Abstract
Background:
Despite a growing body of evidence reporting the deleterious mechanical and oncological complications of biopsy of hepatic malignancy, a small but significant number of patients undergo the procedure prior to specialist surgical referral. Biopsy has been shown to result in poorer longterm survival following resection and advances in modern imaging modalities provide equivalent, or better, diagnostic accuracy.
Methods:
The literature relating to needle-tract seeding of primary and secondary liver cancers was reviewed. MEDLINE, EMBASE and the Cochrane Library were searched for case reports and series relating to the oncological complications of biopsy of liver malignancies. Current non-invasive diagnostic modalities are reviewed and their diagnostic accuracy presented.
Results:
Biopsy of malignant liver lesions has been shown to result in poorer longterm survival following resection and does not confer any diagnostic advantage over a combination of non-invasive imaging techniques and serum tumour markers.
Conclusions:
Given that chemotherapeutic advances now often permit downstaging and subsequent resection of ‘unresectable’ disease, the time has come to abandon biopsy of solid lesions outside the setting of a specialist multi-disciplinary team meeting (MDT).
Keywords: liver malignancy, biopsy, tumour seeding
Introduction
Liver resection is now well established as a safe and efficacious treatment associated with minimal mortality and longterm survival benefits in correctly selected primary and secondary hepatic malignancies.1 Advances in chemotherapeutic agents, operative techniques and surgical technology have combined with better understanding of tumour biology and greater use of radiological interventions, such as portal vein embolization, to allow a constant widening of the criteria that define the boundaries for resection.2 An effective diagnostic paradigm is essential in assessing resectable solid liver lesions and, despite growing evidence demonstrating its detrimental effects,3 which include the seeding of 1000–100 000 cells along each needle tract,4 biopsy is still being used in the diagnostic pathway for such lesions. Sadly, a significant number of patients with potentially resectable liver disease still undergo biopsy of the lesion prior to referral to a hepatobiliary surgical unit. Although unusual, tumour seeding along the biopsy needle tract can convert a potentially curable case to one with disseminated peritoneal disease, whilst adding virtually nothing to the diagnostic pathway.
A combination of modern imaging techniques, the use of serum tumour markers and clinical acumen negates a requirement for biopsy in almost all cases. Given its well-documented mechanical and oncological complications, the time has come for biopsy to be dropped from preoperative assessment.
A review of the potential dangers of biopsy along with the non-invasive techniques available for the characterization of solid hepatic lesions is presented.
Materials and methods
MEDLINE, EMBASE and Cochrane Library searches were performed to identify reported cases and case series of needle tract seeding following biopsy of solid liver tumours. Reports of seeding after biopsy of colorectal liver metastasis and hepatocellular carcinoma (HCC) were discovered. These reports were reviewed and summarized along with the evidence for potential mechanical complications of liver biopsy; these data are presented in combination with information on the current utility of modern imaging techniques and serum tumour markers.
Mechanical complications of liver biopsy
Although it is often considered a relatively benign and straightforward procedure, liver biopsy is not without risk of mechanical complication. The British Society of Gastroenterology published guidance on the use of liver biopsy in 1999.5 Overall mortality associated with biopsy was quoted as being 19% at 3 months6 and was mainly related to the underlying diagnosis leading to biopsy. Mortality that is specifically related to the biopsy procedure is mainly caused by haemorrhage5 and rates vary between institutions, with an apparent relationship to volume of practice. Haemorrhage-related mortality in the specialist setting of the Mayo Clinic was 0.11%,7 but rose to up to three times higher (0.33%) in an audit of UK district general hospitals.6
The UK guidelines also seek to quantify the morbidity associated with liver biopsy and suggest an incidence of minor complications of 5.9%.8 Pain was the most commonly cited complication and was reported in 30% of cases.6 Significant haemorrhage, resulting in a drop in haemoglobin of >2 g/dl occurs in up to 0.5% of procedures,9 with up to 23% having intrahepatic or subcapsular haematomas, detectable by ultrasound at 24 h post-biopsy.10
Haemobilia may be expected in 0.05% of cases5 and other significant complications, including bile leak, sepsis, pancreatitis, local infections or pneumothorax, in 0.23%.11–13 These seemingly small risks must be considered in the light of studies that suggest a diagnostic accuracy for fine-needle aspiration biopsy of 69.0–80.6% and for core biopsy of 86–88% and an overall accuracy of 90.7%14,15 using both techniques combined. Similar and better results are achievable by non-invasive techniques, as detailed below.
Oncological complications of biopsy of colorectal liver metastases
Four case reports detailing a total of six patients,16–19 along with three small case series of five,20 seven21 and 173 patients have described cases of tumour dissemination following biopsy of colorectal liver metastasis, whether performed surgically or percutaneously (Table 1).
Table 1.
Summary of published literature relating to the complications of biopsy of colorectal liver metastases
| Authors | Year | Design | n | Findings |
|---|---|---|---|---|
| Al-Leswas et al.16 | 2008 | Case report | 2 | 2 cases of implantation |
| Jones et al.3 | 2005 | Retrospective review | 17 | 19% seeding rate; poorer longterm survival after biopsy |
| Rodgers et al.21 | 2003 | Retrospective review | 7 | 16% risk of seeding irrespective of route of biopsy |
| Metcalfe et al.18 | 2004 | Case report | 1 | Biopsy added nothing to diagnostic pathway |
| Ohlsson et al.20 | 2002 | Case series | 5 | 10% seeding rate |
| Scheele & Altendorf-Hofmann19 | 1990 | Case report | 2 | Seeding following biopsy of resectable lesion |
| Ferrucci et al.17 | 1979 | Case report | 1 | First documented case of tract seeding |
The reported incidence of seeding in the three case series ranges from 10% in the smallest series of five patients20 to 19% in the largest series of 17.3
The largest series,3 which also had the longest follow-up (minimum of 4 years), described a total of 598 patients who had undergone liver resection with curative intent for colorectal liver metastasis. Ninety (15.1%) of these 598 patients had undergone preoperative biopsy, of which 50 had been performed at the time of a previous laparotomy and 40 by a percutaneous technique under radiological guidance. Despite attempts to resect deposits within the needle tract with a macroscopic radical clearance of tumour, a significant risk to longterm survival was observed, with a 4-year survival rate of 32.5% in the biopsy group vs. 46.7% in the control group. Analysis of the two groups showed that preoperative biopsy was an independent predictor of survival following resection of colorectal liver metastasis (P= 0.001) (Fig. 1).
Figure 1.
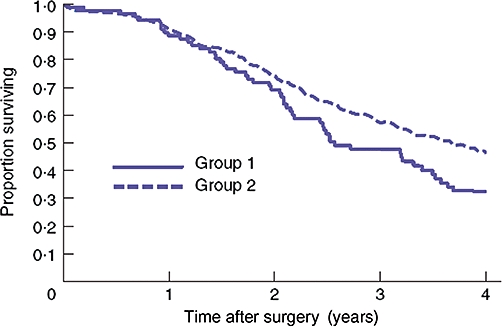
Kaplan–Meier survival curve comparing survival after liver resection in patients who underwent biopsy of suspected colorectal metastases (Group 1, biopsy) and those who did not (Group 2, no biopsy) (Jones et al. 20053)
In the 508 patients who did not undergo preoperative biopsy, seven benign lesions were encountered. In one patient, a clearly benign haemangioma was identified at laparotomy and was not resected, leaving a total of six patients (1.2% of the overall group) to undergo resection of what were ultimately proven to be benign lesions.
Similar effects with tumour dissemination have been shown in smaller case series. A study of 43 patients who underwent preoperative biopsy revealed a seeding rate of 16%21 and a further study of 51 patients demonstrated a seeding rate of 10%.20
Given the clarity of the message that biopsy can lead to dissemination and that such dissemination has a deleterious effect on longterm survival, it is indeed depressing that although the first case report was filed as long ago as 1979, a further two cases were reported as recently as 2008.16
Oncological complications of biopsy in suspected hepatocellular carcinoma
Similar effects of tumour dissemination both within the liver and elsewhere have been reported following biopsies in HCC, with reported seeding rates of 1.6–5.1%22–29 and sites of extrahepatic spread including the peritoneum, ribs, muscle and skin. A systematic review of eight previous case reports and series suggests an overall seeding rate of 2.7%30 (Table 2).
Table 2.
Summary of published literature relating to the complications of biopsy of hepatocellular carcinoma
| Authors | Year | Design | n | Findings |
|---|---|---|---|---|
| Qua et al.26 | 2008 | Case report | 1 | Seeding after biopsy |
| Liu et al.25 | 2007 | Case report | 5 | 2 of 5 converted from potentially curative to palliative by seeding |
| Kosugi et al.24 | 2004 | Case series | 6 | 1.6% seeding rate |
| Durand et al.22 | 2001 | Retrospective review | 2 | 1.6% seeding rate |
| Kim et al.23 | 2000 | Case series | 7 | 3.4% seeding rate |
| Takamori et al.27 | 2000 | Case report | 3 | 5.1% seeding rate |
| Chapoutot et al.28 | 1999 | Case report | 4 | 2.7% seeding rate |
| Huang et al.29 | 1996 | Case series | 9 | 2.1% seeding rate |
| Silva et al.30 | 2008 | Review | 26 | 2.7% overall seeding rate |
Furthermore, at least one study has again identified preoperative biopsy as an independent predictor of poor outcome with an observed rate of peritoneal recurrence of 12.5% following biopsy vs. 1.6% with no biopsy. Five-year disease-free survival has also been deleteriously affected, with reported rates of 24% following biopsy and 52% without biopsy.31
Despite these reported cases, specialist agreement on abandoning biopsy for HCC is less universal and, indeed, several authors advocate its use. The majority of controversy surrounds two small subsets of patients, the first of which demonstrate small nodules (1–2 cm) against a background of cirrhosis and the second of which exhibit larger tumours which fall outside transplant criteria.
In the case of small lesions, which, with current imaging resolution, are impossible to distinguish from regenerative nodules, some enthusiasts have advocated biopsy on the basis of a relatively low seeding rate.32 It is widely accepted, however, that HCCs > 2 cm in size can be reliably identified by their imaging characteristics of hypervascularity in an arterial phase with washout in the early or delayed venous phase on either computed tomography (CT) or magnetic resonance imaging (MRI).33,34 Interval surveillance scanning therefore remains a reasonable alternative to biopsy in patients with small, indeterminate nodules and would seem particularly prudent, given that the risk of seeding following biopsy is not related to the size of the lesion.35
For larger tumours that fall outside current transplant criteria there is emerging evidence that certain biological features on biopsy (such as low grade, a high degree of differentiation or lack of vascular invasion36) can suggest a good prognosis, which may justify an expansion of transplant criteria. One- and 3-year survival rates of 84.2% and 67.4%, respectively, have been reported for tumours that fall outwith the Milan criteria but which are without vascular invasion.37 However, these potential advantages must be balanced against data that suggest that both the degree of differentiation and the presence of vascular invasion can be significantly underestimated on a needle core biopsy compared with the explanted specimen.38
These tumours are, by definition, otherwise untransplantable and the degree of underlying cirrhosis usually precludes resection. In such cases, the patient's outlook is bleak and therefore biopsy is justified39 if it is carried out with a view to selecting patients for a therapy that would otherwise be unavailable and is performed within the setting of a clinical trial.
Non-invasive diagnosis of metastatic malignancy
Recent advances in imaging technologies coupled with the information provided by serum tumour markers mean that the majority of resectable liver lesions can be accurately characterized and a definitive diagnosis reached without the need for biopsy.40,41 Such an approach carries the obvious advantage of avoiding the potential for biopsy complications, but it does require a paradigm shift in the approach to diagnosis and, particularly, in the approach to treatment. Malignant lesions, especially, can be identified with sufficient accuracy to justify resection without a proven tissue diagnosis and, as a result of the low morbidity and mortality now associated with such procedures, their advantages greatly outweigh those of biopsy, given its risk for oncological complications.1
Imaging remains the mainstay of diagnosis, with serum markers providing useful support of the diagnosis of malignancy when elevated, but with significant risk of producing false negatives. Carcinoembryonic antigen (CEA) can be expected to be raised in 62% of cases of colorectal liver metastases and cancer antigen (CA) 19-9 is likely to be elevated in just 40%.42 The choice of imaging modality must be tailored to individual circumstances in terms of local expertise and likely underlying diagnosis. MRI seems to confer the greatest degree of sensitivity and specificity.41,43 All techniques remain extremely operator-dependent, however, and it is important that resectable lesions are investigated in high-volume specialist units by an established protocol. The final decision regarding treatment, whether by resection or not, may then be decided by an appropriate multidisciplinary team.
Detection and diagnosis of liver metastases
Metastatic liver disease is usually detected either as part of a focused surveillance programme following resection of a primary tumour (most often of colorectal origin) or else as a de novo presentation with a previously undiagnosed primary. In both groups the initial imaging modality is usually either CT or ultrasonography; respective levels of sensitivity and specificity of 40% and 63% for ultrasound and 89% and 89% for CT have been reported.44 Although clear guidelines for follow-up are yet to be decided, CT is to be favoured for cancer surveillance,45 with routine CEA measurement adding respective sensitivity and specificity of 80% and 86% following resection of a colorectal primary46 and similar sensitivity of 79% for Ca15-3 in breast cancer.47
Following detection, MRI offers the greatest accuracy in terms of characterization and definitive diagnosis.41 In addition, there is clinical research interest in contrast ultrasound and an emerging role for 18F-FDG positron emission tomography (PET) CT.
Magnetic resonance techniques for the characterization of liver metastases
Magnetic resonance imaging has consistently been shown to be more specific for diagnosing metastatic liver disease than both ultrasound and CT and is considered by most units as the reference method for preoperative evaluation of resectable disease.48–52 Issues relating to the resolution of small lesions of <1 cm have been resolved to a degree by advances in technological aspects, image phasing and weighting and the use of contrast agents, with a result that lesions >5 mm can now be accurately characterized.41,53–56
Despite these advances, MRI remains highly operator-dependant and scans must be optimally planned to maximize their value. This will allow maximum exploitation of the various benefits to be attained by combining weighting and phase of scanning, along with the use of contrast agents.
Unenhanced sequences will give important information on fluid composition, with T2 weighting distinguishing solid from fluid-filled lesions41 (Fig. 2). In- and out-of-phase (chemical shift) imaging with T1 weighting will allow the differentiation of solid lesions from focal fatty changes as a result of the reduced signal intensity on out-of-phase images from fatty liver.57
Figure 2.
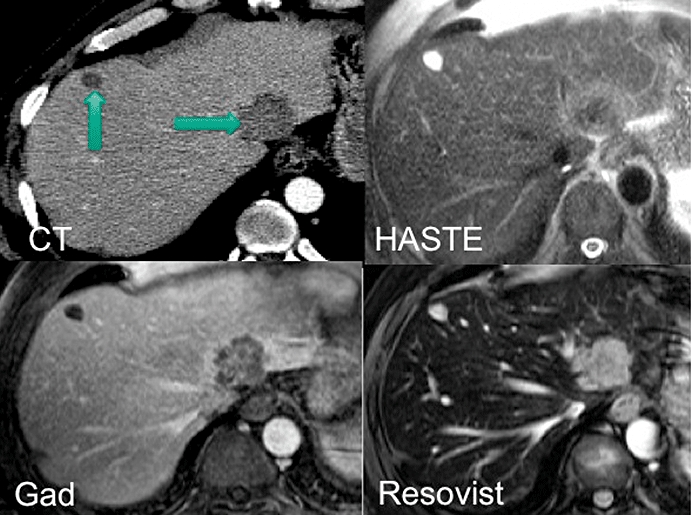
Images obtained by computed tomography (CT) with venous contrast, T2-weighted HASTE magnetic resonance imaging (MRI), gadolinium-enhanced MRI and resovist-enhanced MRI. The comparative images show a simple cyst in segment 4b (CT and GAD images), displaying a typically high signal on T2 images and a metastasis in segment 2 (HASTE and resovist images). Gadolinium, which is an extracellular contrast agent, produces a typical ‘ring-enhancement’ of metastases, whereas resovist, which is a liver-specific agent, reduces the signal from normal parenchyma, which leads to a relatively increased signal from the metastasis
The addition of contrast agents has enabled the characterization of sub-centimetre lesions58 and there are a variety of agents in clinical use. Each agent demonstrates specific properties, which can be used to distinguish small lesions from the background parenchyma, and relies on modern hardware to provide sufficiently thin slices to detect such lesions. Dual contrast imaging is optimal for the characterization of focal liver lesions, whereby a combination of agents that will accumulate in both the extracellular fluid and within the liver cells themselves is used. Differing characteristics in uptake by the various lesions will thus facilitate differentiation.
Gadolinium accumulates in the extracellular space and will delineate hypervascular lesions in the arterial phase, which exhibit rapid accumulation of contrast41 which can be compared with slower uptake by the background normal parenchyma. This contrast is also useful for detecting smaller surface lesions, which become apparent on delayed images with suppression of the surrounding fat of both the background parenchyma and adjacent abdominal wall59 (Fig. 2).
Simple cysts can be classically differentiated by their non-progressive enhancement and haemangiomata by centripetal discontinuous and progressive enhancement.
Liver-specific contrast agents have also been developed. These will target either hepatocytes, producing positive enhancement of normal parenchyma on T1 images (such as gadobente or mangafodipir) or Kuppfer cells, (the supermagnetic iron oxide [SPIO] agents), producing signal loss on T2 images. Malignant liver lesions lack the presence of functioning hepatocytes; these agents allow the differentiation of tumours that otherwise possess similar gadolinium perfusion characteristics to normal liver.
Gadobenate and gadoxetic acid have the extra utility of a biphasic enhancement profile in that they behave like extracellular gadolinium in the early phase and become liver-specific in later phases. The timing of image acquisition is therefore of essential importance and is specific to the contrast agent being used.
The differential uptake of contrast by normal liver and solid lesions has resulted in improved resolution of small lesions with liver-specific MRI compared with unenhanced MRI, gadolinium-enhanced MRI and contrast CT,48,50,52,55 particularly with lesions >1 cm60 (Fig. 2).
Lesions develop progressively from microscopic deposits and inevitably there will be a limit to the resolution at which a positive diagnosis can be made. Given that the current limit is set as low as 5 mm, an expectant approach with interval scanning remains a safe compromise for the characterization of lesions <5 mm in size and has a negligible potential impact on outcome.
Contrast ultrasound for detection of liver metastases
Contrast ultrasound is advantaged by having no radiation dose compared with CT and is relatively quick to perform compared with a full MRI assessment. The use of micro-bubble contrast has been shown to increase the sensitivity of plain ultrasound from 60% to 82%.61
18F-FDG PET CT
Owing to the high background metabolic activity of the liver, PET CT can be difficult to interpret and the majority of studies suggest that MRI remains more sensitive for the characterization of intrahepatic lesions.62 There is certainly an emerging role for PET CT, however, particularly in the detection of extrahepatic disease in non-colorectal liver metastases from primary tumours that are known to commonly metastasize to other sites, such as renal, breast and ovarian carcinoma and malignant melanoma. It is also the senior author's practice to use PET CT preoperatively in cases of colorectal liver metastases with poor prognoses or where resection would be especially challenging. PET CT can be expected to detect extrahepatic disease that is not detected on contrast-enhanced CT, which can change the surgical approach in patients with colorectal liver metastases in up to 17% of cases.63 As it becomes more widely available, its role in the preoperative staging of extrahepatic disease can be expected to expand.
Preoperative imaging for hepatocellular carcinoma
Magnetic resonance imaging again represents the reference standard among tests for the characterization of HCC; a meta-analysis has suggested sensitivity and specificity of 81% and 85%, respectively, which exceed the reported equivalents of both ultrasound (60% and 97%) and CT (68% and 93%).43 The costs and time required for full liver protocol scanning, however, currently prohibit the use of MRI as a screening tool and concerns regarding repeated doses of radiation have resulted in the widespread use of ultrasound with serum AFP (alpha-fetoprotein) level for routine surveillance. An AFP level >400 ng/ml is generally accepted as a diagnostic cut-off, although it is recognized that around 30% of HCC measuring <4 cm are not expected to have an AFP < 200 ng/ml and that up to 20% of HCCs do not produce AFP, irrespective of their size.64
Targeted MRI characterization of nodules in known cirrhotics is therefore advocated65 and has been shown to justify surgical treatment, whether by resection or transplantation, on the basis of radiological appearance alone and without recourse to biopsy.40,66,67
Conclusions
Biopsy of resectable liver malignancies, whether primary or secondary tumours, has been shown to be associated with significant risk for both mechanical and oncological complications. In both groups, preoperative biopsy is associated with a poorer longterm outcome. Advances in imaging techniques have permitted preoperative characterization of solid lesions with a diagnostic accuracy equivalent to or greater than that of tissue biopsy and a maximum reported accuracy of 97.9% in a combination of contrast ultrasound, CT and MRI.68 In cases of true diagnostic uncertainty with small lesions, we would advocate a ‘trial of time’ with early interval surveillance.
Primary surgical treatment, whether by resection or transplantation, has been well described and safely performed on the basis of non-invasive investigation alone, with a minimal false-positive rate in the resected specimen.
As chemotherapeutic advances allowing downstaging,69 staged liver resections and portal vein embolization70 all permit resection of what would hitherto have been considered as inoperable disease, the role of biopsy shrinks even further and should not be performed without specialist MDT discussion.
Preoperative biopsy is therefore not only unnecessary, but damages the longterm outcome for patients with resectable disease and must be strongly discouraged. Specialist units offering surgical treatment will have access to the necessary imaging modalities and therefore solid liver lesions should be referred for tertiary assessment prior to biopsy.
Conflicts of interest
None declared.
References
- 1.Gonzalez HD, Figueras J. Practical questions in liver metastases of colorectal cancer: general principles of treatment. HPB (Oxford) 2007;9:251–258. doi: 10.1080/13651820701457992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun YS, Vauthey JN. Extending the frontiers of resectability in advanced colorectal cancer. Eur J Surg Oncol. 2007;33(Suppl. 2):52–58. doi: 10.1016/j.ejso.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Jones OM, Rees M, John TG, Bygrave S, Plant G. Biopsy of resectable colorectal liver metastases causes tumour dissemination and adversely affects survival after liver resection. Br J Surg. 2005;92:1165–1168. doi: 10.1002/bjs.4888. [DOI] [PubMed] [Google Scholar]
- 4.Ryd W, Hagmar B, Eriksson O. Local tumour cell seeding by fine-needle aspiration biopsy. A semi-quantitative study. Acta Pathol Microbiol Immunol Scand [A] 1983;91:17–21. doi: 10.1111/j.1699-0463.1983.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 5.Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut. 1999;45(Suppl. 4):IV1, IV11. doi: 10.1136/gut.45.2008.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36:437–441. doi: 10.1136/gut.36.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major haemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396–1400. doi: 10.1016/0016-5085(90)91167-5. [DOI] [PubMed] [Google Scholar]
- 8.Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology. 1978;74:103–106. [PubMed] [Google Scholar]
- 9.Knauer CM. Percutaneous biopsy of the liver as a procedure for outpatients. Gastroenterology. 1978;74:101–102. [PubMed] [Google Scholar]
- 10.Minuk GY, Sutherland LR, Wiseman DA, MacDonald FR, Ding DL. Prospective study of the incidence of ultrasound-detected intrahepatic and subcapsular haematomas in patients randomized to 6 or 24 h of bed rest after percutaneous liver biopsy. Gastroenterology. 1987;92:290–293. doi: 10.1016/0016-5085(87)90119-3. [DOI] [PubMed] [Google Scholar]
- 11.Caturelli E, Ghittoni G, Roselli P, De Palo M, Anti M. Fine-needle biopsy of focal liver lesions: the hepatologist's point of view. Liver Transpl. 2004;10(2) Suppl. 1:26–29. doi: 10.1002/lt.20037. [DOI] [PubMed] [Google Scholar]
- 12.Giorgio A, Tarantino L, de Stefano G, et al. Complications after interventional sonography of focal liver lesions: a 22-year single-centre experience. J Ultrasound Med. 2003;22:193–205. doi: 10.7863/jum.2003.22.2.193. [DOI] [PubMed] [Google Scholar]
- 13.Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991;178:253–258. doi: 10.1148/radiology.178.1.1984314. [DOI] [PubMed] [Google Scholar]
- 14.Nyman RS, Cappelen-Smith J, Brismar J, von Sinner W, Kagevi I. Yield and complications in ultrasound-guided biopsy of abdominal lesions. Comparison of fine-needle aspiration biopsy and 1.2-mm needle core biopsy using an automated biopsy gun. Acta Radiol. 1995;36:485–490. [PubMed] [Google Scholar]
- 15.Stewart CJ, Coldewey J, Stewart IS. Comparison of fine-needle aspiration cytology and needle core biopsy in the diagnosis of radiologically detected abdominal lesions. J Clin Pathol. 2002;55:93–97. doi: 10.1136/jcp.55.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Leswas D, O'Reilly DA, Poston GJ. Biopsy of solid liver tumours: adverse consequences. Hepatobiliary Pancreat Dis Int. 2008;7:325–327. [PubMed] [Google Scholar]
- 17.Ferrucci JT, Wittenberg J, Margolies MN, Carey RW. Malignant seeding of the tract after thin-needle aspiration biopsy. Radiology. 1979;130:345–346. doi: 10.1148/130.2.345. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe MS, Bridgewater FH, Mullin EJ, Maddern GJ. Useless and dangerous – fine-needle aspiration of hepatic colorectal metastases. BMJ. 2004;328:507–508. doi: 10.1136/bmj.328.7438.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheele J, Altendorf-Hofmann A. Tumour implantation from needle biopsy of hepatic metastases. Hepatogastroenterology. 1990;37:335–337. [PubMed] [Google Scholar]
- 20.Ohlsson B, Nilsson J, Stenram U, Akerman M, Tranberg KG. Percutaneous fine-needle aspiration cytology in the diagnosis and management of liver tumours. Br J Surg. 2002;89:757–762. doi: 10.1046/j.1365-2168.2002.02111.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodgers MS, Collinson R, Desai S, Stubbs RS, McCall JL. Risk of dissemination with biopsy of colorectal liver metastases. Dis Colon Rectum. 2003;46:454–458. 458–459. doi: 10.1007/s10350-004-6581-6. discussion. [DOI] [PubMed] [Google Scholar]
- 22.Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol. 2001;35:254–258. doi: 10.1016/s0168-8278(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 23.Kim SH, Lim HK, Lee WJ, Cho JM, Jang HJ. Needle-tract implantation in hepatocellular carcinoma: frequency and CT findings after biopsy with a 19.5-gauge automated biopsy gun. Abdom Imaging. 2000;25:246–250. doi: 10.1007/s002610000025. [DOI] [PubMed] [Google Scholar]
- 24.Kosugi C, Furuse J, Ishii H, et al. Needle tract implantation of hepatocellular carcinoma and pancreatic carcinoma after ultrasound-guided percutaneous puncture: clinical and pathologic characteristics and the treatment of needle tract implantation. World J Surg. 2004;28:29–32. doi: 10.1007/s00268-003-7003-y. [DOI] [PubMed] [Google Scholar]
- 25.Liu YW, Chen CL, Chen YS, Wang CC, Wang SH, Lin CC. Needle tract implantation of hepatocellular carcinoma after fine-needle biopsy. Dig Dis Sci. 2007;52:228–231. doi: 10.1007/s10620-006-9354-3. [DOI] [PubMed] [Google Scholar]
- 26.Qua CS, Wong CH, Goh KL. Tumour seeding following percutaneous needle biopsy of hepatocellular carcinoma. Singapore Med J. 2008;49:e8–e11. [PubMed] [Google Scholar]
- 27.Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000;6:67–72. doi: 10.1002/lt.500060103. [DOI] [PubMed] [Google Scholar]
- 28.Chapoutot C, Perney P, Fabre D, et al. Needle-tract seeding after ultrasound-guided puncture of hepatocellular carcinoma. A study of 150 patients. Gastroenterol Clin Biol. 1999;23:552–556. [PubMed] [Google Scholar]
- 29.Huang GT, Sheu JC, Yang PM, Lee HS, Wang TH, Chen DS. Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma – a study based on 420 patients. J Hepatol. 1996;25:334–338. doi: 10.1016/s0168-8278(96)80120-6. [DOI] [PubMed] [Google Scholar]
- 30.Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 31.Young AL, Malik HZ, Abu-Hilal M, et al. Large hepatocellular carcinoma: time to stop preoperative biopsy. J Am Coll Surg. 2007;205:453–462. doi: 10.1016/j.jamcollsurg.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 32.Durand F, Belghiti J, Paradis V. Liver transplantation for hepatocellular carcinoma: role of biopsy. Liver Transpl. 2007;13(11) Suppl. 2:17–23. doi: 10.1002/lt.21326. [DOI] [PubMed] [Google Scholar]
- 33.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 34.Torzilli G, Minagawa M, Takayama T, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–893. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 35.Rizzi PM, Kane PA, Ryder SD, et al. Accuracy of radiology in detection of hepatocellular carcinoma before liver transplantation. Gastroenterology. 1994;107:1425–1429. doi: 10.1016/0016-5085(94)90545-2. [DOI] [PubMed] [Google Scholar]
- 36.Broelsch CE, Frilling A, Malago M. Should we expand the criteria for liver transplantation for hepatocellular carcinoma – yes, of course! J Hepatol. 2005;43:569–573. doi: 10.1016/j.jhep.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Suh KS, Cho EH, Lee HW, Shin WY, Yi NJ, Lee KU. Liver transplantation for hepatocellular carcinoma in patients who do not meet the Milan criteria. Dig Dis. 2007;25:329–333. doi: 10.1159/000106913. [DOI] [PubMed] [Google Scholar]
- 38.Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumour grade using needle biopsy: impl ications for transplant eligibility. Ann Surg. 2007;245:435–442. doi: 10.1097/01.sla.0000250420.73854.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marsh JW, Dvorchik I. Should we biopsy each liver mass suspicious for hepatocellular carcinoma before liver transplantation? Yes. J Hepatol. 2005;43:558–562. doi: 10.1016/j.jhep.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Compagnon P, Grandadam S, Lorho R, et al. Liver transplantation for hepatocellular carcinoma without preoperative tumour biopsy. Transplantation. 2008;86:1068–1076. doi: 10.1097/TP.0b013e318187754c. [DOI] [PubMed] [Google Scholar]
- 41.Ward J. New MR techniques for the detection of liver metastases. Cancer Imaging. 2006;6:33–42. doi: 10.1102/1470-7330.2006.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar Y, Pinedo IR, Tapuria N, Zabron A, Davidson BR. A comparison of tumour M2-PK with carcinoembryonic antigen and CA19-9 in patients undergoing liver resection for colorectal metastases. Eur J Gastroenterol Hepatol. 2008;20:1006–1011. doi: 10.1097/MEG.0b013e3282f857a7. [DOI] [PubMed] [Google Scholar]
- 43.Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513–523. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 44.Quaia E, D'Onofrio M, Palumbo A, Rossi S, Bruni S, Cova M. Comparison of contrast-enhanced ultrasonography versus baseline ultrasound and contrast-enhanced computed tomography in metastatic disease of the liver: diagnostic performance and confidence. Eur Radiol. 2006;16:1599–1609. doi: 10.1007/s00330-006-0192-7. [DOI] [PubMed] [Google Scholar]
- 45.Papagrigoriadis S. Follow-up of patients with colorectal cancer: the evidence is in favour but we are still in need of a protocol. Int J Surg. 2007;5:120–128. doi: 10.1016/j.ijsu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Irvine T, Scott M, Clark CI. A small rise in CEA is sensitive for recurrence after surgery for colorectal cancer. Colorectal Dis. 2007;9:527–531. doi: 10.1111/j.1463-1318.2007.01176.x. [DOI] [PubMed] [Google Scholar]
- 47.Lauro S, Trasatti L, Bordin F, et al. Comparison of CEA, MCA, CA 15-3 and CA 27-29 in follow-up and monitoring therapeutic response in breast cancer patients. Anticancer Res. 1999;19:3511–3515. [PubMed] [Google Scholar]
- 48.Hagspiel KD, Neidl KF, Eichenberger AC, Weder W, Marincek B. Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5 T with dynamic CT, intraoperative US, and percutaneous US. Radiology. 1995;196:471–478. doi: 10.1148/radiology.196.2.7617863. [DOI] [PubMed] [Google Scholar]
- 49.Lencioni R, Della Pina C, Bruix J, et al. Clinical management of hepatic malignancies: ferucarbotran-enhanced magnetic resonance imaging versus contrast-enhanced spiral computed tomography. Dig Dis Sci. 2005;50:533–537. doi: 10.1007/s10620-005-2469-0. [DOI] [PubMed] [Google Scholar]
- 50.Muller RD, Vogel K, Neumann K, et al. SPIO-MR imaging versus double-phase spiral CT in detecting malignant lesions of the liver. Acta Radiol. 1999;40:628–635. doi: 10.3109/02841859909175600. [DOI] [PubMed] [Google Scholar]
- 51.Semelka RC, Cance WG, Marcos HB, Mauro MA. Liver metastases: comparison of current MR techniques and spiral CT during arterial portography for detection in 20 surgically staged cases. Radiology. 1999;213:86–91. doi: 10.1148/radiology.213.1.r99oc3386. [DOI] [PubMed] [Google Scholar]
- 52.Seneterre E, Taourel P, Bouvier Y, et al. Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology. 1996;200:785–792. doi: 10.1148/radiology.200.3.8756932. [DOI] [PubMed] [Google Scholar]
- 53.Kim MJ, Kim JH, Chung JJ, Park MS, Lim JS, Oh YT. Focal hepatic lesions: detection and characterization with combination gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology. 2003;228:719–726. doi: 10.1148/radiol.2283020735. [DOI] [PubMed] [Google Scholar]
- 54.Poeckler-Schoeniger C, Koepke J, Gueckel F, Sturm J, Georgi M. MRI with superparamagnetic iron oxide: efficacy in the detection and characterization of focal hepatic lesions. Magn Reson Imaging. 1999;17:383–392. doi: 10.1016/s0730-725x(98)00180-5. [DOI] [PubMed] [Google Scholar]
- 55.Reimer P, Jahnke N, Fiebich M, et al. Hepatic lesion detection and characterization: value of non-enhanced MR imaging, superparamagnetic iron oxide-enhanced MR imaging, and spiral CT-ROC analysis. Radiology. 2000;217:152–158. doi: 10.1148/radiology.217.1.r00oc31152. [DOI] [PubMed] [Google Scholar]
- 56.Ward J, Chen F, Guthrie JA, et al. Hepatic lesion detection after superparamagnetic iron oxide enhancement: comparison of five T2-weighted sequences at 1.0 T by using alternative-free response receiver operating characteristic analysis. Radiology. 2000;214:159–166. doi: 10.1148/radiology.214.1.r00ja21159. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell DG. Focal manifestations of diffuse liver disease at MR imaging. Radiology. 1992;185:1–11. doi: 10.1148/radiology.185.1.1523289. [DOI] [PubMed] [Google Scholar]
- 58.Ward J, Guthrie JA, Wilson D, et al. Colorectal hepatic metastases: detection with SPIO-enhanced breath-hold MR imaging – comparison of optimized sequences. Radiology. 2003;228:709–718. doi: 10.1148/radiol.2283020376. [DOI] [PubMed] [Google Scholar]
- 59.Low RN, Barone RM, Lacey C, Sigeti JS, Alzate GD, Sebrechts CP. Peritoneal tumour: MR imaging with dilute oral barium and intravenous gadolinium-containing contrast agents compared with unenhanced MR imaging and CT. Radiology. 1997;204:513–520. doi: 10.1148/radiology.204.2.9240546. [DOI] [PubMed] [Google Scholar]
- 60.Ward J, Robinson PJ, Guthrie JA, et al. Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology. 2005;237:170–180. doi: 10.1148/radiol.2371041444. [DOI] [PubMed] [Google Scholar]
- 61.Konopke R, Bunk A, Kersting S. Contrast-enhanced ultrasonography in patients with colorectal liver metastases after chemotherapy. Ultraschall Med. 2008;29(Suppl 4):203–209. doi: 10.1055/s-2008-1027795. [DOI] [PubMed] [Google Scholar]
- 62.Bipat S, van Leeuwen MS, Comans EF, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis – meta-analysis. Radiology. 2005;237:123–131. doi: 10.1148/radiol.2371042060. [DOI] [PubMed] [Google Scholar]
- 63.Kong G, Jackson C, Koh DM, et al. The use of 18F-FDG PET/CT in colorectal liver metastases – comparison with CT and liver MRI. Eur J Nucl Med Mol Imaging. 2008;35:1323–1329. doi: 10.1007/s00259-008-0743-z. [DOI] [PubMed] [Google Scholar]
- 64.Taketa K, Endo Y, Sekiya C, et al. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419–5423. [PubMed] [Google Scholar]
- 65.Tang Y, Yamashita Y, Arakawa A, et al. Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of gadolinium- and ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 1999;172:1547–1554. doi: 10.2214/ajr.172.6.10350287. [DOI] [PubMed] [Google Scholar]
- 66.Horigome H, Nomura T, Saso K, Itoh M, Joh T, Ohara H. Limitations of imaging diagnosis for small hepatocellular carcinoma: comparison with histological findings. J Gastroenterol Hepatol. 1999;14:559–565. doi: 10.1046/j.1440-1746.1999.01915.x. [DOI] [PubMed] [Google Scholar]
- 67.Saada J, Bhattacharya S, Dhillon AP, et al. Detection of small hepatocellular carcinomas in cirrhotic livers using iodized. oil computed tomography. Gut. 1997;41:404–407. doi: 10.1136/gut.41.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torzilli G, Olivari N, Del Fabbro D, et al. Indication and contraindication for hepatic resection for liver tumours without fine-needle biopsy: validation and extension of an Eastern approach in a Western community hospital. Liver Transpl. 2004;10(2) Suppl. 1:30–33. doi: 10.1002/lt.20051. [DOI] [PubMed] [Google Scholar]
- 69.Bismuth H, Adam R, Levi F, et al. Resection of non-resectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. 520–522. doi: 10.1097/00000658-199610000-00009. discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–175. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]


