermann M, Duckers H, Bleie O, Dudek D, Botker HE, von Birgelen C, D'Amico D, Hutchinson T, Zambanini A, Mastik F, van Es GA, van der Steen AF, Vince DG, Ganz P, Hamm CW, Wijns W, Zalewski A. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 34.Shi Y, Zalewski A, Macphee CH, Dawson M. Selective inhibition of lipoprotein-associated phospholipase A2 attenuates markers of plaque vulnerability in humans. Circulation. 2007;116:1. [Google Scholar]
- 35.Wilensky RL, Shi Y, Mohler ER, III, Hamamdzic D, Burgert ME, Li J, Postle A, Fenning RS, Bollinger JG, Hoffman BE, Pelchovitz DJ, Yang J, Mirabile RC, Webb CL, Zhang L, Zhang P, Gelb MH, Walker MC, Zalewski A, Macphee CH. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nature Med. 2008;14:1059–1066. doi: 10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atik B, Johnston SC, Dean D. Correlation of lipoprotein-associated phospholipase A2 serum activity and tissue protein (Lp-PLA2) with carotid tissue macrophages and Chlamydophila pneumoniae infection among patients at tisk for stroke. Stroke. 2008;39:716. [Google Scholar]