Abstract
Arabidopsis (Arabidopsis thaliana) plants subjected to water deficit, sodium chloride (NaCl), or abscisic acid treatments were shown to exhibit a significant increase in the amount of leaf cuticular lipids. These stress treatments led to increases in cuticular wax amount per unit area of 32% to 80%, due primarily to 29% to 98% increases in wax alkanes. Of these treatments, only water deficit increased the total cutin monomer amount (by 65%), whereas both water deficit and NaCl altered the proportional amounts of cutin monomers. Abscisic acid had little effect on cutin composition. Water deficit, but not NaCl, increased leaf cuticle thickness (by 49%). Electron micrographs revealed that both water-deprived and NaCl-treated plants had elevated osmium accumulation in their cuticles. The abundance of cuticle-associated gene transcripts in leaves was altered by all treatments, including those performed in both pot-grown and in vitro conditions. Notably, the abundance of the ECERIFERUM1 gene transcript, predicted to function in alkane synthesis, was highly induced by all treatments, results consistent with the elevated alkane amounts observed in all treatments. Further, this induction of cuticle lipids was associated with reduced cuticle permeability and may be important for plant acclimation to subsequent water-limited conditions. Taken together, these results show that Arabidopsis provides an excellent model system to study the role of the cuticle in plant response to drought and related stresses, and its associated genetic and cellular regulation.
The plant cuticle is a lipidic layer of cutin intermeshed and coated with waxes that covers essentially all aerial organs and functions to restrict transpiration. By this mechanism, the cuticle is thought to play a critical role in plant drought tolerance through its ability to postpone the onset of cellular dehydration stress during drought (Jenks, 2002; Goodwin and Jenks, 2005; Kosma and Jenks, 2007; Samuels et al., 2008). During severe water deficit, when stomata close, it has been proposed that nanoscale diffusion pathways traversing the cuticle become the primary conduit of plant water loss. These pathways are defined by long-chain aliphatic waxes assembled as water-inaccessible crystalline wax domains within the cutin matrix of the cuticle membrane, and these crystalline domains are thought to be a major determinant of cuticle permeability (Riederer and Schreiber, 1995; Kerstiens, 1996; Burghardt and Riederer, 2006; Schreiber, 2006; Kosma and Jenks, 2007). Typical plant waxes are comprised of a homologous series of primary alcohols, aldehydes, alkanes, fatty acids, esters, and sometimes cyclic compounds like triterpenoids and sterols (Jetter et al., 2006). Cuticular wax alkanes, alcohols, and aldehydes have been shown to confer greater resistance to water diffusion, in an artificial membrane, than either very-long-chain fatty acids or the triterpenoids oleanolic and ursolic acid (Grncarevic and Radler, 1967). Arabidopsis (Arabidopsis thaliana) cutin is comprised of C16 and C18 fatty acids and their oxygenated derivatives. Oxygenations occur as midchain (C8, C9, or C10) hydroxyl or ω-hydroxyl groups and can be linked through ester bonds. Cutin monomers may be linked directly to each other or through esterification to glycerol (Kolattukudy, 2001a, 2001b; Graça et al., 2002; Pollard et al., 2008). To date however, the contribution of specific cutin monomers to cuticle permeability is completely unexplored. Nonetheless, mutants defective in one or more components of the cutin polyester such as att1, bdg, hth, lacs2, and wax2, have significant alteration of their cuticle permeability (Lolle et al., 1998; Schnurr et al., 2004; Goodwin and Jenks, 2005; Kurdyukov et al., 2006; Bessire et al., 2007), indicating that properties of the cutin polyester or specific monomers likewise play an important role in establishing the water-barrier properties of the cuticle membrane. Although many hypotheses have been set forth to explain the function of cuticle lipid composition and structure in plant drought tolerance, rigorous experimentation that confirm these hypotheses have yet to be reported.
Recent studies establish that many plants respond to water deficit stress through increased cuticular wax deposition (Shepherd and Wynne Griffiths, 2006; Kosma and Jenks, 2007). For example, plants like tree tobacco (Nicotiana glauca), sesame (Sesamum indicum), soybean (Glycine max), and rose (Rosa × hybrida) possessed more leaf wax per unit area after short periods of water deficit (Jenks et al., 2001; Cameron et al., 2006; Kim et al., 2007a, 2007b). In some studies, stress-induced increases in wax amount were associated with major reductions in leaf water-loss rates (Williams et al., 1999, 2000; Cameron et al., 2006). It is still unclear however, which stress-induced changes in the cuticle are most critical for reducing foliar water loss and drought acclimation. Water deficiency also stimulates production of the phytohormone abscisic acid (ABA), which in turn leads to rapid responses like stomatal closure (Bartels and Sunkar, 2005) and the induction of abiotic stress-responsive genes (Shinozaki and Yamaguchi-Shinozaki, 2007). To date it is unclear whether ABA is involved in abiotic stress-induced cuticle lipid accumulation. Data mining of gene expression databases has recently implicated several cuticle-associated genes as ABA responsive (Kosma and Jenks, 2007), however, only a small number of these have been experimentally verified (Hooker et al., 2002; Duan and Schuler, 2005). Though Arabidopsis typically produces very low amounts of leaf wax and cutin, and is a short-lived ephemeral of assumed limited stress adaptation, we report here that cuticle production and associated gene expression in Arabidopsis is nevertheless highly responsive to water deficit and related treatments including ABA. This report demonstrates the potential of Arabidopsis as an effective model system for use in elucidating determinants of cuticle function in plant drought tolerance.
RESULTS
Impact of Water Deficit, Sodium Chloride, and ABA on Cuticular Wax
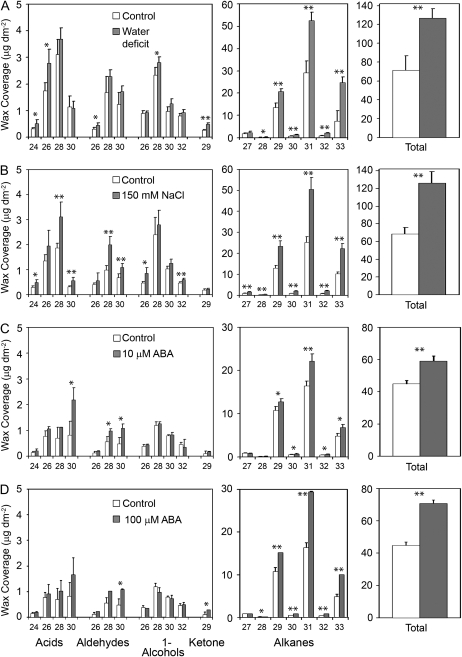
The wax composition of rosette leaves from plants subjected to 150 mm sodium chloride (NaCl), water deficit, or 10 μm or 100 μm ABA treatments was measured to assess the influence of water deficit and associated treatments on wax composition. Waxes of water deficit-treated plants were sampled approximately 8 d after water was withheld. NaCl and ABA treatments were applied three times over an 8 d period. Control plants were subirrigated with water every 3 d, with waxes sampled on the same day as corresponding stress treatments. At the end of the stress treatment period, water deficit-treated plants had a relative water content (RWC) of approximately 60%, the 150 mm-treated plants had a RWC of approximately 79%, the ABA-treated plants RWC was approximately 93%, whereas control plants had a RWC of approximately 95%.
All treatments resulted in a significant increase in total wax amount per unit leaf area (Fig. 1), with the most striking increase observed on NaCl-treated and water deficit-treated plants, which had 80% (t(6) = 7.62, P = 0.0068) and 75% (t(6) = 6.04, P = 0.0009) more wax than control plants, respectively. ABA treatment also led to a significant increase in total wax amount, with 32% (t(4) = 6.38, P = 0.0031) and 54% (t(4) = 9.61, P = 0.0007) increases observed on plants that were sprayed with 10 μm or 100 μm ABA, respectively. The leaves used for wax extraction from water deficit- and NaCl-treated plants had a smaller surface area compared to control plants (31% and 42% smaller, respectively), whereas ABA-treated plants did not have smaller leaves. In addition, we observed significant shifts in the proportion of specific wax constituents. As such, the changes in wax profiles resulting from these stress treatments were not due to leaf area effects alone, but most likely involve wax metabolic responses.
Figure 1.
Cuticular wax profiles of Arabidopsis rosette leaves from plants subjected to water deficit (A), 150 mm NaCl (B), 10 μm ABA (C), or 100 μm ABA (D). ABA-treated plants and their respective controls were greenhouse grown. Methanol was used to dissolve ABA, which was diluted to treatment concentrations with distilled, deionized water. Control plants for ABA treatments were sprayed with distilled, deionized water containing an equivalent amount of methanol. NaCl-treated, water deficit-treated, and their respective control plants were grown in a growth room. Bars represent mean values ± sd (n = 3–4, the experiments were repeated once, twice in the case of water deficit, with similar results). Asterisks denote significant differences (*, P < 0.05; **, P < 0.01) as determined by Student's t tests or Satterthwaite t tests.
All treatments shifted the proportional distribution of wax classes. In general, alkanes accounted for the observed large increases in total wax amount, with 98% (t(6) = 7.98, P = 0.0002) and 93% (t(6) = 6.79, P = 0.0005) increases observed on NaCl-treated and water deficit-treated plants, respectively, and 29% (t(4) = 4.27, P = 0.0129) and 69% (t(4) = 9.61, P = 0.0007) increases on 10 μm and 100 μm ABA-treated plants, respectively (Fig. 1). In general, the increased leaf alkane content was attributable, primarily, to increases in the very-long-chain (C29, C31, and C33) constituents (Fig. 1). Minor but significant increases were observed in other wax class amounts such as fatty acids (t(6) = 2.98, P = 0.0247) and aldehydes (t(6) = 4.67, P = 0.0034) on NaCl-treated plants, ketones (t(6) = 9.88; P < 0.0001) on water deficit-treated plants, and aldehydes (10 μm ABA, t(4) = 3.52, P = 0.0245; 100 μm ABA, t(4) = 4.15, P = 0.0142) on ABA-treated plants. Nonetheless, in all cases, alkanes represented the largest absolute increase of any given wax class.
Impact of Water Deficit, NaCl, and ABA on Cutin Monomer Composition
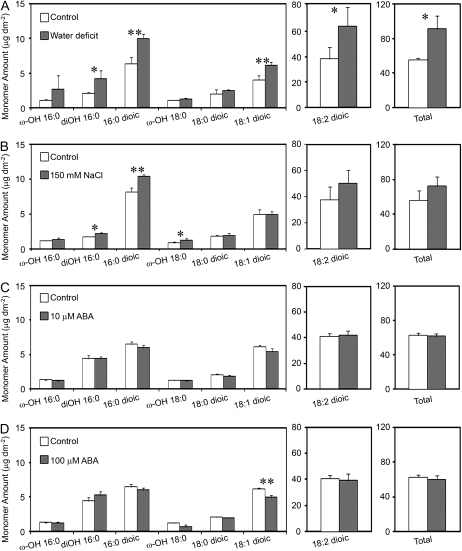
Cutin monomers were sampled from water deficit-, NaCl-, and ABA-treated plants using the same treatments as described above for wax sampling. Water deficit led to a 65% (t(5) = 3.28, P = 0.022) increase in total cutin per unit leaf area, largely manifested as a 67% increase (t(5) = 2.57, P = 0.0498) in C18:2 dioic acids (Fig. 2). Additionally, water deficit significantly increased C16:0 dioic acid (57%; t(5) = 6.51, P = 0.0013), C18:1 dioic acid (52%; t(5) = 5.28, P = 0.0033), and C16:0 (9)10,16-dihydroxy acid amounts (104%; t(3.08) = 3.94, P = 0.0277). The 150 mm NaCl treatment did not result in an increase in the total amount of cutin monomers (Fig. 2). However, salt-treated plants did have significantly higher amounts of C16:0 dioic acids (t(5) = 7.35, P = 0.0007), C16:0 (9)10,16-dihydroxy acids (t(5) = 4.46, P = 0.0007), and C18:0 18-hydroxy acids (t(5) = 3.03, P = 0.0289). ABA treatments did not significantly increase the amounts of total measured cutin or any individual constituent (Fig. 2).
Figure 2.
Cutin monomer profiles of Arabidopsis rosette leaves from plants subjected to water deficit (A), 150 mm NaCl (B), 10 μm ABA (C), or 100 μm ABA (D). ABA-treated plants and their respective controls were greenhouse grown. Methanol was used to dissolve ABA, which was diluted to treatment concentrations with distilled, deionized water. Control plants for ABA treatments were sprayed with distilled, deionized water containing an equivalent amount of methanol. NaCl-treated, water deficit-treated, and their respective control plants were grown in a growth room. Bars represent mean values ± sd (n = 3–4, the experiments were repeated once with similar results). Asterisks denote significant differences (*, P < 0.05; **, P < 0.01) as determined by Student's t tests or Satterthwaite t tests.
Impact of Water Deficit and NaCl on Cuticle Ultrastructure
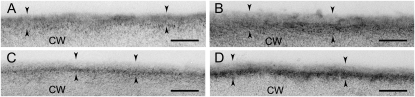
Transmission electron microscopy (TEM) analysis revealed that water deficit-treated plants had thicker cuticles than unstressed control plants (Fig. 3). On average, controls from separate experiments of water deficit-treated and NaCl-treated plants had leaf cuticles 55.3 ± 14.8 nm and 55.7 ± 8.1 nm thick, respectively. Water deficit-treated and 150 mm NaCl-treated plants had leaf cuticles 82.3 ± 10.6 nm and 66.0 ± 12.1 nm thick, respectively, representing 49% and 18% increases in cuticle thickness, respectively. Water deficit-treated and salt-treated plants also exhibited higher accumulations of osmium in the cuticle, especially the cuticular layer, as revealed by the darker appearance of treated cuticles in the micrographs (Fig. 3).
Figure 3.
Leaf cuticle membrane (between arrowheads) from leaf number four of control plants (A and C), water deficit-treated (B), 150 mm NaCl-treated (D) plants. The cuticle is divided into an outermost, electron-translucent layer, the cuticle proper, and an innermost, electron-dense, darker-staining layer, the cuticular layer, that is most evident in leaves of stressed plants (B and D). CW, Cell wall; scale bars = 100 nm.
Impact of Water Deficit, NaCl, and ABA on the Expression of Genes Associated with Cuticle Production
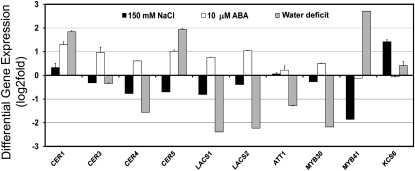
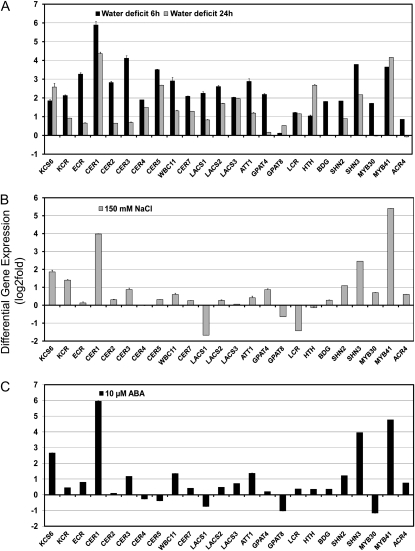
Transcript profiling from leaves of pot-grown plants showed that several cuticle genes (selected based on compositional analysis) were quite responsive to water deficit, salt, and ABA (Fig. 4). ECERIFERUM1 (CER1), CER5, and MYB41 exhibited significant increases in transcript abundance in water deficit-treated plants, whereas CER4, LACS1, LACS2, ATT1, and MYB30 showed significantly lower expression (Fig. 4). In salt-exposed plants, only KCS6/CER6 was induced 24 h after the potting medium was subirrigated with 150 mm NaCl; whereas MYB41 had notably lower transcript abundance (Fig. 4). ABA treatment caused (24 h after treatment) an over 2-fold increase in CER1 transcript abundance, but also increased the abundance of CER3/WAX2, CER5, and LACS2 transcripts by about 2-fold (with the LACS1 expression level falling just below the 2-fold increase).
Figure 4.
Stress profiling of cuticle gene transcripts from stress treatments with pot-grown plants. Modulation of cuticle gene expression was determined in rosette leaves of approximately 15-d-old Arabidopsis plants subjected to different stress conditions including water deficit, 150 mm NaCl for 24 h, or 10 μm ABA for 24 h. The gene expression level was determined by quantitative RT-PCR analysis. Results are presented as differential relative transcript abundance. The data represent the means ± sd of three replicates. KCS6 = CER6.
As a comparison to studies using pot-grown plants, cuticle-associated gene expression studies were also performed on plants grown in vitro, and exposed to analogous water deficit, NaCl, and ABA treatments as in the pot-grown plants (Fig. 5). For in vitro water deficit treatments, petri dish lids were removed, exposing plants to the dry atmosphere; RNA extraction was performed 6 and 24 h later. The 6 h water deficit caused an approximately 2-fold or higher increase in 22 of the 24 cuticle-associated gene transcripts, except GPAT8 and ACR4, whereas the 24 h treatment increased, by approximately 2-fold or higher, transcript abundance of 14 of the 24 genes, including KCS6, KCR, CER1, CER4, CER5, WBC11, CER7, LACS2, LACS3, ATT1, LCR, HTH, SHN3, and MYB41 (Fig. 5). CER1 was increased most by the in vitro water deficit treatment. The NaCl treatments caused a approximately 2-fold increase in KCS6, KCR, CER1, CER3, SHN2, SHN3, and MYB41 (Fig. 5). The ABA treatment caused an approximately 2-fold increase in KCS6, CER1, CER3, WBC11, ATT1, SHN2, SHN3, and MYB41 (Fig. 5). NaCl treatment reduced abundance of LACS1 and LCR transcripts, whereas ABA treatment led to lower abundance of GPAT8 and MYB30 transcripts.
Figure 5.
Stress profiling of cuticle gene transcripts from in vitro stress treatments. Modulation of cuticle gene expression was determined in 15-d-old Arabidopsis plants subjected to different stress conditions including: water deficit (A), 150 mm NaCl (B), or 10 μm ABA (C). Water deficit treatment consisted of removal of petri dish lids and exposure of plantlets to dry air for 6 or 24 h. All other treatments lasted 24 h. The gene expression level was determined by quantitative RT-PCR analysis. Results are presented as differential relative transcript abundance. The data represent the means ± sd of three replicates. KCS6 = CER6, ECR = CER10.
Association of Cuticle Composition with Cuticle Permeability and Plant Drought Acclimation
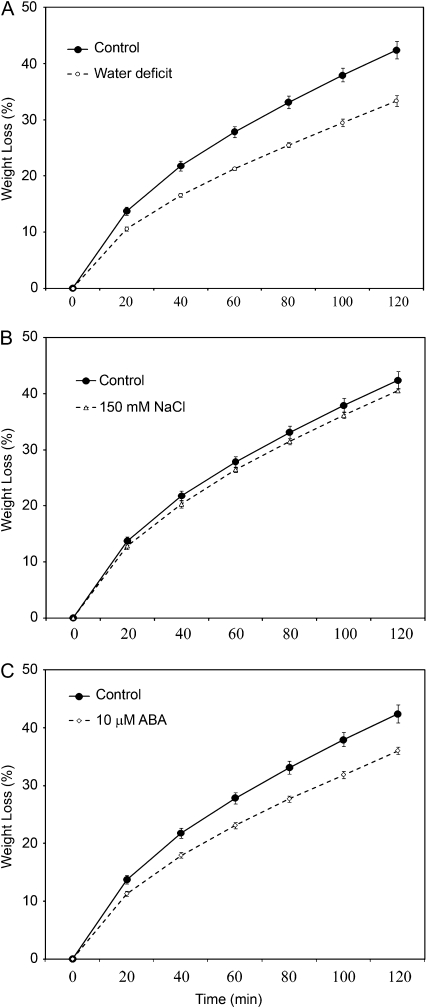
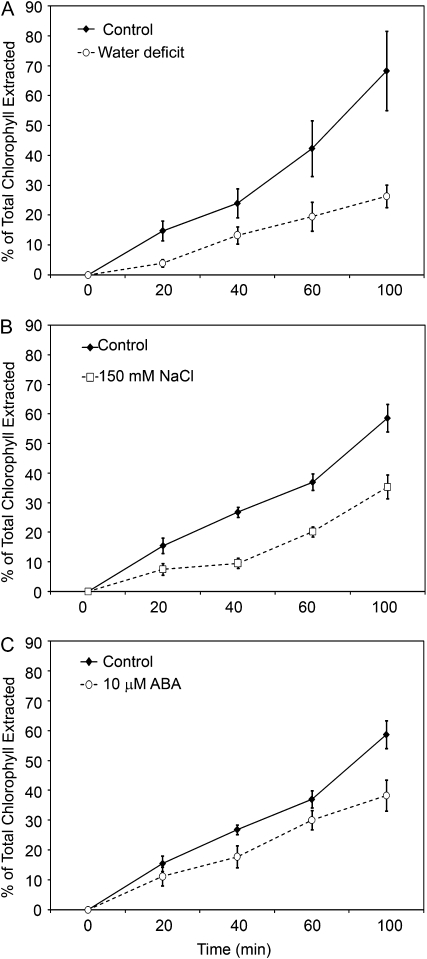
To assess the impact of stress-associated changes in cuticle properties on determinants of plant water status, rosette water-loss and chlorophyll-leaching assays were employed on plants that had received the same treatment schemes as those outlined for wax/cutin sampling. All treatments resulted in a reduced rate of both leaf water loss (Fig. 6) and leaf chlorophyll leaching (Fig. 7). Although the differences were very small, plants subirrigated with 150 mm NaCl could be shown to exhibit the same small reduction in leaf water-loss rate curve in two replicate experiments, suggesting that these differences were significant. It must be considered that supraoptimal levels of salt inflict both hyperosmotic and hyperionic stress on plants (Hasegawa et al., 2000), and that NaCl has also been shown to inhibit dark-induced stomatal closure (Jarvis and Mansfield, 1980). As such, incompletely closed stomata may have influenced the leaf water loss rates of NaCl-treated plants reported here.
Figure 6.
Water-loss rates (expressed as a percentage of initial water-saturated weight) of isolated rosettes from whole plants deprived of water (water deficit; A), subjected to 150 mm NaCl (B), or 10 μm ABA (C). Points represent mean values ± se (n = 5, the experiments were repeated once with similar results).
Figure 7.
Chlorophyll-extraction rates (expressed as a percentage of total chlorophyll extracted after 24 h) of rosettes from plants deprived of water (water deficit; A), subjected to 150 mm NaCl (B), or 10 μm ABA (C). Points represent mean values ± se (n = 4, the experiments were repeated once with similar results).
We also sought to determine if the cuticle changes induced by exposure to water deficit would improve plant tolerance to subsequent water-limited environments (i.e. whether treatments provide drought acclimation). It was observed that plants subjected to water deficit were better able to withstand subsequent water deprivation, compared to previously nonstressed plants, as evidenced by delayed wilting and the maintenance of a higher RWC (Fig. 8).
Figure 8.
Water deprivation acclimation experiment. Approximately 15-d-old plants were exposed to 14 d of water deprivation (dwd) until reaching a RWC of 49% alongside well-watered control plants. Both groups of plants were then rewatered and allowed to recover for 20 h. Plants that recovered from water deficit (acclimated) and control plants (nonacclimated) were then subjected to further water deprivation for up to 15 d post recovery (dpr). Water deficit-acclimated plants were resistant to further water deprivation and were able to maintain a higher RWC compared to nonacclimated plants. ND, Not determined.
DISCUSSION
We report that Arabidopsis plants exposed to a water deficit treatment (wherein irrigation was withheld for approximately 8 d) exhibited a significant increase in total leaf cuticle wax amount of approximately 75% relative to nontreated plants, a value similar to those reported for other plants exposed to water deficit, including both dicotyledonous and graminaceous species (Seiler, 1985; Jefferson et al., 1989; Bondada et al., 1996; Jenks et al., 2001; Samdur et al., 2003; Shepherd and Wynne Griffiths, 2006; Kim et al., 2007a, 2007b; Kosma and Jenks, 2007). The highest reported induction of wax by water deficit occurs on the leaves of tree tobacco, wherein waxes increased by over 150% after exposure to multiple drying events (Cameron et al., 2006). Whether such high wax amounts could be obtained with Arabidopsis using repeated drying cycles is uncertain. Notwithstanding, the wax induction we observed on Arabidopsis is comparable to some of the most responsive plants reported to date. The most notable change in the wax constituent profile of water deficit-treated Arabidopsis leaves is the dramatic increase in alkane constituents, which explains essentially all of the observed increase in the total leaf wax amount. As such, it appears that alkane synthesis is key to this stress response. Besides waxes, we report that water deficit also increases the amount of leaf cutin monomers on Arabidopsis by 65%. Previous studies have not examined cutin monomer responses to water deficits, and so, to our knowledge, this report becomes the first to implicate cutin induction as a drought-responsive adaptation. Unlike wax induction, water deficit increased the amounts of nearly all leaf cutin monomers, indicating that more total cutin, rather than more of any specific cutin constituent, may be of greater importance in this water deficit stress response.
Previous studies in rose (Williams et al., 1999, 2000; Jenks et al., 2001) and tree tobacco (Cameron et al., 2006) provide some evidence that water deficit-induced increases in cuticle wax produce a less water-permeable cuticle, an adaptation that may limit transpiration and delay the onset of cellular dehydration stress during prolonged climatic drought (Kosma and Jenks, 2007). Studies presented here demonstrate that water deficit alters both wax and cutin in Arabidopsis, and produces plants with less permeable cuticles. These induced cuticle changes were associated with delayed wilting in Arabidopsis and the maintenance of normal water status during water deprivation treatments. Nonetheless, a contributory role for osmotic adjustment, stomatal regulation, and other physiological changes in acclimated Arabidopsis, rose, and tree tobacco cannot be ruled out. Moreover, whether plants maintain reduced cuticle permeability for an extended period of time after recovery from water deficit, or whether permeability returns to normal nonstress levels at some time after the stress period, has yet to be explored in Arabidopsis or any plant species. As such, further studies are needed to dissect the contribution of cuticle induction relative to other acclimation responses during the acquisition of stress tolerance. As demonstrated here, Arabidopsis can be a preeminent model for these studies.
The distribution, size, and number of wax crystalline domains within the cuticle are thought to define tortuous paths for the diffusion of water, and thereby serve as major determinants of cuticle permeability (Riederer and Schreiber, 1995; Kerstiens, 1996; Buchholz, 2006; Burghardt and Riederer, 2006; Schreiber, 2006; Kosma and Jenks, 2007). A strong negative relationship exists between polymer crystallinity and membrane/coating permeability to water (Klute and Franklin, 1958; Lasoski and Cobbs,1959; Sangaj and Malshe, 2004). Wax alkanes, primary alcohols, and aldehydes are known to confer greater resistance to water movement in artificial membranes than fatty acids and triterpenoids, and among these, alkanes may form the most impermeable crystalline regions (Grncarevic and Radler, 1967). Whether the increased alkane amounts we observe on Arabidopsis following water deficit contribute to the formation of less permeable crystalline structures in Arabidopsis leaf cuticles awaits further study using spectroscopic, calorimetric, atomic force, and NMR approaches. The Arabidopsis species itself, along with the large collection of diverse cuticle mutants now available (Kosma and Jenks, 2007), may provide an excellent model system to explore these relationships.
As another issue that requires further study, it is yet unclear what role cutin plays in cuticle permeability. Numerous cutin monomer-deficient mutants in Arabidopsis have been reported that show large elevations in cuticle permeability, and from these studies and others (Kosma and Jenks, 2007), it has been postulated that the cutin matrix provides a kind of framework within which waxes are deposited and crystallized. Potentially, mutations that destroy the cutin framework severely disrupt wax crystallization, and subsequently the cuticle's permeability barrier function. Our results, which show a significant increase in total leaf cutin monomers and an increase in the thickness of both the cuticle proper and cuticular layer of the Arabidopsis cuticle after water deficit, lead us to speculate that drought acclimation involves synthesis of a larger cutin framework that supports more concentrated intracuticular packing of wax crystalline regions. As another consideration, essentially all cutin monomers possess carboxyl or hydroxyl functional groups capable of participating in ester or hydrogen bonds. In synthetic polymers, increased cross-linking between monomers is thought to reduce permeability by decreasing segment mobility and the availability of hydrogen-bonding sites within the polymer (Sangaj and Malshe, 2004). Increased cross-linking of the cutin polymer may prohibit hydrogen bonding of water molecules to unlinked, oxygenated cutin functional groups and in this way slow the diffusion of water. Whether the induced cuticle changes we report here influence the structure of the cutin matrix or internal cross-linking in a way that alters water diffusion pathways in water deficit-stressed Arabidopsis is completely unknown.
We implicate the three genes CER1, CER5, and MYB41 as important water deficit-inducible cuticle-associated genes, which is consistent with their proposed roles in alkane synthesis (Aarts et al., 1995; Jenks et al., 1995), wax export (Pighin et al., 2004), and regulation of wax synthesis (Cominelli et al., 2008), respectively. Interestingly, five cuticle-associated transcripts (CER4, LACS1, LACS2, ATT1, and MYB30) were less abundant in plants experiencing water deficit, whereas only three were elevated by water deficit. The fact that CER4 was not more abundant seems logical, as the 1-alcohols are thought to be synthesized by the CER4 protein and were reduced, as a proportion of total waxes, by water deficit treatment. However, both LACS1 and LACS2 genes (Lü et al., 2009) and the ATT1 gene (Xiao et al., 2004), thought to encode important metabolic enzymes involved in wax and/or cutin synthesis, had reduced transcript abundance. These results suggest then that the Arabidopsis' water deficit response involves the activation of specific regions of cuticle lipid pathways required for stress adaptation, and the limitation of others. How the changed expression of these genes might contribute to plant survival in drought is an important question for future study.
Previous studies (Hooker et al., 2002; Duan and Schuler, 2005), as well as gene expression data mined from publicly accessible databases (Kosma and Jenks, 2007), have suggested that several cuticle-associated genes are responsive to ABA and cuticle-associated genes like CER6 and ATT1 are reported to contain ABA-responsive element-like elements in their promoters (Hooker et al., 2002; Duan and Schuler, 2005). Whereas we did not observe elevated cutin with ABA treatment, we did record elevated waxes on leaves treated with low and high levels of ABA, with a preferential increase in alkanes as was observed for water deficit. The CER1 transcript was increased most by our ABA treatments. Taken together, these studies suggest that ABA may be necessary for the activation of several cuticle genes, particularly CER1, during water deficit. Comprehensive analysis of gene expression and cuticle composition responses in ABA-associated mutants (Finkelstein et al., 2002; Nambara and Marion-Poll, 2005) may yield valuable information on the requirement of ABA for cuticle stress responses.
Few studies have examined NaCl or other salts in plant cuticle induction. Nevertheless, Suaeda maritima shows a 60% increase in leaf cuticle membrane thickness after salt treatment, and this was associated with a 35% reduction in transpiration rate (Hajibagheri et al., 1983). Although we did not observe a significant increase in cuticle membrane thickness or total cutin monomer amount of salt-treated Arabidopsis, we did observe increased osmium accumulation in cuticles from micrographs of salt-stressed leaves, a decrease in cuticle permeability, increased wax, and, consistent with observations from public databases (Kosma and Jenks, 2007), significant induction of the gene KCS6/CER6 by salt treatments. The KCS6/CER6 gene is thought to function in wax synthesis, and as such, may have contributed to the altered cuticle properties we observed on our salt-treated plants. Whether other treatments at different time points would reveal the involvement of additional genes requires further study. Moreover, salt stress has both an osmotic and ionic component, and so it might be hypothesized that the observed cuticle changes contribute to water conservation by plants growing in salt-saturated soils and/or the shedding of salt spray from foliage via improved cuticle barrier properties. Further study is now clearly warranted.
A higher proportion of genes were induced with in vitro-grown Arabidopsis plants than were induced in pot-grown plants exposed to comparable water deficit treatments, especially at the 6 h time point following exposure to dry air (including those reported by Joubès et al., 2008). This is likely due to the fact that in vitro plantlets have quite poorly developed, diminutive, and often discontinuous cuticles (Wetzstein and Sommer, 1982; Wardle et al., 1983; Hazrika, 2003), creating a need to rapidly synthesize a whole and normally functioning cuticle more adequately suited to the lower humidity levels of ex vitro conditions. By comparison, pot-grown plants with a fully formed cuticle apparently require the induction of only certain genes when water becomes more limited, such as CER1, that target synthesis of a less permeable or drought-acclimated cuticle. Just as for many other treatments, CER1 was induced more than other genes in the in vitro plants exposed to dry air, strengthening our previous stated hypothesis that CER1 is a very important drought-responsive gene.
Collectively, our results demonstrate that Arabidopsis plants respond to water deficit treatment by increasing the deposition of both leaf cuticular waxes and cutin monomers, and increasing the thickness and osmiophilicity of the cuticle membrane. Likewise, NaCl and ABA induce leaf waxes, but in contrast to water deficit have little effect on cutin monomers. The larger increase in waxes versus cutin monomers on treated leaves, particularly the predominant increase in wax alkanes, fits well with previous models that implicate waxes as critical determinants of cuticle permeability (Buchholz, 2006; Burghardt and Riederer, 2006; Kosma and Jenks, 2007). Results here also reveal that stress-induced accumulation of cuticle lipids involves both accumulation and reduction of specific gene transcripts, with CER1 being conspicuously elevated in most treatments. Although these findings are consistent with a hypothesis that cuticle induction provides an adaptive mechanism for plant acclimation to drought, research is still needed to elucidate the cellular and molecular-genetic mechanisms that underlie cuticle-associated stress responses.
MATERIALS AND METHODS
Plant Growth Conditions and Water Deficit, NaCl, and ABA Treatments for Wax and Cutin Chemistry, TEM Analysis, Epidermal Permeability, Water Deprivation Acclimation, and Pot-Grown Gene Expression Experiments
Arabidopsis (Arabidopsis thaliana; ecotype Columbia-0) seeds were stratified for 3 to 4 d at 4°C, and plants were grown in 3-inch pots of Promix PGX soilless media (Premier Horticulture) at a density of four to six plants per pot. Growth conditions consisted of a growth room at 21°C to 22°C with 30% to 60% relative humidity, a 16/8-h light/dark cycle, and a light intensity of 125 to 150 μmol m−2 s−1 or a greenhouse during the months of September to February with an average temperature of 22°C, an average relative humidity of 68%, and ambient sunlight supplemented with a combination of high-pressure sodium and metal halide lamps contributing approximately 100 μmol m−2 s−1 at plant height on a 16/8-h light/dark cycle. Plants were fertilized once per week for the 2 weeks of growth prior to stress treatments with 1,000 mg per liter 15-5-15 of Miracle Gro© Excel© Cal-Mag (The Scotts Co.) at a pH range of 5.7 to 6.0 with alkalinity reduction achieved via 93% sulfuric acid (Ulrich Chemical) at 0.08 mL per liter.
Fifteen to 16-d-old plants were used for all stress treatments. NaCl stress was imposed by subirrigation with a 150 mm NaCl (Mallinckrodt Baker) solution, allowing 20 to 30 min for absorption, three times over a 7 d period. Control plants for NaCl-treated plants were watered with greenhouse tap water each time NaCl was applied. For water deficit treatments pots were deprived of water until wilting of lower leaves was observed (typically 7–9 d; RWC approximately 60%). ABA treatment consisted of spraying with 10 μm or 100 μm ABA (Sigma-Aldrich) solution three times over a 7 d period. ABA was dissolved in methanol and treatment solutions prepared by dilution, with distilled, deionized water, to the appropriate volume. Control plants for the ABA treatments were sprayed with distilled, deionized water containing equivalent amounts of methanol. For NaCl and ABA treatments, all biochemical and physiological analyses were performed on the 8th d after initial treatment. For gene expression analysis, rosette leaves were harvested 24 h after 150 mm NaCl and 10 μm ABA application; rosette leaves from water-deprived plants were harvested when leaves showed minor wilting (RWC approximately 60%). Leaves from untreated control plants were harvested at the same time as leaves from treated plants.
For water deprivation acclimation studies, plants were grown in a growth chamber at 22°C to 25°C, under a light/dark 8/16-h photoperiod of white light at 185 to 210 μmol m−2 s−1. Fifteen to 16-d-old plants were deprived of water until nearly all plants wilted (typically 14 d) and had reached a RWC of 49%. Control plants grown alongside water deficit-treated plants were watered as needed with tap water. Once water deficit-treated plants had wilted, the soil of both control plants and water deficit-treated plants was saturated with tap water and allowed to recover for 20 h. After 20 h, excess water was poured off both groups of plants. Control (nonacclimated) and water-deprived (acclimated) plants were then subsequently deprived of water until the majority of control plants had wilted (typically 11 d) and had reached a RWC of 56%.
RWC Measurement
Fresh weights of entire rosettes, removed from their roots, were collected on a microbalance. Rosettes were then submerged in distilled, deionized water for 12 h, blotted dry, and saturated fresh weights were collected. Rosettes were then dried in an oven at 80°C until a constant mass was achieved at which point dry weights were collected. Rosette RWC was calculated as described by Barrs and Weatherley (1962). Each replicate consisted of an entire rosette from a single plant. Each replicate was taken from a separate pot. Three to six replicates were used for all analyses.
Cuticular Wax Analysis
Rosette leaf wax composition was determined as described by Chen et al. (2003) with slight modification. Leaves were submerged in hexane for 30 s followed by a short 1 s rinse. Wax extracts were evaporated under N2 gas and derivatized by heating at 100°C for 15 min in N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA; Supelco). Silylated samples were analyzed by gas chromatography (GC) with a Hewlett-Packard 5890 series II gas chromatograph equipped with a flame ionization detector and a 12-m, 0.2-mm HP-1 capillary column with helium as the carrier gas. The oven temperature was programmed with an initial temperature of 80°C and increased at 15°C min−1 to 260°C, at which point the temperature remained unchanged for 10 min. The temperature was then increased at 5°C min−1 to 320°C, at which point the temperature was held for 24 min. Injector and detector temperatures were set at 320°C. Quantification was based on flame ionization detector (FID) peak areas relative to the internal standard hexadecane. Specific correction factors were developed from external standards and applied as described by Jenks et al. (1995). The total amount of cuticular wax was expressed per unit of leaf surface area. Leaf areas were determined by ImageJ software (http://rsb.info.nih.gov/ij/) using digital images of flattened leaves. Molecular identities were determined by electron impact GC/mass spectrometry (Finnigan MAT Thermospray Corp.).
Cutin Monomer Analysis
Leaf cutin monomer content was analyzed based on methods described by Lü et al. (2009). Ground, delipidated, dried leaf tissues were used for all reactions. Depolymerization reactions consisted of 6 mL of 3 n methanolic hydrochloride (Supelco) and 0.45 mL (7%, v/v) methyl acetate (Sigma-Aldrich) at 60°C. Methyl heptadecanoate was used as an internal standard. After 16 h, reactions were allowed to cool to room temperature and terminated by the addition of 6 mL of saturated, aqueous NaCl. Methyl ester monomers were extracted, twice, with 10 mL dichloromethane (Bonaventure et al., 2004). After washing the organic phase three times with 0.9% (w/v) aqueous NaCl, dichloromethane extracts were dried with 2,2-dimethoxypropane (Sigma-Aldrich), and evaporated under nitrogen gas. Monomers were derivatized in pyridine and BSTFA (1:1, v/v) for 15 min at 100°C. Excess pyridine:BSTFA was removed with nitrogen gas, and samples were dissolved in heptane:toluene (1:1, v/v) prior to analysis with a Hewlett-Packard 5890 series II GC equipped with a flame ionization detector (FID) and 12 m, 0.2 mm id HP-1 capillary column with helium as the carrier gas. The GC oven was programmed with an initial temperature of 80°C and increased at 15°C min−1 to 200°C, then increased at 2°C min−1 to 280°C. Injector and detector temperatures were set at 320°C. Quantification was based on uncorrected FID peak areas relative to internal standard methyl heptadecanoate peak area. Areas of rosette leaves were determined by ImageJ software (http://rsb.info.nih.gov/ij/) by using digital images of flattened leaves.
TEM
Leaf cuticle ultrastructure was analyzed by TEM according to methods employed by Chen et al. (2003). Briefly, leaf samples were collected from the middle of the blade between the midvein and the margin of leaf 4. Samples were fixed for 1 h at room temperature in primary fixative containing 2.5% (v/v) glutaraldehyde and 2% (v/v) formaldehyde in 0.05 m phosphate buffer, pH 6.8 (PB; Karnovsky, 1965). Two 40 s microwave treatments (model 3450; Ted Pella) under a vacuum, using the low-power setting, with a 3-min period between the two exposures was used to improve fixation. After fixation, samples were washed with PB and postfixed for 1 h in 2% (v/v) osmium tetroxide in 0.05 m PB. One 40 s microwave treatment under vacuum as described above was applied during osmium fixation. Tissues were than washed with PB and dehydrated through a gradient series of ethanol, infiltrated with Spurr's embedding medium (Electron Microscopy Sciences), and polymerized for 48 h at 60°C. Ultrathin sections (80–100 nm) were prepared from Spurr's resin-embedded samples and mounted on carbon-coated Formvar 100-mesh copper grids (Electron Microscopy Sciences). Sections were air dried, stained at room temperature with 2% (v/v) uranyl acetate for 5 min, and then stained with lead citrate for 3 min before viewing with a Philips EM 400 electron microscope (FEI Co.). Cuticle thickness measurements were made on digitized micrograph images using ImageJ software (http://rsb.info.nih.gov/ij/). Measurements were made from the base of the darker osmium-stained cuticular layer to the outermost edge of the lighter-stained cuticle proper. Three replicates were used for each treatment and the appropriate control. Each replicate was taken from a separate plant from a separate pot. Each replicate consisted of 10 measurements made on two to three sections from the same leaf sample.
Measurement of Epidermal Permeability
To quantify excised rosette water loss, plants were dark acclimated for 3 h prior to measurement. Whole rosettes were excised (from roots) and placed immediately in water (in the dark) and soaked for 60 min to equilibrate water contents. Rosettes removed from soaking were shaken gently and blotted dry to remove excess water, with weights determined gravimetrically every 20 min using a microbalance. Data were expressed as a percentage of the initial water-saturated fresh weight.
Epidermal permeability was also assessed using chlorophyll efflux. Plants were watered and allowed to rehydrate during a 3-h dark-acclimated period prior to measurement. Entire rosettes were collected and immersed in a equal volumes of 80% ethanol in glass scintillation vials. Vials were covered with aluminum foil and agitated gently on a shaker platform. Aliquots of 1 mL were removed every 20 min and 24 h after initial immersion. The amount of chlorophyll extracted into the solution was quantified using a UV-2102 PC spectrophotometer (UNICO) and calculated from UV light absorption at 647 and 664 nm as described by Lolle et al. (1998). Data were expressed as a percentage of the total chlorophyll extracted after 24 h in 80% ethanol.
Plant Material and Growth Conditions for in Vitro Gene Expression Studies
Arabidopsis (ecotype Columbia-0) was used in all experiments. Seeds were either plated on Murashige and Skoog (MS) medium supplemented with 0.7% agar, 2.5 mm MES-KOH, pH 5.7 and plants were grown under long-day conditions (16 h of light, 8 h of darkness) at 22°C, or placed in liquid 0.5× MS medium supplemented with 2% Glc, 20 mm MES-KOH, pH 5.7 and grown under long-day conditions at 22°C on a rotary shaker (120 rpm). The 15-d-old seedlings grown into 0.5× MS medium were osmotically stressed by transferring the plants into new 0.5× MS medium containing 150 mm NaCl or 10 μm ABA for 24 h. For water deficit treatment, the plates containing the seedlings were opened for 6 or 24 h. After 6 or 24 h of treatment, plants were used for RNA extraction.
RNA and cDNA Preparation
RNA from Arabidopsis tissues with the RNeasy plant mini kit (Qiagen). Purified RNA was treated with DNase I using the DNA-free kit (Ambion). First-strand cDNA was prepared from 1 μg of total RNA with the Superscript RT II kit (Invitrogen) and oligo(dT)18 according to the manufacturer's instructions. A 0.66-μL aliquot of the total reaction volume (20 μL) was used as a template in real-time reverse transcription (RT)-mediated PCR amplification.
Real-time (Quantitative) RT-PCR Conditions and Analysis
The PCR amplification was performed with gene-specific primers listed in Supplemental Table S1. PCR efficiency ranged from 95% to 105%. All samples were assayed in triplicate wells. Real-time PCR was performed on a iCycler (Bio-Rad). Samples were amplified in a 25-μL reaction containing 1x SYBR Green Master Mix (Bio-Rad) and 300 nm of each primer. The thermal profile consisted of 1 cycle at 95°C for 3 min 30 s followed by 40 cycles at 95°C for 30 s and at 58°C for 30 s. For each run, data acquisition and analysis was done using the iCycler iQ software (version 3.0a, Bio-Rad). The transcript abundance in treated samples relative to untreated samples was determined using a comparative cycle threshold method. The relative abundance of ACT2 mRNAs in each sample was determined and used to normalize for differences of total RNA amount according to the method described by Vandesompele et al. (2002).
Statistical Analysis
Wax and cutin data were analyzed using SAS 9.1.3 software (SAS Institute Inc.). Student's t tests were used to analyze data when the assumptions of normality and homoscedasticity were met. Welch-Satterthwaite t tests were used to analyze data when assumptions of normality and homoscedasticity were not met.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At1g49240, At1g02205, At4g24510, At5g57800, At4g33790, At1g51500, At1g17840, At3g60500, At1g68530, At1g67730, At3g55360, At2g47240, At1g49430, At1g64400, At4g00360, At1g01610, At4g00400, At2g45970, At1g72970, At1g64670, At5g11190, At5g25390, At3g28910, At4g28110, and At3g59420.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Primers used for quantitative RT-PCR of select cuticle gene.
Supplementary Material
Acknowledgments
We would like to thank Debra Sherman and Chia-Ping Huang of the Purdue University Electron Microscopy Center.
This work was supported by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (grant no. 2006–35304–17323) and, in part, by the Ministère de l'Enseignement Supèrieur et de la Recherche (France; doctoral fellowship to B.B. and A.B.). This work also received support from the Centre National de la Recherche Scientifique and the Université Victor Segalen Bordeaux 2.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Matthew A. Jenks (jenksm@purdue.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aarts MGM, Keijzer CJ, Stiekema WJ, Pereira A (1995) Molecular characterization of the CER1 gene of Arabidopsis involved in epicuticular wax biosynthesis and pollen fertility. Plant Cell 7 2115–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15 413–428 [Google Scholar]
- Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24 23–58 [Google Scholar]
- Bessire M, Chassot C, Jacquat AC, Humphry M, Borel S, Petétot JC, Métreaux JP, Nawrath C (2007) A permeable cuticle in Arabidopsis leads a strong resistance in Botrytis cinera. EMBO J 26 2158–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure G, Beisson F, Ohlrogge J, Pollard M (2004) Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6,cis-9-diene-1,18-dioate as the major component. Plant J 40 920–930 [DOI] [PubMed] [Google Scholar]
- Bondada BR, Oosterhuis DM, Murphy JB, Kim KS (1996) Effect of water stress on the epicuticular wax composition and ultrastructure of cotton (Gossypium hirsutum L.) leaf, bract, and boll. Environ Exp Bot 36 61–67 [Google Scholar]
- Buchholz A (2006) Characterization of the diffusion of non-electrolytes across plant cuticles: properties of the lipophilic pathway. J Exp Bot 57 2501–2513 [DOI] [PubMed] [Google Scholar]
- Burghardt M, Riederer M (2006) Cuticular transpiration. In M Riederer, C Müller, eds, Biology of the Plant Cuticle. Blackwell Publishing, Oxford, pp 292–311
- Cameron KD, Teece MA, Smart LB (2006) Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiol 140 176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XB, Goodwin SM, Boroff VL, Liu XL, Jenks MA (2003) Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 15 1170–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominelli E, Sala T, Calvi D, Gusmaroli G, Tonelli C (2008) Over-expression of the Arabidopsis AtMYB41 gene alters cell expansion and leaf surface permeability. Plant J 53 53–56 [DOI] [PubMed] [Google Scholar]
- Duan H, Schuler M (2005) Differential expression and evolution of the Arabidopsis CYP86A subfamily. Plant Physiol 137 1067–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14 S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin SM, Jenks M (2005) Plant cuticle function as a barrier to water loss. In M Jenks, PM Hasegawa, eds, Plant Abiotic Stress. Blackwell Publishing, Oxford, pp 14–36
- Graça J, Schreiber L, Rodrigues J, Pereira H (2002) Glycerol and glyceryl esters of ω-hydroxyacids in cutins. Phytochemistry 61 205–215 [DOI] [PubMed] [Google Scholar]
- Grncarevic M, Radler F (1967) The effect of wax components on cuticular transpiration-model experiments. Planta 75 23–27 [DOI] [PubMed] [Google Scholar]
- Hajibagheri M, Hall J, Flowers T (1983) The structure of the cuticle in relation to cuticular transpiration in leaves of the halophyte Suaeda maritima (L.) Dum. New Phytol 94 125–131 [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51 463–499 [DOI] [PubMed] [Google Scholar]
- Hazrika BN (2003) Acclimatization of tissue-cultured plants. Curr Sci 85 1704–1712 [Google Scholar]
- Hooker TS, Millar AA, Kunst L (2002) Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis. Plant Physiol 129 1568–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis RG, Mansfield TA (1980) Reduced stomatal responses to light, carbon dioxide and abscisic acid in the presence of sodium ions. Plant Cell Environ 3 279–283 [Google Scholar]
- Jefferson P, Johnson D, Rumbaugh M, Asay K (1989) Water stress and genotypic effects on epicuticular wax production of alfalfa and crested wheatgrass in relation to yield and excised leaf water loss rate. Can J Plant Sci 69 481–490 [Google Scholar]
- Jenks M (2002) Critical issues with the plant cuticle's function in drought tolerance. In AJ Wood, ed, Biochemical & Molecular Responses of Plants to the Environment. Research Signposts, Kerala, India, pp 97–127
- Jenks MA, Andersen L, Teusink RS, Williams MH (2001) Leaf cuticular waxes of potted rose cultivars as affected by plant development, drought and paclobutrazol treatments. Physiol Plant 112 62–70 [DOI] [PubMed] [Google Scholar]
- Jenks MA, Tuttle HA, Eigenbrode SD, Feldmann KA (1995) Leaf epicuticular waxes of the eceriferum mutants in Arabidopsis. Plant Physiol 108 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetter R, Kunst L, Samuels AL (2006) Composition of plant cuticular waxes. In M Riederer, C Müller, eds, Biology of the Plant Cuticle. Blackwell Publishing, Oxford, pp 144–181
- Joubès J, Raffaele S, Bourdenx B, Garcia C, Laroche-Traineau J, Moreau P, Domergue F, Lessire R (2008) The VLCFA elongase gene family in Arabidopsis thaliana: phylogenetic analysis, 3D modelling and expression profiling. Plant Mol Biol 67 547–566 [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27 137A–138A [Google Scholar]
- Kerstiens G (1996) Cuticular water permeability and its physiological significance. J Exp Bot 47 1813–1832 [Google Scholar]
- Kim K, Park S, Jenks M (2007. a) Influence of water deficit on leaf cuticular waxes of soybean. Int J Plant Sci 168 307–316 [Google Scholar]
- Kim KS, Park SH, Jenks MA (2007. b) Changes in leaf cuticular waxes of sesame (Sesamum indicum L.) plants exposed to water deficit. J Plant Physiol 164 1134–1143 [DOI] [PubMed] [Google Scholar]
- Klute CH, Franklin PJ (1958) The permeation of water vapor through polyethylene. J Polym Sci 32 161–176 [Google Scholar]
- Kolattukudy PE (2001. a) Cutin from plants. In Y Doi, A Steinbuchel, eds, Biopolymers, Vol 3a. Wiley-VCH, Münster, Germany, pp 1–35
- Kolattukudy PE (2001. b) Polyesters in higher plants. Adv Biochem Eng Biotechnol 71 1–49 [DOI] [PubMed] [Google Scholar]
- Kosma DK, Jenks MA (2007) Eco-physiological and molecular-genetic determinants of plant cuticle function in drought and salt stress tolerance. In MA Jenks, PM Hasegawa, SM Jain, eds, Advances in Molecular Breeding toward Drought and Salt Tolerant Crops. Springer, Dordrecht, The Netherlands, pp 91–120
- Kurdyukov S, Faust A, Trenkamp S, Bar S, Franke R, Efremova N, Tietjen K, Schreiber L, Saedler H, Yephremov A (2006) Genetic and biochemical evidence for involvement of HOTHEAD in the biosynthesis of long-chain α-,ω-dicarboxylic fatty acids and formation of extracellular matrix. Planta 224 315–329 [DOI] [PubMed] [Google Scholar]
- Lasoski SW, Cobbs WH (1959) Moisture permeability of polymers. I. Role of crystallinity and orientation. J Polym Sci 36 21–33 [Google Scholar]
- Lolle SJ, Hsu W, Pruitt RE (1998) Genetic analysis of organ fusion in Arabidopsis thaliana. Genetics 149 607–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü S, Song T, Kosma DK, Parsons EP, Rowland O, Jenks MA (2009) Arabidopsis CER8 encodes a long-chain acyl CoA synthetase 1 (LACS1) and has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J 59 553–564 [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56 165–185 [DOI] [PubMed] [Google Scholar]
- Pighin JA, Zheng HQ, Balakshin LJ, Goodman IP, Western TL, Jetter R, Kunst L, Samuels AL (2004) Plant cuticular lipid export requires an ABC transporter. Science 306 702–704 [DOI] [PubMed] [Google Scholar]
- Pollard M, Beisson F, Li Y, Ohlrogge J (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13 236–246 [DOI] [PubMed] [Google Scholar]
- Riederer M, Schreiber L (1995) Waxes—the transport barriers of plant cuticles. In RJ Hamilton, ed, Waxes: Chemistry, Molecular Biology and Functions. The Oily Press, Dundee, Scotland, pp 130–156
- Samdur MY, Manivel P, Jain VB, Chikani BM, Gor HK, Desai S, Misra JB (2003) Genotypic differences and water-deficit induced enhancement in epicuticular wax load in peanut. Crop Sci 43 1294–1299 [Google Scholar]
- Samuels AL, Kunst L, Jetter R (2008) Sealing plant surfaces: cuticular wax formation by epidermal cells. Annu Rev Plant Biol 59 683–707 [DOI] [PubMed] [Google Scholar]
- Sangaj NS, Malshe VC (2004) Permeability of polymers in protective organic coatings. Prog Org Coat 50 28–39 [Google Scholar]
- Schnurr J, Shockey J, Browse J (2004) The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 16 629–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L (2006) Characterisation of polar paths of transport in plant cuticles. In M Riederer, ed, Biology of the Plant Cuticle. Blackwell Publishing, Oxford, pp 280–291
- Seiler JR (1985) Morphological and physiological changes in black alder induced by water stress. Plant Cell Environ 8 219–222 [Google Scholar]
- Shepherd T, Wynne Griffiths D (2006) The effects of stress on plant cuticular waxes. New Phytol 171 469–499 [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58 221–227 [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle K, Dobbs EB, Short KC (1983) In vitro acclimatization of aseptically cultured plantlets to humidity. J Am Soc Hortic Sci 108 386–389 [Google Scholar]
- Williams MH, Rosenqvist E, Buchhave M (1999) Response of potted miniature roses (Rosa × hybrida) to reduced water availability during production. J Hortic Sci Biotechnol 74 301–308 [Google Scholar]
- Williams MH, Rosenqvist E, Buchhave M (2000) The effect of reducing production water availability on the post-production quality of potted miniature roses (Rosa × hybrida). Postharvest Biol Technol 18 143–150 [Google Scholar]
- Wetzstein HY, Sommer HE (1982) Leaf anatomy of tissue cultured Liquidambar styraciflua (Hamamelidaceae) during acclimatization. Am J Bot 69 1579–1586 [Google Scholar]
- Xiao FM, Goodwin SM, Xiao YM, Sun ZY, Baker D, Tang XY, Jenks MA, Zhou JM (2004) Arabidopsis CYP86A2 represses Pseudomonas syringae type III genes and is required for cuticle development. EMBO J 23 2903–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.