Abstract
Introduction:
Nomograms are statistical tools designed to predict outcomes. This study evaluates the effects of peri-operative chemotherapy on the accuracy of a prognostic nomogram for disease-specific survival (DSS) after resection of colorectal liver metastases (CRLM) established at Memorial-Sloan Kettering Cancer Center (MSKCC).
Methods:
An external cohort of 203 patients who underwent resection of CRLM between 1996 and 2006 was used to assess the nomogram.
Results:
After median follow-up of 30.4 months (range 0.33–150), Kaplan–Meier (KM) estimates for 3-, 5- and 8-year post-resection DSS were 56%, 41%, and 32%, respectively; similar to nomogram-predicted probabilities for DSS. The concordance index for the nomogram was higher (0.602) than for the Fong colorectal risk score (CRS; 0.533). KM DSS was longer for patients (n= 50) treated with at least 6 months of peri-operative irinotecan or oxaliplatin compared with all other patients (median 66 vs. 40 months, P= 0.06). KM DSS was greater than nomogram predicted DSS for treated patients and less than nomogram predicted DSS for all other patients.
Conclusions:
The CRLM nomogram was validated by an external cohort and more accurately predicted post-resection survival than the commonly used CRS. Differences in observed and nomogram-predicted survival may reflect the effect of treatment factors, such as peri-operative chemotherapy.
Keywords: colorectal liver metastases, nomogram, chemotherapy
Introduction
Several institutions have developed prognostic scoring systems for survival after surgical extirpation of colorectal liver metastases (CRLM) incorporating patient demographics, clinicopathologic tumour characteristics and extent of hepatic resection.1–9 However, the validity of these scoring classifications when applied to external data cohorts has been inconsistent as heterogeneous survival outcomes among patients with similar risk scores have been observed.10–15 For example, some centres have noted significant long-term survival rates after partial hepatectomy among patients with poor predictive risk scores.16 In an effort to improve on these scoring systems, two groups have independently designed prognostic nomograms for survival after resection of CRLM from large hepatic resection databases.17,18 Nomograms are statistical tools that provide probability of a particular outcome and have been developed to predict survival for pancreatic, lung, hepatocellular, prostate, renal cell and oesophageal cancers.19–24 In comparison to traditional scoring systems, advantages of prognostic nomograms include the ability to accurately account for continuous variables [such as number and size of CRLM, carcino-embyronic antigen (CEA) level and disease-free interval from resection of the primary tumour to diagnosis of CRLM] by taking the specific value for these factors into consideration and the ability to allocate greater influence to particular factors in predicting survival after resection. Despite survival benefits of chemotherapy for resectable CRLM observed in prospective randomized controlled trials and large retrospective case series,25–29 neither prognostic nomogram for survival after resection of CRLM account for chemotherapy treatment. The Memorial Sloan-Kettering Cancer Center (MSKCC) nomogram in particular was constructed using an internal population of patients who underwent resection of CRLM from October 1985 to October 1998 – before the widespread use of contemporary chemotherapeutics including oxaliplatin and irinotecan.17 While this nomogram has been validated by an internal cohort,17 external validation of this cohort has not yet been demonstrated. External validation is essential to account for institution-specific factors that may bias results, including particular selection criteria for resection, surgical approaches or patient characteristics. The objectives of this study were to (i) assess the accuracy of the MSKCC nomogram (Fig. 1) for predicting 96-month disease-specific survival (DSS) relative to the Blumgart-Fong colorectal risk score (CRS)1 on an external patient cohort; and (ii) evaluate the effects of peri-operative irinotecan or oxaliplatin treatment on the predictive accuracy of the MSKCC nomogram.
Figure 1.
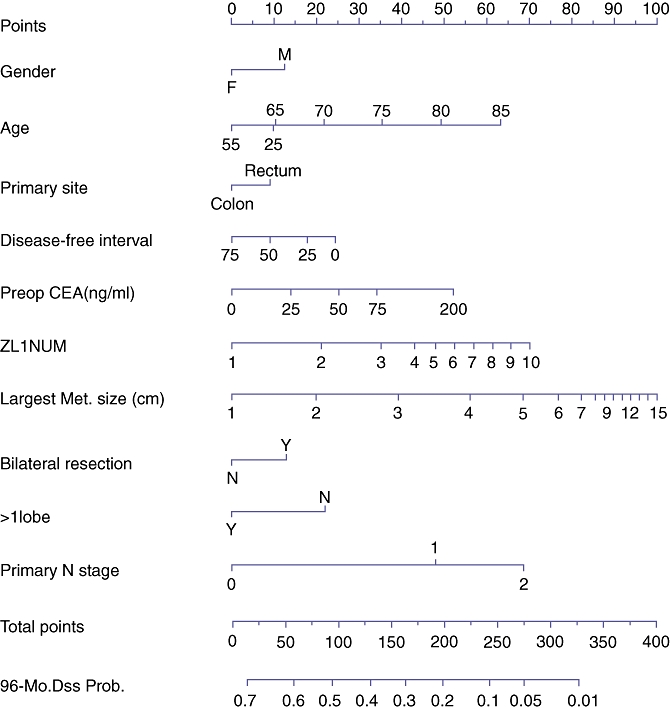
Memorial-Sloan Kettering Cancer Center (MSKCC) prognostic nomogram for predicting 96-month disease-specific survival (DSS) after resection of colorectal liver metastases (CRLM). Reproduced with permission from Kattan et al.17
Methods
After obtaining approval from the Duke University Medical Center (DUMC) Institutional Review Board for Clinical Investigations, patient demographics, clinicopathologic tumour characteristics, treatments and survival outcomes for patients who underwent resection of CRLM were reviewed from a retrospectively collected database. From 1996–2006, 289 patients underwent resection of CRLM. Patients who suffered mortality within 90 days of hepatic resection (n= 10), had gross disease at any location after partial hepatectomy (n= 32), had extra-hepatic metastatic disease at partial hepatectomy (n= 17) and who underwent previous hepatic resection (n= 26) were excluded. These exclusion criteria are similar to those used for creation of the MSKCC nomogram. One patient with both CRLM and hepatocellular carcinoma on pathological examination was also excluded. The 203 remaining patients formed the DUMC external cohort used to validate the MSKCC nomogram. There were no uniform criteria for administration of chemotherapy before or after partial hepatectomy. The specific drug combination used for each patient was at the discretion of the treating medical oncologist. Thus, no particular regimen was selected as first-line therapy. Fluoropyrimidine monotherapy (modalities of treatment included intravenous, oral, or via hepatic arterial infusion) with or without leucovorin was used before 2000. From 2000–2006, combinations of oxaliplatin or irinotecan-based chemotherapy with or without the anti-biologic agents bevacizumab or cetuximab were preferentially utilized, when tolerated. Pre-resection chemotherapy included treatment after discovery of CRLM and before hepatic resection. Post-resection chemotherapy consisted of treatment after partial hepatectomy and before disease recurrence. Peri-operative chemotherapy included treatment before and/or after hepatic resection. Duration of chemotherapy treatment was calculated from the date of initial to final dose of treatment.
Discrete and continuous variables were compared with the χ2- or Fisher's exact tests and the Wilcoxon rank test, respectively. Disease-specific survival (DSS) was calculated using date of death because of disease or date of last follow-up. DSS was estimated using the Kaplan–Meier method. Nomogram validation comprised two activities. First, discrimination was quantified with the concordance index which provides the probability that, in a randomly selected pair of patients in which one patient dies of disease before the other, the patient who died first had the worse predicted outcome from the nomogram. Bootstrapping was used to assess the magnitude of the difference in the predictive accuracy between the nomogram and the Fong colorectal risk score. Second, calibration was assessed by grouping patients by nomgram-predicted probabilities and then comparing the mean nomogram predicted survival of the group with the observed Kaplan–Meier estimate of DSS. All analyses were performed using S-plus 2000 Professional Software (Statistical Sciences, Seattle, WA, USA) or R version 2.8.1(http://www.R-project.org) with the Design and Hmisc libraries added.
Results
While there were similarities in gender, rectal primary tumours and primary lymph node staging, there were several differences in tumour characteristics between the patient cohorts (Table 1). Compared with the MSKCC cohort, DUMC patients had lower pre-resection CEA levels, fewer and smaller CRLM and tended to be younger at hepatic resection. DUMC patients had a shorter disease-free interval from resection of primary tumour to diagnosis of CRLM and less often underwent bilateral and greater than one lobe of hepatic resection. The MSKCC nomogram was validated with the DUMC external patient cohort (Fig. 2). After a median follow-up of 30.2 months, Kaplan–Meier (KM) estimates for 3-, 5- and 8-year post-resection DSS were 56%, 41%, and 32%, similar to nomogram predicted probabilities for DSS (Fig. 3). The estimated KM survival outcomes were similar to that of other large series of partial hepatectomy for colorectal metastases.30–32 The nomogram concordance index was higher than the concordance index for the CRS (0.602 vs. 0.533, P= 0.04). Nomogram predicted survival probabilities were heterogeneous within CRS scores, particularly for the lower scores (Fig. 4).
Table 1.
Demographics, clinicopathologic tumour characteristics and treatments for the patients in the Duke University Medical Center (DUMC) and Memorial-Sloan Kettering Cancer Center (MSKCC) cohorts are compared. Continuous variables are listed as medians with 1st and 3rd quartiles, discrete variables are listed with percentages
| Variables | MSKCC | DUMC | P |
|---|---|---|---|
| n | 1475 | 203 | – |
| Gender | 0.770 | ||
| Male | 853 (57.8%) | 120 (59.1%) | |
| Female | 624 (42.2%) | 83 (40.9%) | |
| Primary tumour | 0.281 | ||
| Colon | 1092 (74.0%) | 158 (77.8%) | |
| Rectum | 383 (26.0%) | 45 (22.2%) | |
| Primary lymph node | 0.1373 | ||
| 0 | 564 (40.1%) | 66 (34.7%) | |
| 1 | 588 (41.8%) | 79 (41.6%) | |
| 2 | 255 (18.1%) | 45 (23.7%) | |
| Pre-operative CEA (ng/ml) | 16.4 (5.7, 64.3) | 8.9 (2.9, 30.1) | <0.0001 |
| Number of CRLM | 2 (1, 3) | 1 (1, 2) | 0.0016 |
| Size of largest CRLM (cm) | 4.1 (2.8, 6.4) | 3.4 (2.2, 5.5) | 0.0002 |
| Age (year) | 62.8 (54.2, 69.6) | 61.0 (51.8, 68.8) | 0.06 |
| Disease-free interval (mo) | 14.4 (4.4,26) | 2 (0, 16) | <0.0001 |
| Bilateral resection | |||
| No | 890 (60.3%) | 150 (73.9%) | 0.0002 |
| Yes | 587 (39.7%) | 53 (26.1%) | |
| >1 Lobe resected | |||
| No | 528 (35.7%) | 133 (65.5%) | <0.0001 |
| Yes | 949 (64.3%) | 70 (34.5%) |
CRLM, colorectal liver metastases; CEA, carcino-embyronic antigen.
Disease-free interval refers to interval from resection of the primary tumour to diagnosis of CRLM.
Figure 2.
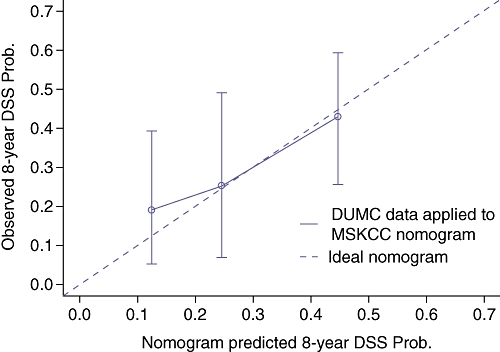
Duke University Medical Center (DUMC) cohort calibration curve – observed disease-specific survival (DSS) versus nomogram predicted DSS. DUMC patients were stratified into three groups based on nomogram predicted DSS – vertical lines with error bars depict the performance of the Memorial-Sloan Kettering Cancer Center (MSKCC) nomogram applied to DUMC patients. The ideal nomogram is depicted by the diagonal line
Figure 3.
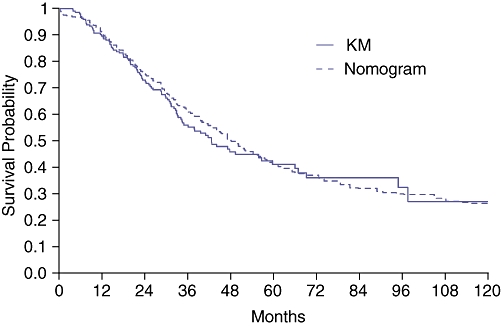
Observed Kaplan–Meier (KM) disease-specific survival (DSS) vs. Memorial-Sloan Kettering Cancer Center (MSKCC) nomogram predicted DSS after resection of colorectal liver metastases (CRLM) for the Duke University Medical Center (DUMC) patient cohort
Figure 4.
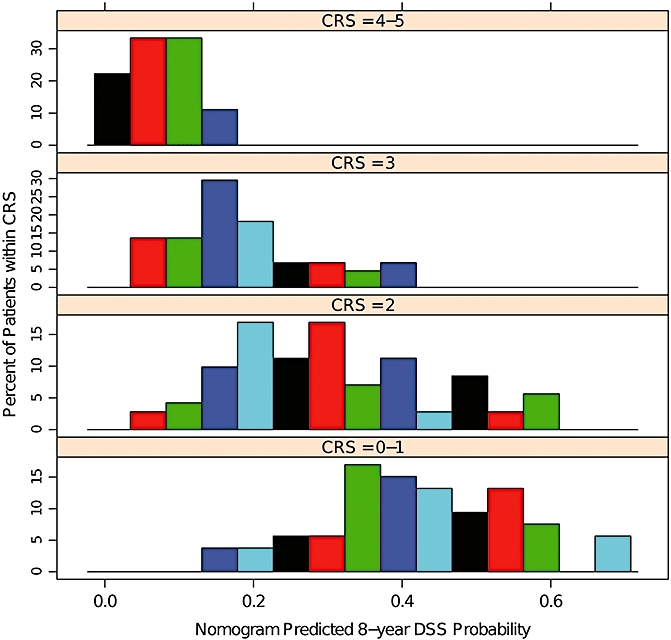
Comparisons of nomogram predicted probabilities of disease-specific survival (DSS) by CRS risk score. Note the heterogeneity for predicted survivals within each colorectal risk score (CRS) score
We focused on those patients treated with at least 6 months of peri-operative irinotecan or oxaliplatin relative to hepatic resection as 6 months was the intended duration of FOLFOX4 chemotherapy among the treatment group in the EORTC 40983 randomized controlled trial.22 Fifty out of 203 (24.6%) patients were treated with peri-operative irinotecan or oxaliplatin-based chemotherapy for a total of at least 6 months. Twenty-four (48%) of these patients were treated with irinotecan, 21 (42%) were treated with oxaliplatin and 5 patients (10%) were treated with both irinotecan and oxaliplatin. Many patients were treated before the widespread use of these agents. Patients were treated by a variety of medical oncologists both inside and outside our institution, and there was no established ‘standard of care’. Seventeen patients (34%) were treated with pre-resection chemotherapy only, 10 (20%) were treated with post-resection chemotherapy only, and 33 (66%) patients were treated with both pre-resection and post-resection chemotherapy. Forty-nine out of 203 patients (24.1%) were treated with peri-operative irinotecan or oxaliplatin for a total of less than 6 months (median treatment duration of three months). Fifty-five out of 203 (27.1%) patients were treated with alternate chemotherapy (most commonly 5-fluorouracil) and 49/203 (24.1%) were treated with no chemotherapy before or after partial hepatectomy. Patients with a follow-up after resection of less than 6 months were excluded from subsequent analyses. Nomogram predicted post-resection DSS was compared with observed DSS for the 50 patients treated with at least 6 months of peri-operative irinotecan or oxaliplatin and the 153 remaining patients (Fig. 5). Observed DSS was greater than nomogram predicted DSS for the 50 patients treated with at least 6 months of peri-operative irinotecan or oxaliplatin at 1, 2 and 3 years after hepatic resection, and less than nomogram predicted DSS for all other patients. A landmark analysis was performed to evaluate the independent effect of duration of peri-operative chemotherapy on DSS. Length of post-resection treatment was truncated at 6 months. Maximal survival benefit from peri-operative irinotecan or oxaliplatin was observed with a duration of 6 months of chemotherapy, although the effect of chemotherapy was not statistically significant (Fig. 6).
Figure 5.
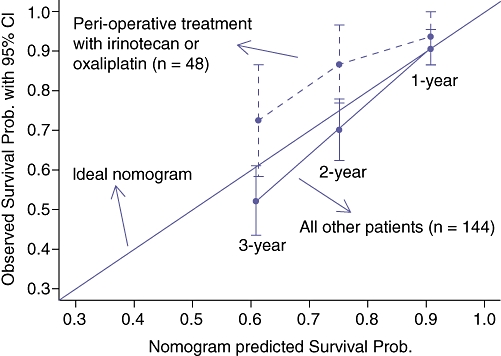
Observed disease-specific survival (DSS) at 1, 2 and 3 years after hepatic resection versus mean nomogram predicted DSS stratified by peri-operative chemotherapy treatment for patients with follow-up after liver resection of at least 6 months. The 48 patients treated with peri-operative irinotecan or oxaliplatin are compared with the other 144 patients. The lines with error bars represent the performance of the Memorial-Sloan Kettering Cancer Center (MSKCC) nomogram applied to the Duke University Medical Center (DUMC) patients. The diagonal line represents the performance of an ideal nomogram
Figure 6.
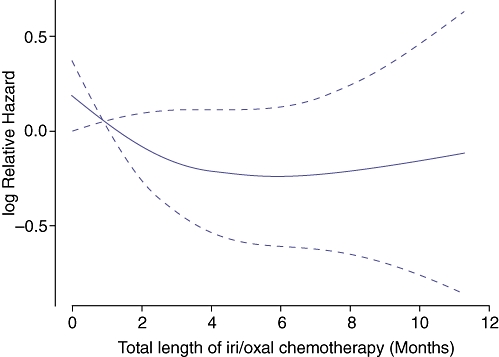
Landmark analysis for hazard ratio for disease-specific survival (DSS) by peri-operative irinotecan or oxaliplatin (iri/oxal) therapy. Post-resection chemotherapy was truncated at 6 months and all patients with follow-up time less than 6 months were excluded form this landmark analysis. The total length of iri/oxal chemotherapy was recalculated as the sum of the pre-resection chemotherapy and truncated post-resection chemotherapy. P= 0.27
Discussion
Enhancements in surgical technique, pre-operative imaging, critical care and understanding of hepatic anatomy have dramatically improved survival after resection of CRLM. Despite these improvements, long-term survival after resection of CRLM is not uniform as recent large case series note 5-year survival of 40–50%.7,27,29,33 In an attempt to better predict prognosis after partial hepatectomy, Kattan et al.17 have designed a prognostic nomogram incorporating patient demographic and clinicopathologic variables. Our report describes the validation of this nomogram by an external cohort (Fig. 2) despite several differences in clinicopathologic tumour characteristics between the DUMC and MSKCC patient cohorts (Table 1). The MSKCC nomogram concordance index when applied to the DUMC cohort was 0.602, slightly lower than the internal validation concordance index of 0.688.17 This implies that for any two randomly selected patients in the DUMC cohort, there is roughly a 60% chance that the MSKCC nomogram predicts the patient with shorter DSS. While superior to the CRS (which had a concordance index only slightly better than chance), there is substantial room for improvement.
There are three potential stages in the overall treatment of patients with CRLM where nomograms may affect clinical decisions: (i) to determine whether resection or chemotherapy should be the initial treatment; (ii) after pre-resection chemotherapy to determine if surgical extirpation should be performed; and (iii) after partial hepatectomy to determine administration of post-resection chemotherapy and ultimate prognosis. As noted by Kanemitsu and Kato,18 different factors affect decision making at each stage. Upon initial diagnosis of CRLM, therapeutic decisions are governed by patient demographics, comorbidity and clinicopathological tumor characteristics identified on pre-operative imaging or prior colorectal resection. Response to pre-resection chemotherapy also determines subsequent operative interventions as some studies have shown that patients with unresponsive or progressive disease after pre-resection chemotherapy treatment have poorer survival after hepatic resection compared with patients with responsive disease.34–36 After surgical extirpation, additional treatment factors (such as extent of resection and width of hepatic resection margins) may also influence the use of post-resection chemotherapy and ultimate prognosis. While useful as a tool for counselling patients regarding prognosis, further modifications to the nomogram – or perhaps creation of specific nomograms for different stages of treatment – are necessary to reach a level of predictive accuracy sufficient for making treatment decisions for individual patients. Additional factors noted above that have been shown to alter selection criteria for resection or are associated with survival after partial hepatectomy could be incorporated to improve nomogram predictive accuracy. These include positron emission tomography (PET),37–42 width of hepatic resection margin,33–46 and response to pre-operative chemotherapy treatment.34–36 In addition, molecular predictors of prognosis and response are actively being sought by many groups.
The EORTC 40983 randomized controlled trial demonstrated benefits in progression-free survival among patients treated with peri-operative FOLFOX4 compared with patients treated with partial hepatectomy alone among patients who ultimately were resected [42.4% (34.0–50.5%) vs. 33.2% (25.3–41.2%), P= 0.025].25 These results have been used by many to support the use of peri-operative chemotherapy for CRLM. Thus, this study was designed to evaluate the effects of similar durations of peri-operative irinotecan or oxaliplatin treatment on the predictive accuracy of the MSKCC nomogram.
In this study, observed Kaplan–Meier survival for patients treated with at least 6 months of peri-operative irinotecan or oxaliplatin in the DUMC cohort at 1, 2 and 3 years after resection was greater than the corresponding nomogram predicted survival whereas corresponding observed survival for all other patients was less than nomogram predicted survival (Fig. 5). Treatment with at least 6 months of peri-operative irinotecan or oxaliplain chemotherapy did not have a statistically significant effect on survival, but more than one-half of the other patients were treated with some type of chemotherapy, which may lead to underestimation of the effect of modern chemotherapy. These analyses do not address the relative value of preoperative versus post-operative chemotherapy but do suggest that differences in observed and nomogram predicted survival rates, which are based purely on demographical and clinicopathological criteria, may be attributable to contemporary chemotherapeutics.
There are several limitations to this retrospective study. The administration of pre-resection and/or post-resection chemotherapy and the specific drug regimens and dosing schedules were all at the discretion of the treating medical oncologists. In our study, the relatively small number of patients who were treated with at least 6 months of irinotecan or oxaliplatin, the subsequent heterogeneity in chemotherapy regimens and durations of treatment and probable biases in selecting patients for chemotherapy treatment (particularly pre-resection chemotherapy) prevent us from making definitive statements concerning the benefits of chemotherapy for CRLM based on this study alone. For example, patients who suffered major complications after hepatic resection, with overall poor performance status or with severe adverse reactions to chemotherapy were likely not to be treated with extended durations of oxaliplatin or irinotecan before or after partial hepatectomy. Thus, it is possible that some of the difference in KM observed and nomogram predicted survival for patients treated with at least 6 months of peri-operative irinotecan or oxaliplatin was as a result of selection bias as opposed to efficacy of chemotherapy treatment. We were also unable to evaluate the specific effects of biological agents as these agents were not uniformly utilized. Another limitation of this study is that some of the clinicopathological data utilized for application to the MSKCC nomogram were based on post-operative pathological examination. Pre-operative treatment may affect certain factors in the nomogram (e.g. maximum tumor size) and may therefore affect nomogram-predicted survival, obscuring a treatment effect in our analysis.
In conclusion, the MSKCC nomogram for DSS after resection of CRLM was validated by an external cohort and more accurately predicted survival than the commonly used CRS. Further refinements to the nomogram will likely improve its predictive accuracy in individual patients. Meanwhile, comparisons of observed to nomogram-predicted survival rates in groups of patients may be useful for the evaluation of treatment factors not incorporated into the nomogram.
Conflicts of interest
None declared.
References
- 1.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gayowski TJ, Iwatsuki S, Madariaga JR, Selby R, Todo S, Irish W, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–710. [PMC free article] [PubMed] [Google Scholar]
- 3.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 4.Cady B, Stone MD, McDermott WV, Jr, Jenkins RL, Bothe A, Jr, Lavin PT, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992;127:561–568. doi: 10.1001/archsurg.1992.01420050085011. [DOI] [PubMed] [Google Scholar]
- 5.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konopke R, Kersting S, Distler M, Dietrich J, Gastmeier J, Heller A, et al. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29:89–102. doi: 10.1111/j.1478-3231.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 7.Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–814. doi: 10.1097/SLA.0b013e318142d964. [DOI] [PubMed] [Google Scholar]
- 8.Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati P. Patterns of recurrence after resection of colorectal liver metastases: prediction by models of outcome analysis. World J Surg. 2001;25:638–644. doi: 10.1007/s002680020138. [DOI] [PubMed] [Google Scholar]
- 9.Rees M, Tekkis PP, Welsh FKS, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 10.Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M, et al. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- 12.Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168–1172. doi: 10.1001/archsurg.139.11.1168. [DOI] [PubMed] [Google Scholar]
- 13.Chen YY, Perera DS, Yan TD, Schmidt LM, Morris DL. Applying Fong's CRS liver score in patients with colorectal liver metastases treated by cryotherapy. Asian J Surg. 2006;29:238–241. doi: 10.1016/S1015-9584(09)60095-6. [DOI] [PubMed] [Google Scholar]
- 14.Mala T, Bøhler G, Mathisen Ø, Bergan A, Søreide O. Hepatic resection for colorectal metastases: can preoperative scoring predict patient outcome? World J Surg. 2002;26:1348–1353. doi: 10.1007/s00268-002-6231-x. [DOI] [PubMed] [Google Scholar]
- 15.Tan MCB, Castaldo ET, Gao F, Chari RS, Linehan DC, Wright JK, et al. A prognostic system applicable to patients with resectable liver metastasis from colorectal carcinoma staged by Postitron Emmision Tomography with [18F]Fluoro-2-Deoxy-Glucose: the role of primary tumor variables. J Am Coll Surg. 2008;206:857–869. doi: 10.1016/j.jamcollsurg.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 17.Kattan MW, Gönen M, Jarnagin WR, DeMatteo R, D'Angelica M, Weiser M, et al. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:282–287. doi: 10.1097/SLA.0b013e31815ed67b. [DOI] [PubMed] [Google Scholar]
- 18.Kanemitsu Y, Kato T. Prognostic models for predicting death after hepatectomy in individuals with hepatic metastases from colorectal cancer. World J Surg. 2008;32:1097–1107. doi: 10.1007/s00268-007-9348-0. [DOI] [PubMed] [Google Scholar]
- 19.Hoang T, Xu R, Schiller JH, Bonomi P, Johnson DH. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol. 2005;23:175–183. doi: 10.1200/JCO.2005.04.177. [DOI] [PubMed] [Google Scholar]
- 20.Kattan MW, Vickers AJ, Yu C, Bianco FJ, Cronin AM, Eastham JA, et al. Preoperative and postoperative nomograms incorporating surgeon experience for clinically localized prostate cancer. Cancer. 2009;115:1005–1010. doi: 10.1002/cncr.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagarde SM, Reitsma JB, Ten Kate FJ, Busch OR, Obertop H, Zwinderman AH, et al. Predicting individual survival after potentially curative esophagectomy for adenocarcinoma of the esophagus or gastroesophageal junction. Ann Surg. 2008;248:1006–1013. doi: 10.1097/SLA.0b013e318190a0a2. [DOI] [PubMed] [Google Scholar]
- 22.Cho CS, Gonen M, Shia J, Kattan MW, Klimstra DS, Jarnagin WR, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg. 2008;206:281–291. doi: 10.1016/j.jamcollsurg.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Bukowski RM, Figlin RA, Hutson TE, Michaelson MD, Kim ST, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2008;113:1552–1558. doi: 10.1002/cncr.23776. [DOI] [PubMed] [Google Scholar]
- 24.Brennan MF, Kattan MW, Klimstra D, Colon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 27.Reddy SK, Zorzi D, Lum YW, Barbas AS, Pawlik TM, Ribero D, et al. Timing of multimodality therapy for resectable synchronous colorectal liver metastases: a retrospective multi-institutional analysis. Ann Surg Oncol. 2008;16:1809–1819. doi: 10.1245/s10434-008-0181-y. [DOI] [PubMed] [Google Scholar]
- 28.Parks R, Gonen M, Kemeny N, Jarnagin W, D'Angelica M, DeMatteo R, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204:753–761. doi: 10.1016/j.jamcollsurg.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 29.Figueras J, Torras J, Valls C, Llado L, Ramos E, Marti-Ragué J, et al. Surgical resection of colorectal liver metastases in patients with expanded indications: a single-center experience with 501 patients. Dis Colon Rectum. 2007;50:478–488. doi: 10.1007/s10350-006-0817-6. [DOI] [PubMed] [Google Scholar]
- 30.Reddy SK, Broadwater G, Niedzwiecki D, Barbas AS, Hurwitz HI, Bendell JC, et al. Multiagent chemotherapy for isolated colorectal liver metastases: a single-centered retrospective study. J Gastrointest Surg. 2009;13:74–84. doi: 10.1007/s11605-008-0617-5. [DOI] [PubMed] [Google Scholar]
- 31.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 32.Viganò L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 33.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornprat P, Jarnagin WR, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Outcome after hepatectomy for multiple (four or more) colorectal metastases in the era of effective chemotherapy. Ann Surg Oncol. 2007;14:1151–1160. doi: 10.1245/s10434-006-9068-y. [DOI] [PubMed] [Google Scholar]
- 35.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blazer DG, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of colorectal liver metastases. J Clin Oncol. 2008;25:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 37.Pawlik TM, Assumpcao L, Vossen JA, Buijs M, Gleisner AL, Schulick R, et al. Trends in nontherapeutic laparotomy rates in patients undergoing surgical therapy for hepatic colorectal metastases. Ann Surg Oncol. 2009;16:371–378. doi: 10.1245/s10434-008-0230-6. [DOI] [PubMed] [Google Scholar]
- 38.Huguet EL, Old S, Praseedom RK, Balan KK, Gibbs P, Jamieson NV. F18-FDG-PET evaluation of patients for resection of colorectal liver metastases. Hepatogastroenterology. 2007;54:1667–1671. [PubMed] [Google Scholar]
- 39.Sorensen M, Mortensen FV, Hoyer M, Vilstrup H, Keiding S. FDG-PET improves management of patients with colorectal liver metastases allocated for local treatment: a consecutive prospective study. Scand J Surg. 2007;96:209–213. doi: 10.1177/145749690709600305. [DOI] [PubMed] [Google Scholar]
- 40.Chua SC, Groves AM, Kayani I, Menezes L, Gacinovic S, Du Y, et al. The impact of 18F-FDG PET/CT in patients with liver metastases. Eur J Nucl Med Imaging. 2007;34:1906–1914. doi: 10.1007/s00259-007-0518-y. [DOI] [PubMed] [Google Scholar]
- 41.Riedl CC, Akhurst T, Larson S, Stanziale SF, Tuorto S, Bhargava A, et al. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med. 2007;48:771–775. doi: 10.2967/jnumed.106.037291. [DOI] [PubMed] [Google Scholar]
- 42.Joyce DL, Wahl RL, Patel PV, Schulick RD, Gearhart SL, Choti MA. Preoperative positron emission tomography to evaluate potentially resectable hepatic colorectal metastases. Arch Surg. 2006;141:1220–1226. doi: 10.1001/archsurg.141.12.1220. [DOI] [PubMed] [Google Scholar]
- 43.Are C, Gonen M, Zazzali K, DeMatteo RP, Jarnagin WR, Fong Y, et al. The impact of margins on outcome after hepatic resection of colorectal metastases. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakai T, Shirai Y, Sakata J, Valera VA, Korita PV. Akazawa K, et al. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastases. Ann Surg Oncol. 2008;15:2472–2481. doi: 10.1245/s10434-008-0023-y. [DOI] [PubMed] [Google Scholar]
- 45.Konopke R, Kersting S, Makowiec F, Gabmann P, Kuhlisch E, Senninger N, et al. Resection of colorectal liver metastases: is a resection margin of 3 mm enough? World J Surg. 2008;32:2047–2056. doi: 10.1007/s00268-008-9629-2. [DOI] [PubMed] [Google Scholar]
- 46.Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B, et al. Influence of surgical margin on the type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008;143:384–393. doi: 10.1016/j.surg.2007.09.038. [DOI] [PubMed] [Google Scholar]


