ADPglucose (ADPGlc) is the substrate for starch synthesis in the plastids of higher plants. The glucosyl moiety is used by starch synthases to elongate the glucans that comprise starch. Recently, there has been renewed debate about the ADPGlc synthesis, with the widely accepted or classical pathway questioned and a controversial new pathway proposed. Published experimental results are not entirely consistent with either pathway. Here we focus on starch synthesis in the Arabidopsis (Arabidopsis thaliana) leaf and present our opinion in the debate.
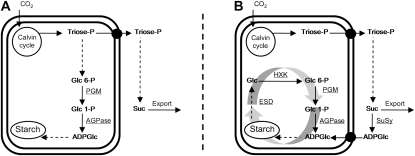
The classical pathway of starch synthesis in Arabidopsis leaves involves the chloroplastic enzymes phosphoglucomutase (PGM), which generates Glc-1-P from Glc-6-P, and ADPGlc pyrophosphorylase (AGPase), which uses Glc-1-P and ATP to generate ADPGlc and inorganic pyrophosphate (Fig. 1A). Mutations affecting either enzyme cause low-starch phenotypes, providing support for their role in starch synthesis. Low-starch mutants lacking plastidial PGM have also been reported for tobacco (Nicotiana tabacum) and pea (Pisum sativum; Hanson and McHale, 1988; Harrison et al., 1998, 2000). However, results at odds with the classical pathway include the report that ADPGlc levels are unaffected in Arabidopsis mutants lacking chloroplastic PGM and AGPase (Muñoz et al., 2005). If the classical pathway of starch synthesis were the only source of ADPGlc, a substantial reduction or an absence of ADPGlc would be expected in both mutants. Furthermore, while frequently described as starchless, several publications report that starch is measurable in pgm (ranging from 1%–3% of the corresponding wild type; Caspar et al., 1985; Kofler et al., 2000; Gibon et al., 2004; Niittylä et al., 2004). Muñoz et al. (2006) reported levels as high as 15% of the corresponding wild-type levels upon the provision of exogenous sugars (although in this experiment the absolute levels in the wild type—7.2 μmol g−1 fresh weight—were only 10%–20% of that typically seen in soil-grown plants). These data have been highlighted as support for a new pathway of starch synthesis whereby cytosolic Suc synthase (SuSy), using ADP and Suc, produces Fru and ADPGlc (Fig. 1B; see also Baroja-Fernández et al., 2004, 2005; Muñoz et al., 2005). This model proposes that ADPGlc is imported into the chloroplast for starch synthesis using a similar transport mechanism to that in the developing cereal endosperm (though in that case ADPGlc is synthesized by a cytosolic form of AGPase; see Tetlow et al., 2004, and refs. therein). If correct, this new model would force a major reconsideration of the pathways and regulation of photosynthetic carbon metabolism. An obvious inconsistency in this newly proposed pathway is the low-starch or starchless phenotypes of mutants lacking chloroplastic PGM and AGPase (pgm and adg1). These enzymes would not be required for starch synthesis as carbon exported from the chloroplast as triose-Ps would be converted to Suc (and thence to ADPGlc) in the cytosol.
Figure 1.
Suggested pathways of starch synthesis in leaves, redrawn from Muñoz et al. (2006). A, Classical model. Starch synthesis is directly linked to the Calvin cycle. All enzymatic reactions to convert triose-P to ADPGlc take place in the chloroplast. Suc synthesis proceeds in the cytosol starting with triose-P exported from the chloroplast. Starch synthesis and degradation do not occur simultaneously. B, New model. Suc is an intermediate of starch synthesis. ADPGlc is primarily synthesized by SuSy. Starch is continuously synthesized and degraded. Plastidial enzymes including PGM function to recycle Glc released by degradation back into starch synthesis. Dashed arrows indicate more than one enzymatic step. HXK, Plastidial hexokinase; ESD, enzymes of starch degradation.
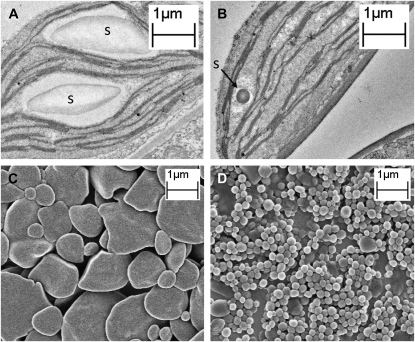
Reassessing the Arabidopsis pgm phenotype using electron microscopy reveals that most leaf mesophyll chloroplast sections did not contain starch granules, but in a few, tiny granules could be observed (Fig. 2, A and B). Tiny granules can also be purified and visualized by scanning electron microscopy (Fig. 2, C and D). Because of the size of the granules, most would be missed when making ultrathin sections. Thus, many or all pgm chloroplasts could contain such granules. The tiny granules are consistent with the very low starch contents measured in this material (0.3% of the wild type; the limit of detection in our analyses was approximately 0.03%, equivalent to 2 μg starch g−1 fresh weight; Fig. 3). The mutation in pgm is a null mutation (Caspar et al., 1985; Periappuram et al., 2000). Thus, there must be another source of either Glc-1-P or ADPGlc, either produced inside the chloroplast, or imported from the cytosol.
Figure 2.
A and B, Transmission electron micrographs of sections of wild-type (A) and pgm (B) leaf mesophyll cells harvested at the end of the day (12-h-day/12-h-night regime, 150 μmol photons m−2 s−1, 20°C constant temperature, 60% relative humidity). Tiny starch granules (s) were seen in pgm chloroplasts (as in B). The frequency of granules in pgm sections was lower than in the wild type, presumably due to the relatively small probability that a given 60- to 100-nm section would include such a tiny feature. C and D, Scanning electron micrographs of starch purified from 5-week-old wild-type (C) and pgm (D) plants. Methods can be found in the article by Streb et al. (2008).
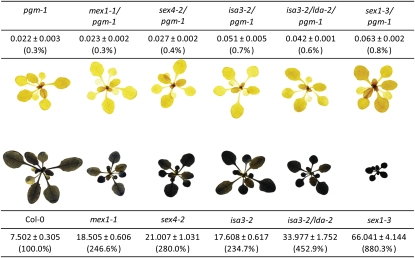
Figure 3.
Iodine staining of starch content together with quantitative measurements (in mg g−1 fresh weight, and as a % of Columbia-0 in parentheses) from four replicate plants, harvested at the end of the day. Growth conditions were as in Figure 2. Plants for iodine staining and quantitative measurements were 30 and 35 d old, respectively. Methods can be found in the article by Smith and Zeeman (2006).
A simple explanation for the low levels of starch in pgm is that the alternative source of substrate limits the rate of starch synthesis. For example, chloroplast envelope transporters for triose-Ps, phosphoenolpyruvate, or xylulose-5-P might, with very low affinity, transport Glc-1-P from the cytosol. Unlike cereal endosperm, there is no evidence for a cytosolic AGPase in Arabidopsis, but the action of SuSy in the cytosol could produce ADPGlc (Delmer, 1972). The adenylate transporter might translocate tiny amounts of ADPGlc, although analysis of the Arabidopsis Brittle1 protein (the closest homolog of the plastidial ADPGlc transporter of maize [Zea mays]) shows that it has a very low affinity for ADPglc, compared with ADP (Kirchberger et al., 2008). The leakage of substrates from the cytosol would still be consistent with the classical model of starch synthesis where the bulk of ADP-Glc is synthesized in the chloroplast. In contrast, the new model proposes that majority of ADPGlc linked to starch synthesis is imported from the cytosol. Therefore, another explanation is needed for the low-starch phenotypes of pgm and adg1. Baroja-Fernández et al. (2004) hypothesized that there is continuous turnover of starch to Glc-6-P during net starch accumulation and that chloroplastic PGM and AGPase work in a salvage capacity, recycling Glc-6-P back into starch (Fig. 1B). Glc-6-P is not frequently considered to be a product of the pathway, but could theoretically be produced by the phosphorylation of Glc if a chloroplast-localized hexokinase is present in mesophyll cells. According to this idea, the rate of starch synthesis is not altered in pgm mutants but chloroplastic recycling does not occur. This implies enormous rates of degradation and recycling in wild-type plants during periods of net starch accumulation such that when the proposed recycling does not occur, up to 99.7% of the carbon entering the starch pool is lost through concomitant degradation (as pgm mutants have 0.3% of the wild-type starch in our experiments). The new model does not explain the fate of the hypothetical starch degradation products in pgm mutants, which would be the equivalent of 30% to 50% of the total photosynthetically fixed carbon, depending on the plant growth conditions. If exported to the cytosol for reentry into the Suc pool, the carbohydrate should again be available for cytosolic ADPGlc production and starch synthesis. Thus, recycling would still occur and pgm should not have such low starch. If degradation products do not reenter the Suc pool, it should be possible to discover their fate, as such large carbohydrate fluxes can easily be measured.
If constant starch degradation and recycling occurs during periods of net starch accumulation, limiting starch degradation in the pgm background should cause a substantial increase in starch content. Current models indicate that the major starch degradation products are maltose and, to a lesser extent, Glc (for review, see Zeeman et al., 2007). Both sugars can diffuse out of the chloroplast for metabolism in the cytosol (Weber et al., 2000; Niittylä et al., 2004). Several mutants with reduced rates of starch breakdown have been isolated (Zeeman et al., 2007). These mutants accumulate excess starch in their leaves (a so-called sex phenotype) and, in some cases, intermediates of starch breakdown. For example, mex1 cannot export maltose, which then accumulates inside the chloroplast during the night (Niittylä et al., 2004; Lu et al., 2006). The sex4 mutant cannot dephosphorylate phosphoglucan intermediates of degradation, and phosphooligosaccharides accumulate during the night (Kötting et al., 2009). Introduction of these mutations into the pgm background does not increase starch content as would be expected if extensive starch turnover were occurring via the actions of these proteins in the pgm mutant. Furthermore, degradation intermediates do not accumulate (Niittylä et al., 2004; Kötting et al., 2009). We have also introduced into the pgm background mutations that block other stages in the starch degradation pathway. Mutants deficient in glucan-hydrolyzing enzymes that debranch amylopectin (isoamylase 3 and limit dextrinase; Delatte et al., 2006) and mutants deficient in glucan, water dikinase, required for the initiation of starch breakdown (the sex1 mutant; Yu et al., 2001), have strong sex phenotypes. However, in all double and multiple mutants, starch increases only marginally. Substantial increases would be expected if extensive starch turnover were occurring via the actions of these enzymes in the pgm mutant. Thus, pgm is epistatic over the mutations affecting starch degradation rather than vice versa (Fig. 3).
Our current view is that the classical pathway of ADPGlc production operates in Arabidopsis and that PGM is needed for primary synthesis of starch. Based on the evidence at hand, we consider it unlikely that there is extensive recycling of starch degradation products during the day. The presence of starch and ADPGlc in pgm is intriguing, and worthy of further investigation. The increased synthesis of Suc caused by an inability to make starch via the plastidial pathway could increase the amount of ADPGlc made by SuSy in the cytosol, explaining the levels observed in pgm. Some of this may leak into the plastid and explain the very low starch phenotype observed. Nonaqueous fractionation techniques to determine subcellular metabolite concentrations could be applied to wild-type and pgm plants to address this possibility directly. However, a recent study in which SuSy isoforms were systematically removed from Arabidopsis leaves showed that when almost all SuSy activity was eliminated, ADP-Glc levels and starch synthesis were similar to the wild type (Barratt et al., 2009). These data argue against SuSy as the primary source of ADPGlc for starch synthesis under normal circumstances.
Two important factors are highlighted by this debate. First, metabolite levels cannot be equated with metabolic fluxes. In this case, either the reported ADPGlc level in pgm is not a good indicator of the flux into starch synthesis (i.e. the classical model) or the starch level itself in pgm is not a good indicator of the flux into starch synthesis (i.e. the new model). Second, data obtained using disparate plant systems and methodologies, while significant to this debate, are conflicting (see Muñoz et al., 2005, 2006; Neuhaus et al., 2005). Such studies cannot always be directly compared and should not be used to derive a consensus view. Differences in metabolic configurations may exist between species and plant organs. Thus, it is necessary to resolve this question unambiguously for a single species (e.g. Arabidopsis leaves), then assess the generality. The fact that low-starch pgm mutants also occur in solanaceous and leguminous plants (Hanson and McHale, 1988; Harrison et al., 1998, 2000) suggests to us that the pathway of starch synthesis in leaves is similar among dicotyledonous plants.
This work was supported by ETH Zurich and the Swiss National Science Foundation (grant no. 3100AO–116434/1), and through the National Centre for Competence in Research—Plant Survival.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Samuel C. Zeeman (szeeman@ethz.ch).
References
- Baroja-Fernández E, Muñoz FJ, Pozueta-Romero J (2005) Response to Neuhaus et al.: no need to shift the paradigm on the metabolic pathway to transitory starch in leaves. Trends Plant Sci 10 156–158 [DOI] [PubMed] [Google Scholar]
- Baroja-Fernández E, Muñoz FJ, Zandueta-Criado A, Morán-Zorzano MT, Viale AM, Alonso-Casajús N, Pozueta-Romero J (2004) Most of ADP-glucose linked to starch biosynthesis occurs outside the chloroplast in source leaves. Proc Natl Acad Sci USA 101 13080–13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt DHP, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, Feil R, Simpson C, Maule AJ, Smith AM (2009) Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc Natl Acad Sci USA 106 13124–13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte T, Umhang M, Trevisan M, Eicke S, Thorneycroft D, Smith SM, Zeeman SC (2006) Evidence for distinct mechanisms of starch granule breakdown in plants. J Biol Chem 281 12050–12059 [DOI] [PubMed] [Google Scholar]
- Delmer DP (1972) Purification and properties of sucrose synthetase from etiolated phaseouls aureus seedlings. J Biol Chem 247 3822–3828 [PubMed] [Google Scholar]
- Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günther M, Stitt M (2004) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39 847–862 [DOI] [PubMed] [Google Scholar]
- Hanson KR, McHale NA (1988) A starchless mutant of Nicotiana sylvestris containing a modified plastid phosphoglucomutase. Plant Physiol 88 838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Hedley CL, Wang TL (1998) Evidence that the rug3 locus of pea (Pisum sativum L.) encodes plastidial phosphoglucomutase confirms that the imported substrate for starch synthesis in pea amyloplasts is glucose-6-phosphate. Plant J 13 753–762 [Google Scholar]
- Harrison CJ, Mould RM, Leech MJ, Johnson SA, Turner L, Schreck SL, Baird KM, Jack PL, Rawsthorne S, Hedley CL, et al (2000) The rug3 locus of pea encodes plastidial phosphoglucomutase. Plant Physiol 122 1187–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger S, Tjaden J, Neuhaus HE (2008) Characterization of the Arabidopsis Brittle1 transport protein and impact of reduced activity on plant metabolism. Plant J 56 51–63 [DOI] [PubMed] [Google Scholar]
- Kofler H, Häusler RE, Schulz B, Gröner F, Flügge UI, Weber A (2000) Molecular characterisation of a new mutant allele of the plastid phosphoglucomutase in Arabidopsis and complementation of the mutant with the wild-type cDNA. Mol Gen Genet 263 978–986 [DOI] [PubMed] [Google Scholar]
- Kötting O, Santelia D, Edner C, Eicke S, Marthaler T, Comparot-Moss S, Chen J, Gentry M, Ritte G, Steup M, et al (2009) SEX4, a glucan phosphatase, dephosphorylates amylopectin at the granule surface during starch breakdown in Arabidopsis leaves. Plant Cell 21 334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Steichen JM, Weise SE, Sharkey TD (2006) Cellular and organ level localization of maltose in maltose-excess Arabidopsis mutants. Planta 224 935–943 [DOI] [PubMed] [Google Scholar]
- Muñoz FJ, Baroja-Fernández E, Morán-Zorzano MT, Viale AM, Etxeberria E, Alonso-Casajús N, Pozueta-Romero J (2005) Sucrose synthase controls both intracellular ADP glucose levels and transitory starch biosynthesis in source leaves. Plant Cell Physiol 46 1366–1376 [DOI] [PubMed] [Google Scholar]
- Muñoz FJ, Zorzano MTM, Alonso-Casajus N, Baroja-Fernandez E, Etxeberria E, Pozueta-Romero J (2006) New enzymes, new pathways and an alternative view on starch biosynthesis in both photosynthetic and heterotrophic tissues of plants. Biocatalysis Biotransform 24 63–76 [Google Scholar]
- Neuhaus HE, Häusler RE, Sonnewald U (2005) No need to shift the paradigm on the metabolic pathway to transitory starch in leaves. Trends Plant Sci 10 154–156 [DOI] [PubMed] [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303 87–89 [DOI] [PubMed] [Google Scholar]
- Periappuram C, Steinhauer L, Barton DL, Taylor DC, Chatson B, Zou J (2000) The plastidic phosphoglucomutase from Arabidopsis: a reversible enzyme reaction with an important role in metabolic control. Plant Physiol 122 1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC (2006) Quantification of starch in plant tissues. Nat Protoc 1 1342–1345 [DOI] [PubMed] [Google Scholar]
- Streb S, Delatte T, Umhang M, Eicke S, Schoderet M, Trevisan M, Reinhard D, Zeeman SC (2008) Starch granule biosynthesis in Arabidopsis is abolished by removal of all debranching enzymes, but restored by the subsequent removal of an endoamylase. Plant Cell 20 3448–3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow IJ, Morell MK, Emes MJ (2004) Recent developments in understanding the regulation of starch metabolism in higher plants. J Exp Bot 55 2131–2145 [DOI] [PubMed] [Google Scholar]
- Weber A, Servaites JC, Geiger DR, Kofler H, Hille D, Gröner F, Hebbeker U, Flügge UI (2000) Identification, purification, and molecular cloning of a putative plastidic glucose translocator. Plant Cell 12 787–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Kofler H, Häusler RE, Hille D, Flügge UI, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, et al (2001) SEX1 is a general regulator of starch degradation in plants and not the chloroplast hexose transporter. Plant Cell 13 1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM (2007) The diurnal metabolism of leaf starch. Biochem J 401 13–28 [DOI] [PubMed] [Google Scholar]