Abstract
Proanthocyanidins (PAs) are secondary metabolites that contribute to the protection of the plant and also to the taste of the fruit, mainly through astringency. Persimmon (Diospyros kaki) is unique in being able to accumulate abundant PAs in the fruit flesh. Fruits of the nonastringent (NA)-type mutants lose their ability to produce PA at an early stage of fruit development, while those of the normal astringent (A) type remain rich in PA until fully ripened. The expression of many PA pathway genes was coincidentally terminated in the NA type at an early stage of fruit development. The five genes encoding the Myb transcription factor were isolated from an A-type cultivar (Kuramitsu). One of them, DkMyb4, showed an expression pattern synchronous to that of the PA pathway genes in A- and NA-type fruit flesh. The ectopic expression of DkMyb4 in kiwifruit (Actinidia deliciosa) induced PA biosynthesis but not anthocyanin biosynthesis. The suppression of DkMyb4 in persimmon calluses caused a substantial down-regulation of the PA pathway genes and PA biosynthesis. Furthermore, analysis of the DNA-binding ability of DkMyb4 showed that it directly binds to the MYBCORE cis-motif in the promoters of the some PA pathway genes. All our results indicate that DkMyb4 acts as a regulator of PA biosynthesis in persimmon and, therefore, suggest that the reduction in the DkMyb4 expression causes the NA-type-specific down-regulation of PA biosynthesis and resultant NA trait.
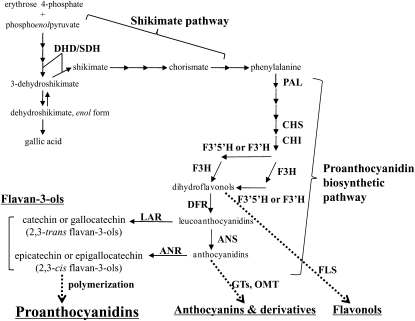
Proanthocyanidins (PAs; also called condensed tannins) are phenolic oligomers that result from the condensation of flavan-3-ol units and are synthesized via the flavonoid pathway (for representative structures, see Fig. 1; Dixon et al., 2005; Lepiniec et al., 2006). Flavonoids generally have protective functions in plants, particularly against herbivores and UV irradiation (Harborne and Grayer, 1993; McMahon et al., 2000; Winkel-Shirley, 2001; Peters and Constabel, 2002). They act as antioxidants with beneficial effects for human health, including protection against free radical, cardiovascular, and metabolic diseases (Bagchi et al., 2000; Cos et al., 2004; Aron and Kennedy, 2008). As a final product of the flavonoid pathway, PA also contributes to the quality of many important plant products, such as wine, teas, some berries, and cocoa (Aron and Kennedy, 2008). PA biosynthesis is controlled by structural genes, which encode enzymes that directly participate in the formation of the biochemical structure, and transcription factors (TFs), which control the expression patterns of structural genes (Koes et al., 2005; Lepiniec et al., 2006). The genetics and biochemical functions of some structural genes in the flavonoid pathway have been well characterized in some plant species (Holton and Cornish, 1995), and recently, much progress has been made in elucidating PA biosynthesis (Dixon et al., 2005; Lepiniec et al., 2006; Aron and Kennedy, 2008).
Figure 1.
Scheme of the PA biosynthetic pathway. ANR, Anthocyanidin reductase; ANS, anthocyanidin synthase; CHI, chalcone isomerase; CHS, chalcone synthase; DFR, dihydroflavonol 4-reductase; DHD/SDH, 3-dehydroquinate dehydratase/shikimate 5-dehydrogenase; F3H, flavanone 3-hydroxylase; F3′H, flavanone 3′-hydroxylase; F3′5′H, flavanone 3′5′-hydroxylase; FLS, flavonol synthase; GTs, glycosyltransferases; LAR, leucoanthocyanidin reductase; OMT, O-methyltransferase; PAL, Phe ammonia lyase.
Recently, Xie et al. (2003) demonstrated that BANYULS functions as an anthocyanidin reductase (ANR), which converts anthocyanidins to 2,3-cis-flavan-3-ols from Arabidopsis (Arabidopsis thaliana), and Tanner et al. (2003) reported the cloning and biochemical characterization of another key enzyme, leucoanthocyanidin reductase (LAR), which converts leucoanthocyanidins to 2,3-trans-flavan-3-ols from Desmodium uncinatum. In Arabidopsis, the transparent testa (tt) and tannin-deficient seed mutants, which have an altered seed coat color, define many reactions in PA biosynthesis and accumulation (Shirley et al., 1992; Abrahams et al., 2002; Lepiniec et al., 2006), and five structural genes of the flavonoid pathway, namely chalcone isomerase (CHI), chalcone synthase (CHS), dihydroflavonol 4-reductase (DFR), flavanone 3-hydroxylase (F3H), and flavanone 3′-hydroxylase (F3′H), as well as ANR, were identified in these mutants (Lepiniec et al., 2006). Other genes involved in PA accumulation, such as TT12 encoding a multidrug and toxic compound extrusion family transporter (Debeaujon et al., 2001), TT19 encoding a glutathione S-transferase (GST; Kitamura et al., 2004), and AUTO-INHIBITED H+-ATPase ISOFORM (AHA10) encoding a proton pump involved in vacuolar biogenesis (Baxter et al., 2005), were identified in the Arabidopsis mutant lines. Furthermore, some TFs such as the Myb-like protein, which regulates transcription of the structural genes involved in PA biosynthesis, have been identified in a few plant species. In Arabidopsis, TT2, a Myb TF that controls the transcription of ANR, DFR, TT12, and AHA10, has been identified (Nesi et al., 2001; Baudry et al., 2004; Sharma and Dixon, 2005; Lepiniec et al., 2006). It was reported that TT2-like Myb TFs in Brassica napus (Wei et al., 2007) and Lotus japonicas (Yoshida et al., 2008) are involved in PA biosynthesis. Furthermore, in grapevine (Vitis vinifera), it was demonstrated that the function and expression patterns of three Myb-like TFs, VvMYBPA1, VvMYBPA2, and VvMYB5b, were involved in PA biosynthesis (Bogs et al., 2007; Deluc et al., 2008; Terrier et al., 2009). In contrast to these findings, however, few spontaneous genetic mutants in PA biosynthesis or accumulation have been reported, although there are many mutants for the biosynthesis of other flavonoids, mainly anthocyanin in plant species, such as apple (Malus domestica; Takos et al., 2006; Espley et al., 2007, 2009), grapevine (Kobayashi et al., 2004), maize (Zea mays; Lloyd et al., 1992; Mol et al., 1998), and petunia (Petunia hybrida; Holton et al., 1993; Brugliera et al., 1999).
The oriental persimmon (Diospyros kaki; 2n = 6x = 90) is one of the major fruit crops in East Asia. It has a unique character in that it accumulates high-Mr PAs that cause strong astringency in fresh fruits; therefore, it is not edible without artificial treatment to remove astringency (Taira, 1996). PAs are overwhelmingly the most abundant phenolic compounds in persimmon fruit and can constitute more than 1% of fruit flesh weight in the mature stage (Taira et al., 1998). There is a spontaneous mutant phenotype that terminates the accumulation of PAs at an early stage of fruit development (Yonemori et al., 2000; Ikegami et al., 2005; Akagi et al., 2009). This nonastringent (NA) mutant phenotype, also called the pollination constant and nonastringent (PCNA) type, loses its astringency naturally on the tree during fruit development, so that fruits are edible without any artificial treatment after harvest. It has been reported that the allelotype of astringent (A) and NA was controlled by a single gene known as the AST/ast allele, and expression of the NA genotype requires homozygous recessive alleles (ast) at the AST locus (Kanzaki et al., 2001; Yamada and Sato, 2002). However, the molecular mechanisms determining the A/NA phenotypes are not fully understood. This situation may be mainly due to technical difficulties caused by the prolonged life cycle and genetic complexity of the hexaploid persimmon.
Previously, using mRNA expression profiling between the A- and NA-type cultivars or breeding lines, it was demonstrated that some structural genes of the PA pathway were synchronously down-regulated in the NA type with coincident termination of PA accumulation (Ikegami et al., 2005; Akagi et al., 2009). In addition, Akagi et al. (2009) suggested that the coincidental down-regulation of the two PA pathway genes, DkF3′5′H and DkANR, was mainly involved in termination of PA biosynthesis in the NA-type fruit. These results suggest that there is a common regulatory factor controlling the transcription of the PA pathway genes, including DkF3′5′H and DkANR, and it is possibly involved in substantial PA biosynthesis in persimmon fruit. In this study, we aimed to identify the TFs involved in PA biosynthesis during fruit development using segregated BC1-like offspring, which are considered to be a genetically homogeneous population. We isolated five Myb homologs from persimmon fruit and found that one of them, DkMyb4, which notably showed the highest expression level among them, was down-regulated in the NA type, as well as many PA pathway genes. To this end, we indicated that DkMyb4 acts as a regulatory gene for PA biosynthesis in developing persimmon fruit. These insights into the molecular mechanism of seasonal PA regulation in this nonmodel plant will contribute to future progress in the study of PA accumulation.
RESULTS
Temporal PA Biosynthesis and the Expression of PA Pathway Genes in A and NA Types
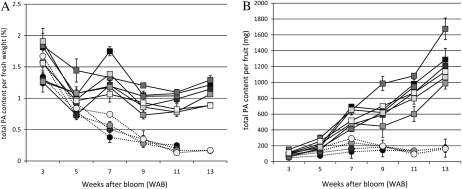
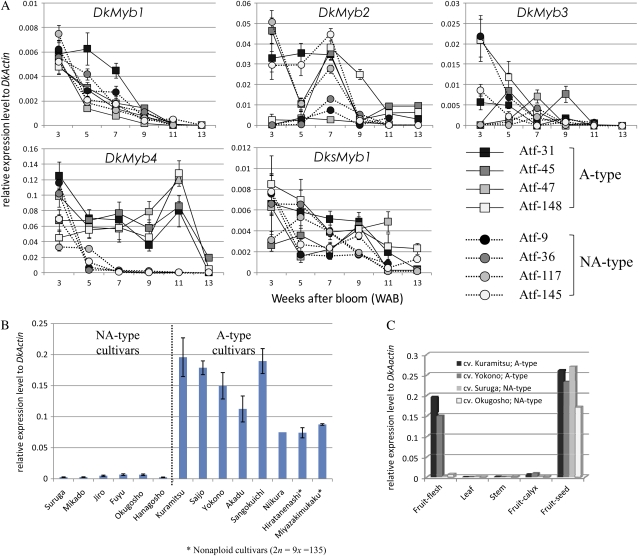
It was previously reported that soluble PA concentration was markedly reduced in the NA type compared with the A type at an early stage of fruit development (Ikegami et al., 2005; Akagi et al., 2009). In this study, we used 11 individuals of a BC1-like offspring, named the Atf line, which were derived from cv Aizu-mishirazu (A type), cv Taishu (NA type), and cv Fuyu (NA type; see “Materials and Methods”), for the analysis of temporal PA accumulation. We confirmed the same tendency for a marked reduction of total PA concentration in the NA type (Fig. 2A). This NA-type-specific reduction in PA concentration started from 5 weeks after bloom (WAB). During this stage, PA concentrations in A-type individuals increased, whereas all of the NA-type individuals showed a marked reduction. The analysis for total PA content per fruit also indicated that NA-type individuals accumulate PA in their fruits like the A type until 5 WAB (P = 0.351 on 3 WAB, P = 0.041 on 5 WAB), but this stopped in the NA-type individuals after 7 WAB (Fig. 2B; P < 0.001). We found no significant difference in fruit weight between A and NA types (data not shown; P > 0.125).
Figure 2.
Development patterns of fruits in A and NA individuals of the Atf line. Circles and dotted lines indicate NA-type individuals, and squares and solid lines indicate A-type individuals. A, Total PA concentration per fresh weight. B, Total PA content per fruit. Data collection started at 3 WAB and continued until 11 or 13 WAB. Error bars indicate sd. We analyzed three independent fruits for each individual of the Atf line (n = 3).
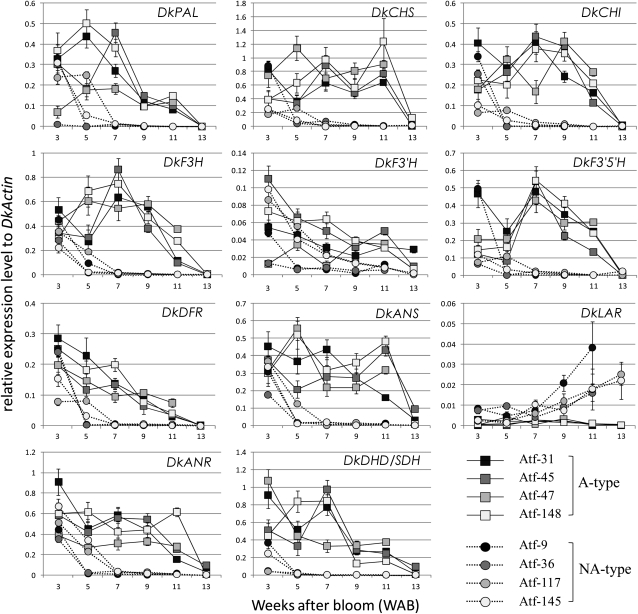
Expression levels of the 10 genes DkPAL, DkCHS, DkCHI, DkF3H, DkF3′H, DkF3′5′H, DkDFR, DkLAR, DkANS, and DkANR that encode the enzymes of the PA biosynthetic pathway (Fig. 1) were measured in fruit flesh in four individuals each of A and NA types by quantitative real-time (qRT)-PCR. We also measured the expression level of DHD/SDH, which is an enzymatic gene of the shikimate pathway (Fig. 1) and is reported to be involved in PA regulation in persimmon fruit (Ikegami et al., 2007; Akagi et al., 2009). Expression of most of the genes (DkPAL, DkCHS, DkCHI, DkF3H, DkF3′5′H, DkDFR, DkANS, and DkANR) was synchronously down-regulated from 5 WAB and was almost below the detection limit after 7 WAB in NA-type individuals (Fig. 3), even though significant differences in their expression levels were not detected at 3 WAB (P > 0.2) between A and NA types. Expression in the A type was gradually down-regulated during the late developmental stages and approached the level of the NA type by 13 WAB (Fig. 3). The mean expression levels of these genes were typically approximately 40- to 10,000-fold higher in the A-type individuals than in the NA type at 7 WAB. The NA-type-specific down-regulation of PA pathway genes was coincident with the reduction in PA content in the NA type (Fig. 2). This result indicates that the NA-type-specific reduction in PA content is due to the phenological and marked down-regulation of the genes of the PA biosynthetic pathway, as described in previous reports by Akagi et al. (2009) and Ikegami et al. (2005).
Figure 3.
qRT-PCR analysis for determining the expression levels of the structural genes involved in PA biosynthesis. Flesh from four A-type (Atf-31, Atf-45, Atf-47, and Atf-148) and NA-type (Atf-9, Atf-36, Atf-117, and Atf-145) individual fruits from 3 to 13 WAB was used for the expression analysis. The expression level is shown as the value relative to expression of DkActin (accession no. AB473616) in each sample. Expression is shown for genes encoding the enzymes PAL, CHS, CHI, F3H, F3′H, F3′5′H, DFR, ANS, LAR, ANR, and DHD/SDH. Error bars indicate sd.
We found that the expression of DkDHD/SDH was also down-regulated in the NA type (Fig. 3). The expression patterns of DkDHD/SDH were largely synchronous with the genes of the flavonoid biosynthetic pathway, but the NA-type-specific down-regulation of DkDHD/SDH occurred earlier than that with the genes of the flavonoid biosynthetic pathway in this analysis. The shikimate pathway provides Phe for both protein synthesis and as a secondary metabolite in higher plants (Nicholson and Hammerschmidt, 1992; Lillo et al., 2008). It has been demonstrated that compromising the shikimate pathway by the transcription inhibition of DHD/SDH leads to stunted growth and a reduction in the major second metabolites such as lignin in tobacco (Nicotiana tabacum) plants (Ding et al., 2007). In persimmon, it has been suggested that the down-regulation of DkDHD/SDH expression reduced the content of PAs in the NA-type fruit (Ikegami et al., 2007, 2009; Akagi et al., 2009). In addition, intermediates from the shikimate pathway serve as substrates for the biosynthesis of gallic acid (Werner et al., 2004; Fig. 1). Gallic acid is essential for the formation of galloylated PA, which is a major esterified form of PA in persimmon fruit. Therefore, it was suggested that the down-regulation of DkDHD/SDH expression also affected the reduction in the content of galloylated PA in the NA-type fruit, as described in a previous report by Akagi et al. (2009).
By contrast, F3′H did not show significant differences in expression patterns between A and NA types from 5 WAB (Fig. 3). A significant reduction in the expression level of DkF3′H was detected only at 11 WAB (P < 0.01), although the expression levels were lower in NA-type individuals than those in the A type. Furthermore, the expression of LAR was considerably up-regulated in the NA type, particularly after 7 WAB (Fig. 3; P < 0.01 on all data points after 7 WAB). The mean expression level of DkLAR was approximately 5- to 40-fold higher in the NA type than in the A type after 7 WAB. The relative expression levels of DkLAR, however, were clearly lower than those of other genes.
Cloning of Myb Homolog Candidates from Persimmon Fruit
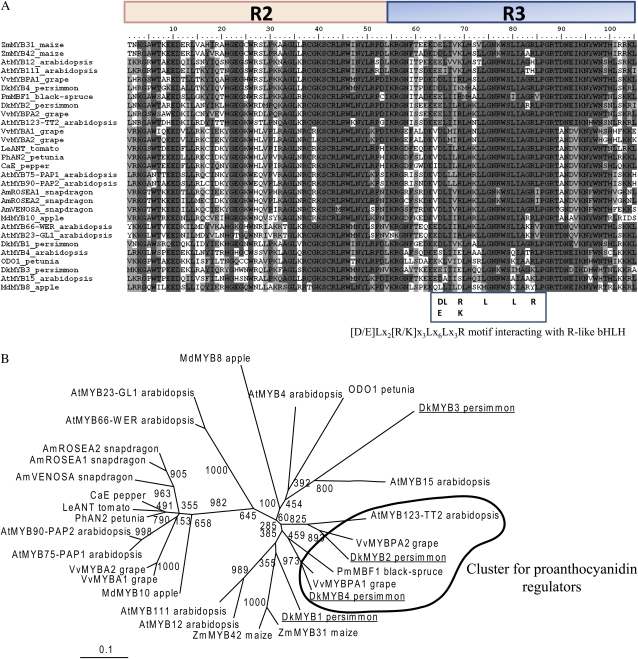
Most of the PA pathway genes were coincidentally down-regulated in fruit flesh of the NA type (Fig. 3), which implies one or more TFs controlling these PA pathway genes. To exploit this possibility, we prepared cDNA from the fruit of A-type cv Kuramitsu at the development stage in which PA biosynthesis was specifically down-regulated in the NA type and used it as a template for PCR to isolate Myb TFs involved in PA regulation. We isolated a partial cDNA fragment of the Myb homolog and screened the cDNA libraries derived from Kuramitsu to obtain the full-length cDNA of this Myb fragment. To this end, we isolated one full-length Myb gene, DkMyb1, which showed homology in BLAST searches to maize ZmMYB31, -42, or subgroup 4 MYBs (Kranz et al., 1998; Fornalé et al., 2006) from other species. We screened approximately 500,000 recombinant clones from the same cDNA libraries under a low-stringency hybridized condition with the R2R3 conserved region of DkMyb1 (Fig. 4A) as a probe and isolated 84 clones of Myb homologs. Their sequence alignment gave five presumably full-length cDNA sequences of DkMyb1, DkMyb2, DkMyb3, DkMyb4, and DksMyb1. We found that DksMyb1 is a small-type Myb TF containing the R3 conserved region, but not R2, and that the others are R2R3-type Myb TFs containing an R2R3 conserved region in their sequences (Stracke et al., 2001). We isolated 59 clones of DkMyb4, 12 of DkMyb2, six of DkMyb1, five of DkMyb3, and two of DksMyb1.
Figure 4.
Comparison of DkMyb1 to -4 and DksMyb1 deduced amino acid sequences with other Myb TFs. A, Protein sequence alignment of the global Myb TFs by phylogenetic analysis. The R2 and R3 domains are shown, and the [D/E]Lx2[R/K]x3Lx6Lx3R motif interacting with R-like bHLH is indicated in the box. B, Phylogenetic analysis displaying the similarities of DkMyb1 to -4 with other Myb TFs. The tree is based on the alignment of the 105 amino acids spanning the R2R3 domain with the ClustalW multiple sequence alignment system (Thompson et al., 1997) using the default parameters of the DNA Data Bank of Japan. The tree was constructed using the neighbor-joining method of TreeView (Page, 1996). The scale bar represents 0.1 substitutions per site, and the numbers next to the nodes are bootstrap values from 1,000 replicates. The GenBank accession numbers of Myb TFs are as follows: AtMYB111 (NP_199744), AtMYB12 (NP_182268), ZmMYB31 (AM156906), ZmMYB42 (AM156908), DkMYB1 (AB503698), DkMYB4 (AB503671), VvMybPA1 (CAJ90831), PmMBF1 (AAA82943), DkMYB2 (AB503699), VvMybPA2 (EU919682), AtMYB123-TT2 (Q9FJA2), AtMYB15 (Y14207), DkMYB3 (AB503670), ODO1 (AAV98200), AtMyb4 (BAA21619), MdMYB8 (DQ267899), AtMYB23-GL1 (NP_189430), AtMYB66-WER (AAF18939), AmROSEA2 (ABB83827), AmROSEA1 (ABB83826), AmVENOSA (ABB83828), CaE (AJ608992), LeANT1 (AAQ55181), PhAN2 (AAF66727), AtMYB90-PAP2 (NP_176813), AtMYB75-PAP1 (NP_176057), VvMYBA1 (BAD18977), VvMYBA2 (BAD18978), and MdMYB10 (ABB84753). [See online article for color version of this figure.]
A phylogenetic analysis of the R2R3 region of these deduced amino acid sequences placed DkMyb2 and DkMyb4 in a cluster of MYB proteins that include the following: Picea mariana PmMBF (Xue et al., 2003), grape VvMYBPA1 and VvMYBPA2 (Bogs et al., 2007; Terrier et al., 2009), and Arabidopsis TT2 (Nesi et al., 2001; Fig. 4B). Three of these Mybs, VvMYBPA1, VvMYBPA2, and TT2, have been characterized as PA regulators (Nesi et al., 2001; Bogs et al., 2007; Terrier et al., 2009). This suggests that DkMyb2 and DkMyb4 also act as regulators of PA biosynthesis. DkMyb2 has 86% identity to VvMYBPA2 and DkMyb4 has 91% to VvMYBPA1 in the R2R3 region (Fig. 4A), but no significant homology was detected in the other coding regions. DkMyb3 was placed in the same clade as petunia ODORANT1 (Verdonk et al., 2005) and Arabidopsis AtMYB15 (Chen et al., 2006), which are regulators of the shikimate pathway. A phylogenetic analysis of the R3 region (data not shown) suggested that DksMyb1 is similar to the R3-type CAPRICE (Lepiniec et al., 2006; Tominaga et al., 2007), which is a repressor of Arabidopsis AtMYB66 (WEREWOLF).
An alignment of deduced amino acid sequences in the R3 region showed that four DkMybs, DkMyb1, DkMyb2, DkMyb4, and DksMyb1, had the amino acid residues [D/E]Lx2[R/K]x3Lx6Lx3R that specifically interacted with the R-like basic helix-loop-helix (bHLH) protein (Fig. 4A, the residues are indicated in the box; Grotewold et al., 2000; Zimmermann et al., 2004). These results suggest that specific R-like bHLH cofactors are needed to act as regulatory factors, as well as other plant Myb TFs (Lepiniec et al., 2006; Espley et al., 2007).
Expression Analysis of Myb Homologs
The expression patterns of DkMyb1, DkMyb2, DkMyb3, and DksMyb1 showed no significant differences between the A and NA types, and these tendencies were inconsistent (Fig. 5A). The expression levels of DkMyb1 and DksMyb1 were gradually reduced in both A- and NA-type individuals. The expression of DkMyb2 and DkMyb3 showed sudden up-regulation in some individuals, but this tendency was observed in both A- and NA-type individuals. However, the expression of DkMyb4, whose level was notably the highest of the five Myb TFs isolated from persimmon fruit flesh, showed NA-type-specific down-regulation from 5 WAB and was almost below the limits of detection after 7 WAB in NA-type individuals (Fig. 5A). This expression pattern of DkMyb4 was similar to that of the genes of the PA biosynthetic pathway, which showed NA-type-specific down-regulation (Fig. 3). These results suggest that DkMyb4 acts as a regulator of PA biosynthesis in fruit flesh of persimmon.
Figure 5.
qRT-PCR analysis of expression levels of the Myb TFs isolated from persimmon fruit. A, Temporal expression for DkMyb1 to -4 and DksMyb1 from 3 to 13 WAB in four individuals of A or NA type. B, Gene expression of DkMyb4 in the fruit flesh of eight A-type cultivars and six NA-type cultivars at 7 WAB. C, Gene expression of DkMyb4 in fruit flesh, leaf, stem, fruit calyx, and fruit seed of two A- and NA-type cultivars at 7 WAB. Error bars indicate sd. [See online article for color version of this figure.]
To test this possibility, we analyzed the expression level of DkMyb4 in A- and NA-type cultivars and their plant organs. We performed qRT-PCR analysis for transcribing DkMyb4 from the fruit flesh of eight A-type and six NA-type cultivars at 7 WAB when the NA-type-specific down-regulation of DkMyb4 and PA biosynthesis were detected in the fruit flesh of the Atf line (Figs. 2A, 3, and 5A). The expression level of DkMyb4 was approximately 20- to 200-fold higher in all A-type cultivars than in NA-type cultivars as well as in the Atf line (Fig. 5B). In addition, this remarkable expression difference between A and NA types was observed only in the fruit flesh and no other plant organs (Fig. 5C). The expression level of DkMyb4 in the leaf, stem, and fruit calyx was considerably lower than that in the fruit flesh and showed no significant differences between A and NA types (P = 0.614 for leaf, P = 0.542 for stem, and P = 0.109 for calyx). In the fruit seeds, DkMyb4 was highly expressed, but no significant difference in expression was detected between the A- and NA-type cultivars (P = 0.655).
Analysis of PA Biosynthesis in CaMV35S-DkMyb4-Transformed Kiwifruit
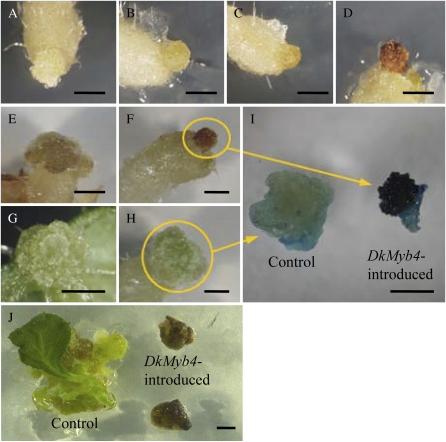
To analyze the function of DkMyb4 in PA biosynthesis in plants, a kiwifruit (Actinidia deliciosa ‘Hayward’) was transformed with DkMyb4 under the transcriptional control of the cauliflower mosaic virus 35S promoter (CaMV35S) and a kanamycin-selective marker. Koshita et al. (2008) demonstrated the effective functional analysis of grape (Vitis labruscana) Myb TF VlMYBA1-2, the regulator of anthocyanin biosynthesis, by kiwifruit transformation (Hayward). The anthocyanin and PA biosynthetic pathways share almost all structural genes except for the last catalytic steps (Fig. 1; Koes et al., 2005; Lepiniec et al., 2006). These facts indicated that the activity of Myb TFs regulating PA biosynthesis could be analyzed by transformation of kiwifruit. The regenerated calluses of kanamycin-positive transformants were pigmented with red 2 months after the infection (Fig. 6, A–F), whereas control plants transformed with an empty vector consistently showed no pigmentation (Fig. 6, G and H). p-Dimethylaminocinnamaldehyde (DMACA) staining (Li et al., 1996) showed high PA accumulation on the surface cells of the transformed callus, while there was only slight PA accumulation in the control callus (Fig. 6I). In addition, we analyzed anthocyanin accumulation with HPLC, comparing the VlMYBA1-2-introduced plants that accumulate a quantity of anthocyanin (Koshita et al., 2008) and the DkMyb4-introduced plants. The major anthocyanin of VlMYBA1-2-introduced plants was detected as cyanidin 3-O-(2-O-β-xylosyl)-β-glucoside, as reported by Koshita et al. (2008). On the other hand, no anthocyanins were detected in DkMyb4-introduced plants (Supplemental Fig. S1) as well as control plants. These results show that the expression of DkMyb4 induces PA biosynthesis in plant cells and suggest that red pigmentation in the surface of transformants is not due to anthocyanin but presumably oxidized PAs, as in the seed coat of Arabidopsis (Shirley et al., 1992; Abrahams et al., 2002; Lepiniec et al., 2006). Control plants had regenerated shoots 7 months after transformation, but the calluses transformed by CaMV35S-DkMyb4 stopped developing and became brown (Fig. 6J).
Figure 6.
Functional analysis of DkMyb4 with kiwifruit transformation. Ectopic expression of DkMyb4-induced PA accumulation in the kiwifruit callus. Growth of the DkMyb4-introduced callus is shown after the in vitro infection of leaves with Agrobacterium. A, Four weeks. B, Six weeks. C, Eight weeks. D, Ten weeks. E and F, Three months. G and H, Control callus with empty vector after 3 months. I, DkMyb4-introduced and control calluses at 3 months after infection are stained with DMACA. The strong blue coloration in the DkMyb4-introduced callus indicates the accumulation of a large amount of PA in the surface cells. J, DkMyb4-introduced and control plants at 6 months after infection. The control callus regenerated, while the DkMyb4-introduced callus is brown and growth has stopped. Bars = 2 mm.
Transformation of Persimmon with CaMV35S-Antisense DkMyb4
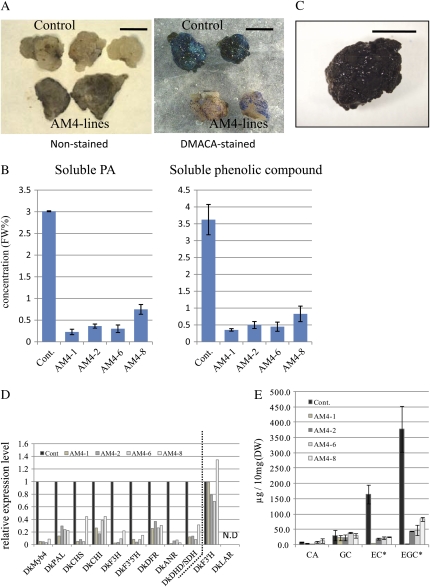
To test the function of DkMyb4 in a homologous system, persimmon (cv Fujiwaragosho) was transformed with CaMV35S-sense DkMyb4 or CaMV35S-antisense DkMyb4 and a kanamycin-selective marker (see “Materials and Methods”). We preliminarily confirmed that the persimmon callus (Fujiwaragosho) expresses a quantity of DkMyb4 and markedly accumulates soluble PAs. Therefore, we introduced sense or antisense DkMyb4 into persimmon calluses to analyze the effect of DkMyb4 expression levels on PA biosynthesis. Some regenerated calluses transformed by CaMV35S-antisense DkMyb4, named AM4 lines, whose expression level of DkMyb4 clearly declined, showed a slight accumulation of PA in the surface cells when stained by DMACA (Li et al., 1996), whereas control plants transformed with an empty vector consistently showed high accumulation of PA (Fig. 7A). The same tendencies were observed in the content of soluble PA with methanol extraction as well as with the DMACA (Li et al., 1996) and Folin-Ciocaltaeu (Oshida et al., 1996) methods (Fig. 7B). Almost all calluses transformed by CaMV35S-sense DkMyb4 turned black and stopped growing immediately after transformation (Fig. 7C). We could not precisely measure the PA content in these transformants by methanol extraction or DMACA staining because of the small quantity of blackened callus.
Figure 7.
Functional analysis of DkMyb4 with persimmon transformation. Some of the persimmon calluses into which antisense DkMyb4 had been introduced are regenerated and designated the AM4 lines. A, The AM4 lines and control callus at 2 months after the in vitro infection of leaves with Agrobacterium (left) and stained with DMACA (right). Calluses of the AM4 lines clearly showed less blue staining than controls. This indicates lower PA accumulation in the surface cells of the AM4 line calluses. Bars = 10 mm. B, Soluble PA content (left) and soluble phenol compound content (right) in the AM4 line calluses and control callus. FW, Fresh weight. C, Sense DkMyb4-introduced persimmon callus. All sense DkMyb4 lines have stopped growing and turned black. Bar = 2 mm. D, Gene expression in the AM4 line and control calluses. The structural genes of the PA biosynthetic pathway and DkDHD/SDH are measured. The transcription levels of the genes showing the NA-type-specific down-regulation (Fig. 3) were lower in the AM4 lines than those in the controls. Expression of DkLAR was not detected (N.D). E, PA subunit composition in the AM4 line and control (Cont.) callus. The contents of EC and EGC were significantly lower in the AM4 lines than those in the controls. The contents of catechin (CA) and gallocatechin (GC) were not changed in the AM4 lines. Error bars indicate sd. We analyzed each individual with three replications by HPLC (n = 3). DW, Dry weight. * The galloylated EC and EGC were also detected slightly in both AM4 lines and controls and contained in EC and EGC, respectively, in E.
qRT-PCR expression analysis in the calluses of four AM4 lines (AM4-1, -2, -6, and -8) revealed the down-regulation of DkMyb4 accompanied by a proportional reduction in the expression levels of some structural genes of the PA biosynthetic pathway: DkPAL, DkCHS, DkCHI, DkF3H, DkF3′5′H, DkDFR, and DkANR (Fig. 7D). These are specifically the down-regulated genes in the NA-type fruit (Fig. 3). Expression levels of DkDHD/SDH, which showed the NA-type-specific reduction (Fig. 3), were also reduced in proportion to the reduction in those of DkMyb4. However, expression levels of DkF3′H and DkLAR, which did not show the NA-type-specific reduction in the fruit flesh (Fig. 3 for DkF3′H and DkLAR), were not influenced by the DkMyb4 expression levels (Fig. 7D).
These expression changes of PA pathway genes in AM4 lines were clearly reflected in the composition of PA. HPLC analysis of the PA subunits following acid catalysis in the presence of excess phloroglucinol (Kennedy and Jones, 2001; Akagi et al., 2009) showed that the content of epicatechin (EC) and especially epigallocatechin (EGC), which are both synthesized via ANR (Fig. 1), were notably reduced in all AM4 lines, whereas the contents of catechin and gallocatechin, which are synthesized via LAR (Fig. 1), were much lower than those of EC and EGC and not significantly influenced (Fig. 7E; Supplemental Fig. S2). Galloylated EC and EGC (EC-3-O-gallate and EGC-3-O-gallate) were also detected, but the amounts were much lower than those of other PA components and were not affected in AM4 lines (data not shown). These observations indicate that the reduction in total PA content caused by the suppression of DkMyb4 expression in the AM4 lines (Fig. 7C) was mainly due to the reduction in the contents of EC and EGC, and DkLAR, whose expression was not regulated by DkMyb4, did not affect PA biosynthesis in the AM4 lines. All of these results indicated that DkMyb4 directly or indirectly acts as a regulator for the PA pathway genes and controls PA biosynthesis in persimmon.
Identification of Target Motifs and Genes of DkMyb4
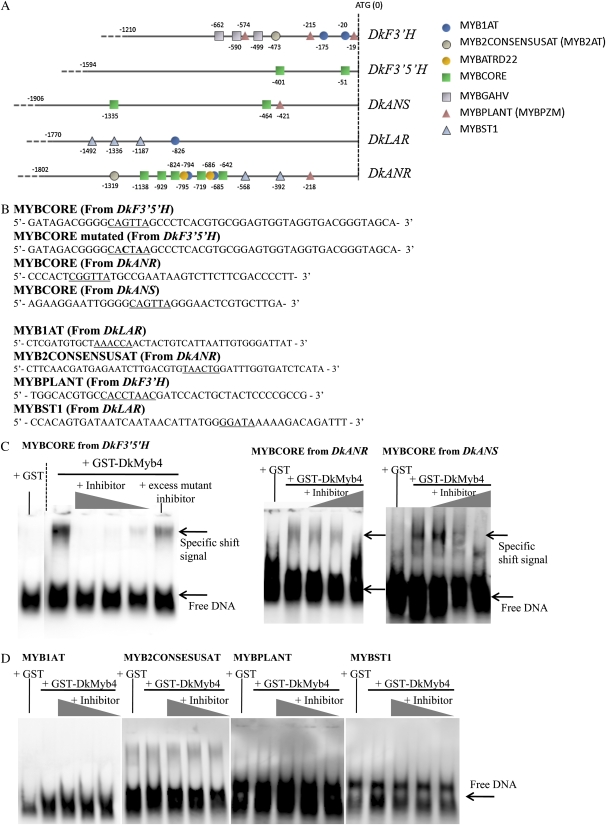
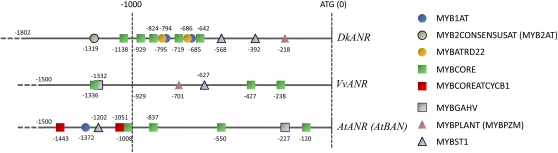
To identify the motifs or genes directly targeted by the DkMyb4 protein, we performed an electrophoretic mobility shift assay (EMSA) with the recombinant GST-DkMyb4 fusion protein expressed in Escherichia coli. We obtained the GST-DkMyb4 fusion protein from E. coli (BL21) cells transformed by pDEST15-DkMyb4, which encodes an N-terminal in-frame fusion of DkMyb4 with a GST tag (see “Materials and Methods”), and confirmed the solubility of this fusion protein. GST-DkMyb4 was easily soluble when expressed at 18°C (data not shown). We isolated the promoter regions of DkF3′H, DkF3′5′H, DkANS, DkLAR, and DkANR (Fig. 1) and used their oligonucleotide fragments containing MYB-binding cis-motifs as the DNA probes. The MYB-binding cis-motifs were detected in the database of plant cis-acting regulatory DNA elements (PLACE; Higo et al., 1999). Finally, seven groups of the MYB-binding cis-motif were identified in the promoter regions of the five genes (Fig. 8A). We tested the four groups that were highly conserved for two genes among the five as candidates for the DkMyb4-binding cis-motif. These five groups were as follows: WAACCA sequence, the MYB1AT motif; YAACKG (TAACTG), the MYB2CONSENSUS (MYB2AT) motif; CNGTTR, the MYBCORE motif; MACCWAMC (CCWACC), the MYBPLANT (MYBPZM) motif; and GGATA, the MYBST1 motif (Urao et al., 1993; Solano et al., 1995; Tamagnone et al., 1998; Planchais et al., 2002; Abe et al., 2003).
Figure 8.
MYB-binding cis-motifs in the promoter region of the PA pathway genes in persimmon (Kuramitsu) and the ability of the DkMyb4 fusion protein to bind to them. A, MYB-binding motifs in the promoters of DkF3′H, DkF3′5′H, DkANS, DkLAR, and DkANR detected in the sense coding strand using the PLACE database (http://www.dna.affrc.go.jp/PLACE/signalup.html; Higo et al., 1999). B, Sequences for oligonucleotides used in EMSA analysis. The MYB-binding motifs are shown as the underlined nucleotides. The mutated oligonucleotide sequences of the MYBCORE cis-motif are shown in boldface (G and T to C and A). C, EMSA using DkMyb4 fusion protein and the MYBCORE cis-motif derived from the promoter region of DkF3′5′H (top). The binding signals are reduced in proportion to the amount of nonlabeled MYBCORE inhibitor at 20-, 50-, or 150-fold excess versus the DIG-labeled oligonucleotide probe, but 150-fold excess nonlabeled mutated MYBCORE inhibitor does not significantly decrease the binding signal. The binding signals were detected also in EMSA using DkMyb4 fusion protein and the MYBCORE cis-motif derived from the promoter region of DkANR and DkANS (bottom). D, EMSA using DkMyb4 fusion protein and the MYB1AT, MYB2CONSENSUS, MYBPLANT, and MYB1ST motifs. Significant binding signals are not detected in any of these.
The purified GST-DkMyb4 fusion protein was examined for its ability to bind to an oligonucleotide containing the MYB-binding cis-motifs by EMSA. To analyze the specificity of the cis-motif-binding activity, we used nonlabeled mutated oligonucleotides and nonlabeled normal oligonucleotides as probe inhibitors. The mutated oligonucleotides contained two or three point mutations in the MYB-binding cis-motifs, as shown in Figure 8B. The GST-DkMyb4 fusion protein bound to an oligonucleotide containing the MYBCORE motif occurred in the DkF3′5′H promoter region (Fig. 8C). The nonlabeled oligonucleotides containing the normal MYBCORE motif competed with the labeled MYBCORE probe and reduced the binding signal between GST-DkMyb4 and MYBCORE probes in proportion to its concentration. However, a quantity of nonlabeled oligonucleotides containing the mutated MYBCORE motif did not significantly reduce the binding signal. This inability of GST-DkMyb4 to bind to oligonucleotides containing the mutated MYBCORE motif suggests that DkMyb4 recognizes the MYBCORE motif in the probe sequences. DkMyb4 also showed an ability to bind to other MYBCORE motifs in the promoter regions of DkANR and DkANS as well as DkF3′5′H (Fig. 8C) but not to the other four MYB-binding cis-motifs, MYB1AT, MYB2CONSENSUSAT (MYB2AT), MYBPLANT (MYBPZM), and MYBST1, in any promoter regions (Fig. 8D). The promoter regions of DkLAR have MYB1AT and MYBST1 motifs, but not the MYBCORE motif, at least up to 1,773 bp upstream from the ATG start codon (Fig. 8A). This suggests that DkMyb4-independent expression levels of DkLAR in the fruit and callus (Figs. 3 and 5A for fruit and Fig. 7D for callus) are due to the inability of DkMyb4 to bind to the promoter region of DkLAR. Also, we could not find the MYBCORE motif in the promoter region of DkF3′H, where expression showed no significant correlation with DkMyb4 expression (Figs. 3 and 5A for fruit and Fig. 7C for callus). Taken together, these results suggest that DkMyb4 directly binds to the MYBCORE cis-motif in the promoter region of the PA pathway genes and also that the presence of MYBCORE cis-motif in the promoter region is possibly responsible for the DkMyb4-dependent activation of their expression in persimmon fruit.
DISCUSSION
DkMyb4 Acts as a Regulator of PA Pathway Genes
Myb TFs generally act as regulators for the second metabolite pathway in plant species. Myb TFs have been studied through coloration due to anthocyanin biosynthesis in many plant species, such as Arabidopsis (Borevitz et al., 2000), apple (Takos et al., 2006; Espley et al., 2007, 2009), grapevine (Kobayashi et al., 2002, 2004), maize (Lloyd et al., 1992; Mol et al., 1998), petunia (Mol et al., 1998; Quattrocchio et al., 1999), and strawberry (Fragaria species; Aharoni et al., 2001). However, only a few Myb TFs have been reported to regulate PA biosynthesis (Nesi et al., 2001; Bogs et al., 2007; Mellway et al., 2009; Terrier et al., 2009). In this study, we showed that DkMyb4, a Myb TF, functions as a PA regulator by transformation in kiwifruit and persimmon (Figs. 6 and 7). The correlation between the expression level of DkANR, which is a PA-specific gene, and the expression level of DkMyb4 (Figs. 3 for DkANR, 5A for DkMyb4, and 7D), with the ability to bind to MYBCORE motifs in the promoter region of DkANR and DkMyb4 (Fig. 8C), suggests that DkMyb4 directly activates DkANR transcription. The expression correlation and binding ability with DkMyb4 (DkMyb4) were also observed in DkANS and DkF3′5′H (Figs. 3 for DkF3′5′H and DkANS, 5A for DkMyb4, and 8C for binding ability with DkMyb4); these were shared with anthocyanin, flavonol, and the PA biosynthetic pathway (Fig. 1). This result indicates that DkMyb4 directly activates the PA-specific genes and also other genes of the flavonoid pathway.
On the other hand, one of the two PA-specific genes, DkLAR, showed expression completely not correlated to that of DkMyb4 in persimmon fruit (Figs. 3 for DkLAR and 5A for DkMyb4). As described, the expression of VvMYBPA1 in grapevine activates only one of the two LAR genes (Bogs et al., 2007). In addition, in Medicago truncatula, slight activation of LAR expression was observed when compared with ANR expression after overexpression of a Myb TF, TT2 (Pang et al., 2008), and the expression level of LAR did not correlate with PA accumulation (Pang et al., 2007). These results support the possibility that DkLAR is not regulated by DkMyb4 in persimmon.
The coincident and synchronous down-regulation of DkMyb4 and many structural genes of the flavonoid pathway, including DkDHD/SDH in the NA type (Figs. 3 and 4), suggested that DkMyb4 acts as a direct or indirect regulator of them. Ectopic suppression of DkMyb4 expression in persimmon calluses, causing a proportional reduction in the expression levels of the identical flavonoid pathway genes (Fig. 7D), indicated at least indirect regulation by DkMyb4. This is supported by the results showing that VvMYBPA1, which is a grapevine Myb TF phylogenetically close to DkMyb4 (Fig. 4), regulates the expression of VvANR and one of the two LAR genes in grapevine, VvLAR1, which are PA-specific genes, and the expression of other structural genes of the flavonoid pathway (Bogs et al., 2007) and also the phenylpropanoid and/or shikimate pathways, including DHD/SDH (Terrier et al., 2009). However, we have not identified the promoter regions of the early flavonoid pathway genes, such as PAL or CHS; therefore, we could not confirm whether DkMyb4 directly regulates their expression. The early flavonoid pathway, including the phenylpropanoid and/or shikimate pathways, can affect the biosynthesis of a diverse class of secondary metabolites (i.e. pigment, lignin, phytoalexins, and plant hormones; Herrmann, 1995; Lillo et al., 2008). Hence, applications of TFs that can regulate the general flavonoid pathway will contribute to studies of the metabolic engineering of plants for both plant and human health, as discussed previously (Dixon, 2005; Sharma and Dixon, 2005; Yu and McGonigle, 2005).
MYBCORE, the Target Motif of DkMyb4, Is a General Key cis-Element for PA Biosynthesis in Plant Species
There are various target motifs of plant Myb TFs, such as MYBCORE (Urao et al., 1993; Solano et al., 1995), MYBPLANT (Grotewold et al., 1994; Tamagnone et al., 1998), MYB2CONSENSUSAT (Abe et al., 2003), in the PLACE database for plant cis-motifs (Higo et al., 1999). The ability of Myb TF binding to them depends on the sequences of the R2 region (Koes et al., 2005). Therefore, it is suggested that the Myb TFs with conserved homologous R2 sequences have the same binding ability. The R2 regions of Myb TFs phylogenetically placed in the same cluster of DkMyb4, such as VvMYBPA1, VvMYBPA2, and TT2 (AtMYB123; Fig. 4B), showed high sequence identity to DkMyb4, 88% to VvMYBPA1, 65% to VvMYBPA2, and 66% to TT2 (Fig. 4A). Their detailed target motifs have not been identified. Our results suggest that they can bind to the MYBCORE cis-motif as well as DkMyb4. Their functions were partially or entirely consistent, and all of them acted as regulators for ANR, which is a PA-specific gene (Fig. 1; Nesi et al., 2001; Bogs et al., 2007; Terrier et al., 2009). It has also been reported that VvMYBPA1 acts as a regulator of ANR in Arabidopsis (Bogs et al., 2007). Their conserved functions among plant species suggest that these Myb TFs have the same DNA-binding ability. Furthermore, the promoter of ANR contains the same MYB-binding cis-motifs targeted by them. The comparative analyses of the promoter regions of ANR orthologs in persimmon (DkANR; accession no. 195284), grapevine (VvANR; accession no. CAD91911), and Arabidopsis (AtANR; accession no. AT1G61720) using the PLACE database showed that they have many MYBCORE cis-motifs that are highly conserved (Fig. 9). In addition, only the MYBCORE cis-motif among the MYB-binding cis-motifs was conserved in all three promoter regions of ANR at 1 kb upstream from the ATG start codon. Taken together, these results suggest that the regulation of ANR by Myb TFs depends on the presence of the MYBCORE cis-motif in the promoter region of ANR and the ability of Myb TFs to bind to the MYBCORE cis-motif in plant species. This hypothesis was supported by a previous study of the promoter of AtANR in Arabidopsis. Debeaujon et al. (2003) demonstrated that a minimal promoter of AtANR necessary for expression in PA-accumulating cells contains only the MYBCORE cis-motif among the MYB-binding cis-motifs. Furthermore, in grapevine, all promoter regions of the genes activated by VvMYBPA1, which regulate PA biosynthesis in grapevine, VvANR, VvCHI, VvLAR1, VvF3′5′H, and VvLDOX, contain the MYBCORE cis-motifs. These results are consistent with our results that DkANR, DkANS, and DkF3′5′H, which contain the MYBCORE cis-motifs in the promoter region, are regulated by DkMyb4, but DkF3′H and DkLAR, which do not contain the MYBCORE cis-motifs in the promoter region, are not regulated by DkMyb4. Therefore, it is suggested that the MYBCORE cis-motif is widely conserved in the promoter regions of general PA pathway genes in plant species, and this cis-motif is a key factor in transcription activation by the Myb TFs regulating the PA biosynthetic pathway.
Figure 9.
MYB-binding cis-motifs in the promoter region of ANR orthologs from persimmon (cv Kuramitsu), grapevine (cv Pinot Noir), and Arabidopsis. Analysis was performed using the PLACE database as described in the legend to Figure 8.
Application of Ectopic DkMyb4 Expression
The ectopic overexpression of VvMYBPA1 in Arabidopsis plants induced elevated biosynthesis of PA in the roots, hypocotyls, and apical meristems (Bogs et al., 2007). These transformed plants, however, died before they fully developed their first leaves because of the accumulation of excess PAs. Similar toxic effects of PA accumulation were observed in Arabidopsis by constitutive coexpression of TT2, PAP1, and the maize regulatory gene leaf color, which resulted in PA formation in roots and leaves and eventual death of the plants (Sharma and Dixon, 2005). In our study, the activation of PA biosynthesis in kiwifruit leaves and regenerated calluses in vitro by ectopic DkMyb4 expression caused termination of callus growth and adventitious bud regeneration (Fig. 6). These results are presumably due to both the toxic effects of PA and disturbance by PA oxidization, because previous reports showed that phenolic compounds and their oxidation cause the browning of plant tissue and physically disturb regeneration (Madhusudhanan and Rahiman, 2000; Tang and Newton, 2004; Johkan et al., 2008). The transformation of many fruit crops, such as apple, grapevine, kiwifruit, and persimmon, depends on adventitious bud regeneration from leaves or calluses in vitro (Maheswaran et al., 1992; Matsuta et al., 1993; Scorza et al., 1995; Tao et al., 1997; Koshita et al., 2008). However, our results suggested that it is difficult to regenerate transgenic plants with DkMyb4 via adventitious buds in vitro. It would be interesting to determine whether the ectopic expression of DkMyb4 in forage plants, such as alfalfa (Medicago sativa) and clover (Trifolium repens), can induce PA accumulation in leaves, because there is great interest in engineering PAs in forage crops to reduce the risk of pasture bloat in ruminants (Dixon et al., 2005).
Involvement of DkMyb4 in the Astringency Trait in Persimmon Fruit Flesh
Our results suggested that DkMyb4 acts as a regulator of the PA pathway genes and controls PA biosynthesis in persimmon. Therefore, this suggests the possibility that the fruit flesh-specific down-regulation of DkMyb4 expression in the NA type causes a significant reduction in PA content and the resultant NA trait in fruits. Previously, it was reported that the expression levels of DkF3′5′H and DkANR substantially affect the PA content and composition of developing fruits of the A and NA types, whereas that of DkLAR does not (Akagi et al., 2009). Our results suggest the direct regulation of DkF3′5′H and DkANR transcription by DkMyb4, but no regulation of DkLAR transcription, with the suppression of DkMyb4 in persimmon calluses (Fig. 7D) and EMSA (Fig. 8). Furthermore, the significant reduction in EGC content caused by suppression of DkMyb4 in AM4 lines (Fig. 8E) is consistent with that in NA-type fruits, which is the main reason for the NA trait of the NA type (Akagi et al., 2009). These all support the possibility that the down-regulation of DkMyb4 expression causes the suppression of DkF3′5′H and DkANR expression, resulting in a significant reduction in EGC content in fruit flesh of the NA type.
In this study, we also isolated DkMyb2, another MYB TF, which is in a phylogenetic cluster containing MYB proteins regulating the PA pathway (Fig. 4B). However, we detected no correlation between the expression level of DkMyb2 and PA biosynthesis (Figs. 2 and 5A). In addition, we could not detect the ability of DkMyb2 to bind to the MYBCORE cis-motif (T. Akagi, unpublished data). This implied that DkMyb2 could not act as a direct regulator of DkF3′5′H, because the promoter region of DkF3′5′H contained only the MYBCORE cis-motifs among the MYB-binding motifs detected using the PLACE database (Fig. 8A). These results suggest that the expression level of DkMyb2 is not involved in the substantial PA accumulation in developing persimmon fruit. Other Myb TFs, or TFs such as bHLH and/or WD repeat, may also possibly regulate the PA pathway genes in persimmon fruit flesh and be involved in the astringency trait. In the future, further analysis, such as ectopic expression of DkMyb4 in NA-type fruit flesh, will show whether there is involvement of phenological activity of DkMyb4 in the astringency trait in persimmon fruit.
CONCLUSION
To determine the nature of the genetic basis of PA biosynthesis in persimmon fruit, we analyzed temporal expression levels of the structural genes of the PA biosynthetic pathway. Almost all of their expression specifically showed coincident down-regulation in the NA type. We identified a Myb TF, DkMyb4, that showed a synchronous expression pattern to the genes of the PA biosynthetic pathway. DkMyb4 recognized the MYBCORE cis-motif in EMSA analysis and could act as a regulator for PA pathway genes in the transformation of kiwifruit and persimmon. These results indicate that DkMyb4 acts as a direct regulator of the PA pathway genes and controls PA biosynthesis in persimmon. This suggests the possibility that phenological down-regulation of DkMyb4 causes the NA-type-specific down-regulation of PA biosynthesis and determines the NA trait. An insight into the phenological mechanisms regulating PA biosynthesis will greatly contribute to the modification of the PA-derived trait in fruits by controlling environmental conditions.
MATERIALS AND METHODS
Plant Materials
To analyze temporal PA accumulation and gene expression in persimmon (Diospyros kaki) fruit, the fruit flesh of 11 individuals in a BC1-like offspring named the Atf line was collected in 2009. The Atf line was derived from a cross between cv Fuyu (NA type) and cv 275-13 (A type). The A-type parent 275-13 was derived from a cross between cv Taishu (NA type) and cv Aizu-mishirazu (A type). The offspring were grown and maintained in the orchard of Kyoto University (Kyoto) and the Department of Grape and Persimmon Research, National Institute of Fruit Tree Science (Akitsu). Three fruits of each individual were sampled at 2-week intervals from 3 WAB until 11 or 13 WAB, at which time PA accumulation in the fruit is almost completed, as reported previously (Ikegami et al., 2005; Akagi et al., 2009).
To isolate Myb TF homologs in persimmon fruit, we used the fruit of an A-type cv Kuramitsu sampled at 5 and 7 WAB in 2007 in the orchard of Kyoto University. For additional expression analysis on DkMyb4, we sampled fruit flesh and leaves of 14 cultivars of the A type (Akadu, Hiratanenashi, Kuramitsu, Miyazakimukaku, Nikura, Saijyo, Sangokuichi, and Yokono) and the NA type (cultivars Fuyu, Hanagosho, Jiro, Mikado, Okugosho, and Suruga) at 7 WAB in the orchard of Kyoto University.
In all these experiments, fruit flesh was diced into small pieces (approximately 1 cm × 0.7 cm × 0.5 cm), frozen in liquid nitrogen, and stored at −80°C until analyzed.
Analysis for PA and Anthocyanin Accumulation
Soluble PAs were extracted from 50 mg of finely ground dried materials in 25 mL of 80% methanol at room temperature. Insoluble PAs were extracted from the residue in 25 mL of 1% (v/v) HCl-methanol for 1 h at 60°C according to the previous report by Taira et al., 1998). We examined the PA content using the DMACA method (Li et al., 1996). We also examined soluble phenolic compounds using the Folin-Ciocalteau method (Oshida et al., 1996). PA or phenolic compound concentration per fresh weight was expressed as (+)-catechin equivalents.
The presence of PAs in transformed plant callus tissue was detected by staining the tissues with DMACA solution (1% DMACA and 1% 6 n HCl in methanol) according to the previous report by Li et al. (1996). Plant callus tissue was decolorized in 5 mL of 30% acetic acid in ethanol for 12 h. The tissues were washed with 75% ethanol and then stained blue in DMACA solution for 1 min.
The PA subunit composition in transformed plant callus tissue was analyzed using HPLC following acid catalysis in the presence of excess phloroglucinol (Kennedy and Jones, 2001) according to the previous report by Akagi et al. (2009). Briefly, soluble PAs were extracted from 10 mg of finely ground dried materials in 1 mL of 70% acetone containing 0.1% ascorbic acid for 24 h at room temperature. The supernatant was used for acid-catalyzed cleavage of the PAs in the presence of excess phloroglucinol according to the method of Downey et al. (2003). These reactions were stopped with 200 mm sodium acetate (twice the amount of the phloroglucinol reagent), and then a vanillin solution (1 mg of vanillin in 5 mL of 1% [v/v] HCl-methanol) of the same volume as the reagent was added as an external standard. Samples were run on a reverse-phase HPLC apparatus (LC2010; Shimadzu) using a Wakosil-II 5C18 RS analytical column (4 mm × 250 mm) protected by a guard column containing the same material. Elution was performed with two solvents, 0.2% (v/v) aqueous acetic acid (solvent A) and methanol (solvent B), using the following elution program: initially 1% B for 30 min, increasing to 15.5% for 35 min, then to 45% for 35 min, followed by washing with 100% B for 15 min, and a return to the initial conditions (1% B). The analysis was performed by detecting A280 at 30°C with a flow rate of 1 mL min−1. For each measurement, three replications of each sample were analyzed.
Anthocyanins in kiwifruit (Actinidia deliciosa) plants were extracted with 5% aqueous acetic acid. We analyzed anthocyanin content according to the previous report by Koshita et al. (2008) with some modifications. Samples were run on a reverse-phase HPLC apparatus (LC2010) using a Shim-pack VP-ODS analytical column (4.6 mm × 150 mm; Shimadzu) at 40°C with a flow rate of 1 mL min−1 by detecting A240–580. A linear gradient of 10% to 50% solvent B (1.5% H3PO4, 40% acetonitrile, and 50% acetic acid) in solvent A (1.5% H3PO4) was run over 40 min.
Identification of Myb TF Homologs
Total RNA was isolated from 0.2 g of frozen flesh tissue using the hot borate method (Wan and Wilkins, 1994). cDNA was synthesized from 1 μg of total RNA using a SMART PCR cDNA Synthesis Kit (Clontech). The degenerate primers for Myb TFs isolated from persimmon fruit were designed by an alignment of R2R3 nucleotide sequences of the Myb TFs involved in flavonoid regulation in other plant species. The full lengths of Myb TFs were obtained by screening the cDNA libraries of Kuramitsu. The cDNA libraries were constructed using the SMART cDNA Library Construction Kit (Clontech) using 2.0 μg of total RNA of Kuramitsu sampled at 5 or 7 WAB in 2007, according to the manufacturer's instructions, and positive clones were screened with digoxigenin (DIG)-labeled probes (Roche Diagnostics).
The phylogenetic analysis of the R2R3 conserved region was performed using the ClustalW multiple sequence alignment system (Thompson et al., 1997) using the default parameters of the DNA Data Bank of Japan, and the tree was constructed using the neighbor-joining method of TreeView (Page, 1996).
Expression Analysis by qRT-PCR
Expression analysis was performed by qRT-PCR. Total RNA was isolated as described above. cDNAs were synthesized from approximately 1 μg of total RNA using SuperScript III transcriptase (Invitrogen) and adopter primer. The primer pairs for amplification were designed based on the conserved sequences of five isolated Myb TFs (DkMyb1, DkMyb2, DkMyb3, DkMyb4, and DksMyb1) and the structural genes of the flavonoid biosynthetic pathway (DkANR, DkANS, DkCHI, DkCHS, DkDFR, DkF3H, DkF3′H, DkF3′5′H, and DkLAR; Ikegami et al., 2005, 2007; Nakagawa et al., 2008; Akagi et al., 2009). We also detected DHD/SDH, which is reportedly involved in PA regulation (Ikegami et al., 2007; Akagi et al., 2009). All primers were designed using conserved sequences of them with the Primer Express software (version 2.1; Applied Biosystems), and the sequences are listed in Supplemental Table S1. An aliquot of 1:5 diluted pools of cDNA was used in the PCR as a template. Expression levels were assayed using an ABI Prism 7900HT (Applied Biosystems) with the SYBR Green system and SYBR Pre-mix Ex-Taq (TaKaRa). All reactions were performed in a total volume of 25 μL well−1, comprising 12.5 μL of SYBR-Pre mix, 9 μL of sterilized distilled water, 1 μL of each detection primer (5 μm), 0.5 μL of Rox dye, and 1 μL of template cDNA. The standard amplification protocol consisted of an initial denaturing step at 95°C for 30 s, followed by 40 cycles at 95°C for 10 s, 57°C for 5 s, and 72°C for 15 s.
For each expression, the average threshold cycle (Ct) was automatically determined by ABI Prism 7900HT as the default state. Ct was defined as the point at which fluorescence rises appreciably above the background. For each measurement, independent standard curves were constructed, and at least three replications of each sample were analyzed. The mean Ct of three replications was used for each sample. Standard curves for the target genes and a housekeeping gene (DkActin) were obtained by the amplification of a serially diluted mixture of cDNA samples with six dilution points. The gene quantification method was based on the relative expression of the target gene versus the reference gene (Actin). In addition, the relative expression levels among target genes were determined approximately using equal quantities of the PCR products of the target genes for standardization.
Plant Transformations with DkMyb4
The ORF of DkMyb4 was amplified by PCR from persimmon (Kuramitsu) cDNA derived from fruit flesh sampled at 7 WAB in 2007 using Prime STAR HS (TaKaRa) polymerase, with the two primer sets DkMyb-startF-TOPO (5′-CACCATGGGAAGAGCTCCTTGTTGT-3′) and DkMyb4-stopR (5′-GGATCCGAAACTACAACTAAGATTCAGAG-3′) for cloning of the sense strand DkMyb4 and DkMyb4-startF (5′-CGTCACAAATATCAGAAGAAGAGATG-3′) and DkMyb4-stopR-TOPO (5′-CACCGAAACTACAACTAAGATTCAGAG-3′) for cloning of the antisense strand DkMyb4. The sequence in boldface (CACC) is the additional sequence necessary for directional cloning in the following procedure. The two PCR fragments generated were subcloned into the entry vector pENTR/D-TOPO (Invitrogen) by TOPO. The cloning procedure followed the manufacturer's instructions. Finally, we cloned the sense or antisense DkMyb4 ORF into pGWB2 (Nakagawa et al., 2007) to obtain pGWB2-SSDkMyb4 and pGWB2-ASDkMyb4, where the sense and antisense DkMyb4 were under the control of the CaMV35S promoter, using the Gateway Cloning System with LR Clonase (Invitrogen). The nucleotide sequences were determined using CEQ8000 version 7.0 (Beckman Coulter). pGWB2-SSDkMyb4 and pGWB2-ASDkMyb4 were transformed into the Agrobacterium tumefaciens strain C58C1 by electroporation.
Transformations of persimmon (Fujiwaragosho) and kiwifruit (Heyward) were performed with A. tumefaciens according to the methods reported previously by Tao et al. (1997) for persimmon and by Koshita et al. (2008) and Matsuta et al. (1993) for kiwifruit. Pieces of leaf were infected with the transformed A. tumefaciens, and callus tissue was regenerated on solidified Murashige and Skoog medium containing kanamycin (50 mg L−1) as a selective antibiotic.
Isolation of cis-Motifs in the Promoter Region of Structural Genes of the Flavonoid Pathway
The genomic fosmid library derived from Diospyros lotus, which is one of the closest diploid relatives of persimmon (Yonemori et al., 2008), was constructed using the CopyControl Fosmid Library Production Kit (Epicentre) by a modified version of the manufacturer's protocol. Randomly sheared and end-repaired genomic DNA (20–30 μg) was run on a 0.3% agarose gel. Thereafter, DNA fragments of about 40 kb in length were extracted using RECOCHIP (TaKaRa). The extracted size-selected DNA was then purified by chloroform extraction and ligated into pCC1FOS. The inserts in the plasmids were then packaged into phage λ (Epicentre). We screened clones containing the homologous ORFs of DkANS, DkANR, DkF3′H, DkF3′5′H, and DkLAR from the genomic fosmid library. The sequences of each promoter region were determined, and then specific primers amplifying them were designed (Supplemental Table S2). The promoter regions in persimmon (Kuramitsu) were amplified using PrimeSTAR HS (TaKaRa) and directly sequenced using Exo-Sap IT (GE Healthcare Bio-Sciences) and CEQ8000 version 7.0 (Beckman Coulter). Many single nucleotide polymorphisms were detected in these promoter sequences, because the hexaploid persimmon (2n = 6x = 90) has six alleles at each locus. We only detected cis-motifs in their consensus sequences. Analysis of the promoter regions was performed using the PLACE database (http://www.dna.affrc.go.jp/PLACE/signalup.html; Higo et al., 1999).
Production and Purification of DkMyb4-GST Fusion Proteins
The sense-strand DkMyb4 ORF amplified by the primer set DkMyb-startF-TOPO and DkMyb4-stopR was subcloned into the entry vector pENTR/D-TOPO (Invitrogen) by the TOPO cloning procedure as described above. To express the GST-tagged recombinant DkMyb4 protein in Escherichia coli, the sense-strand DkMyb4 was cloned into the vector pDEST15 (Invitrogen) to give pDEST15-DkMyb4, which encodes an N-terminal in-frame fusion of DkMyb4 with the GST tag, using the Gateway Cloning System with LR Clonase (Invitrogen). The resultant plasmid was transformed into E. coli (BL21) cells. The transformants were precultured at 37°C for 16 h in LB medium containing 100 μg mL−1 ampicillin. A 3-mL volume of the preculture was inoculated into 300 mL of fresh LB medium containing the same antibiotic. After incubation at 37°C, until the A600 reached 0.2, isopropyl β-d-thiogalactopyranoside was added to the broth to a final concentration of 0.3 mm, followed by further incubation at 18°C for 24 h. The cells were harvested by centrifugation at 4,000 rpm for 15 min and stored at −80°C before further processing. Collected cells were resuspended in phosphate buffer saline containing 1% Triton X-100 (PBST), and the insoluble bacterial debris was removed by centrifugation (15,000 rpm for 15 min). After dithiothreitol was added to the resulting supernatant to a final concentration of 1 mm, the soluble fraction was loaded onto a 3-mL Glutathione Sepharose 4B column (GE Healthcare Bio-Sciences) that was preequilibrated with PBST. The column was washed with 15 column volumes of PBST. The recombinant protein, which was bound to the affinity column, was eluted with 15 mL of 50 mm Tris-HCl buffer (pH 9.5) containing 50 mm reduced glutathione. The eluted fraction was collected and concentrated using a Y30 membrane (Amicon; Millipore), and glycerol was added to a final concentration of 10% (v/v) before being stored at −80°C.
EMSA Analyses
Oligonucleotide probes were labeled by DIG-ddUTP using DIG Oligonucleotide 3′-End Labeling Kit, 2nd Generation (Roche). The DNA-binding reaction was allowed to proceed for 20 min at 25°C in 20 μL of the two binding buffers (5% glycerol, 4 mm KCl, 5 mm MgCl2, 1 mm EDTA, 25 mm HEPES/KOH, pH 7.5, and 5% glycerol, 50 mm KCl, 0.5 mm dithiothreitol, 0.5 mm EDTA, 25 mm HEPES/KOH, pH 8.0), according to previous reports by Urao et al. (1993) and Hartmann et al. (2005), containing 4 ng of a DIG-labeled oligonucleotide probe and 400 ng of purified GST-DkMyb4 fusion protein. Competition experiments were performed by adding unlabeled competitor oligonucleotides at 20-, 50-, or 150-fold excess versus the DIG-labeled oligonucleotide probe. The bound complexes were subjected to electrophoresis on native 5% polyacrylamide gels in 0.5% Tris-borate-EDTA buffer, pH 8.0, at 90 V for 60 to 80 min at room temperature and then transferred to a nylon membrane (Biodine-Plus; Pall). The DIG-labeled signals were detected using an anti-DIG-alkaline phosphate conjugate, a chemiluminescent substrate (CDP-Star; Roche), and a chemiluminometer (LAS3000 mini; Fujifilm).
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: DkDHD/SDH (AB472375), DkPAL (AB472364), DkCHS (AB472365), DkCHI (AB472367), DkF3H (AB472368), DkF3′H (AB472369), DkF3′5′H (AB472676), DkDFR (AB472371), DkANS (AB472677), DkLAR (AB472681), DkANR (AB195284), DkMyb1 (AB503698), DkMyb2 (AB503699), DkMyb3 (AB503700), DkMyb4 (AB503701), DksMyb1 (AB503702), and DkActin (AB473616).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. HPLC chromatogram of anthocyanins from transformed kiwifruit calluses.
Supplemental Figure S2. HPLC chromatogram of PA cleavage products from the AM4-1 calluses.
Supplemental Table S1. Information for primers used in qRT-PCR analysis.
Supplemental Table S2. Information for primers used in promoter analysis.
Supplementary Material
Acknowledgments
We are grateful to Dr. Tsuyoshi Nakagawa (Department of Molecular and Functional Genomics, Center for Integrated Research in Science, Shimane University) for providing the pGWB2 vector.
This work was supported by a Grant-in Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (grant no. 19380019).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Keizo Yonemori (keizo@kais.kyoto-u.ac.jp).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams S, Tanner GJ, Larkin PJ, Ashton AR (2002) Identification and biochemical characterization of mutants in the proanthocyanidin pathway in Arabidopsis. Plant Physiol 130 561–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, De Vos CHR, Wein M, Sun Z, Greco R, Kroon A, Mol JNM, O'Connell AP (2001) The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J 28 319–332 [DOI] [PubMed] [Google Scholar]
- Akagi T, Ikegami A, Suzuki Y, Yoshida J, Yamada M, Sato A, Yonemori K (2009) Expression balances of structural genes in shikimate and flavonoid biosynthesis cause a difference in proanthocyanidin accumulation in persimmon (Diospyros kaki Thunb.) fruit. Planta 230 899–915 [DOI] [PubMed] [Google Scholar]
- Aron PM, Kennedy JA (2008) Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res 52 79–104 [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, Joshi SS, Pruess HG (2000) Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology 148 187–197 [DOI] [PubMed] [Google Scholar]
- Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L (2004) TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 39 366–380 [DOI] [PubMed] [Google Scholar]
- Baxter IR, Young JC, Armstrong G, Foster N, Bogenschutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF (2005) A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci USA 102 2649–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogs J, Jaffé FW, Takos AM, Walker AR, Robinson SP (2007) The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol 143 1347–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12 2383–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugliera F, Barri-Rewell G, Holton TA, Mason JG (1999) Isolation and characterization of a flavonoid 3′-hydroxylase cDNA clone corresponding to the Ht1 locus of Petunia hybrida. Plant J 19 441–451 [DOI] [PubMed] [Google Scholar]
- Chen Y, Xiangbo Z, Wei W, Chen Z, Gu H, Qu LJ (2006) Overexpression of the wounding-responsive gene AtMYB15 activates the shikimate pathway in Arabidopsis. J Integr Plant Biol 48 1084–1095 [Google Scholar]
- Cos P, De Bruyne T, Hermans N, Apers S, Berghe DV, Vlietinck AJ (2004) Proanthocyanidins in health care: current and new trends. Curr Med Chem 11 1345–1359 [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Nesi N, Perez P, Devic M, Grandjean O, Caboche M, Lepiniec L (2003) Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and roll in seed development. Plant Cell 15 2514–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Peeters AJ, Leon-Kloosterziel KM, Koornneef M (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13 853–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc L, Bogs J, Walker AR, Ferrier T, Decendit A, Merillon JM, Robinson SP, Barrieu F (2008) The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol 147 2041–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Hofius D, Hajirezaei MR, Fernie AR, Bornke F, Sonnewald U (2007) Functional analysis of the essential bifunctional tobacco enzyme 3-dehydroquinate dehydratase/shikimate dehydrogenase in transgenic tobacco plants. J Exp Bot 58 2053–2067 [DOI] [PubMed] [Google Scholar]
- Dixon RA (2005) Engineering of plant natural product pathways. Curr Opin Plant Biol 8 329–336 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Xie DY, Sharma SB (2005) Proanthocyanidins: a final frontier in flavonoid research? New Phytol 165 9–28 [DOI] [PubMed] [Google Scholar]
- Downey MO, Harvey JS, Robinson SP (2003) Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust J Grape Wine Res 9 15–27 [Google Scholar]
- Espley R, Brendolise C, Chagné D, Kutty-Amma S, Green S, Volz R, Putterill J, Schouten HJ, Gardiner SE, Hellens RP, et al (2009) Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. Plant Cell 21 168–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espley R, Hellens R, Putterill J, Stevenson D, Kutty-Amma S, Allan A (2007) Red coloration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 49 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornalé S, Sonbol FM, Maes T, Capellades M, Puigdoménech P, Rigau J, Caparrós-Ruiz D (2006) Down-regulation of the maize and Arabidopsis thaliana caffeic acid O-methyl-transferase genes by two new maize R2R3-MYB transcription factors. Plant Mol Biol 62 809–823 [DOI] [PubMed] [Google Scholar]
- Grotewold E, Drummond BJ, Bowen B, Peterson T (1994) The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell 76 543–553 [DOI] [PubMed] [Google Scholar]
- Grotewold E, Sainz MB, Tagliani L, Hernandez JM, Bowen B, Chandler VL (2000) Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proc Natl Acad Sci USA 97 13579–13584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB, Grayer RJ (1993) Flavonoids and insects. In JB Harborne, ed, The Flavonoids: Advances in Research Since 1986. Chapman & Hall, London, pp 589–618
- Hartmann U, Sagasser M, Mehrtens F, Stracke R, Weisshaar B (2005) Differential combinatorial interactions of cis-acting element recognized by R2R3-MYB, BZIP, and BHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Mol Biol 57 155–171 [DOI] [PubMed] [Google Scholar]
- Herrmann KM (1995) The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol 107 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton TA, Brugliera F, Lester DR, Tanaka Y, Hyland GD, Menting JGT, Lu CY, Farcy E, Stevenson TW, Cornish EC (1993) Cloning and expression of cytochrome-P450 gene controlling flower color. Nature 366 276–279 [DOI] [PubMed] [Google Scholar]
- Holton TA, Cornish EC (1995) Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7 1071–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami A, Akagi T, Potter D, Yamada M, Sato A, Yonemori K, Kitajima A, Inoue K (2009) Molecular identification of 1-Cys peroxiredoxin and anthocyanidin/flavonol 3-O-galactosyltransferase from proanthocyanidin-rich young fruits of persimmon (Diospyros kaki Thunb.). Planta 230 841–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami A, Eguchi S, Kitajima A, Inoue K, Yonemori K (2007) Identification of genes involved in proanthocyanidin biosynthesis of persimmon (Diospyros kaki) fruit. Plant Sci 172 1037–1047 [Google Scholar]
- Ikegami A, Kitajima A, Yonemori K (2005) Inhibition of flavonoid biosynthetic gene expression coincides with loss of astringency in pollination-constant, non-astringent (PCNA)-type persimmon fruit. J Hortic Sci Biotechnol 80 225–228 [Google Scholar]
- Johkan M, Mori G, Mitsukuni K, Mishiba K, Morikawa T, Oda M (2008) In vivo shoot regeneration promoted by shading the cut surface of the stem in tomato plants. HortScience 43 220–222 [Google Scholar]
- Kanzaki S, Sato A, Yamada M, Yonemori K, Sugiura A (2001) Identification of molecular markers linked to the trait of natural astringency loss Japanese persimmon (Diospyros kaki) fruit. J Am Soc Hortic Sci 126 51–55 [Google Scholar]
- Kennedy JA, Jones GP (2001) Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J Agric Food Chem 49 1740–1746 [DOI] [PubMed] [Google Scholar]
- Kitamura S, Shikazono N, Tanaka A (2004) TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J 37 104–114 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Goto-Yamamoto N, Hirochika H (2004) Retrotransposon-induced mutations in grape skin color. Science 304 982. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ishimaru M, Hiraoka K, Honda C (2002) Myb related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta 215 924–933 [DOI] [PubMed] [Google Scholar]
- Koes R, Verweij W, Quattrocchio F (2005) Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10 237–242 [DOI] [PubMed] [Google Scholar]
- Koshita Y, Kobayashi S, Ishimaru M, Funamoto Y, Shiraishi M, Azuma A, Yakushikji H, Nakayama M (2008) An anthocyanin regulator from grapes, VlmybA1-2, produces reddish-purple plants. J Jpn Soc Hortic Sci 77 33–37 [Google Scholar]
- Kranz HD, Denekamp M, Greco R, Jin H, Leyva A, Meissner RC, Petroni K, Urzainqui A, Bevan M, Martin C, et al (1998) Towards functional characterisation of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J 16 263–276 [DOI] [PubMed] [Google Scholar]
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonids. Annu Rev Plant Biol 57 405–430 [DOI] [PubMed] [Google Scholar]
- Li YG, Tanner G, Larkin P (1996) The DMACA-HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J Sci Food Agric 70 89–101 [Google Scholar]
- Lillo C, Lea US, Ruoff P (2008) Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ 31 587–601 [DOI] [PubMed] [Google Scholar]
- Lloyd AM, Walbot V, Davis RW (1992) Arabidopsis and Nicotiana anthocyanin production activated by maize regulators R and C1. Science 258 1773–1775 [DOI] [PubMed] [Google Scholar]
- Madhusudhanan K, Rahiman BA (2000) The effect of activated charcoal supplemented media to browning of in vitro cultures of Piper species. Biol Plant 43 297–299 [Google Scholar]
- Maheswaran G, Welander M, Hutchinson JF, Graham MW, Richards D (1992) Transformation of apple rootstock M26 with Agrobacterium tumefaciens. J Plant Physiol 139 560–568 [Google Scholar]
- Matsuta N, Iketani H, Hayashi T (1993) Transformation in grape and kiwifruit. In T Hayashi, M Omura, NS Scott, eds, Techniques on Gene Diagnosis and Breeding in Fruit Trees. Fruit Tree Research Station, Tsukuba, Japan, pp 184–192
- McMahon LR, McAllister TA, Berg BP, Majak W, Acharya SN, Popp JD, Coulman BE, Wang Y, Cheng KJ (2000) A review of the effects of forage condensed tannins on ruminal fermentation and bloat in grazing cattle. Can J Plant Sci 80 469–485 [Google Scholar]
- Mellway RD, Tran LT, Prouse MB, Campbell MM, Constabel CP (2009) The wound-, pathogen-, and ultraviolet B-responsive MYB134 gene encodes an R2R3 MYB transcription factor that regulates proanthocyanidin synthesis in poplar. Plant Physiol 150 924–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol J, Grotewold E, Koes R (1998) How genes paint flowers and seeds. Trends Plant Sci 3 212–217 [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K (2007) Development of series of Gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104 34–41 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nakatsuka A, Yano K, Yasugahira S, Nakamura R, Sun N, Itai A, Suzuki T, Itamura H (2008) Expressed sequence tags from persimmon at different development stages. Plant Cell Rep 27 931–938 [DOI] [PubMed] [Google Scholar]
- Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L (2001) The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13 2099–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RL, Hammerschmidt R (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30 369–389 [Google Scholar]
- Oshida MK, Yonemori K, Sugiura A (1996) On the nature of coagulated tannins in astringent-type persimmon fruit after an artificial treatment of astringency removal. Postharvest Biol Technol 8 317–327 [Google Scholar]
- Page R (1996) Tree View: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12 357–358 [DOI] [PubMed] [Google Scholar]
- Pang Y, Peel GJ, Sharma SB, Tang Y, Dixon RA (2008) A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. Proc Natl Acad Sci USA 105 14210–14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Peel GJ, Wright E, Wang Z, Dixon RA (2007) Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol 145 601–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DJ, Constabel CP (2002) Molecular analysis of herbivore-induced condensed tannin synthesis: cloning and expression of dihydroflavonol reductase from trembling aspen (Populus tremuloides). Plant J 32 701–712 [DOI] [PubMed] [Google Scholar]
- Planchais S, Perennes C, Glab N, Mironov V, Inzé D, Bergounioux C (2002) Characterization of cis-acting element involved in cell cycle phase-independent activation of Arath;CycB1;1 transcription and identification of putative regulatory proteins. Plant Mol Biol 50 109–125 [DOI] [PubMed] [Google Scholar]
- Quattrocchio F, Wing J, van der Woude K, Souer E, de Vetten N, Mol J, Koes R (1999) Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. Plant Cell 11 1433–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza R, Cordts JM, Ramming DW, Emershad RL (1995) Transformation of grape (Vitis vinifera L.) zygotic-derived somatic embryos and regeneration of transgenic plants. Plant Cell Rep 14 589–592 [DOI] [PubMed] [Google Scholar]
- Sharma SB, Dixon RA (2005) Metabolic engineering of proanthocyanidins by ectopic expression of transcription factors in Arabidopsis thaliana. Plant J 44 62–75 [DOI] [PubMed] [Google Scholar]
- Shirley BW, Hanley S, Goodman HM (1992) Effects of ionizing radiation on a plant genome: analysis of two Arabidopsis transparent testa mutations. Plant Cell 4 333–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Nieto C, Avila J, Canas L, Diaz I, Paz-Ares J (1995) Dual DNA binding specificity of a petal epidermis-specific MYB transcription factor (MYB.Ph3) from Petunia hybrida. EMBO J 14 1773–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4 447–456 [DOI] [PubMed] [Google Scholar]
- Taira S (1996) Astringency in persimmon. In HF Linskens, JF Jackson, eds, Fruit Analysis. Springer-Verlag, Berlin, pp 97–110
- Taira S, Matsumoto N, Ono M (1998) Accumulation of soluble and insoluble tannins during fruit development in nonastringent and astringent persimmon. J Jpn Soc Hortic Sci 67 572–576 [Google Scholar]
- Takos AM, Jaffe FW, Jacob SR, Bogs J, Robinson SP, Walker AR (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142 1216–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Merida A, Parr A, Mackay S, Culianez-Macia FA, Roberts K, Martin C (1998) The AmMYB308 and AmMYB330 transcription factors from Antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. Plant Cell 10 135–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Newton RJ (2004) Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in Virginia pine (Pinus virginiana Mill.). Plant Sci 167 621–628 [Google Scholar]
- Tanner GJ, Francki KT, Abrahams S, Watson JM, Larkin PJ, Ashton AR (2003) Proanthocyanidin biosynthesis in plants: purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA. J Biol Chem 278 31647–31656 [DOI] [PubMed] [Google Scholar]
- Tao R, Dandekar AM, Sandra LU, Vail PV, Tebbets JS (1997) Engineering genetic resistance against insects in Japanese persimmon using the cryIA(c) gene of Bacillus thuringiensis. J Am Soc Hortic Sci 122 764–771 [Google Scholar]
- Terrier T, Torregrosa L, Ageorges A, Vialet S, Verries C, Cheynier V, Romieu C (2009) Ectopic expression of VvMybPA2 promotes proanthocyanidin biosynthesis in Vitis vinifera L. and suggests additional targets in the pathway. Plant Physiol 149 1028–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson F, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL-X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga R, Iwata M, Okada K, Wada T (2007) Functional analysis of the epidermal-specific MYB genes CAPRICE and WEREWOLF in Arabidopsis. Plant Cell 19 2264–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Yamaguchi-Shinozaki K, Urao S, Shinozaki K (1993) An Arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence. Plant Cell 5 1529–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk JC, Haring MA, Tunen AJ, Scuurink RC (2005) ODORANT1 regulates fragrance biosynthesis in petunia flowers. Plant Cell 17 1612–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Wilkins TA (1994) A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal Biochem 223 7–12 [DOI] [PubMed] [Google Scholar]
- Wei YL, Li JN, Lu J, Tang ZL, Pu DC, Chai YR (2007) Molecular cloning of Brassica napus TRANSPARENT TESTA 2 gene family encoding potential MYB regulatory proteins of proanthocyanidin biosynthesis. Mol Biol Rep 34 105–120 [DOI] [PubMed] [Google Scholar]
- Werner RA, Rossmann A, Schwarz C, Bacher A, Schmidt HL, Eisenreich W (2004) Biosynthesis of gallic acid in Rhus typhina: discrimination between alternative pathways from natural oxygen isotope abundance. Phytochemistry 65 2809–2813 [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B (2001) Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie DY, Sharma SB, Paiva NL, Ferreira D, Dixon RA (2003) Role of anthocyanidin reductase, encoded by BANYULS in plant flavonid biosynthesis. Science 299 396–399 [DOI] [PubMed] [Google Scholar]
- Xue B, Charest PJ, Devantier Y, Rutledge RG (2003) Characterization of a MYBR2R3 gene from black spruce (Picea mariana) that shares functional conservation with maize C1. Mol Genet Genomics 270 78–86 [DOI] [PubMed] [Google Scholar]
- Yamada M, Sato A (2002) Segregation for fruit astringency type in progenies derived from crosses of ‘Nishimura-wase’ × pollination constant non-astringent genotypes in Oriental persimmon (Diospyros kaki Thunb). Sci Hortic (Amsterdam) 92 107–111 [Google Scholar]
- Yonemori K, Honsho C, Kanzaki S, Ino H, Ikegami A, Kitajima A, Sugiura A, Parfitt DE (2008) Sequence analyses of the ITS regions and the mat K gene for determining phylogenetic relationships of Diospyros kaki (persimmon) with other wild Diospyros (Ebenaceae) species. Tree Genet Genomes 4 149–158 [Google Scholar]
- Yonemori K, Sugiura A, Yamada M (2000) Persimmon genetics and breeding. In J Janick, ed, Plant Breeding Reviews 19. John Wiley & Sons, New York, pp 191–225
- Yoshida K, Iwasaka R, Kaneko T, Sato S, Tabata S, Sakuta M (2008) Functional differentiation of Lotus japonicus TT2s, R2R3-MYB transcription factors comprising a multigene family. Plant Cell Physiol 49 157–169 [DOI] [PubMed] [Google Scholar]
- Yu O, McGonigle B (2005) Metabolic engineering of isoflavone biosynthesis. Adv Agron 86 147–190 [Google Scholar]
- Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF (2004) Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40 22–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.