Abstract
The activation of Rubisco in vivo requires the presence of the regulatory protein Rubisco activase. To elucidate its role in maintaining CO2 assimilation rate at high temperature, we examined the temperature response of CO2 assimilation rate at 380 μL L−1 CO2 concentration (A380) and Rubisco activation state in wild-type and transgenic tobacco (Nicotiana tabacum) with reduced Rubisco activase content grown at either 20°C or 30°C. Analyses of gas exchange and chlorophyll fluorescence showed that in the wild type, A380 was limited by ribulose 1,5-bisphosphate regeneration at lower temperatures, whereas at higher temperatures, A380 was limited by ribulose 1,5-bisphosphate carboxylation irrespective of growth temperatures. Growth temperature induced modest differences in Rubisco activation state that declined with measuring temperature, from mean values of 76% at 15°C to 63% at 40°C in wild-type plants. At measuring temperatures of 25°C and below, an 80% reduction in Rubisco activase content was required before Rubisco activation state was decreased. Above 35°C, Rubisco activation state decreased slightly with more modest decreases in Rubisco activase content, but the extent of the reductions in Rubisco activation state were small, such that a 55% reduction in Rubisco activase content did not alter the temperature sensitivity of Rubisco activation and had no effect on in vivo catalytic turnover rates of Rubisco. There was a strong correlation between Rubisco activase content and Rubisco activation state once Rubisco activase content was less that 20% of wild type at all measuring temperatures. We conclude that reduction in Rubisco activase content does not lead to an increase in the temperature sensitivity of Rubisco activation state in tobacco.
The catalytic sites of Rubisco must be activated for CO2 fixation to take place. This requires the carbamylation of a Lys residue at the catalytic sites to allow the binding of Mg2+ and ribulose 1,5-bisphosphate (RuBP; Andrews and Lorimer, 1987). Rubisco activase facilitates carbamylation and the maintenance of Rubisco activity by removing inhibitors such as tight-binding sugar phosphates from Rubisco catalytic sites in an ATP-dependent manner (Andrews, 1996; Spreitzer and Salvucci, 2002; Portis, 2003; Parry et al., 2008). The activity of Rubisco activase is regulated by the ATP/ADP ratio and redox state in the chloroplast (Zhang and Portis, 1999; Zhang et al., 2002; Portis, 2003).
In many plant species, Rubisco activation state decreases at high temperature in vivo (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004b; Cen and Sage, 2005; Yamori et al., 2006b; Makino and Sage, 2007). However, it is unclear what the primary mechanisms underlying the inhibition of Rubisco activation are and whether Rubisco deactivation limits CO2 assimilation rate at high temperature. It has been proposed that Rubisco activation state decreases at high temperature, because the activity of Rubisco activase is insufficient to keep pace with the faster rates of Rubisco inactivation at high temperatures (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004a, 2004c; Kim and Portis, 2006). In in vitro assays using purified Rubisco and Rubisco activase, the activity of Rubisco activase was sufficient for the activation of Rubisco at the optimum temperature but not at high temperatures (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004a, 2004c). ATP hydrolysis activity of Rubisco activase in vitro has varying temperature optima among species (e.g. 25°C in Antarctic hairgrass [Deschampsia antarctica] and spinach [Spinacia oleracea] but 35°C in tobacco [Nicotiana tabacum] and cotton [Gossypium hirsutum]), and Rubisco activase more readily dissociates into inactive forms at high temperature, causing a loss of Rubisco activase capacity (Crafts-Brandner and Law, 2000; Salvucci and Crafts-Brandner, 2004b). Moreover, the rates of inhibitor formation by misprotonation of RuBP during catalysis increased at higher temperatures (Salvucci and Crafts-Brandner, 2004c; Kim and Portis, 2006). CO2 assimilation rates and plant growth were improved under heat stress in transgenic Arabidopsis expressing thermotolerant Rubisco activase isoforms generated by either gene-shuffling technology (Kurek et al., 2007) or chimeric Rubisco activase constructs (Kumar et al., 2009). These results support the view that the reduction of Rubisco activase activity limits the Rubisco activation and, therefore, the CO2 assimilation rates at high temperatures.
It has also been suggested that the decrease in CO2 assimilation rate at high temperatures is caused by a limitation of RuBP regeneration capacity (e.g. electron transport capacity) rather than by Rubisco deactivation per se (Schrader et al., 2004; Wise et al., 2004; Cen and Sage, 2005; Makino and Sage, 2007; Kubien and Sage, 2008). These groups suggest that Rubisco deactivation at high temperature may be a regulatory response to the limitation of one of the processes contributing to electron transport capacities. For example, at high temperature, protons can leak through the thylakoid membrane, impairing the coupling of ATP synthesis to electron transport (Pastenes and Horton, 1996; Bukhov et al., 1999, 2000). As the electron transport capacity becomes limiting, ATP/ADP ratios and the redox potential of the chloroplast decline, causing a loss of Rubisco activase activity and, in turn, a reduction in the Rubisco activation state (Zhang and Portis, 1999; Zhang et al., 2002; Sage and Kubien, 2007). Based on this understanding, the decline in the Rubisco activation state at high temperature may be a regulated response to a limitation in electron transport capacity rather than a consequence of a direct effect of heat on the integrity of Rubisco activase.
Temperature dependence of CO2 assimilation rate shows a considerable variation with growth temperature (Berry and Björkman, 1980; Hikosaka et al., 2006; Sage and Kubien, 2007). Plants grown at low temperature generally exhibit higher CO2 assimilation rates at low temperatures compared with plants grown at high temperature, but they exhibit lower rates at high temperature. Furthermore, both the temperature response of Rubisco activation state and the limiting step of CO2 assimilation rate (a Rubisco versus RuBP regeneration limitation) have been shown to differ depending on growth temperature (Hikosaka et al., 1999; Onoda et al., 2005; Yamori et al., 2005, 2006a, 2006b, 2008). This suggests that the regulation of Rubisco activation state could also differ in plants grown at different growth temperatures. Here, we analyzed the effects of Rubisco activase content on Rubisco activation state and CO2 assimilation rate at leaf temperatures ranging from 15°C to 40°C in tobacco grown under two different temperature regimes (day/night temperatures of 20°C/15°C or 30°C/25°C). We used wild-type and transgenic tobacco with a range of reductions in Rubisco activase content to examine the dependence of Rubisco activation on Rubisco activase content over the range of leaf temperatures (Mate et al., 1993, 1996).
RESULTS
Temperature Acclimation of Photosynthesis in the Wild Type
Growth temperature had a large effect on leaf properties. Leaf mass per area and contents of Rubisco, cytochrome f, and chlorophyll were greater in wild-type plants grown at 20°C compared with 30°C. Contents of Rubisco and cytochrome f were 25.7% and 27.7% greater in 20°C-grown plants than in 30°C-grown plants, respectively (Table I). Thus, the cytochrome f/Rubisco ratio did not change with growth temperature. On the other hand, Rubisco activase contents were similar irrespective of growth temperatures, such that the activase/Rubisco ratio was greater in 30°C-grown plants. Assuming molecular masses of 42 kD for the Rubisco activase monomer and 550 kD for Rubisco, the activase/Rubisco ratio (mol mol−1) averaged 0.91 and 1.32 in 20°C- and 30°C-grown plants, respectively. Although the activase/Rubisco ratio was greater in 30°C-grown plants than in 20°C-grown plants, Rubisco activation was slightly greater in 20°C- compared with 30°C-grown wild-type plants (Tables I and II; Fig. 1).
Table I.
Quantification of photosynthetic components
Leaf mass per area, contents of Rubisco activase, Rubisco, cytochrome f, and chlorophyll, and chlorophyll a/b ratio were quantified. We classified plants into three groups with respect to Rubisco activase contents in 20°C- and 30°C-grown plants: the wild type (20°C), 123.2 to 160.0 mg m−2; plants with intermediate Rubisco activase contents (20°C), 37.1 to 94.6 mg m−2; plants with low Rubisco activase contents (20°C), 8.0 to 12.4 mg m−2; the wild type (30°C), 136.4 to 165.0 mg m−2; plants with intermediate Rubisco activase contents (30°C), 32.1 to 99.8 mg m−2; plants with low Rubisco activase contents (30°C), 7.5 to 13.5 mg m−2. Activase content (μmol m−2) was calculated as a monomer of 42 kD, whereas Rubisco content (μmol m−2) was calculated as a hexadecamer of 550 kD. Data represent means ± se; n = 4. Different letters show significant differences (Tukey-Kramer multiple comparison test; P < 0.05). Asterisks next to wild-type (20°C) values indicate significant differences between data in wild-type plants grown at 20°C and 30°C (Student's t test): * P < 0.05, ** P < 0.01, *** P < 0.001.
| Component | 20°C-Grown Plants |
30°C-Grown Plants |
||||
|---|---|---|---|---|---|---|
| Wild Type | Intermediate | Low | Wild Type | Intermediate | Low | |
| Leaf mass per area (g m−2) | 26.3 ± 0.7a** | 25.7 ± 0.9 a | 26.0 ± 0.7 a | 20.3 ± 0.8 a | 20.5 ± 0.3 a | 20.5 ± 0.7 a |
| Activase (mg m−2) | 142.6 ± 8.3a | 63.6 ± 11.9 b | 10.3 ± 1.2 c | 154.2 ± 6.7 a | 68.5 ± 15.4 b | 10.2 ± 1.4 c |
| Activase (μmol m−2) | 3.39 ± 0.20a | 1.51 ± 0.28 b | 0.24 ± 0.03 c | 3.67 ± 0.16 a | 1.63 ± 0.37 b | 0.24 ± 0.03 c |
| Rubisco (g m−2) | 2.06 ± 0.03a*** | 1.88 ± 0.07 a | 1.89 ± 0.08 a | 1.53 ± 0.04 a | 1.47 ± 0.08 a | 1.64 ± 0.08 a |
| Rubisco (μmol m−2) | 3.75 ± 0.06a*** | 3.42 ± 0.10 a | 3.44 ± 0.14 a | 2.79 ± 0.07 a | 2.67 ± 0.15 a | 2.98 ± 0.15 a |
| Activase/Rubisco (mol mol−1) | 0.91 ± 0.06a** | 0.45 ± 0.09 b | 0.07 ± 0.01 c | 1.32 ± 0.06 a | 0.61 ± 0.14 b | 0.08 ± 0.01 c |
| Cytochrome f (μmol m−2) | 1.95 ± 0.07a*** | 2.00 ± 0.05 a | 1.90 ± 0.12 a | 1.41 ± 0.02 a | 1.33 ± 0.04 a | 1.30 ± 0.02 a |
| Chlorophyll (μmol m−2) | 532.4 ± 25.2a* | 511.3 ± 22.9 a | 462.1 ± 10.5 a | 433.3 ± 14.4 a | 448.2 ± 41.4 a | 403.1 ± 12.2 a |
| Chlorophyll a/b | 3.73 ± 0.14a | 3.81 ± 0.13 a | 3.72 ± 0.03 a | 3.68 ± 0.02 a | 3.54 ± 0.12 a | 3.75 ± 0.19 a |
Table II.
Physiological characteristics in plants grown at 20°C or 30°C
CO2 assimilation rate at 1,500 μmol photons m−2 s−1 and 380 μL L−1 CO2 concentration (A380), the Rubisco activation state, the NADP-MDH activation state, and in vivo catalytic turnover rate of Rubisco were analyzed. The catalytic turnover rate of Rubisco was calculated from gross CO2 assimilation rates (A380 + dark respiration) and Rubisco carbamylated site contents. Dark respiration was measured after a 10-h dark period. Different letters show significant differences (Tukey-Kramer multiple comparison test; P < 0.05).
| Characteristic | 25°C |
40°C |
||||
|---|---|---|---|---|---|---|
| Wild Type | Intermediate | Low | Wild Type | Intermediate | Low | |
| 20°C-grown plants | ||||||
| A380 (μmol CO2 m−2 s−1) | 19.3 ± 0.3 a | 17.7 ± 0.5 a | 8.00 ± 0.95 b | 16.7 ± 0.5 a | 12.0 ± 0.76 b | 2.36 ± 0.99 c |
| Rubisco activation (%) | 81.2 ± 0.4 a | 75.9 ± 2.3 a | 55.3 ± 3.3 b | 64.0 ± 0.6 a | 51.2 ± 2.2 b | 37.9 ± 2.2 c |
| NADP-MDH activation (%) | 51.5 ± 3.9 a | 49.9 ± 2.0 a | 50.5 ± 2.6 a | 23.7 ± 1.7 a | 26.5 ± 1.2 a | 26.7 ± 0.8 a |
| In vivo catalytic turnover rate of Rubisco (s−1) | 0.949 ± 0.055 a | 0.986 ± 0.038 a | 0.656 ± 0.084 b | 1.23 ± 0.08 a | 1.29 ± 0.09 a | 0.737 ± 0.158 b |
| 30°C-grown plants | ||||||
| A380 (μmol CO2 m−2 s−1) | 16.0 ± 0.4 a | 15.8 ± 0.3 a | 8.56 ± 1.77 b | 15.1 ± 0.4 a | 12.6 ± 0.6 a | 3.92 ± 1.25 b |
| Rubisco activation (%) | 75.8 ± 0.5 a | 70.9 ± 2.0 a | 50.0 ± 2.7 b | 62.3 ± 0.7 a | 53.2 ± 1.7 b | 37.5 ± 1.9 c |
| NADP-MDH activation (%) | 61.0 ± 3.6 a | 62.8 ± 2.9 a | 59.5 ± 1.0 a | 38.0 ± 3.4 a | 41.2 ± 2.1 a | 37.2 ± 0.9 a |
| In vivo catalytic turnover rate of Rubisco (s−1) | 1.11 ± 0.07 a | 1.20 ± 0.10 a | 0.849 ± 0.121 b | 1.55 ± 0.10 a | 1.60 ± 0.08 a | 1.00 ± 0.13 b |
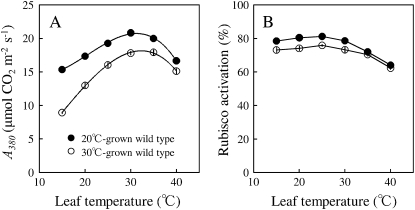
Figure 1.
Temperature dependence of CO2 assimilation rate at 1,500 μmol photons m−2 s−1 and 380 μL L−1 CO2 concentration (A380; A) and the Rubisco activation state (B) in wild-type plants grown at 20°C/15°C (black symbols) and 30°C/25°C (white symbols). The optimum temperatures for A380 were 31.6°C ± 0.7°C and 33.2°C ± 0.6°C in 20°C- and 30°C-grown plants, respectively. Data represent means ± se; n = 4.
The temperature response of CO2 assimilation rate at 380 μL L−1 (A380) and Rubisco activation state was different depending on growth temperature (Fig. 1). The optimum temperature of A380 was higher in 30°C-grown plants than in 20°C-grown plants. A380 measured at 20°C in 20°C-grown plants was similar to A380 measured at 30°C in 30°C-grown plants. This is known as the temperature homeostasis of photosynthesis and has been observed in other species (Hikosaka et al., 2006; Yamori et al., 2008, 2009). Rubisco activation state below 25°C was high and constant irrespective of growth temperatures, whereas above 30°C, Rubisco activation state decreased both in 20°C-grown plants and 30°C-grown plants. The extent of reduction in Rubisco activation state at high temperature was slightly greater in 20°C-grown plants than in 30°C-grown plants. Rubisco activation state at 40°C decreased by 17.1% ± 0.8% in 20°C-grown plants and by 13.5% ± 1.1% in 30°C-grown plants (Table II).
Relationships between Rubisco Activase Content, CO2 Assimilation Rate, and Rubisco Activation State
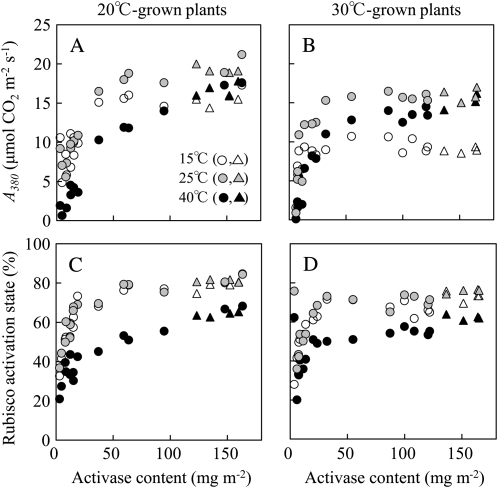
Figure 2 shows the relationship between A380 and Rubisco activation state and Rubisco activase content at 15°C, 25°C, and 40°C (for all measured leaf temperatures, see Supplemental Figs. S1 and S2; for the dependence of A380 and Rubisco activation state on activase/Rubisco ratio, see Supplemental Fig. S3). Progeny of several primary transformants were used to generate the observed variation of activase content. At 15°C and 25°C, an 80% reduction in Rubisco activase content was required before A380 or Rubisco activation state was decreased in both 20°C- and 30°C-grown plants, whereas at 40°C, A380 and Rubisco activation state decreased slightly, with a more modest decrease in Rubisco activase content, particularly in 20°C-grown plants. In both 20°C- and 30°C-grown plants, A380 and Rubisco activation state were drastically decreased when Rubisco activase content was less than 20% of wild-type levels, irrespective of leaf temperatures (Fig. 2).
Figure 2.
CO2 assimilation rate at 380 μL L−1 CO2 concentration (A380; A and B) and the Rubisco activation state (C and D) as a function of Rubisco activase contents in wild-type (triangles) and antisense (circles) lines. Plants were grown at 20°C/15°C (A and C) or 30°C/25°C (B and D). A380 and Rubisco activation state was determined at leaf temperatures of 15°C, 25°C, and 40°C.
We classified plants into three groups with respect to Rubisco activase contents in 20°C- and 30°C-grown plants (Table I; Fig. 2): for the wild type (20°C), 123.2 to 160.0 mg m−2; for intermediate Rubisco activase contents (20°C), 37.1 to 94.6 mg m−2; for low Rubisco activase contents (20°C), 8.0 to 12.4 mg m−2; for the wild type (30°C), 136.4 to 165.0 mg m−2; for intermediate Rubisco activase contents (30°C), 32.1 to 99.8 mg m−2; for low Rubisco activase contents (30°C), 7.5 to 13.5 mg m−2. The contents of Rubisco, cytochrome f, and chlorophyll were similar between wild-type and antisense lines in both 20°C- and 30°C-grown plants (Table I). NADP-malate dehydrogenase (MDH) activation state, which is indicative of the redox status in the chloroplast (Scheibe and Stitt, 1988), was greater at 25°C compared with 40°C but was also similar between wild-type and antisense lines, irrespective of growth temperatures (Table II). Thus, there were no apparent differences between the leaf properties of wild-type and antisense lines, other than their Rubisco activase contents, that could confound interpretation of their temperature response of CO2 assimilation and Rubisco activation state.
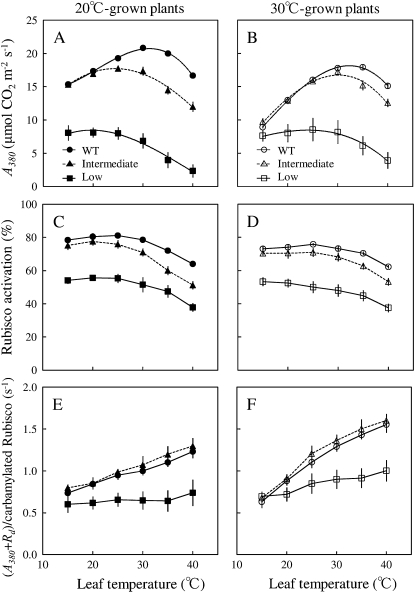
When comparing the three groups of plants, it is apparent that the temperature dependence of Rubisco activation is not very different, although there was a slightly greater decline in Rubisco activation state from 25°C to 40°C in plants with intermediate and low activase levels (Fig. 3; Table II). The in vivo catalytic turnover rate of Rubisco, which was estimated from gross photosynthetic rate (A380 + dark respiration) and the carbamylated active site content of Rubisco, increased as expected with temperature and was similar between the wild type and plants with intermediate Rubisco activase levels (Fig. 3). This demonstrates that the differences in Rubisco activation state quantitatively accounted for differences in observed changes in CO2 assimilation rate. In plants with low activase content, in vivo turnover rates were reduced, suggesting inhibitor binding to Rubisco catalytic sites (He et al., 1997).
Figure 3.
Temperature responses of A380 (A and B), Rubisco activation state (C and D), and in vivo catalytic turnover rate of Rubisco (E and F) in plants grown at 20°C/15°C (black symbols) and 30°C/25°C (white symbols). Three groups were classified with respect to Rubisco activase contents (Table I): wild type (WT; circles), plants with intermediate Rubisco activase contents (triangles), and plants with low Rubisco activase contents (squares). The catalytic turnover rate of Rubisco was calculated from gross CO2 assimilation rates (A380 + dark respiration) and Rubisco carbamylated site content. Dark respiration was measured after a 10-h dark period. The optimum temperatures for A380 in 20°C-grown plants were 31.6°C ± 0.7°C, 25.3°C ± 1.5°C, and 18.2°C ± 1.3°C in wild-type, intermediate, and low Rubisco activase plants, respectively. Optimum temperatures for A380 in 30°C-grown plants were 33.2°C ± 0.6°C, 29.9°C ± 1.0°C, and 24.1°C ± 1.2°C in wild-type, intermediate, and low Rubisco activase plants, respectively. Data represent means ± se; n = 4.
Limiting Step of the CO2 Assimilation Rate
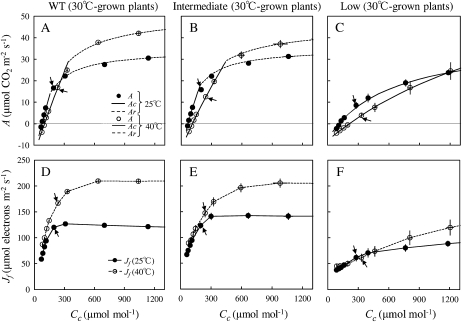
CO2 response curves of CO2 assimilation rate (A) and chloroplast electron transport rates estimated from chlorophyll fluorescence (Jf) were measured to determine under what conditions A380 was limited by Rubisco or RuBP regeneration (Fig. 4 for plants grown at 30°C/25°C; Supplemental Fig. S4 for plants grown at 20°C/15°C). Chloroplast CO2 concentration (Cc) was calculated from intercellular CO2 (Ci) as described in “Materials and Methods.” A-Cc responses were then fitted to the C3 photosynthesis model (Farquhar et al., 1980). In the wild type and plants with intermediate activase content, the curves showed a transition from Rubisco-limited A (Ac) at lower CO2 concentrations to RuBP regeneration-limited A (Ar) at higher CO2 concentrations at all leaf temperatures irrespective of growth temperature (Fig. 4, A, B, D, and E). In plants with low activase content, CO2 assimilation rate was always limited by Rubisco (Fig. 4, C and F).
Figure 4.
A to C, CO2 response of CO2 assimilation rate (A) measured at 25°C and 40°C and 1,500 μmol photons m−2 s−1 in wild-type (WT) plants (A), plants with intermediate Rubisco activase contents (B), and plants with low Rubisco activase contents (C) grown at 30°C/25°C. The explanation of plant classification is given in Table I. A at 25°C is shown as black circles, whereas A at 40°C is shown as white circles. Rubisco-limited A (Ac; solid lines) was estimated from Equation 1, whereas RuBP regeneration-limited A (Ar; dotted lines) was estimated from Equation 2. D to F, Electron transport rate from chlorophyll fluorescence (Jf) as a function of chloroplast CO2 concentration (Cc) at 25°C and 40°C in wild-type plants (D), plants with intermediate Rubisco activase contents (E), and plants with low Rubisco activase contents (F) grown at 20°C/15°C. Jf at 25°C is shown as black circles, whereas Jf at 40°C is shown as white circles. Arrows show measurements made at 380 μL L−1 CO2 concentration. Data represent means ± se; n = 4.
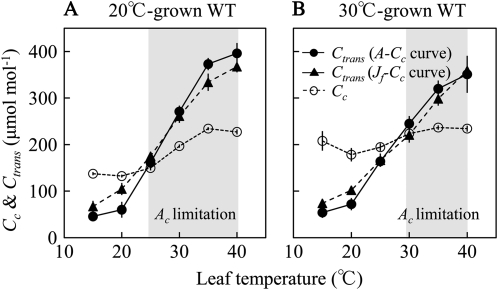
The Cc measured under Ca = 380 μmol mol−1 was compared with the chloroplast CO2 concentration at which the transition from Rubisco to RuBP regeneration limitation occurs (Ctrans). Ctrans was estimated from two methods. First, the A-Cc curve was analyzed for Ctrans (A-Cc curve), based on the C3 photosynthesis model (see Eq. 3 below; Fig. 4, A–C). Second, Jf-Cc curves were analyzed to obtain the chloroplast CO2 concentration above which electron transport rate (Jf) is constant [Ctrans (Jf-Cc curve); Fig. 4, D–F]. Ctrans values estimated from these two independent methods were similar and increased with temperature (Fig. 5). The analysis showed that A380 was limited by Rubisco above 25°C in 20°C-grown wild-type plants (Fig. 5A) and above 30°C in 30°C-grown wild-type plants (Fig. 5B). On the other hand, A380 was limited by RuBP regeneration below 20°C in 20°C-grown wild-type plants (Fig. 5A) and below 25°C in 30°C-grown wild-type plants (Fig. 5B). The difference between the two growth temperatures was primarily due to differences in Cc measured under Ca = 380 μmol mol−1 rather than to differences in the relationship between Ctrans and temperature (Fig. 5).
Figure 5.
Temperature dependence of chloroplast CO2 concentration (Ctrans) at which the transition from Rubisco (Ac) to RuBP regeneration (Ar) limitation occurs (black symbols) and chloroplast CO2 concentration (Cc) for CO2 assimilation rate measured at Ca = 380 μmol mol−1 (white symbols) in 20°C-grown wild-type (WT; A) and 30°C-grown wild-type (B) plants. Ctrans was analyzed from two methods. First, A-Cc curve was analyzed for Ctrans (A-Cc curve; black circles), based on the C3 photosynthesis model (Eq. 3). Second, Jf-Cc curve was analyzed to obtain the chloroplast CO2 concentration at which electron transport rate (Jf) remains constant [Ctrans (Jf-Cc curve); black triangles]. Cc for A380 less than the Ctrans indicates that CO2 assimilation is limited by Ar, whereas Cc for A380 above Ctrans indicates that CO2 assimilation is limited by Ac (gray areas). Data represent means ± se; n = 4.
DISCUSSION
Rubisco Activity Limits CO2 Assimilation Rate at High But Not at Low Temperature in Tobacco
There has been controversy in the literature regarding what causes the decline in CO2 assimilation rate at high temperature and the role that Rubisco activase plays in the regulation of CO2 assimilation under these conditions. Our measurements of CO2 response curves of CO2 assimilation rate and chloroplast electron transport rates estimated from chlorophyll fluorescence (Jf) provided two independent means to assess the limitations and showed that in tobacco, A380 was limited by RuBP regeneration only at low temperature, whereas at high temperature A380 was limited by Rubisco (Fig. 5). Thus, decreases in Rubisco activation state reduce the potential of CO2 assimilation rates at high temperature. For some species, Rubisco limitation has been observed at high temperature (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004a, 2004c; Yamori et al., 2006a, 2006b, 2008); however, for other species, RuBP regeneration has been reported as the major limitation at high temperature (Wise et al., 2004; Cen and Sage, 2005; Makino and Sage, 2007). There is considerable species variation in the limiting step of CO2 assimilation rate (Yamori et al., 2010). Since A380 at low temperature was limited by RuBP regeneration in 20°C- and 30°C-grown plants (Fig. 5), Rubisco activation state did not affect A380. Cen and Sage (2005) suggested that Rubisco activation state decreased at low temperature due to a regulatory feedback reflecting limitations in the RuBP regeneration capacity. However, some studies found little evidence for decreases in Rubisco activation state at low temperatures in spinach (Yamori et al., 2006b), rice (Oryza sativa; Makino and Sage, 2007), and tobacco (Kubien and Sage, 2008; this study). Therefore, at lower temperatures, Rubisco activation state is maintained at high levels in many plant species and does not limit CO2 assimilation rate.
Tobacco plants acclimated to low growth temperature with an increase in leaf thickness and several photosynthetic components (Table I), but there was no change in the balance between Rubisco capacity and electron transport capacity, and this was also reflected by the fact that the Ctrans, the chloroplast CO2 at which the transition from Rubisco to RuBP regeneration limitation occurs, was independent of growth temperature (Fig. 5). Interestingly, Rubisco activase contents did not vary with growth temperature. Thus, the activase/Rubisco ratio was greater in 30°C-grown plants than in 20°C-grown plants, but Rubisco activation was nevertheless slightly less in 30°C-grown plants (Table I; Fig. 1). The observed variation in Rubisco activation state with temperature was small (approximately 15%) and was also less than what has been observed for some other species (Salvucci and Crafts-Brandner, 2004b; Cen and Sage, 2005; Kim and Portis, 2005; Yamori et al., 2005). The fact that CO2 assimilation rate in tobacco is Rubisco limited at high temperature makes it an ideal experimental system to examine the relationship between Rubisco activase content, Rubisco activation state, and CO2 assimilation rate.
Regulation of Rubisco Activation State and CO2 Assimilation Rate at High and Low Temperatures
The dependence of Rubisco activation state on Rubisco activase content at the various leaf temperatures (Fig. 2; Supplemental Fig. S2) is very similar to previous observations in tobacco made at 25°C (Mate et al., 1996) and in Flaveria bidentis, a C4 dicot (Hendrickson et al., 2008). That is, when Rubisco activase is present at levels less than 20% of the wild type, both Rubisco activation and CO2 assimilation rate are severely reduced, but a 50% reduction in Rubisco activase levels has only minor effects on Rubisco activation, in particular at low temperature. Since there was a slight increase in the dependence on activase content above 35°C in the low-temperature-grown plants, the role of Rubisco activase may be more important in 20°C-grown plants compared with 30°C-grown plants.
It has been proposed that Rubisco activation state decreases at high temperature, because the activity of Rubisco activase is insufficient to keep pace with the faster rates of Rubisco inactivation at high temperatures (Crafts-Brandner and Salvucci, 2000; Salvucci and Crafts-Brandner, 2004a, 2004c; Kim and Portis, 2006), but the fact that the temperature dependence of Rubisco activation state is not strongly linked to Rubisco activase content argues against this hypothesis (Figs. 2 and 3). The mode of action of Rubisco activase is to remove tight binding inhibitors from Rubisco uncarbamylated and carbamylated catalytic sites (Portis, 2003), and it has been shown that the rate of inhibitor formation increases with temperature (Salvucci and Crafts-Brandner, 2004a; Kim and Portis, 2006). In our experiments, the in vivo turnover rates (estimated from gross photosynthetic rate [A380 + dark respiration] and the carbamylated active site content of Rubisco) did not differ between wild-type plants and plants with intermediate activase levels (Fig. 3). Thus, there was no evidence that CO2 assimilation rate was reduced at high temperature due to inhibitor buildup at carbamylated sites. On the other hand, in plants with the low Rubisco activase content, in vivo turnover rate was reduced, particularly at high temperature (Fig. 3; Table II), similar to what had previously been observed by He et al. (1997).
There appears to be no compelling link between Rubisco activase content and the temperature dependence of Rubisco activation state, and this raises the question of what regulates Rubisco activation state at high temperature. Several plant species express two activase isoforms, the longer (α) and shorter (β) forms (Spreitzer and Salvucci, 2002). Since the α-form is subjected to redox regulation via thioredoxin, reductions in ATP/ADP levels and the redox potential of the chloroplast could cause a down-regulation of activase activity and, in turn, a reduction in the activation state of Rubisco (Zhang and Portis, 1999; Spreitzer and Salvucci, 2002; Zhang et al., 2002). However, several plant species (e.g. tobacco, tomato [Solanum lycopersicum], and maize [Zea mays]) express only the non-redox-regulated β-form of activase (Salvucci et al., 1987; Qian and Rodermel, 1993). Nevertheless, in transgenic tobacco, which reduced amounts of cytochrome b/f complex, Rubisco activation state at 25°C changed with the redox status in the chloroplast stroma but not with the ATP/ADP ratio (Price et al., 1998; Ruuska et al., 2000). Thus, it appears that Rubisco activase activity in tobacco may also be regulated by the thioredoxin system by an as-yet-unknown mechanism. This is supported by our results that the NADP-MDH activation state was decreased at high temperature as well as Rubisco activation state (Table II), as was also reported by Schrader et al. (2004). Moreover, Kim and Portis (2006) showed that low stromal Mg2+ concentration reduced Rubisco activation state at high temperature. Moderate heat stress can have very large effects on thylakoid reactions in tobacco and induce increases in proton conductance and ion movement (Zhang et al., 2009). Thus, if in vivo Mg2+ concentration is reduced at higher temperatures, it is also possible that a decrease in the Rubisco activation state could be due to a change in Mg2+ concentration. These factors could also be the cause for the deactivation of Rubisco at high temperature. It should be noted that, even when A380 at high temperature was limited by Rubisco and not by RuBP regeneration (Fig. 5), the levels of various regulatory metabolites (e.g. ATP and NADPH) and ionic concentration in stroma (e.g. Mg2+) can be changed by the photosynthetic electron transport and membrane leakiness. We conclude that the decrease in Rubisco activation state at high temperature is not solely influenced by Rubisco activase activity.
CONCLUSION
At low temperature, Rubisco activation state was maintained at high levels and did not limit CO2 assimilation rate in both 20°C- and 30°C-grown plants. On the other hand, Rubisco activation state decreased at high temperature and limited CO2 assimilation rate both in 20°C- and 30°C-grown plants. However, the temperature response of Rubisco activation was not strongly dependent on Rubisco activase content, suggesting that other processes also modulate Rubisco activation. Our results indicate that a selective enhancement of Rubisco activase capacity could partly enhance photosynthesis at high temperature, but this enhancement would generally be small.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Tobacco (Nicotiana tabacum ‘Wisconsin 38’) plants and the progeny of several primary transformants of anti-activase tobacco (lines A36, A37, A41, and A49) were grown in controlled environmental growth cabinets (Mate et al., 1993, 1996; He et al., 1997). Plants were grown at irradiance of 200 μmol m−2 s−1 with a photoperiod of 12 h and CO2 concentration of 3,000 μmol mol−1. The day/night air temperatures were either 20°C/15°C or 30°C/25°C, and the relative humidity was 70%. Plants were grown in 5-L pots in garden mix containing approximately 2 g L−1 slow-release fertilizer (Osmocote; 15:4.8:10.8:1.2 nitrogen:phosphorus:potassium:magnesium and trace elements boron, copper, iron, manganese, molybdenum, and zinc; Scotts Australia) and watered daily. The low irradiance and high CO2 were selected to minimize the differences in the growth rate of plants and the capacity of CO2 assimilation at the growth condition.
Gas-Exchange and Fluorescence Measurements
CO2 gas exchange of leaves was measured with a portable gas-exchange system (LI-6400, Li-COR). The whole portable gas-exchange system was enclosed in a temperature-controlled cabinet (Yamori et al., 2005). The CO2 assimilation rate (A) versus intercellular CO2 concentration (Ci) was measured at high light intensity of 1,500 μmol photons m−2 s−1 under several measurement temperatures. Then, the chloroplast CO2 concentration (Cc) was estimated with an assumption that the mesophyll conductance to CO2 diffusion (gm) at 25°C was 0.234 mol m−2 s−1 in 20°C-grown plants and 0.202 mol m−2 s−1 in 30°C-grown plants, based on the relationship between gm and A at 25°C (gm = 0.012 × A; Evans and von Caemmerer, 1996). We used the temperature dependence of gm in tobacco (Bernacchi et al., 2002). Measurements of temperature response were initiated at 15°C, and leaf temperature was subsequently increased in 5°C intervals to 40°C. The leaf was allowed to equilibrate for at least 15 min before data were recorded at each measurement leaf temperature. The vapor pressure deficit was kept under 3.0 kPa even at the highest leaf temperature of 40°C.
Chlorophyll a fluorescence was also determined simultaneously with gas exchange during the temperature response measurements by an integrated fluorescence chamber head (LI-6400, and LI-6400-40 leaf chamber fluorometer; LI-COR). After measurements of the quantum yield of PSII (ΦPSII; Genty et al., 1989), the rate of linear electron transport (Jf) was determined as Jf = ΦPSII × f × I × αleaf, where f is the fraction of absorbed light reaching PSII (assumed 0.5 for C3 plants; Ogren and Evans, 1993), I is incident photon flux density, and αleaf is leaf absorptance. Leaf absorptance to the red and blue light-emitting diode light source of the LI-6400 was measured with an integrating sphere and spectroradiometer (LI-1800; LI-COR): for the wild type (30°C), 0.89 ± 0.01; for antisense lines (30°C), 0.89 ± 0.02; for the wild type (20°C), 0.90 ± 0.01; for antisense lines (20°C), 0.89 ± 0.01.
Analyses of the Limiting Step of the Photosynthetic Rate at 380 μL L−1
A-Cc curves were fitted with the C3 photosynthesis model (Farquhar et al., 1980). When CO2 assimilation is limited by the capacity of Rubisco to consume RuBP, the CO2 assimilation rate (Ac) is expressed as:
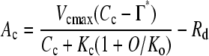 |
(1) |
where Vcmax (μmol m−2 s−1) is the maximum rate of Rubisco carboxylation on the leaf area basis, Kc (μmol mol−1) and Ko (mmol mol−1) are the Km values for CO2 and O2, respectively, Cc (μmol mol−1) and O (mmol mol−1) are chloroplastic CO2 and O2 concentrations, respectively, Γ* (μmol mol−1) is the CO2 compensation point in the absence of day respiration, and Rd (μmol m−2 s−1) is the day respiration rate. When CO2 assimilation is limited by the RuBP regeneration rate, the CO2 assimilation rate (Ar) is expressed as:
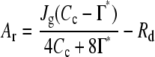 |
(2) |
where Jg (μmol m−2 s−1) is the rate of electron transport. Temperature dependencies of Rubisco kinetics were obtained in tobacco from Bernacchi et al. (2002). Fitting was performed with the software Kaleidagraph (Synergy Software), and Vcmax and Jg were estimated from CO2 assimilation rate at low CO2 concentration and at high CO2 concentration, respectively.
The chloroplast CO2 concentration at which the transition from Rubisco to RuBP regeneration limitation occurs (Ctrans) was determined as:
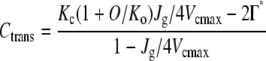 |
(3) |
(von Caemmerer and Farquhar, 1981; von Caemmerer, 2000). Moreover, the Jf-Cc curve was also analyzed to obtain the chloroplast CO2 concentration at which electron transport rate (Jf) remains constant [Ctrans (Jf-Cc curve)], since the Ctrans (Jf-Cc curve) indicates the transition point from Rubisco to RuBP regeneration limitation (von Caemmerer and Farquhar, 1981). We analyzed which limits the CO2 assimilation rate at 380 μmol mol−1 CO2 concentration (A380). The Cc measured under Ca = 380 μmol mol−1 was compared with Ctrans. If Cc for A380 is less than Ctrans, it indicates a limitation by RuBP regeneration, whereas if Cc for A380 is greater than Ctrans, it indicates a limitation by Rubisco. At Ctrans, a colimitation by RuBP regeneration and Rubisco exists.
Determinations of Total and Carbamylated Rubisco Active Sites
Samples used for the Rubisco activation assay were collected from a leaf equilibrated at steady-state conditions in the gas-exchange chamber. After gas exchange had reached the steady-state rate for at least 30 min at a given leaf temperature, the leaf in the chamber was taken and immediately frozen in liquid N2. Rubisco catalytic sites and Rubisco activation state were determined by the stoichiometric binding of [14C]carboxy-arabinitol-P2, as described by Ruuska et al. (1998), using a CO2 free extraction buffer containing 50 mm Bicine-NaOH buffer (pH 8.0), 2 mm MgCl2, 5 mm dithiothreitol (DTT), 2 mm EDTA, 1.5% (w/v) polyvinylpyrrolidone, and 1.5% (v/v) protease inhibitor cocktail (Sigma).
Determinations of NADP-MDH Activation State
The activation state of chloroplast NADP-MDH was assayed according to the method of Scheibe and Stitt (1988) and Ruuska et al. (2000) with minor modifications. Frozen leaf tissue (1.0 cm2) was homogenized in ice-cold extraction buffer (bubbled with humidified nitrogen gas) consisting of 50 mm sodium acetate (pH 6.0), 10 mm MgSO4, 1 mm EDTA, 4.0 mm DTT, 0.1% (v/v) Triton X-100, 1.5% (w/v) polyvinylpyrrolidone, and 1.5% (v/v) protease inhibitor cocktail. The crude extract was centrifuged at 10,000g for 5 min at 4°C, and the initial activity of NADP-MDH was assayed immediately in assay buffer (bubbled with humidified nitrogen gas) consisting of 100 mm Tris-HCl, pH 8.0, 1 mm EDTA, 1 mm DTT, 0.2 mm NADPH, 2 mm oxaloacetic acid, and 50 μL of the supernatant. The decline in A340 was monitored. To obtain the activity of the fully reduced enzyme, a portion of the supernatant was incubated in 250 mm Tris-HC1 (pH 9.0) and 125 mm DTT at room temperature for 20 min.
Determinations of Rubisco Activase, Cytochrome f, and Chlorophyll
Immediately after the measurements of gas exchange, leaf discs of 0.5 cm2 were taken, immersed in liquid nitrogen, and stored at −80°C until determinations of Rubisco activase, cytochrome f, and chlorophyll. The frozen leaf sample was ground in liquid nitrogen and homogenized in an extraction buffer containing 50 mm HEPES-KOH buffer (pH 7.8), 5 mm DTT, 10 mm MgCl2, 1 mm EDTA, 1.5% (w/v) polyvinylpyrrolidone, 0.1% (v/v) Triton X-100, and 1.5% (v/v) protease inhibitor cocktail. Rubisco activase was quantified by immunoblotting with anti-activase antibody (Mate et al., 1996). The cytochrome f content was quantified from two combination methods, by immunoblotting with anti-cytochrome f antibody (Baroli et al., 2008) and by the hydroquinone-reduced, ferricyanide-oxidized difference spectrum of the thylakoid membranes (Bendall et al., 1971; Yamori et al., 2005). For measurements of the hydroquinone-reduced, ferricyanide-oxidized difference spectrum, the difference spectrum at 554 nm was recorded with a dual-beam spectrophotometer (model 557; Perkin-Elmer). The millimolar extinction coefficient of 20 mm−1 cm−1 was used. Chlorophyll was extracted in 80% (v/v) acetone and determined by the procedure of Porra et al. (1989).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. A380 as a function of Rubisco activase contents.
Supplemental Figure S2. Rubisco activation state as a function of Rubisco activase contents.
Supplemental Figure S3. A380 and Rubisco activation state as a function of the activase/Rubisco ratio.
Supplemental Figure S4. CO2 response of CO2 assimilation rate and electron transport rate in plants grown at 20°C/15°C.
Supplementary Material
Acknowledgments
We thank Dr. John Evans and Dr. Shunichi Takahashi for their generous advice.
This work was supported by a grant from the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad (to W.Y.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Wataru Yamori (wataru.yamori@biochem.tohoku.ac.jp).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Andrews TJ (1996) The bait in the Rubisco mousetrap. Nat Struct Biol 3 3–7 [DOI] [PubMed] [Google Scholar]
- Andrews TJ, Lorimer GH (1987) Rubisco: structure, mechanisms, and prospects for improvement. In MD Hatch, NK Boardman, eds, The Biochemistry of Plants: A Comprehensive Treatise, Vol 10, Photosynthesis. Academic Press, New York, pp 131–218
- Baroli I, Price GD, Badger MR, von Caemmerer S (2008) The contribution of photosynthesis to the red light response of stomatal conductance. Plant Physiol 146 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall DS, Davenport HE, Hill R (1971) Cytochrome components in chloroplasts of the higher plants. Methods Enzymol 23 327–344 [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, Von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance: implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130 1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JA, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31 491–543 [Google Scholar]
- Bukhov NG, Samson G, Carpentier R (2000) Nonphotosynthetic reduction of the intersystem electron transport chain of chloroplasts following heat stress: steady-state rate. Photochem Photobiol 72 351–357 [PubMed] [Google Scholar]
- Bukhov NG, Wiese C, Neimanis S, Heber U (1999) Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59 81–93 [Google Scholar]
- Cen YP, Sage RF (2005) The regulation of ribulose-1,5-bisphosphate carboxylase activity in response to variation in temperature and atmospheric CO2 partial pressure in sweet potato. Plant Physiol 139 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Law RD (2000) Effect of heat stress on the inhibition and recovery of ribulose-1,5-bisphosphate carboxylase/oxygenase activation state. Planta 212 67–74 [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA 97 13430–13435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149 78–90 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990 87–92 [Google Scholar]
- He Z, von Caemmerer S, Hudson GS, Price GD, Badger MR, Andrews TJ (1997) Ribulose-1,5-bisphosphate carboxylase/oxygenase activase deficiency delays senescence of ribulose-1,5-bisphosphate carboxylase/oxygenase but progressively impairs its catalysis during tobacco leaf development. Plant Physiol 115 1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson L, Sharwood R, Ludwig M, Whitney SM, Badger MR, von Caemmerer S (2008) The effects of Rubisco activase on C4 photosynthesis and metabolism at high temperature. J Exp Bot 59 1789–1798 [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57 291–302 [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Murakami A, Hirose T (1999) Balancing carboxylation and regeneration of ribulose bisphosphate in leaf photosynthesis: temperature acclimation in an evergreen tree, Quercus myrsinaefolia. Plant Cell Environ 22 841–849 [Google Scholar]
- Kim K, Portis AR Jr (2005) Temperature dependence of photosynthesis in Arabidopsis plants with modifications in Rubisco activase and membrane fluidity. Plant Cell Physiol 46 522–530 [DOI] [PubMed] [Google Scholar]
- Kim K, Portis AR Jr (2006) Kinetic analysis of the slow inactivation of Rubisco during catalysis: effects of temperature, O2 and Mg++. Photosynth Res 87 195–204 [DOI] [PubMed] [Google Scholar]
- Kubien DS, Sage RF (2008) The temperature response of photosynthesis in tobacco with reduced amounts of Rubisco. Plant Cell Environ 31 407–418 [DOI] [PubMed] [Google Scholar]
- Kumar A, Li C, Portis AR Jr (2009) Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth Res 100 143–153 [DOI] [PubMed] [Google Scholar]
- Kurek I, Chang TK, Bertain SM, Madrigal A, Liu L, Lassner MW, Zhu G (2007) Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 19 3230–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Sage RF (2007) Temperature response of photosynthesis in transgenic rice transformed with ‘sense’ or ‘antisense’ rbcS. Plant Cell Physiol 48 1472–1483 [DOI] [PubMed] [Google Scholar]
- Mate CJ, Hudson GS, von Caemmerer S, Evans JR, Andrews TJ (1993) Reduction of ribulose bisphosphate carboxylase activase levels in tobacco (Nicotiana tabacum) by antisense RNA reduces ribulose bisphosphate carboxylase carbamylation and impairs photosynthesis. Plant Physiol 102 1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mate CJ, von Caemmerer S, Evans JR, Hudson GS, Andrews TJ (1996) The relationship between CO2-assimilation rate, Rubisco carbamylation and Rubisco activase content in activase-deficient transgenic tobacco suggests a simple model of activase action. Planta 198 604–613 [DOI] [PubMed] [Google Scholar]
- Ogren E, Evans JR (1993) Photosynthetic light-response curves. I. The influence of CO2 partial pressure and leaf inversion. Planta 189 180–190 [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T (2005) The balance between RuBP carboxylation and RuBP regeneration: a mechanism underlying the interspecific variation in acclimation of photosynthesis to seasonal change in temperature. Funct Plant Biol 32 903–910 [DOI] [PubMed] [Google Scholar]
- Parry MAJ, Keys AJ, Madgwick PJ, Carmo-Silva AE, Andralojc PJ (2008) Rubisco regulation: a role for inhibitors. J Exp Bot 59 1569–1580 [DOI] [PubMed] [Google Scholar]
- Pastenes C, Horton P (1996) Effect of high temperature on photosynthesis in bean. II. CO2 assimilation and metabolite contents. Plant Physiol 112 1253–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975 384–394 [Google Scholar]
- Portis AR Jr (2003) Rubisco activase: Rubisco's catalytic chaperone. Photosynth Res 75 11–27 [DOI] [PubMed] [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Siebke K, Anderson JM, Badger MR (1998) Photosynthesis is strongly reduced by antisense suppression of chloroplastic cytochrome bf complex in transgenic tobacco. Aust J Plant Physiol 25 445–452 [Google Scholar]
- Qian J, Rodermel SR (1993) Ribulose-1,5-bisphosphate carboxylase/oxygenase activase cDNAs from Nicotiana tabacum. Plant Physiol 102 683–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska SA, Andrews TJ, Badger MR, Hudson GS, Laisk A, Price GD, von Caemmerer S (1998) The interplay between limiting processes in C3 photosynthesis studied by rapid-response gas exchange using transgenic tobacco impaired in photosynthesis. Aust J Plant Physiol 25 859–870 [Google Scholar]
- Ruuska SA, Andrews TJ, Badger MR, Price GD, von Caemmerer S (2000) The role of chloroplast electron transport and metabolites in modulating Rubisco activity in tobacco: insights from transgenic plants with reduced amounts of cytochrome b/f complex or glyceraldehyde 3-phosphate dehydrogenase. Plant Physiol 122 491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30 1086–1106 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. a) Mechanism for deactivation of Rubisco under moderate heat stress. Physiol Plant 122 513–519 [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. b) Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134 1460–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ (2004. c) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120 179–186 [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Werneke JM, Ogren WL, Portis AR Jr (1987) Purification and species distribution of Rubisco activase. Plant Physiol 84 930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R, Stitt M (1988) Comparison of NADP-malate dehydrogenase activation, QA reduction and O2 evolution in spinach leaves. Plant Physiol Biochem 26 473–481 [Google Scholar]
- Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TD (2004) Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant Cell Environ 27 725–735 [Google Scholar]
- Spreitzer RJ, Salvucci ME (2002) Rubisco: interactions, associations and the possibilities of a better enzyme. Annu Rev Plant Biol 53 449–475 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Collingwood, Australia
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153 376–387 [DOI] [PubMed] [Google Scholar]
- Wise RR, Olson AJ, Schrader SM, Sharkey TD (2004) Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ 27 717–724 [Google Scholar]
- Yamori W, Noguchi K, Hanba YT, Terashima I (2006. a) Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Physiol 47 1069–1080 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hikosaka K, Terashima I (2009) Cold tolerant crop species have greater temperature homeostasis of leaf respiration and photosynthesis than cold sensitive species. Plant Cell Physiol 50 203–215 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Hikosaka K, Terashima I (2010) Phenotypic plasticity in photosynthetic temperature acclimation among crop species with different cold tolerances. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Yamori W, Noguchi K, Kashino Y, Terashima I (2008) The role of electron transport in determining the temperature dependence of the photosynthetic rate in spinach leaves grown at contrasting temperatures. Plant Cell Physiol 49 583–591 [DOI] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28 536–547 [Google Scholar]
- Yamori W, Suzuki K, Noguchi K, Nakai M, Terashima I (2006. b) Effects of Rubisco kinetics and Rubisco activation state on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Environ 29 1659–1670 [DOI] [PubMed] [Google Scholar]
- Zhang N, Kallis RP, Ewy RG, Portis AR Jr (2002) Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc Natl Acad Sci USA 99 3330–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Portis AR Jr (1999) Mechanism of light regulation of Rubisco: a specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Proc Natl Acad Sci USA 96 9438–9443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Cruz JA, Kramer DM, Magallanes-Lundback ME, Dellapenna D, Sharkey TD (2009) Moderate heat stress reduces the pH component of the transthylakoid proton motive force in light-adapted, intact tobacco leaves. Plant Cell Environ 32 1538–1547 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.