Abstract
The phytohormone abscisic acid (ABA) is known to be a negative regulator of legume root nodule formation. By screening Lotus japonicus seedlings for survival on an agar medium containing 70 μm ABA, we obtained mutants that not only showed increased root nodule number but also enhanced nitrogen fixation. The mutant was designated enhanced nitrogen fixation1 (enf1) and was confirmed to be monogenic and incompletely dominant. The low sensitivity to ABA phenotype was thought to result from either a decrease in the concentration of the plant's endogenous ABA or from a disruption in ABA signaling. We determined that the endogenous ABA concentration of enf1 was lower than that of wild-type seedlings, and furthermore, when wild-type plants were treated with abamine, a specific inhibitor of 9-cis-epoxycarotenoid dioxygenase, which results in reduced ABA content, the nitrogen fixation activity of abamine-treated plants was elevated to the same levels as enf1. We also determined that production of nitric oxide in enf1 nodules was decreased. We conclude that endogenous ABA concentration not only regulates nodulation but also nitrogen fixation activity by decreasing nitric oxide production in nodules.
Many legumes establish nitrogen-fixing root nodules following reciprocal signal exchange between the plant and rhizobia (Hayashi et al., 2000; Hirsch et al., 2003). The host plant produces chemical compounds, frequently flavonoids, which induce rhizobial nod genes, whose products are involved in the synthesis and secretion of Nod factor. Perception of this chitolipooligosaccharide by the host plant results in the triggering of a signal transduction cascade that leads to root hair deformation and curling and subsequent cortical cell divisions, which establish the nodule primordium. The rhizobia enter the curled root hair cell and nodule primordial cells through an infection thread. Eventually, the rhizobia are released into nodule cells, enclosed within a membrane, and differentiate into nitrogen-fixing bacteroids that reduce atmospheric nitrogen into ammonia. In return, the host plant supplies photosynthetic products, to be used as carbon sources, to the rhizobia (Zuanazzi et al., 1998; Hayashi et al., 2000).
The host plant is known to be important for regulating the number of nodules established on its roots. For example, hypernodulating mutants such as nitrate-tolerant symbiotic1 (nts1; Glycine max), hypernodulation aberrant root formation1 (har1; Lotus japonicus), super numeric nodules (sunn; Medicago truncatula), and symbiosis29 (sym29; Pisum sativum) disrupt the balance between supply and demand by developing excessive root nodules (Oka-Kira and Kawaguchi, 2006). Grafting experiments demonstrated that leaf tissue is a principal source of the systemic signals contributing to the autoregulation of nodulation (Pierce and Bauer, 1983; Kosslak and Bohlool, 1984; Krusell et al., 2002; Nishimura et al., 2002b; van Brussel et al., 2002; Searle et al., 2003; Schnabel et al., 2005). The Nts1, Har1, Sunn, and Sym29 genes encode a receptor-like kinase similar to CLAVATA1, which regulates meristem cell number and differentiation (Krusell et al., 2002; Nishimura et al., 2002a; Searle et al., 2003; Schnabel et al., 2005).
Phytohormones are also known to regulate nodulation (Hirsch and Fang, 1994). For example, ethylene is a well-known negative regulator of nodulation, influencing the earliest stages from the perception of Nod factor to the growth of infection threads (Nukui et al., 2000; Oldroyd et al., 2001; Ma et al., 2003). The ethylene-insensitive mutant sickle1 (skl1) of M. truncatula has a hypernodulating phenotype (Penmetsa and Cook, 1997). Skl1 is homologous to Ethylene insensitive2 of Arabidopsis (Arabidopsis thaliana), which is part of the ethylene-signaling pathway (Alonso et al., 1999; Penmetsa et al., 2008). In contrast, cytokinin is a positive regulator of nodulation. The cytokinin-insensitive mutant hyperinfected1 (loss of function) of L. japonicus and the spontaneous nodule formation2 (gain of function) mutants of M. truncatula provide genetic evidence demonstrating that cytokinin plays a critical role in the activation of nodule primordia (Gonzalez-Rizzo et al., 2006; Murray et al., 2007; Tirichine et al., 2007).
Abscisic acid (ABA), added at concentrations that do not affect plant growth, also negatively regulates nodulation in some legumes (Phillips, 1971; Cho and Harper, 1993; Bano et al., 2002; Bano and Harper, 2002; Suzuki et al., 2004; Nakatsukasa-Akune et al., 2005; Liang et al., 2007). Recently, M. truncatula overexpressing abscisic acid insensitive1-1, a gene that encodes a mutated protein phosphatase of the type IIC class derived from Arabidopsis and that suppresses the ABA-signaling pathway (Leung et al., 1994; Hagenbeek et al., 2000; Gampala et al., 2001; Wu et al., 2003), was shown to exhibit ABA insensitivity as well as a hypernodulating phenotype (Ding et al., 2008).
In this study, we isolated a L. japonicus (Miyakojima MG20) mutant that showed an increased root nodule phenotype and proceeded to carry out its characterization. This mutant, named enhanced nitrogen fixation1 (enf1), exhibits enhanced symbiotic nitrogen fixation activity. Most legume nitrogen fixation activity mutants, such as ineffective greenish nodules1 (ign1), stationary endosymbiont nodule1, and symbiotic sulfate transporter1 (sst1), are Fix− (Suganuma et al., 2003; Krusell et al., 2005; Kumagai et al., 2007).
RESULTS
Isolation of the enf1 Mutant
To obtain ABA-insensitive or low-sensitive mutants of L. japonicus, we treated Miyakojima MG20 with ethyl methanesulfonate to induce base substitutions randomly in the genome. M3 seeds were sown on an agar-solidified medium containing 70 μm ABA, a concentration that inhibits the germination of wild-type MG20 seeds. M4 plants obtained by the screening were inoculated with rhizobia (Mesorhizobium loti MAFF303099), and the number of nodules per plant was counted 35 d after inoculation (DAI). Mutant No. 12 not only formed more root nodules than did the wild-type MG20 plants but, surprisingly, also exhibited increased nitrogen fixation activity per plant. Both mutant phenotypes were stably inherited in the M4 and M5 generations. Back-crossing mutant No. 12 to wild-type MG20 yielded 153 F2 progeny, from which a line that showed the highest nitrogen fixation activity and numerous nodules per plant was derived. This line was designated enf1.
Symbiotic enf1 Phenotypes
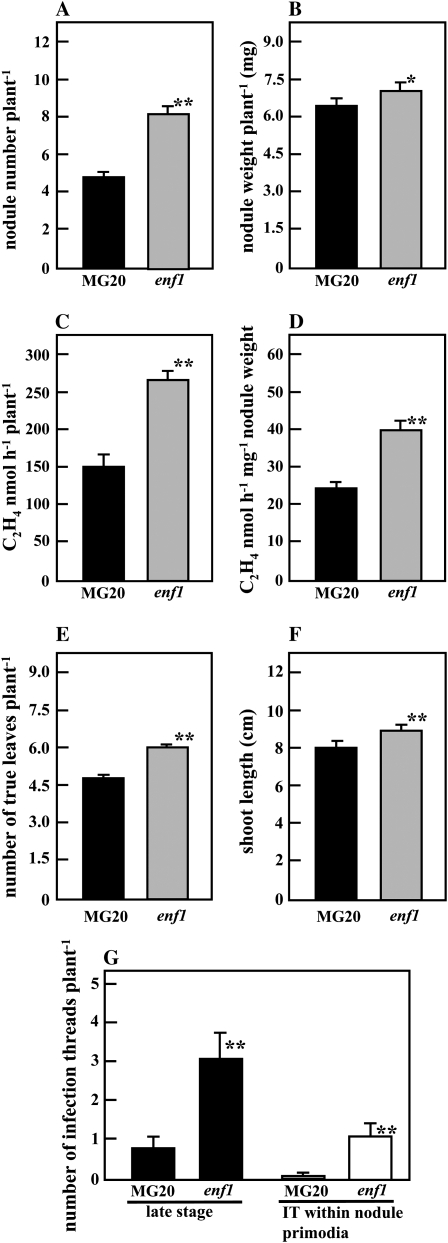
Figure 1 illustrates the nodulation phenotypes of MG20 and enf1 L. japonicus at 28 DAI. The number of nodules formed on enf1 roots was approximately 1.7 times greater than that of MG20; concomitantly, the weight of nodules per enf1 plant also increased slightly (Figs. 1 and 2, A and B). In general, hypernodulating mutants tend to form smaller nodules than wild-type plants (Wopereis et al., 2000), and this is also the case for the enf1 mutant. Surprisingly, however, the nitrogen fixation activity per enf1 plant was elevated 1.8 times over that of the wild-type plants (Fig. 2C). Moreover, based on the fact that nitrogen fixation activity per unit of enf1 nodule weight was also increased 1.7 times (Fig. 2D), we concluded that the increased nitrogen fixation activity was not solely due to the enhanced number of root nodules.
Figure 1.
Symbiotic and growth phenotypes of the L. japonicus enf1 mutant. M. loti-inoculated plants were grown in pots containing vermiculite, which was watered with B&D medium. Wild-type MG20 (left) and enf1 (right) L. japonicus plants 28 DAI are shown. Bar = 1 cm.
Figure 2.
Analysis of enf1 symbiotic phenotypes. M. loti-inoculated plants were grown for 28 d in pots containing vermiculite and watered with B&D medium (A–F) or grown on B&D agar-solidified (1.5%, w/v) medium for 12 d (G). The plants on the agar plates were inoculated with M. loti NZP2037 carrying plasmid pHC60 (G). A, Nodule number per plant. B, Nodule weight per plant. C and D, Acetylene reduction activity per plant (C) and per nodule weight (D). E, Number of true leaves per plant. F, Shoot length. G, The number of infection threads (IT) per plant at 12 DAI. Black bars indicate MG20, and gray bars indicate enf1 (A –F). In G, black bars indicate later stages of infection thread formation, whereas white bars refer to the initiation of nodule formation. At least 40 plants (A–F) and 17 to 20 plants (G) were examined in each experiment. Error bars represent se. Statistical significance is indicated by asterisks (* P < 0.05, ** P < 0.01, by Student's t test).
ABA is known to inhibit the early stages of nodulation. Therefore, we investigated infection thread formation in enf1 from 4 to 12 DAI. Although infection pockets were detected (4 DAI) earlier in MG20 root hairs compared with the enf1 mutant (data not shown), elongated infection threads were more common in enf1 root hairs at later stages of development (8–12 DAI; black bars in Fig. 2G). Furthermore, infection threads were detected in nodule primordia more frequently in enf1 compared with MG20 (white bars in Fig. 2G).
Nonsymbiotic enf1 Phenotypes
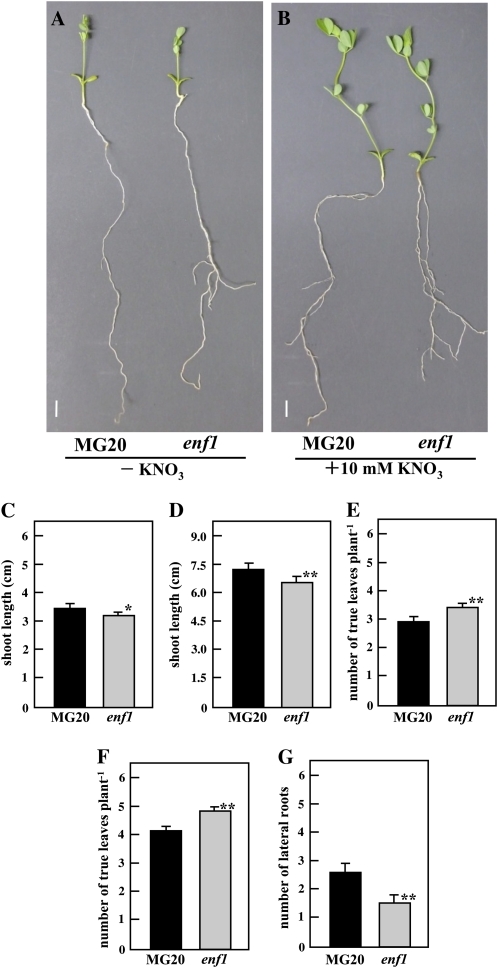
In addition to the symbiotic phenotypes just described, enf1 had more true leaves and exhibited greater shoot length at 28 DAI than the MG20 plants (Fig. 2, E and F). To confirm that enf1's vigorous growth was due to an increase in nitrogen supplied from nodules, MG20 and enf1 plants without rhizobial inoculation were grown in the presence (10 mm KNO3) or absence of nitrogen. Figure 3, A and B, illustrates data derived from MG20 and enf1 plants 21 d after sowing (DAS). Surprisingly, under conditions of nitrogen sufficiency or nitrogen inefficiency in the absence of rhizobia, enf1 shoot length was statistically lower than that of MG20 (Fig. 3, C and D), a result completely opposite to that observed for plants inoculated with M. loti (Fig. 2F). These results indicate that rhizobial inoculation of the enf1 mutant has a greater effect on the shoot growth than it does on wild-type plant.
Figure 3.
Nonsymbiotic phenotypes of the enf1 mutant. A to F, Plants were grown for 21 d in vermiculite-filled pots supplied with B&D medium with or without 10 mm KNO3. G, Plants were grown on B&D agar-solidified (1.5%, w/v) medium. A, MG20 (left) and enf1 (right) grown with no nitrogen. B, MG20 (left) and enf1 (right) grown with 10 mm KNO3. C and D, Shoot length resulting from growth without KNO3 (C) or with 10 mm KNO3 (D). E and F, Number of true leaves per plant in medium without KNO3 (E) or with 10 mm KNO3 (F). G, Number of lateral roots per plant at 24 DAS. At least 30 plants were used in each experiment. Error bars indicate se, and statistical significance is indicated by asterisks (* P < 0.05, ** P < 0.01, by Student's t test).
The number of true leaves in enf1 plants was also increased (usually by one) compared with MG20 plants under both (+)nitrogen and (–)nitrogen conditions (Fig. 3, E and F), which was similar to the result observed for inoculated plants (Fig. 2E).
Because ABA is known to affect lateral root formation in L. japonicus (Suzuki et al., 2004), the number of lateral roots of 24-d-old (24 DAS) MG20 and enf1 were counted. Following inoculation with M loti, only a few lateral roots were observed, so that a significant difference between MG20 and enf1 could not be detected (data not shown). However, the number of lateral roots developed on uninoculated enf1 was significantly decreased compared with MG20 without M. loti (Fig. 3G).
Low Sensitivity of enf1 to ABA
Because enf1 was isolated as an ABA low-sensitivity mutant, we tested seed germination on an agar medium containing 70 μm ABA. Two independent experiments of 40 seeds each demonstrated that enf1 seeds germinated better than the wild-type seeds (MG20 average = 6.25%, enf1 average = 17.5%). No difference was observed in seed germination between MG20 and enf1 in agar medium without ABA (data not shown).
We next examined whether ABA had any toxicity effects on the growth of MG20 and enf1. When M. loti-inoculated MG20 plants were grown in the presence of 0.5 μm ABA for 17 d, most of the young leaves at the shoot tip turned brown and shriveled (78.7%; 48 of 61 plants). In contrast, although some yellowing of enf1 leaves occurred, the shoot tip showed very little dieback (9.6%; five in 52 plants; Fig. 4). These results strongly suggest that enf1 is not as sensitive to exogenous ABA as is the wild-type MG20.
Figure 4.
Effects of 0.5 μm ABA on the growth of enf1. A, MG20 at 17 DAI with M. loti. B, enf1 at 17 DAI with M. loti. Shown are untreated MG20 and enf1 (left) and treated with 0.5 μm ABA (right). C and D, Shoot tips of MG20 (C) and enf1 (D) following 0.5 μm ABA treatment. Bars = 1 cm.
Low Endogenous Concentration of ABA in enf1
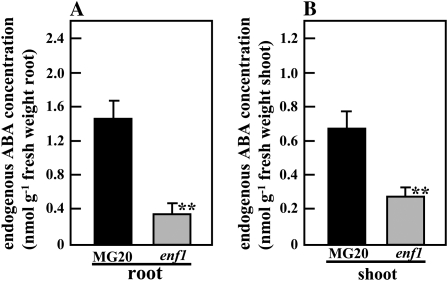
We measured the endogenous ABA concentration of enf1 at 21 DAI and found that it was four or 2.5 times lower in nodulated roots and shoots, respectively, compared with wild-type nodulated roots and shoots (Fig. 5).
Figure 5.
Endogenous concentration of ABA in enf1. M. loti-inoculated plants were grown for 21 d on vermiculite-filled pots supplied with B&D medium. Endogenous concentrations of ABA in MG20 and enf1 roots (A) and shoots (B) are shown. The data represent averages ± se of six independent experiments derived from eight different plants. Statistical significance is indicated by asterisks (** P < 0.01, by Student's t test).
Analysis of enf1 in Long-Term Growth Experiments with M. loti
We found little difference between the enf1 mutant and wild-type L. japonicus in terms of seed set rate, pod length, and the number of seeds per pod at 98 DAI (Table I). However, the fresh and dry weights of 100 seeds of enf1 were significantly larger than the comparable number of wild-type seeds, suggesting that enf1 may result in increased seed yield (Table I). Because the nitrogen fixation activity per enf1 plant was elevated 1.8 times over that of the wild-type plants, we measured seed nitrogen content. As shown in Table I, the nitrogen content per dry weight of enf1 seeds was significantly larger than that of MG20. Based on seed dry weight, the total nitrogen content per seeds was calculated to be increased more than 18%.
Table I.
Growth parameters of wild-type (MG20) and enf1 L. japonicus
Indicated parameters of MG20 and enf1 were analyzed at 98 DAI. The data represent averages ± se of 28 (MG20) or 33 (enf1) individual plants, except for total nitrogen of seeds. The data of total nitrogen of seeds represent averages ± se of six independent experiments derived from 50 different seeds. Statistical significance is indicated by asterisks (** P < 0.01, by Student's t test).
| Parameter | MG20 | enf1 |
|---|---|---|
| No. of flowers per plant | 11.4 ± 0.6 | 10.7 ± 0.6 |
| No. of pods per plant | 6.7 ± 0.3 | 6.5 ± 0.4 |
| Seed set (%) | 65.7 ± 2.6 | 69.2 ± 3.0 |
| Pod length (mm) | 30.1 ± 0.4 | 30.6 ± 0.4 |
| No. of seeds per pod | 21.4 ± 0.5 | 22.7 ± 0.5 |
| Fresh weight of 100 seeds (mg) | 116.8 ± 2.4 | 130.4 ± 1.4** |
| Dry weight of 100 seeds (mg) | 108.8 ± 2.2 | 122.2 ± 1.3** |
| Nitrogen content of seeds (μg mg−1 dry weight) | 58.4 ± 0.7 | 61.8 ± 0.7** |
Growth at 72 DAS of enf1 mutant and wild-type plants in field soil-filled pots was also analyzed. Although no M. loti inoculum was added, root nodules were formed by indigenous rhizobia on all plants tested. As shown in Table II, the number of root nodules, their dry weight, the dry weight of entire plants including pods and seeds, as well as total nitrogen content of the enf1 mutant were significantly larger than the comparable parameters for MG20. These results suggest that a larger amount of nitrogen is fixed in the enf1 mutant than in wild-type plants.
Table II.
Growth and symbiotic parameters of wild type (MG20) and enf1 L. japonicus
Uninoculated plants were grown for 72 d using field soil-filled pots supplied with water in the Saga University greenhouse (average temperature was 24.7°C). Nodules were formed by indigenous rhizobia on the roots of all plants tested. For total nitrogen content, entire plants, including pods and seeds, were analyzed. The data represent averages ± se of five individual plants. Statistical significance is indicated by asterisks (* P < 0.05, by Student's t test).
| Parameter | MG20 | enf1 |
|---|---|---|
| No. of root nodules per plant | 155.0 ± 14.3 | 253.4 ± 30.9* |
| Dry weight of nodules (mg plant−1) | 29.9 ± 4.9 | 55.2 ± 6.9* |
| Dry weight of entire plant (mg plant−1) | 3,640.9 ± 213.0 | 4,310.8 ± 192.2* |
| Total nitrogen content (mg plant−1) | 85.2 ± 2.6 | 108.5 ± 8.9* |
We also determined in long-term growth experiments that M. loti-inoculated enf1 plants flowered significantly later than the wild-type plants: 54.4 ± 1.1 versus 62.1 ± 0.8 DAI (Table III). The flowering time of enf1 plants grown in 10 mm KNO3 without M. loti inoculation was also investigated. Under these conditions, enf1 showed a significantly earlier flowering time compared with wild-type plants (i.e. 50.9 ± 1.4 versus 58.6 ± 1.7 DAS; Table III).
Table III.
Flowering time of MG20 and enf1
In long-term growth experiments, flowering time was investigated. The data represent averages ± se. Statistical significance is indicated by asterisks (** P < 0.01, by Student's t test).
| Flowering Time | |
|---|---|
| −KNO3 with M. loti | |
| MG20 (n = 33) | 54.4 ± 1.1a |
| enf1 (n = 36) | 62.1 ± 0.8a ** |
| +KNO3 without M. loti | |
| MG20 (n = 36) | 58.6 ± 1.7b |
| enf1 (n = 37) | 50.9 ± 1.4b ** |
DAI.
DAS.
enf1 Is Monogenic and Shows Incomplete Dominance
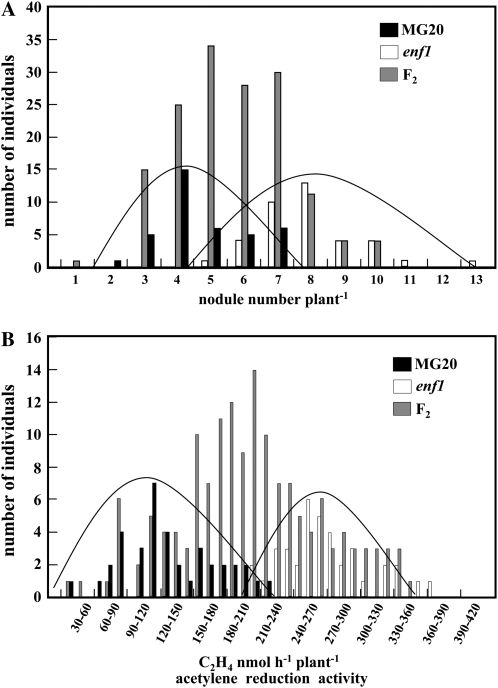
To confirm that the described phenotypes were due to a mutation in a single gene, a segregation analysis of 152 F2 progeny derived from the back-crossed MG20 and No. 12 L. japonicus was carried out using a histogram analysis of root nodule number and nitrogen fixation activity. The mean value of the F1 progeny was found to be in the middle range. Similarly, the mean value of the F2 group was also in the middle of the averages of MG20 and enf1 (data not shown). These results suggested that enf1 was incompletely dominant, but it was difficult to distinguish a segregation ratio of 1:2:1 between enf1 and MG20 because the differences observed were smaller than those shown by other symbiotic mutants (Nishimura et al., 2002a; Suganuma et al., 2003; Krusell et al., 2005; Oka-Kira et al., 2005; Kumagai et al., 2007).
To determine an accurate segregation ratio, we created histograms for root nodule number and nitrogen fixation activity (Fig. 6). Thirty-eight MG20 plants and the same number of enf1 plants were examined, because if the mutation is the result of incomplete dominance, the expected number of the MG20 type or of the enf1 type in an F2 progeny consisting of 152 plants should be 38. First, the number of overlaps between the F2 progeny and enf1 or MG20 types was determined. This number was defined as the experimental value (Table IV). The experimental value of the intermediate type was obtained by subtracting the experimental value of the MG20 and enf1 types from the number of F2 progeny (e.g. for root nodule number, 152 − [37 + 34] = 81). Thus, the segregation ratio based on nodule number for the different types was MG20:intermediate:enf1 = 37:81:34 (χ2 = 0.78; Table IV, top). For nitrogen fixation activity, the segregation ratio was MG20:intermediate:enf1 = 33:84:35 (χ2 = 1.74; Table IV, bottom). These results closely fit the expected value of 1:2:1; thus, we conclude that the mutation resulting in enf1 is monogenic and incompletely dominant.
Figure 6.
Histograms of MG20, enf1, and F2 progeny. Histograms of nodule number per plant (A) and of acetylene reduction activity per plant (B) are shown. Black bars indicate MG20, white bars indicate enf1, and gray bars indicate F2 progeny.
Table IV.
Genetic analysis of F2 progeny of MG20 and enf1 L. japonicus
Segregation analyses were carried out using histograms for nodule number and nitrogen fixation activity. The χ2 tests were carried out using experimental values (segregation ratio) based on nodule number and nitrogen fixation activity.
| Experimental Value | Expected Value | χ2 | P | |
|---|---|---|---|---|
| Root nodule no. | ||||
| MG20 type | 37 | 38 | ||
| enf1 type | 34 | 38 | 0.78 | 0.68 |
| MG20 × enf1 intermediate | 81 | 76 | ||
| Nitrogen fixation activity | ||||
| MG20 type | 33 | 38 | ||
| enf1 type | 35 | 38 | 1.74 | 0.42 |
| MG20 × enf1 intermediate | 84 | 76 |
Effect of Endogenous ABA on Nitrogen Fixation Activity
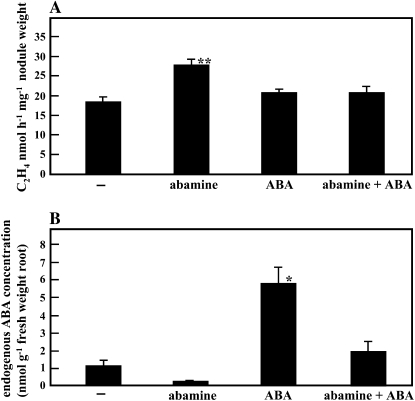
Because enf1 had a low endogenous ABA concentration, we hypothesized that the decrease in ABA concentration caused the elevation of nitrogen fixation activity. To test this hypothesis, we treated wild-type plants at 28 DAI with 20 μm abamine, a specific inhibitor of ABA synthesis (Han et al., 2004). After a 3-d treatment period, acetylene reduction activity was measured. Such short treatment periods of abamine are not expected to induce new nodule development. In MG20 roots treated with abamine, the endogenous concentration of ABA decreased to about one-fourth of that of control plants, and at the same time, the acetylene reduction activity per nodule weight was significantly up-regulated (Fig. 7). These results strongly suggest that the decrease in endogenous ABA concentration in enf1 was responsible for the increased levels of nitrogen fixation activity. On the other hand, plants treated with 0.5 μm ABA or with both ABA and abamine showed no difference in nitrogen fixation activity from the control plants.
Figure 7.
Effects of ABA concentration on nitrogen fixation activity. M. loti-inoculated plants were grown for 21 d on vermiculite-filled pots supplied with B&D medium. Plant roots at 28 DAI were treated with 0.5 μm ABA, 20 μm abamine, both ABA and abamine, or were untreated (B&D medium control) for 3 d. A, Acetylene reduction activity per nodule weight. B, ABA concentration in root. At least 15 plants were used in acetylene reduction assay. Four different plants were used for measurement of ABA concentration, and three repeats were performed. Error bars indicate the se, and the significance of differences between untreated control and treated values was determined by the two-tailed multiple t test with Bonferroni correction following ANOVA (three comparisons in four groups): * P < 0.05, ** P < 0.01.
Expression Analysis of Nitrogen Fixation Activity-Related Genes
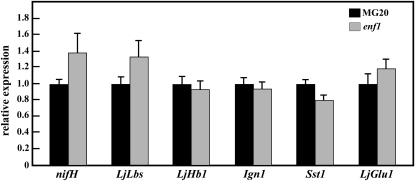
To investigate the mechanism whereby nitrogen fixation activity was elevated in enf1, we analyzed the relative expression of several already identified nitrogen fixation activity-related genes in enf1-derived nodules at 28 DAI. We found no significant difference in the amount of relative expression of either LjLbs, the three class 2 hemoglobin (Hb) genes of L. japonicus (Nagata et al., 2008), or nifH, a rhizobial gene encoding nitrogenase (Shimoda et al., 2009), compared with wild-type plants (Fig. 8). Moreover, the amount of relative expression of other genes including LjHb1, one of the class 1 Hb genes of L. japonicus (Uchiumi et al., 2002; Shimoda et al., 2009), of Ign1, a gene encoding a protein that consists of ankyrin repeats with transmembrane regions (Kumagai et al., 2007), of Sst1, a gene encoding the nodule-specific sulfuric acid transporter (Krusell et al., 2005), and of LjGlu1, a gene coding for β-1,3-glucanase (Suzuki et al., 2008), was not significantly different from that observed in wild-type nodules (Fig. 8).
Figure 8.
Relative amounts of nitrogen fixation-related gene transcripts in enf1 nodules at 28 DAI. Transcript amounts of nifH, LjLbs, LjHb, Ign1, Sst1, and LjGlu1 were normalized against sigA (used as an internal control for nifH) and ATP synthase (internal control for LjLbs, LjHb, Ign1, Sst1, and LjGlu1) transcripts. The mean value of expression in wild-type plants was set to 1. The data represent averages ± se of three independent experiments of nodules derived from five different plants.
Production of Nitric Oxide in enf1 Nodules
In plants, nitric oxide (NO) is a key component of the ABA signaling pathway (García-Mata and Lamattina, 2002; Neill et al., 2002). NO serves as a signal molecule that induces plant defense, whereas symbiotic rhizobia induce transient NO production in the roots of host plants (Durner et al., 1998; Dordas et al., 2003; Nagata et al., 2008). Furthermore, inhibition of nitrogenase activity by NO production in nodules results in decreased nitrogen fixation activity (Trinchant and Rigaud, 1982). Recently, L. japonicus overexpressing LjHb1, a gene that encodes a class 1 Hb that exhibits an extremely high affinity for oxygen, demonstrated enhanced nitrogen fixation activity by decreasing NO production (Shimoda et al., 2009).
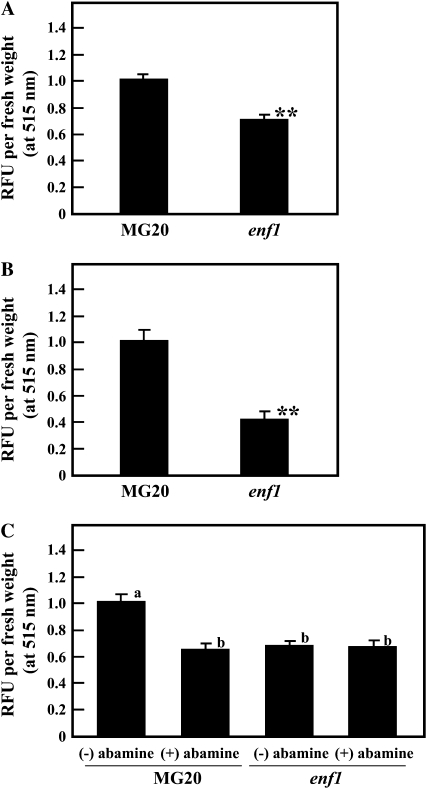
NO production in root nodules formed by enf1 at 21 and 28 DAI was examined using the fluorescent dye diaminofluorescein-FM (DAF-FM), a NO-specific detector, and relative fluorescence unit (RFU) values were estimated. The RFU values of enf1 nodules at 21 DAI were clearly decreased compared with that of MG20; this trend was more obvious at 28 DAI (Fig. 9, A and B). Moreover, the effect of reduced ABA concentration caused by treatment with abamine on NO production was analyzed. When nodules formed on the roots of 28-d-old plants were treated, the RFU value of the enf1 mutant was almost the same for (−)abamine and (+)abamine-treated plants, whereas the RFU value of abamine-treated MG20 plants was significantly reduced compared with nontreated MG20 (Fig. 9C). These results strongly suggest that decreased production of NO caused by the low concentration of ABA in enf1 nodules was responsible for the increase in nitrogen fixation activity.
Figure 9.
NO production in nodules. A and B, Quantification of NO produced in nodules of MG20 and enf1 at 21 DAI (A) and 28 DAI (B). C, Quantification of NO in nodules that were treated with abamine. Nodules on the root of 28-d-old plants were treated with 20 μm abamine for 3 d. RFU values per nodule fresh weight at 515 nm, normalized against MG20 plants, are shown. The data represent averages ± se of three independent experiments derived from nodules of six to eight plants. In A and B, statistical significance is indicated by asterisks (** P < 0.01, by Student's t test). In C, the significance of differences among four groups was determined by the two-tailed multiple t test with Bonferroni correction following ANOVA (six comparisons in four groups), and the different letters refer to significant differences at P < 0.01.
DISCUSSION
In this study, we isolated a mutant designated enf1 that exhibited decreased sensitivity to exogenous ABA and that was also reduced in endogenous ABA concentration. We found that the enf1 mutant not only produced more root nodules but also showed elevated nitrogen fixation activity.
In our earlier work, we provided evidence showing that ABA was a negative regulator of L. japonicus nodulation (Suzuki et al., 2004). High endogenous ABA levels inhibited nodule number, whereas low concentrations promoted root nodule formation. As described herein, endogenous ABA levels were lower in enf1 L. japonicus compared with wild-type plants (Fig. 5); concomitantly, nodule numbers increased to 1.7 times greater than those of the wild type (Fig. 2A). Based on this correlation, we conclude that the increase in root nodule number in enf1 is caused by the lowered concentration of endogenous ABA compared with wild-type plants. ABA is believed to regulate early nodulation stages negatively by inhibiting Nod factor signaling, bacterial infection, and nodule initiation (Miwa et al., 2006; Ding et al., 2008). On the other hand, M. loti-inoculated enf1 mutants had more infection sites and developed more infection threads than wild-type roots (Fig. 2G), strongly suggesting a correlation between decreased endogenous ABA levels and the increased occurrence of infection events. We conclude that the earliest stages of nodule development are not as strongly inhibited in enf1 as they are in wild-type MG20.
Other phenotypic differences from wild-type plants, such as shoot length and number of true leaves, can also be attributed to a reduction in endogenous ABA content. Overall, enf1 shoot length was less than that of MG20 grown in 10 mm KNO3 or without nitrogen (Fig. 3, C and D). However, enf1 shoot length increased compared with that of the wild-type controls following inoculation with M. loti (Fig. 2F). Such a result may be explained by the fact that nodules on enf1 mutant roots supply additional nitrogen to the plants. Alternatively, the results may be explained by the lowered ABA concentration caused by the mutation, with a concomitant change in the levels of other growth factors, which also affect nodulation. The most likely candidate would be GA3, which is involved in nodulation (Maekawa et al., 2009) but also in controlling shoot length (Ueguchi-Tanaka et al., 2005) and flowering (Blazquez and Weigel, 2000). Based on the evidence on hand, we favor the first hypothesis.
L. japonicus is a model legume used to elucidate the physiological functions of leguminous crops, for example, soybean (Glycine max), using molecular biology techniques. If this knowledge is to be applied from the model plant to crop species, it is important to investigate yield in long-term growth experiments after inoculation with a rhizobial symbiont. Although some yield parameters were the same for both enf1 and wild-type plants, both the dry weight and nitrogen content of 100 seeds and entire enf1 plants were significantly larger compared with those of wild-type seeds and plants (Tables I and II), strongly suggesting that the enf1 mutation has the potential to bring about increased yield. The augmentation of the weight and nitrogen content of the enf1 plants most likely reflects the increased nitrogen supplied by the additional enf1 nodules and the concomitant increase in nitrogen fixation activity.
We hypothesize that the increase in nitrogen fixation activity of enf1 results from the decrease in endogenous concentration of ABA. An earlier paper reported that application of 100 μm ABA to roots inhibited nitrogen fixation activity in P. sativum (Gonzalez et al., 2001). Because this concentration of ABA may have adverse growth effects, we applied 0.5 μm ABA and 20 μm abamine (an inhibitor of 9-cis-epoxycarotenoid dioxygenase, an ABA synthesis gene). No negative effects following a 3-d treatment on wild-type L. japonicus growth on agar medium were observed. Wild-type plants treated with abamine had a reduced endogenous ABA concentration in roots, to about one-fourth of the level of control plants. However, nitrogen fixation activity was elevated to about 170% over the nontreated controls (Fig. 7). This result phenocopies enf1, which shows decreased endogenous ABA concentration as well as elevated nitrogen fixation activity. Applying 0.5 μm ABA did not result in a further increase in nitrogen fixation activity, even though the endogenous ABA concentrations are presumed to increase (Fig. 7).
To get a better understanding of how endogenous ABA controls nitrogen fixation activity, we looked at the expression of various markers for nitrogen fixation such as nifH and LjLbs (Shimoda et al., 2009) in enf1 nodules at 28 DAI and found no significant difference in expression for either gene (Fig. 8). The expression levels in other nitrogen fixation activity-related genes, such as Ign1 (Kumagai et al., 2007), Sst1 (Krusell et al., 2005), LjHb1 (Shimoda et al., 2009), and LjGlu1 (Suzuki et al., 2008), also showed no significant differences (Fig. 8). Thus, these genes are unlikely to be downstream of the pathway affected by the enf1 mutation.
NO is known as a strong inhibitor of nitrogen fixation activity (Trinchant and Rigaud, 1982) as well as a signal component in the ABA signaling pathway (García-Mata and Lamattina, 2002; Neill et al., 2002). The decreasing concentration of endogenous ABA in enf1 was correlated with elevated nitrogen fixation activity without enhanced nifH gene expression (Figs. 2D and 8) and with reduced NO production in the root nodule (Fig. 9). This result phenocopies L. japonicus overexpressing LjHb1, which shows decreased NO production as well as increased nitrogen fixation activity without elevated nifH gene expression (Shimoda et al., 2009). Therefore, we conclude that the increased nitrogen fixation activity in enf1 was due to the decrease in NO production by root nodules. Moreover, ABA is known to negatively regulate early nodulation stages. Our results demonstrate that the decreased endogenous ABA levels of enf1 resulted in an increase in nodule number as a consequence of more infection events, in this way enhancing nitrogen fixation activity.
Other reports link ABA and nodulation in the studies of mutants such as lateral root organ-defective (latd), sensitivity to ABA-1 (sta-1), and Beyma (Liang et al., 2007; Ding et al., 2008; Biswas et al., 2009). Although latd, Beyma, and enf1 showed reduced or less sensitivity to ABA, sensitivity to ABA in sta-1 differed depending on developmental processes. For lateral root formation, sta-1 was less sensitive to ABA than enf1, and the number was reduced compared with control plants in both mutants (Fig. 3G). Although the emergence of lateral roots increased, in latd mutants, the number of elongated roots decreased because of a root apical meristem defect (Bright et al., 2005). In terms of nodulation and nodule function, enf1 plants exhibit advantageous phenotypes (i.e. enhanced nodule number and nitrogen fixation), whereas the other mutants show negative phenotypes. The reduced nodulation of sta-1 is the result of a hypersensitivity to ABA, the development of nodules in latd is arrested due to the nodule meristem abortion, and nitrogen fixation activity in latd and Beyma are decreased. Overall, because the regulation of root nodule formation by ABA is likely to be polygenic, the identification of genes responsible for this regulation is essential for understanding the involvement of ABA.
Until now, the majority of symbiotic mutants that have been described represent loss of or defects in root nodule formation (Schauser et al., 1999; Stracke et al., 2002; Radutoiu et al., 2003; Imaizumi-Anraku et al., 2005; Murray et al., 2007). Many of these mutants induce nodules that are Fix− (Suganuma et al., 2003; Krusell et al., 2005; Kumagai et al., 2007). Although reports of mutants that show increased root nodule number (Penmetsa and Cook, 1997; Nishimura et al., 2002b; Oka-Kira et al., 2005; Magori et al., 2009) or spontaneous root nodule formation (Tirichine et al., 2006a, 2006b, 2007) exist, mutations whereby nitrogen fixation activity is elevated without deleterious effects on plant growth and development have been limited. One exception is the L. japonicus root-determined hypernodulation1 (rdh1) mutant, which also exhibits a hypernodulation and enhanced nitrogen fixation phenotype (Ishikawa et al., 2008). However, the RDH1 gene is unlikely to be the same as the ENF1 gene, because the phenotype of rdh1 is inherited in a monogenic, recessive manner, whereas enf1 is monogenic but incompletely dominant. Moreover, except for the nodulation phenotype, no other differences were observed in growth traits between rdh1 and wild-type plants (Ishikawa et al., 2008).
In this report, we have shown that mutating the ENF1 gene leads to an elevation of nitrogen fixation activity and to enhanced biomass production without accompanying adverse growth effects. Therefore, this gene should be an important target for molecular breeding. To determine the identity of the ENF1 gene, we have initiated mapping experiments by employing backcrossed F2 plants of enf1 and Gifu. Our future work will identify the gene responsible for these positive growth effects.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Lotus japonicus Miyakojima MG20 mutants were generated by ethyl methanesulfonate treatment. Seeds that germinated on agar medium containing 70 μm ABA, which inhibits wild-type seed germination, were selected as ABA low-sensitivity candidates from a total of 4,000 M3 seeds. The No. 12 mutant line, which displayed increased nodule number and nitrogen fixation activity, was isolated from these candidates. The No. 12 mutant was then back-crossed to MG20 to investigate the mutant phenotypes in greater detail. From 153 F2 progeny, a mutant designated enf1, which displayed the highest nitrogen fixation activity and nodule number per plant, was isolated.
Seeds were surface-sterilized by immersion in sodium hypochlorite (2% [v/v] containing 0.1% [v/v] Tween 20) for 20 min and rinsed several times with sterile distilled water. After overnight imbibition, the swollen seeds were sown on vermiculite-filled pots that were watered with B&D medium (nitrogen-deficient; Broughton and Dilworth, 1971) with or without 10 mm KNO3 or with or without 1.0 × 107 cells mL−1 Mesorhizobium loti MAFF303099 (Keele et al., 1969; Kaneko et al., 2000; Saeki and Kouchi, 2000), which had been grown in yeast mannitol liquid medium (Keele et al., 1969). In this method, the stage of plant for inoculation was day 1. The plants were grown at 24°C under 16-h-light/8-h-dark conditions at a light intensity of 150 μmol m−2 s−1. Seeds were also sown on 0.8% (w/v) agar medium, and the plates were incubated at 24°C in the dark. After 3 d, germinated seedlings were transplanted onto B&D medium containing 1.5% (w/v) agar and concomitantly inoculated with rhizobia at a concentration of 1.0 × 107 cells per plant. The plants were grown at 24°C under 16-h-light/8-h-dark conditions at a light intensity of 150 μmol m−2 s−1.
Measurement of Dry Weights and Total Nitrogen Contents
Plant samples were dried at 80°C for 3 d and then weighed. Nitrogen content of seeds was measured by micro-Kjeldahl and indophenol methods (Cataldo et al., 1974). For the analysis of total nitrogen content, soil obtained from a field within Saga University was used without sterilization. Imbibed seeds were sown on field soil-filled Wagner pots (16 cm diameter × 19 cm height) and supplied with water, and the plants were grown for 72 d in the Saga University greenhouse (average temperature was 24.7°C) without additional rhizobial inoculation.
Measurement of ABA Concentration of enf1
Plants were grown for 21 d in vermiculite-filled pots supplied with B&D medium, inoculated with M. loti, and after harvest frozen rapidly in liquid nitrogen. ABA concentration was measured by the Phytodetek ABA enzyme immunoassay test kit (Agdia) as described previously (Suzuki et al., 2004).
Acetylene Reduction Assay
A plant was grown for 28 d in a vermiculite-filled pot supplied with B&D medium, inoculated with M. loti, placed in a 34-mL test tube, which was covered with a rubber serum cap, and degassed for 30 s. Acetylene that was diluted five times was injected in the tube, which was then incubated in a growth chamber at 25°C. After 2 h of incubation, the amount of ethylene formed was determined by gas chromatography (Suzuki et al., 2008).
Observation of Sensitivity of Plants to ABA Treatment
To study germination rate, seeds imbibed for 2 h were sown on 0.8% (w/v) agar medium containing 70 μm (±)ABA [(2-cis,4-trans)-5-(1-hydroxy-2,6,6-trimethyl-4-oxo-2-cyclohexen-1-yl)-3-methyl-2,4-pentadienoic acid; Sigma Aldrich]. The plates were placed upside down and incubated at 24°C in the dark. After 3 d, the rate of seed germination was measured using root emergence as the criterion for germination. To study plant responses to exogenous ABA, seeds were sown on vermiculite-filled pots supplied with B&D medium containing 0.5 μm ABA, which had been inoculated with M. loti. The degree of leaf yellowing/browning and shoot tip shriveling was measured 17 DAI.
Observation of Infection Threads Using Fluorescence Microscopy
Seeds were sown on 0.8% (w/v) agar medium and incubated at 24°C in the dark. After 3 d, germinated seedlings were transplanted onto 1.5% (w/v) agar-solidified B&D medium or 1% (w/v) Phytagar-solidified one-quarter-strength Hoagland medium (Machlis and Torrey, 1956) without nitrogen and inoculated with M. loti strain NZP2037 (Scott et al., 1996) carrying the GFP plasmid pHC60 (Cheng and Walker, 1998). The seedlings were then grown at 24°C under 16-h-light/8-h-dark conditions at a light intensity of 100 μmol m−2 s−1 for 4 to 12 d. The roots of plants were rinsed three times with water and observed by fluorescence microscopy. Wavelengths for excitation and emission were 460 to 495 and over 510 nm, respectively.
ABA and Abamine Treatment
Plants were grown for 28 d on vermiculite-filled pots supplied with B&D medium that had been inoculated with M. loti. Roots were treated 28 DAI with 0.5 μm ABA and 20 μm abamine, with abamine alone, with ABA alone, or with B&D medium (control) for 3 d. Abamine is an inhibitor of 9-cis-epoxycarotenoid dioxygenase (Han et al., 2004).
Isolation of Total RNA from L. japonicus
Plants were quick-frozen in liquid N2 and stored at –80°C. Total RNA was prepared using the Plant Total RNA Extraction miniprep system (Viogene). DNaseI treatment was performed using deoxyribonuclease RT-Grade (Wako). RNA was then precipitated by ethanol in the presence of ethachinmate (Wako) as a carrier and was resuspended in RNase-free water.
Analysis of Gene Expression by Real-Time Reverse Transcription-PCR
To quantify the relative amount of transcripts derived from nitrogen fixation-related genes, real-time reverse transcription (RT)-PCR was performed in the One Step SYBR PrimeScript RT-PCR Kit (TaKaRa). The enzymatic reactions were performed using a LightCycler 1.5 Instrument (Roche). After the RT reaction at 42°C for 5 min followed by heating at 95°C for 10 s, the target gene was amplified in 40 cycles of 95°C for 5 s and 60°C for 20 s. The sequences of primers were as follows: for MlnifH, 5′-TCCAAGCTCATCCACTTCGTG-3′ and 5′-AGTCCGGCGCATACTGGATTA-3′; for MlsigA, 5′-GCCCTCTGCTCGACCTTTCC-3′ and 5′-AGCATCGCCATCGTGTCCTC-3′; for LjHb1, 5′-CCTTTGGAGGAGAACCCCAA-3′ and 5′-AGACTGCTGATTCACAAGTCATG-3′; for Ign1, 5′-TGCATTAACCAGAGACCACAA-3′ and 5′-GTGAACGTCTTTCTTAATCTGAGTA-3′; for LjLbs, 5′-TCTGGRCCYAMGCAYAGTC-3′ and 5′-CRTCRCGWGTCAGTSCAAAA-3′; for LjSst1, 5′-TCTTGGACTGGTCTTCCTTGCT-3′ and 5′-TGGTCTCTTGTTCCTCACGTGT-3′; for LjGlu1, 5′-GCTGTCGGTAACGAAGTTCC-3′ and 5′-TCATAGCCGGGAGAACTGAC-3′; for LjATPsyn, 5′-ACATGCTTGCACCATACCAA-3′ and 5′-TCCCCAACTCCAGCAAATAC-3′.
The MlsigA gene for nodules and LjATPsyn for L. japonicus were used for normalizing the results of real-time RT-PCR (Nakagawa and Kawaguchi, 2006; Shimoda et al., 2009).
Quantification of NO by Fluorescence Spectrophotometry
A stock solution of DAF-FM (7 mm in dimethyl sulfoxide) was diluted 1,000-fold in water before use. Detached root nodules of L. japonicus at 21 and 28 DAI were soaked in the DAF-FM solution for 1 min. To analyze the effect of abamine on the production of NO, nodules on the roots of 28-d-old plants were covered by filter paper containing 20 μm abamine for 3 d. Then, the detached nodules were soaked in DAF-FM solution for 1 min. The RFU of the DAF-FM solution was measured using a NanoDrop ND-3300 fluorospectrometer (NanoDrop Technologies). The wavelengths for excitation and emission were 470 and 515 nm, respectively.
Acknowledgments
We thank Professor Tadao Asami (University of Tokyo) for providing the abamine. L. japonicus Miyakojima MG20 seeds were provided by the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (grant no. 19580016 to A.S.), the Takano Life Science Research Foundation (grant to A.S.), and the Sumitomo Foundation (grant to A.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Akihiro Suzuki (azuki@cc.saga-u.ac.jp).
Open Access articles can be viewed online without a subscription.
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284 2148–2152 [DOI] [PubMed] [Google Scholar]
- Bano A, Harper JE (2002) Plant growth regulators and phloem exudates modulate root nodulation of soybean. Funct Plant Biol 29 1299–1307 [DOI] [PubMed] [Google Scholar]
- Bano A, Harper JE, Auge RM, Neuman DS (2002) Changes in phytohormone levels following inoculation of two soybean lines differing in nodulation. Funct Plant Biol 29 965–974 [DOI] [PubMed] [Google Scholar]
- Biswas B, Chan PK, Gresshoff PM (2009) A novel ABA insensitive mutant of Lotus japonicus with a wilty phenotype displays unaltered nodulation regulation. Mol Plant 2 487–499 [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Weigel D (2000) Integration of floral inductive signals in Arabidopsis. Nature 404 889–892 [DOI] [PubMed] [Google Scholar]
- Bright LJ, Liang Y, Mitchell DM, Harris JM (2005) The LATD gene of Medicago truncatula is required for both nodule and root development. Mol Plant Microbe Interact 18 521–532 [DOI] [PubMed] [Google Scholar]
- Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo DA, Schrader LE, Youngs VL (1974) Analysis by digestion and colorimetric assay of total nitrogen in plant tissues high in nitrate. Crop Sci 14 854–856 [Google Scholar]
- Cheng HP, Walker GC (1998) Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J Bacteriol 180 5183–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MJ, Harper JE (1993) Effect of abscisic acid application on root isoflavonoid concentration and nodulation of wild-type and nodulation mutant soybean plants. Plant Soil 153 145–149 [Google Scholar]
- Ding Y, Kalo P, Yendrek C, Sun J, Liang Y, Marsh JF, Harris JM, Oldroyd GE (2008) Abscisic acid coordinates Nod factor and cytokinin signaling during the regulation of nodulation in Medicago truncatula. Plant Cell 20 2681–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordas C, Hasinoff BB, Igamberdiev AU, Manac'h N, Rivoal J, Hill RD (2003) Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J 35 763–770 [DOI] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessing D (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA 95 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampala SS, Hagenbeek D, Rock CD (2001) Functional interactions of lanthanum and phospholipase D with the abscisic acid signaling effectors VP1 and ABI1-1 in rice protoplasts. J Biol Chem 276 9855–9860 [DOI] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128 790–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EM, Galvaz L, Arrese-Igor C (2001) Abscisic acid induces a decline in nitrogen fixation that involves leghaemoglobin, but is independent of sucrose synthase activity. J Exp Bot 52 285–293 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S, Crespi M, Frugier F (2006) The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18 2680–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbeek D, Quatrano RS, Rock CD (2000) Trivalent ions activate abscisic acid-inducible promoters through an ABI1-dependent pathway in rice protoplasts. Plant Physiol 123 1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S-Y, Kitahata N, Sekimata K, Saito T, Kobayashi M, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K, Yoshida S, Asami T (2004) A novel inhibitor of 9-cis-epoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiol 135 1574–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Imaizumi-Anraku H, Akao S, Kawaguchi M (2000) Nodule organogenesis in Lotus japonicus. J Plant Res 112 489–495 [Google Scholar]
- Hirsch AM, Bauer WD, Bird DM, Cullimore J, Tyler B, Yoder JI (2003) Molecular signals and receptors: controlling rhizosphere interactions between plants and other organisms. Ecology 84 858–868 [Google Scholar]
- Hirsch AM, Fang Y (1994) Plant hormones and nodulation: What's the connection? Plant Mol Biol 26 5–9 [DOI] [PubMed] [Google Scholar]
- Imaizumi-Anraku H, Takeda N, Charpentier M, Perry J, Miwa H, Umehara Y, Kouchi H, Murakami Y, Mulder L, Vickers K, et al (2005) Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature 433 527–531 [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Yokota K, Li YY, Wang Y, Liu CT, Suzuki S, Aono T, Oyaizu H (2008) Isolation of a novel root-determined hypernodulation mutant rdh1 of Lotus japonicus. Soil Sci Plant Nutr 54 259–263 [Google Scholar]
- Kaneko T, Nakamura Y, Sato S, Asamizu E, Kato T, Sasamoto S, Watanabe A, Idesawa K, Ishikawa A, Kawashima K, et al (2000) Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res 7 331–338 [DOI] [PubMed] [Google Scholar]
- Keele BB, Hamilton PB, Elkan GH (1969) Glucose catabolism in Rhizobium japonicum. J Bacteriol 97 1184–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak RM, Bohlool BB (1984) Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol 75 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell L, Krause K, Ott T, Desbrosses G, Kramer U, Sato S, Nakamura Y, Tabata S, James EK, Sandal N, et al (2005) The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 17 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F, et al (2002) Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420 422–426 [DOI] [PubMed] [Google Scholar]
- Kumagai H, Hakoyama T, Umehara Y, Sato S, Kaneko T, Tabata S, Kouchi H (2007) A novel ankyrin-repeat membrane protein, IGN1, is required for persistence of nitrogen-fixing symbiosis in root nodules of Lotus japonicus. Plant Physiol 143 1293–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J (1994) Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264 1448–1452 [DOI] [PubMed] [Google Scholar]
- Liang YJ, Mitchell DM, Harris JM (2007) Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Dev Biol 304 297–307 [DOI] [PubMed] [Google Scholar]
- Ma W, Guinel FC, Glick BR (2003) Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl Environ Microbiol 69 4396–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlis L, Torrey JG (1956) Plants in Action: A Laboratory Manual of Plant Physiology. WH Freeman, New York
- Maekawa T, Maekawa-Yoshikawa M, Takeda N, Imaizumi-Anraku H, Murooka Y, Hayashi M (2009) Gibberellin controls the nodulation signaling pathway in Lotus japonicus. Plant J 58 183–194 [DOI] [PubMed] [Google Scholar]
- Magori S, Oka-Kira E, Shibata S, Umehara Y, Kouchi H, Hase Y, Tanaka A, Sato S, Tabata S, Kawaguchi M (2009) TOO MUCH LOVE, a root regulator associated with the long-distance control of nodulation in Lotus japonicus. Mol Plant Microbe Interact 22 259–268 [DOI] [PubMed] [Google Scholar]
- Miwa H, Sun J, Oldroyd GE, Downie JA (2006) Analysis of Nod-factor-induced calcium signaling in root hairs of symbiotically defective mutants of Lotus japonicus. Mol Plant Microbe Interact 19 914–923 [DOI] [PubMed] [Google Scholar]
- Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K (2007) A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315 101–104 [DOI] [PubMed] [Google Scholar]
- Nagata M, Murakami E, Shimoda Y, Shimoda-Sasakura F, Kucho K, Suzuki A, Abe M, Higashi S, Uchiumi T (2008) Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol Plant Microbe Interact 21 1175–1183 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kawaguchi M (2006) Shoot-applied MeJA suppresses root nodulation in Lotus japonicus. Plant Cell Physiol 47 176–180 [DOI] [PubMed] [Google Scholar]
- Nakatsukasa-Akune M, Yamashita K, Shimoda Y, Uchiumi T, Abe M, Aoki T, Kamizawa A, Ayabe S, Higashi S, Suzuki A (2005) Suppression of root nodule formation by artificial expression of the TrEnodDR1 (coat protein of white clover cryptic virus 1) gene in Lotus japonicus. Mol Plant Microbe Interact 18 1069–1080 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128 13–16 [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, et al (2002. a) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420 426–429 [DOI] [PubMed] [Google Scholar]
- Nishimura R, Ohmori M, Kawaguchi M (2002. b) The novel symbiotic phenotype of enhanced-nodulating mutant of Lotus japonicus: astray mutant is an early nodulating mutant with wider nodulation zone. Plant Cell Physiol 43 853–859 [DOI] [PubMed] [Google Scholar]
- Nukui N, Ezura H, Yuhashi KI, Yasuta T, Minamisawa K (2000) Effects of ethylene precursor and inhibitors for ethylene biosynthesis and perception on nodulation in Lotus japonicus and Macroptilium atropurpureum. Plant Cell Physiol 41 893–897 [DOI] [PubMed] [Google Scholar]
- Oka-Kira E, Kawaguchi M (2006) Long-distance signaling to control root nodule number. Curr Opin Plant Biol 9 496–502 [DOI] [PubMed] [Google Scholar]
- Oka-Kira E, Tateno K, Miura K, Haga T, Hayashi M, Harada K, Sato S, Tabata S, Shikazono N, Tanaka A, et al (2005) klavier (klv), a novel hypernodulation mutant of Lotus japonicus affected in vascular tissue organization and floral induction. Plant J 44 505–515 [DOI] [PubMed] [Google Scholar]
- Oldroyd GE, Engstrom EM, Long SR (2001) Ethylene inhibits the Nod factor signal transduction pathway of Medicago truncatula. Plant Cell 13 1835–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR (1997) A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275 527–530 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Uribe P, Anderson J, Lichtenzveig J, Gish JC, Nam YW, Engstrom E, Xu K, Sckisel G, Pereira M, et al (2008) The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J 55 580–595 [DOI] [PubMed] [Google Scholar]
- Phillips DA (1971) Abscisic acid inhibition of root nodule initiation in Pisum sativum. Planta 100 181–190 [DOI] [PubMed] [Google Scholar]
- Pierce M, Bauer WD (1983) A rapid regulatory response governing nodulation in soybean. Plant Physiol 73 286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Gronlund M, Sato S, Nakamura Y, Tabata S, Sandal N, et al (2003) Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425 585–592 [DOI] [PubMed] [Google Scholar]
- Saeki K, Kouchi H (2000) The Lotus symbiont, Mesorhizobium loti: molecular genetic techniques and application. J Plant Res 113 457–465 [Google Scholar]
- Schauser L, Roussis A, Stiller J, Stougaard J (1999) A plant regulator controlling development of symbiotic root nodules. Nature 402 191–195 [DOI] [PubMed] [Google Scholar]
- Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J (2005) The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol 58 809–822 [DOI] [PubMed] [Google Scholar]
- Scott DB, Young CA, Collins-Emerson JM, Terzaghi EA, Rockman ES, Lewis PE, Pankhurst CE (1996) Novel and complex chromosomal arrangement of Rhizobium loti nodulation genes. Mol Plant Microbe Interact 9 187–197 [DOI] [PubMed] [Google Scholar]
- Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, Gresshoff PM (2003) Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299 109–112 [DOI] [PubMed] [Google Scholar]
- Shimoda Y, Shimoda-Sasakura F, Kucho K, Kanamori N, Nagata M, Suzuki A, Abe M, Higashi S, Uchiumi T (2009) Overexpression of class 1 plant hemoglobin genes enhances symbiotic nitrogen fixation activity between Mesorhizobium loti and Lotus japonicus. Plant J 57 254–263 [DOI] [PubMed] [Google Scholar]
- Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Szczyglowski K, et al (2002) A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417 959–962 [DOI] [PubMed] [Google Scholar]
- Suganuma N, Nakamura Y, Yamamoto M, Ohta T, Koiwa H, Akao S, Kawaguchi M (2003) The Lotus japonicus Sen1 gene controls rhizobial differentiation into nitrogen-fixing bacteroids in nodules. Mol Genet Genomics 269 312–320 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Akune M, Kogiso M, Imagama Y, Osuki K, Uchiumi T, Higashi S, Han SY, Yoshida S, Asami T, et al (2004) Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol 45 914–922 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamashita K, Ishihara M, Nakahara K, Abe M, Kucho K, Uchiumi T, Higashi S, Arima S (2008) Enhanced symbiotic nitrogen fixation by Lotus japonicus containing an antisense β-1,3-glucanase gene. Plant Biotechnol 25 357–360 [Google Scholar]
- Tirichine L, Imaizumi-Anraku H, Yoshida S, Murakami Y, Madsen LH, Miwa H, Nakagawa T, Sandal N, Albrektsen AS, Kawaguchi M, et al (2006. a) Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441 1153–1156 [DOI] [PubMed] [Google Scholar]
- Tirichine L, James EK, Sandal N, Stougaard J (2006. b) Spontaneous root-nodule formation in the model legume Lotus japonicus: a novel class of mutants nodulates in the absence of rhizobia. Mol Plant Microbe Interact 19 373–382 [DOI] [PubMed] [Google Scholar]
- Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, Asamizu E, Tabata S, Stougaard J (2007) A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315 104–107 [DOI] [PubMed] [Google Scholar]
- Trinchant JC, Rigaud J (1982) Nitrite and nitric oxide as inhibitors of nitrogenase from soybean bacteroids. Appl Environ Microbiol 44 1385–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiumi T, Shimoda Y, Tsuruta T, Mukoyoshi Y, Suzuki A, Senoo K, Sato S, Kato T, Tabata S, Higashi S, et al (2002) Expression of symbiotic and nonsymbiotic globin genes responding to microsymbionts on Lotus japonicus. Plant Cell Physiol 43 1351–1358 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow T, Hsing YC, Kitano H, Yamaguchi I, et al (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437 693–698 [DOI] [PubMed] [Google Scholar]
- van Brussel AA, Tak T, Boot KJ, Kijne JW (2002) Autoregulation of root nodule formation: signals of both symbiotic partners studied in a split root system of Vicia sativa subsp. nigra. Mol Plant Microbe Interact 15 341–349 [DOI] [PubMed] [Google Scholar]
- Wopereis J, Pajuelo E, Dazzo FB, Jiang Q, Gresshoff PM, de Bruijn FJ, Stougaard J, Szczyglowski K (2000) Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J 23 97–114 [DOI] [PubMed] [Google Scholar]
- Wu Y, Sanchez JP, Lopez-Molina L, Himmelbach A, Grill E, Chua NH (2003) The abi1-1 mutation blocks ABA signaling downstream of cADPR action. Plant J 34 307–315 [DOI] [PubMed] [Google Scholar]
- Zuanazzi JA, Clergeot PH, Quirion JC, Husson HP, Kondorosi A, Ratet P (1998) Production of Sinorhizobium meliloti nod gene activator and repressor flavonoids from Medicago sativa roots. Mol Plant Microbe Interact 11 784–794 [Google Scholar]