Abstract
Background:
The surgeon's contribution to patients with localized pancreatic adenocarcinoma (PAC) is a margin negative (R0) resection. We hypothesized that a prediction rule based on pre-operative imaging would maximize the R0 resection rate while reducing non-therapeutic intervention.
Methods:
The prediction rule was developed using computed tomography (CT) and endoscopic ultrasound (EUS) data from 65 patients with biopsy-proven PAC who underwent attempted resection. The rule classified patients as low or high risk for non-R0 outcome and was validated in 78 subsequent patients.
Results:
Model variables were: any evidence of vascular involvement on CT; EUS stage and EUS size dichotomized at 2.6 cm. In the validation cohort, 77% underwent resection and 58% achieved R0 status. If only patients in the low-risk group underwent surgery, the prediction rule would have increased the resection rate to 92% and the R0 rate to 73%. The R0 rate was 40% higher in low-risk compared with high-risk patients (P < 0.001). High risk was associated with a 67% rate of non-curative surgery (unresectable disease and metastases).
Conclusion:
The prediction rule identified patients most likely to benefit from resection for PAC using pre-operative CT and EUS findings. Model predictions would have increased the R0 rate and reduced non-therapeutic interventions.
Keywords: pancreatic adenocarcinoma, resectability, prediction model, R0, margin negative
Introduction
Margin-negative (R0) surgical resection is the only treatment for pancreatic adenocarcinoma demonstrating actual 5-year survival.1 As a result, the criteria for identifying patients with localized pancreatic cancer for resection are currently the most critical aspect of treatment for early stage disease. Published indicators of resectability and borderline resectability have achieved the status of an expert consensus definition despite the absence of a rigorous prospective validation of their accuracy at predicting R0 outcome or the potential survival benefit of surgical intervention.2 Conversely, the post-operative morbidity and negative survival impact of non-therapeutic surgical interventions such as ‘open and close’ procedures for unexpected metastases as well as bypass procedures for unresectable disease have not been evaluated in a prospective clinical trial of surgical treatment using intention-to-treat methodology. This knowledge deficit has reduced formal risk-benefit analysis of surgical treatment for patients with localized pancreatic adenocarcinoma to a simplistic ‘surgery/no surgery’ decision.
The current definition of ‘resectable’ pancreatic cancer requires: the absence of extrapancreatic disease; no evidence of tumour extension to the superior mesenteric artery or celiac axis as defined by an intact fat plane between the tumour and the adjacent visceral artery; a patent superior mesenteric-portal vein confluence; and no encasement of the portal or superior mesenteric vein. These criteria are a strictly ‘operational’ definition of resectability based on anatomic factors favouring a technically safe surgical procedure. They are routinely applied by surgeons selecting patients likely to benefit from successful attempted resection, but there is limited data stratifying the risks of surgical exploration particularly for non-R0 outcomes such as margin-positivity or metastatic disease. Furthermore, individualized treatment planning for pancreatic cancer patients requires a consideration of additional survival factors including the risk of early cancer progression, tumour biology, post-operative morbidity and mortality, and the relative merits of alternative strategies such as neoadjuvant-combined modality therapy.
We therefore tested the hypothesis that a prediction rule could be developed and validated to maximize the rate of R0 resection among patients with localized pancreatic cancer using a combination of imaging characteristics identified on pre-operative computed tomography (CT) and endoscopic ultrasound (EUS) studies. Multidetector, contrast-enhanced CT and EUS are complimentary modalities routinely used to determine respectability,3,4 and imaging predictors of unresectable disease have been reasonably well established.5,6 However, combining the two to develop and validate a prediction rule for resectability has not been performed. Establishing evidence-based criteria for surgical intervention in early stage pancreatic cancer may standardize treatment decisions across institutions, reduce the risk of non-therapeutic operations and permit a definition of resectable pancreatic cancer based on empirical outcomes rather than technical safety.
Methods
All study procedures were approved by the Institutional Review Board of the University of Pittsburgh. We analysed a de-identified dataset of patients with pathologically-confirmed pancreatic adenocarcinoma who underwent surgery at the University of Pittsburgh Medical Center (UPMC) between 2002–2007. The data included: age and gender, serum biochemistry, clinical reports of pre-operative CT scans and EUS, operative reports, surgical pathology, post-operative outcomes, the provision of adjuvant chemotherapy and/or chemoradiation, as well as oncologic follow-up. CT and EUS findings were abstracted directly from the clinical reports that were available to the surgeon pre-operatively and used to formulate the original treatment plan for potentially-resectable pancreatic cancer. Post-hoc analysis of these images was not performed.
The generation and testing of the prediction rule utilized independent training and validation cohorts of patients that underwent both helical pancreas mass protocol CT and EUS performed by a dedicated pancreatic endosonographer during their initial evaluation for pancreatic cancer. Patients referred for neoadjuvant therapy for any reason were excluded. The training cohort consisted of 65 patients who underwent surgical exploration for pancreatic adenocarcinoma between 2002–2005 at UPMC. Recursive partitioning of CT, EUS and pathologic data was then used to create a prediction rule (classification tree) classifying patients into two risk categories for R0 resectability (high/low). The ‘tree’ function in Splus 2000 (MathSoft Inc., Seattle, WA, USA) was used to develop a series of candidate prediction rules, and the final prediction rule was selected using cross-validation to compare the misclassification rates of candidate rules. In cross-validation, 90% of the data are used for training and 10% are used to compute the misclassification rate; the procedure is repeated with all possible non-overlapping 10% samples of the data.
The classification tree with the smallest misclassification rate utilized EUS and CT variables (Fig. 1). These variables are (i) a binary variable indicating any evidence of arterial or venous vascular involvement on CT; (ii) the combination of EUS T and N data to assign a pre-operative stage according to the criteria of the American Joint Committee on Cancer (AJCC) 6th edn,7 and (iii) a binary variable indicating whether the largest EUS tumour dimension was greater than 2.6 cm. The prediction rule is the following: a patient is considered a good candidate for R0 resection (low risk) and should undergo surgery as primary therapy if (i) the EUS stage is ≤1A, or (ii) if there is no vascular involvement on CT and EUS stage is either 1B or 2A, or (iii) if there is no vascular involvement on CT and EUS stage is 2B and higher but the largest tumour dimension is ≤2.6 cm; otherwise, a patient is a poor candidate for R0 resection (high risk). Evidence for vascular involvement by CT scan includes minimal abutment of the superior mesenteric or hepatic arteries without extension to the celiac axis, as well as any pre-operative suspicion that the tumour involves the superior mesenteric-portal vein confluence despite the possibility of venous resection and reconstruction. Patients with more extensive arterial encasement or venous occlusion were referred for neoadjuvant protocols and were not included in this study. For the purposes of this prediction rule, operative findings of metastatic or locally advanced disease as well as positive resection margins were considered treatment failures.
Figure 1.
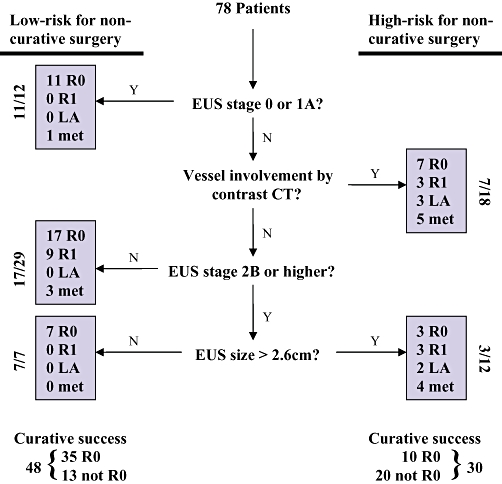
Classification tree and surgical outcomes after application of the prediction rule. R0 margin-negative resection; R1 margin-positive; LA locally advanced unresectable disease; met metastases
The resulting prediction rule was next applied to a validation cohort of 78 patients who underwent surgical exploration for what was considered resectable pancreatic adenocarcinoma between 2005–2007, all of whom were staged with CT and EUS. The assessment of resectability was made by individual surgeons at this high-volume tertiary referral centre for pancreatic cancer. Patients with suspected metastases and those who had received neoadjuvant therapy for any reason were again excluded. The cohort was retrospectively classified into high-risk and low-risk groups for curative resection using the model, and outcomes were analysed based on that classification. All patients that underwent surgery for potentially-resectable pancreatic cancer were included in the analysis regardless of treatment outcome. No patients were withdrawn from analysis, and the outcome assessments were obtained in a like and unbiased manner for all patients. The high- and low-risk groups were compared in terms of R0 and overall resectability, the incidence of metastatic or locally-advanced disease at operation and margin positivity. Final clinical and pathologic staging were also compared, with staging based upon pathology for resected specimens. For unresected patients, in keeping with the spirit of the AJCC staging system, patients were declared Stage 3 if they had an unresectable primary tumour even in the absence of pathologic confirmation that the celiac axis or superior mesenteric artery was involved. Unreconstructable venous involvement by tumour was also categorized Stage 3. Liver or peritoneal metastases and nodal involvement outside the limits of normal resection (e.g. a positive para-aortic node) were considered Stage 4.
Statistical analysis
The prediction rule sensitivity, specificity and predictive values for R0 resection were calculated using standard methods. Categorical data were analysed using contingency table methods, applying Fisher's exact test. Wilcoxon's rank-sum test was used to compare the stage and nodal distributions between groups. For curative surgery, ratios between the two groups with 95% confidence intervals were also calculated. In addition, survival curves from date of surgery were estimated using the Kaplan–Meier method and the distributions compared with the log-rank test. Two-sided tests were used and results considered statistically significant at the P < 0.05 level. Data analyses were carried out with Stata 10.1 (StataCorp LP, College Station, TX, USA), SAS 9.1.3 (SAS Institute Inc, Cary, NC, USA) and StatXact 4.0.1 (Cytel Software Corp, Cambridge, MA, USA).
Results
The demographic profile of the validation cohort was typical for pancreatic cancer; the average age at surgery was 68 ± 11 years (mean ± SD) with 40 (51%) men and 38 (49%) women. The majority of tumours were located in the pancreatic head (60/78, 77%) compared with the pancreatic body/tail (18/78, 23%). The median tumour diameter based on pre-operative imaging was 2.6 cm (0.8 to 6.1 cm) among head lesions and 2.7 cm (0.7 to 5.9 cm) in the body/tail.
The observed resection rate among study subjects was 77% (60/78) (Table 1). Of resected patients, 45 (75%) underwent pancreaticoduodenectomy with 5 (11%) requiring portal vein resection and reconstruction (PVR). Fifteen patients (25%) received distal pancreatectomy. Thirteen patients had unexpected metastases at the time of exploration and did not undergo resection, and five had unresectable locally-advanced disease. On final pathology, the median diameter of lesions in the pancreatic head was 3 cm (range 1–7.5) compared with 3.5 cm (range 1–8.2) in the pancreatic body/tail. The majority of resected tumours (58%, 35/60) were node positive. Forty-five patients had negative surgical margins and 15 patients had microscopically positive margins (R1), 4 of whom had two sites of microscopic residual disease on final pathology. Two out of five (40%) patients requiring PVR had an R0 result. No patients had grossly positive margins (R2 resection). The margin most frequently involved by tumour was the retroperitoneal/SMA margin (8/19 positive margins, 42%), followed by the radial pancreatic margin adjacent to the portal vein (6/19, 32%) and the pancreatic transection margin (5/19, 26%).
Table 1.
Demographic characteristics and pathologic outcomes of 78 patients explored for ‘resectable’ pancreatic cancer. Outcomes were analyzed on an intention-to-treat basis as all patients underwent surgery with curative intent
| Characteristic | Number of patients (%) n= 78 | |
|---|---|---|
| Tumour location | Head | 60 (77) |
| Body/Tail | 18 (23) | |
| Surgical outcome | Resected | 60 (76) |
| R0 | 45 (58) | |
| R1 | 15 (19) | |
| Not Resected | 18 (23) | |
| Locally advanced | 5 (6) | |
| Metastatic | 13 (17) | |
| AJCC stage, 6th edn. | 1A | 3 (4) |
| 1B | 5 (6) | |
| 2A | 17 (22) | |
| 2B | 35 (45) | |
| 3 | 5 (6) | |
| 4 | 13 (17) | |
| Adjuvant chemotherapy | 48 (76)a | |
| Adjuvant radiation | 16 (27)b | |
Data for adjuvant chemotherapy were available for 63 patients.
Data for adjuvant radiation were available for 60 patients.
The majority of patients with available data received adjuvant chemotherapy (48/63, 76%) whereas fewer received adjuvant radiation (16/60, 27%). There was no significant association between the location of the tumour and the surgical outcome (Table 2). The overall resectability rates for cancers in the proximal and distal pancreas were similar (75% vs. 83%, respectively), as were the R0 (57% vs. 61%) and R1 (18% vs. 22%) resection rates. At the time of survival analysis, 44 (56%) patients had died with median follow-up among survivors of 17.1 months (range 1.5–31.9 months).
Table 2.
Surgical treatment and outcomes stratified by tumour location
| Surgical outcome |
Tumour location, No. (%) |
P | ||
|---|---|---|---|---|
| Head (n= 60) | Body/Tail (n= 18) | |||
| Resectability rate | 45 (75) | 15 (83) | 0.54 | |
| R0 | 34 (56) | 11 (61) | 0.38 | |
| R1 | 11 (18) | 4 (22) | ||
| Locally advanced | 3 (5) | 2 (11) | ||
| Metastatic disease | 12 (20) | 1 (5) | ||
| AJCC stage | 1A | 2 (3) | 1 (5) | 0.04 |
| 1B | 1 (2) | 4 (22) | ||
| 2A | 13 (22) | 4 (22) | ||
| 2B | 29 (48) | 6 (33) | ||
| 3 | 3 (5) | 2 (11) | ||
| 4 | 12 (20) | 1 (6) | ||
| Adjuvant chemotherapya | 38 (76) | 10 (77) | 1.00 | |
| Adjuvant radiationb | 12 (26) | 4 (31) | 0.73 | |
| Median survival, months | 18.2 | 15.3 | 0.89 | |
Data for adjuvant chemotherapy were known for 50 head and 13 body/tail lesions.
Data for adjuvant radiation were known for 47 head and 13 body/tail lesions.
Figure 1 shows the surgical outcomes of patients grouped by predicted risk. Summary data are shown in Table 3. The observed rate of R0 resection for the entire 78 patient cohort was 58%. Overall, the prediction rule demonstrated 71% accuracy, 78% sensitivity and 61% specificity for R0 resection. The low-risk group had a significantly greater chance of R0 resection compared with the high-risk group (73% vs. 33% R0, P= 0.0009). Furthermore, the overall resection rate (R0 + R1) among low-risk patients was 92% compared with 53% in the high-risk group (P < 0.0002). Additional operative findings distinguishing the two risk groups included a greater proportion of unresectable, locally-advanced tumours (17% vs. 0%, P= 0.007) as well as unexpected metastatic disease (30% vs. 8%, P= 0.026) in the high-risk group. Of the five portal vein resections performed, two were in the low and three in the high-risk groups, respectively. The rate of margin positivity in resected patients was greater in the high-risk group but did not achieve statistical significance (38% vs. 20%, P= 0.195).
Table 3.
Surgical and pathologic outcomes stratified by risk classification
| Outcome measure |
Predicted risk groupNo. (%) |
P | ||
|---|---|---|---|---|
| Low-risk (n= 48) | High-risk (n= 30) | |||
| Resectability Rate | 44 (92) | 16 (53) | <0.001 | |
| R0 | 35 (73) | 10 (33) | 0.001 | |
| R1 | 9 (19) | 6 (20) | 0.892 | |
| Locally-advanced | 0 (0) | 5 (17) | 0.007 | |
| Metastatic | 4 (8) | 9 (30) | 0.026 | |
| AJCC Stage | 1A | 3 (6) | 0 (0) | 0.002 |
| 1B | 3 (6) | 2 (6) | ||
| 2A | 13 (27) | 4 (13) | ||
| 2B | 25 (52) | 10 (33) | ||
| 3 | 0 (0) | 5 (17) | ||
| 4 | 4 (8) | 9 (30) | ||
| Median number of total nodes per specimen if resected (range) | 10 (3–34) | 12 (4–41) | 0.30 | |
| Median number of positive nodes if Stage 2B (range) | 2 (1–10) | 3 (1–10) | 0.81 | |
| Mean node ratio if Stage 2B (±SD) | 0.33 (±0.23) | 0.27 (±0.24) | 0.50 | |
| Adjuvant chemotherapya | 32 (78) | 16 (73) | 0.76 | |
| Adjuvant radiationb | 9 (23) | 7 (33) | 0.54 | |
| Tumour location, head | 38 (79) | 22 (73) | 0.55 | |
Data for adjuvant chemotherapy were known for 41 low-risk and 22 high-risk lesions.
Data for adjuvant radiation were known for 39 low-risk and 21 high-risk lesions.
High predicted risk of surgical failure corresponded to more advanced stages of disease on final surgical pathology (Table 3). The high-risk group demonstrated a significant distribution towards higher AJCC stage, with 14/30 patients having stage ≥3 compared with 4/48 in the low-risk group (P < 0.0001). This finding reflects the greater proportion of locally-advanced and metastatic tumours in the high-risk group. However, the risk prediction did not differentiate between patients at high and low risk of nodal involvement (62% vs. 57%, P= 0.69). There were no significant differences between the low and high-risk groups in the median number of nodes harvested (low: 10, range 3–34; high: 12, range 4–41), median numbers of positive lymph nodes (low: 2, range 1–10; high: 3, range 1–10) or lymph node ratios among resected patients with node positive disease (low: mean 0.33 ± 0.23 SD; high: 0.27 ± 0.24 SD).
The risk classification also correlated with post-operative overall survival. Median survival for the entire validation cohort was 17.9 months (95% CI 12.8 to 22.5), with estimated 1- and 2-year survivals of 66% (95% CI 55% to 77%) and 36% (95% CI 22% to 50%), respectively. Median survival of the low-risk group was 20.3 months compared with 12.1 months in the high-risk group (P= 0.02). Regarding prognostic factors, tumour location was similar between risk groups with 79% (38/48) of patients in the low-risk group having tumours in the pancreatic head compared with 73% (22/30) of patients in the high-risk group (P= 0.59). Likewise, rates of adjuvant chemotherapy were similar between high- and low-risk prediction groups (73% vs. 78%, P= 0.76).
Discussion
This study validates a prediction rule identifying a subset of patients with potentially resectable pancreatic cancer that are at high risk of non-curative surgical outcomes. Unlike consensus definitions of resectability, this decision rule quantifies the likelihood of treatment failure in individual patients based on readily obtainable clinical parameters including EUS tumour diameter, presence of local invasion and nodal involvement by EUS, as well as signs of vascular abutment on CT. Patients at high predicted risk had significantly greater rates of unresectable locally-advanced as well as metastatic disease at the time of surgical exploration with correspondingly lower rates of overall resectability and R0 resection. Furthermore, unfavourable risk classification was associated with more advanced AJCC stages on final pathology and shorter overall survival. The classification tree formalizes the selection of patients most likely to benefit from pancreatic resection for adenocarcinoma. Ultimately, the model also presents a new definition of borderline resectable pancreatic cancer based on the statistical risk of treatment failure rather than the anatomic relationships between a tumour and the adjacent vasculature.2
We used recursive partitioning to formulate the prediction rule. This regression technique is a practical alternative to traditional methods such as logistic regression analysis. Although nomograms can distil a logistic regression model into a user-friendly decision-support tool, recursive partitioning creates a classification tree which mimics clinical decision making and simplifies risk stratification. The current risk model is composed solely of imaging parameters that are available to the surgeon at the time of preoperative decision making. The model is therefore more clinically applicable than published prognostic nomograms for pancreatic cancer that rely on data available only after surgery has been performed.8
Other groups have created scoring systems and used multivariable techniques to predict resectability of localized pancreatic cancer.6,9 However, the resulting predictive models in the surgical literature are rarely validated. Without prospective validation, predictive models are not expected to perform as well when applied to data independent of the training set from which they were derived. By comparison, our analysis was validated in a population of patients completely independent of the ‘training’ set of patients. The validation cohort represented the 2005–2007 time period in our registry when improvements in imaging, staging and selection of operative candidates might reduce the apparent effectiveness of a prediction model derived from the older data between 2002–2005. Moreover, we did not perform a retrospective expert review of EUS or CT imaging that might inadvertently bias application of the model or provide specialized information unavailable to the surgeon at the time of the decision to attempt resection. Consequently, data for the analysis were abstracted directly from the original EUS and CT reports to reproduce the staging information available pre-operatively and without knowledge of the surgical outcomes.
Because the recursive model relies heavily upon accurate EUS staging, the experience of endosonographers may limit the broader applicability of this study's findings to other practices. However, our data showed EUS to have 80% sensitivity and 93% specificity for vascular invasion; with 71% positive predictive value for nodal involvement. These findings are comparable to data published in the gastroenterology literature.6,10
The logical question is whether patients with apparently resectable tumours but features that when critically examined indicate a high risk of non-R0 outcome should be considered a novel indication for neoadjuvant therapy. Using intention-to-treat analysis, the published R0 resection rate after neoadjuvant therapy for resectable pancreatic cancer ranges from 50% to 66% at experienced centres as a result of disease progression, intolerance to therapy and loss to follow up.11–13 This rate is not different from the overall R0 rate after primary surgical therapy observed in the current study but is significantly higher than the 33% R0 rate observed among patients at high predicted risk. Conceptually, neoadjuvant therapy may improve the outcome of surgery for patients at risk for treatment failure by sterilizing the eventual surgical margin and treating unrecognized systemic disease earlier in the patient's care.14 The high-risk group is an ideal population for a randomized trial of neoadjuvant therapy given the statistically evident risk of treatment failure with surgery and conventional adjuvant chemotherapy.15 Reduced median survival in the high-risk group provides solid ethical justification for conducting neoadjuvant clinical trials in a group of patients currently receiving surgical intervention. By stratifying risk, the prediction rule proposed by this study may establish better endpoints in the design and evaluation of such trials.
In conclusion, this validated prediction rule establishes a new evidence-based definition of ‘resectable’ pancreatic cancer based on surgical outcomes and overall survival. The model utilizes readily available clinical parameters derived from EUS and CT and demonstrated 71% accuracy, 78% sensitivity and 61% specificity for R0 resection. In order to improve the quality of surgical management for pancreatic cancer we must better understand the efficacy of therapies and diagnostic studies intended to increase resectability as one contributor towards increasing long-term survival. The implications of a validated model to predict R0 resection on future clinical investigations, particularly alternative treatment strategies such as the neoadjuvant approach for patients at high risk of early progression, justifies a multicentre trial to validate these conclusions.
Conflicts of interest
None declared.
References
- 1.Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, et al. Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 2008;12:701–706. doi: 10.1007/s11605-007-0384-8. [DOI] [PubMed] [Google Scholar]
- 2.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Long EE, Van Dam J, Weinstein S, Jeffrey B, Desser T, Norton JA. Computed tomography, endoscopic, laparoscopic, and intra-operative sonography for assessing resectability of pancreatic cancer. Surg Oncol. 2005;14:105–113. doi: 10.1016/j.suronc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Virtue MA, Mallery S, Li R, Sielaff TD. Clinical utility of endoscopic ultrasound in solid pancreatic mass lesions deemed resectable by computer tomography. JOP. 2008;9:167–171. [PubMed] [Google Scholar]
- 5.Bao PQ, Johnson JC, Lindsey EH, Schwartz DA, Arildsen RC, Grzeszczak E, et al. Endoscopic ultrasound and computed tomography predictors of pancreatic cancer resectability. J Gastrointest Surg. 2008;12:10–16. doi: 10.1007/s11605-007-0373-y. discussion 16. [DOI] [PubMed] [Google Scholar]
- 6.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 7.Greene FL. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002. [Google Scholar]
- 8.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yovino S, Darwin P, Daly B, Garofalo M, Moesinger R. Predicting unresectability in pancreatic cancer patients: the additive effects of CT and endoscopic ultrasound. J Gastrointest Surg. 2007;11:36–42. doi: 10.1007/s11605-007-0110-6. [DOI] [PubMed] [Google Scholar]
- 10.DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753–763. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]
- 11.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 12.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 13.White RR, Xie HB, Gottfried MR, Czito BG, Hurwitz HI, Morse MA, et al. Significance of histological response to preoperative chemoradiotherapy for pancreatic cancer. Ann Surg Oncol. 2005;12:214–221. doi: 10.1245/ASO.2005.03.105. [DOI] [PubMed] [Google Scholar]
- 14.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner TB, Grabenbauer GG, Meyer T, Golcher H, Sauer R, Hohenberger W. Primary resection versus neoadjuvant chemoradiation followed by resection for locally resectable or potentially resectable pancreatic carcinoma without distant metastasis. A multi-centre prospectively randomised phase II-study of the Interdisciplinary Working Group Gastrointestinal Tumours (AIO, ARO, and CAO) BMC Cancer. 2007;7:41. doi: 10.1186/1471-2407-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]


