Abstract
Expansins are cell wall proteins associated with the process of plant growth. However, investigations in which expansin gene expression has been manipulated throughout the plant have often led to inconclusive results. In this article, we report on a series of experiments in which overexpression of expansin was targeted to specific phases of leaf growth using an inducible promoter system. The data indicate that there is a restricted window of sensitivity when increased expansin gene expression leads to increased endogenous expansin activity and an increase in leaf growth. This phase of maximum expansin efficacy corresponds to the mid phase of leaf growth. We propose that the effectiveness of expansin action depends on the presence of other modulating factors in the leaf and we suggest that it is the control of expression of these factors (in conjunction with expansin gene expression) that defines the extent of leaf growth. These data help to explain some of the previously observed variation in growth response following manipulation of expansin gene expression and highlight a potential linkage of the expression of modifiers of expansin activity with the process of exit from cell division.
Expansins were initially identified as cell wall proteins that had the ability to promote the extension of plant tissue in vitro (McQueen-Mason et al., 1992). Further work on these proteins and the genes encoding them has revealed a picture in which, although a general correlation with growth has often been substantiated, it is clear that control of growth is a much more complex process than the control of expression of a single protein type (for review, see Cosgrove, 2000; Lee et al., 2001; Li et al., 2003). In addition, although it is clear that expansins play a role in many growth processes, there are a number of open questions about exactly how expansins contribute to these processes. First, we still have a very limited understanding of the molecular mechanism of expansin action. Efforts to identify classical enzymatic activities associated with expansins have proven fruitless (McQueen-Mason and Cosgrove, 1995; Li and Cosgrove, 2001) and the remaining, somewhat speculative, interpretation is that expansins intercalate within carbohydrate matrices in the cell wall, leading to transient loosening of noncovalent interactions and, thus, the ability of these matrices to move relative to each other (McQueen-Mason and Cosgrove., 1994). In addition, by unlocking aspects of the molecular architecture of the cell wall, expansins may allow access of other cell wall proteins/enzymes to particular substrates. Depending on the nature of these other proteins/enzymes, expansin activity could thus be associated not only with growth processes, but also with cell wall modifications linked with differentiation. Such a mechanism would help to explain observations (described below) that the effectiveness of expansin action appears to be context dependent and is not only associated with changes in plant growth but also with differentiation.
Various analyses have revealed that expansins are present in a wide range of plants, including bryophytes, ferns, angiosperms, and conifers (Hutchison et al., 1999; Kim et al., 2000; Schipper et al., 2002). Moreover, they are generally encoded by relatively large gene families whose members often show distinct patterns of gene expression (Kende et al., 2004). Some of these expression patterns correlate with growth processes, such as root growth (Wu et al., 1996), internode growth (Cho and Kende, 1997), leaf growth (Muller et al., 2007), and cotton (Gossypium hirsutum) fiber growth (Ruan et al., 2001), whereas others correlate with events of differentiation, such as fruit ripening (Rose et al., 1997; Brummell et al., 1999b), grass tiller formation (Reidy et al., 2001), and endosperm breakdown (Chen and Bradford, 2000). In addition, some novel nonplant expansin activities have been identified that suggest that pathogens may induce altered cell wall structure via an expansin-mediated mechanism (Qin et al., 2004). Since in vitro assays have suggested that the activities of expansins extracted from different sources tend to be similar (Cosgrove, 2000), it has been proposed that this tissue, organ, and environmental specificity of expression pattern reflects a specialized role for expansins in specific contexts rather than any major difference in activity of the protein. As stated above, this specific function may depend on the presence (or absence) of tissue-specific cofactors, the nature of which is as yet unclear.
In addition to biochemical approaches to understanding expansin function, numerous groups have undertaken transgenic experiments to alter expansin gene expression in plants to observe the outcome on plant phenotype. Although some successes with antisense strategies have been reported (Brummell et al., 1999a; Cho and Cosgrove, 2000), the encoding of expansin by large gene families means that genetic redundancy poses a significant problem for such approaches (e.g. Schipper et al., 2002). Simple overexpression strategies to alter expansin activity may also be difficult to interpret. For example, when expansins were constitutively overexpressed throughout Arabidopsis (Arabidopsis thaliana), tomato (Solanum lycopersicum), and rice (Oryza sativa) plants, the outcomes tended to be pleiotropic, including a decrease in overall plant growth (Cho and Cosgrove, 2000; Rochange et al., 2001; Choi et al., 2003). However, when altered expansin expression was targeted more specifically to a particular tissue or organ, then more easily interpretable results were obtained. For example, when altered expansin expression was directed to the developing leaf petiole in Arabidopsis, altered leaf growth was observed (Cho and Cosgrove, 2000), consistent with the idea that expansins promote growth, and when inducible expression of expansin was targeted throughout rice plants, quantitative changes in growth were observed (Choi et al., 2003). The results of these experiments indicate that expansin gene expression can be used as a tool to modulate growth, but that the timing and spatial extent of expression can have a significant influence on the phenotype observed. Again, these data support the hypothesis that the effectiveness of expansin in promoting specific growth or differentiation events is dependent on the presence of particular tissue- or developmental-specific cofactors. So far, little progress has been made on the identification and characterization of these cofactors.
In previous work, we reported on the characterization of transgenic lines of tobacco (Nicotiana tabacum) in which a cucumber (Cucumis sativus) expansin (CsEXP1) could be induced by application of a chemical inducer (anhydrotetracycline [Ahtet]). In these experiments, we targeted expansin overexpression to localized regions of either the shoot apical meristem or very young leaf primordia, which led to localized promotion of growth (Pien et al., 2001), consistent with the idea that expansins play a role in the endogenous mechanism of leaf initiation (Reinhardt et al., 1998). However, when inductions were performed throughout the plant the resulting phenotypes were variable and difficult to interpret (S. Pien and A. Fleming, unpublished data), in line with other reports (Rochange et al., 2001). To investigate the possibility that this variable response reflected a differential sensitivity to expansin in different tissues at different stages of development, we performed a series of experiments (reported here) in which overexpression of expansin was targeted to specific stages of leaf growth. Our data indicate that the efficacy of expansin action depends on the presence of other factors that are present in a developmentally controlled fashion, so that increased expansin gene expression is only effective in promoting leaf growth during a specific developmental period of leaf growth. This period corresponds to the inflection point of relative growth rate (RGR) and, thus, to the phase of maximum leaf growth rate. An intriguing article by Cookson et al. (2005) reported on potential correlations between various parameters of leaf growth and final leaf size. They found that the best predictor of final leaf size was the maximum value of absolute leaf growth rate. Thus, the experiments reported here identify a novel, developmental control of expansin efficacy in the regulation of leaf growth, investigate the reported correlation between maximal leaf expansion rate and leaf size, and provide an insight into potential means of controlling leaf growth.
RESULTS
Gene Expression Can Be Targeted to Specific Phases of Leaf Growth
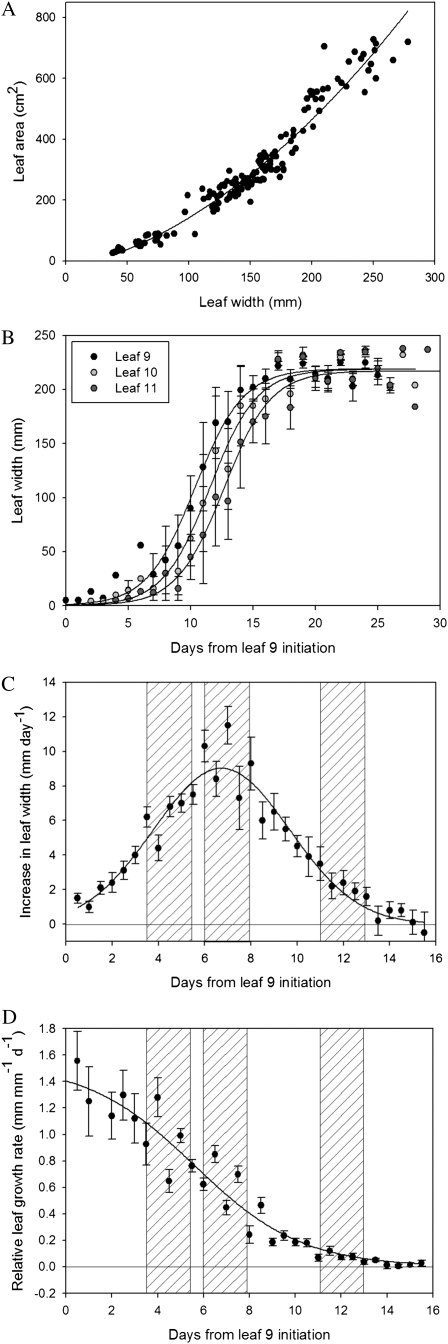
The characterization of the outcome of elevated expansin activity on leaf growth first requires knowledge of the normal growth curves of the leaves to be manipulated. Although leaf area is a good measure of growth, accurate measurement of leaf area normally requires dissection of the leaf from the plant, clearly a destructive intervention. Therefore, we first established whether leaf width (which can be easily measured with minimal interference to the plant) could be used as a proxy for leaf area. Figure 1A shows that during the phase of tobacco leaf development investigated here (leaf area of approximately 35–280 cm2), leaf width and area were strongly correlated (Pearson's product moment = 0.959, P < 0.001). In the experiments described below, leaf width was used to stage leaves prior to induction and to measure the initial growth responses before final measurements of leaf area.
Figure 1.
Analysis of leaf growth. A, Correlation of leaf width and area during development. Each point represents the measurement of a single leaf sacrificed at a different stage of development (Pearson's product moment = 0.959, P < 0.001, n = 40). B, Growth curves of tobacco leaves 9, 10, and 11. Each point represents the mean width value calculated from measurements of five leaves for leaf number 9, 10, or 11. Error bars = se of the mean. Sigmoidal curves have been fitted to the average data. C, Absolute growth rates during development of leaf 9. Each point represents mean values for the increase in leaf width/day calculated from measurements on 10 leaves. Error bars = se of the mean. The hatched areas indicate defined phases of early, mid, and late growth. D, Relative leaf growth rate during development of leaf 9. Each point represents mean values for the relative increase in leaf width/day calculated from measurements on 10 leaves. Error bars = se of the mean. The hatched areas indicate defined phases of early, mid, and late growth.
Figure 1B shows the change in leaf width with time for three sequential leaves during tobacco development (leaves 9, 10, and 11; these data are taken from an analysis of growth of the first 25 leaves produced by plants under our growth conditions, shown in Supplemental Fig. S1). Leaves 9, 10, and 11 show comparable and consistent growth curves, and thus represent suitable targets for investigation. All the experiments and analyses described below were performed on leaves 9 (treated) or 10 (untreated control data).
The growth curves shown in Figure 1B have classical sigmoidal forms. This allowed us to define three growth phases. First, an early phase when the absolute expansion rate (E) is increasing (approximately d3–d5 after initiation for leaf 9, as shown in Fig. 1C); a mid phase encompassing the period of maximum absolute rate of leaf growth (Emax; approximately d6–d8 after initiation for leaf 9), and a late phase (d11–d13 after initiation for leaf 9) during which the absolute rate of leaf expansion is decreasing. These phases can be distinguished by boundary values of leaf width so that leaves of width 20 to 25 mm are at the beginning of the early growth phase, those with width 60 to 70 mm are at the beginning of the mid growth phase, and those of width 150 to 160 mm are at the beginning of the late growth phase.
As well as absolute changes in leaf size, the data in Figure 1C can be expressed as RGRs (Fig. 1D). Thus, during the early stages of development leaves display a high rate of relative growth that subsequently gradually declines until approaching zero as the leaf reaches maturity. Superimposition of the growth phases defined by absolute growth rates indicates that both early and mid growth phases represent periods when the RGR is declining, whereas the late growth phase represents leaves where the RGR is approaching zero.
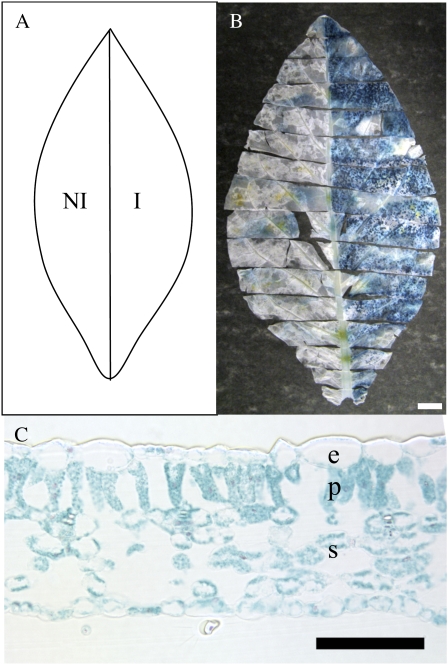
Our strategy was to use previously generated transgenic plants (Pien et al., 2001) in which the expression of either a cucumber expansin (CsEXP1) or a reporter gene (GUS) can be induced by supply of the chemical Ahtet. These transgenic lines (Tet∷EXP and Tet∷GUS) were used in the experiments described below. However, even allowing for the careful growth staging described in Figure 1 and highly controlled growth conditions, initial experiments indicated that there was still plant-to-plant variation in leaf growth that might obscure quantitative changes in leaf growth occurring as a result of our interventions. Thus, comparison of the growth curves of leaf 9 of R7 plants (the genetic background into which the Tet∷EXP and Tet∷GUS constructs were transformed), Tet∷EXP and Tet∷GUS plants indicated a variance in curve parameters (Supplemental Fig. S2). To minimize the effect of this inherent variation in leaf growth, we devised a scheme so that individual leaves could be analyzed with one half of a leaf being induced and the other half being mock treated. Since our preliminary data indicated that there was no significant difference in growth rate between the two halves of leaves (delineated by the mid vein), this approach provided an internal control that obviated differences due to plant-to-plant variation in growth. This scheme is shown schematically in Figure 2A and in the following analysis statistical comparisons are made using paired half-leaf data.
Figure 2.
Half-leaf GUS induction. A, Schematic representation of half-leaf induction. The left side of each leaf was treated with a control solution and was designated noninduced (NI), whereas the right side was treated with 200 μg/mL Ahtet and was designated induced (I). B, GUS activity (blue) visualized in a late growth phase Tet∷GUS leaf 5 d after half-leaf induction (as depicted in A). Bar = 20 mm. C, Cross section of an induced mid phase Tet∷GUS leaf, 5 d after induction and visualization of GUS activity (blue). e, Epidermis; p, palisade mesophyll; s, spongy mesophyll. Bar = 100 μm.
Plants were grown and selected for the size of leaf 4 at the start of the experiment to create a pool of developmentally equivalent staged leaves for manipulation and growth analysis. When the target leaf for manipulation (leaf 9) had reached a particular growth phase (as determined by leaf-width measurement), one half of the leaf was induced with an Ahtet solution (induced half-leaf), whereas the other half of the leaf was treated with a mock solution (noninduced half-leaf). Application of the inducer Ahtet was performed once every 24 h, leading to a total of three applications over a 48-h period. Leaves were sacrificed 48 h after the final Ahtet application or allowed to grow until plant flowering prior to analysis. To investigate the spatial and temporal restriction of gene induction using this approach, a number of Tet∷GUS plants were analyzed and the extent of GUS activity visualized (as described in “Materials and Methods”). As shown in Figure 2B, half-leaf application of Ahtet led to a restriction of GUS activity generally to the half of the leaf where the Ahtet was applied, although there was some low-level signal apparent in some areas of the noninduced half-leaf. Analysis of cross sections of induced tissue indicated that all cell layers and types within the leaf were induced to express the GUS gene following Ahtet application (Fig. 2C).
Induction of Expansin Gene Expression Elicits an Increase in Leaf Area Only during the Mid Phase of Leaf Growth
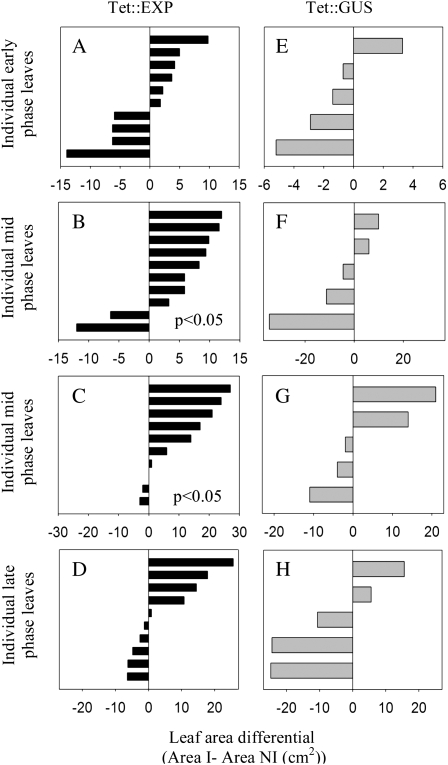
In a first series of experiments, Tet∷EXP and Tet∷GUS plants were grown and leaf 9 was treated with Ahtet in a comparable fashion, so that for each experiment there was a series of leaves (genotype either Tet∷EXP or Tet∷GUS) in which one half of each leaf was either induced or noninduced at the beginning of either the early, mid, or late growth phase. Either 2 d after the final application of Ahtet or at flowering, the area of each induced half-leaf was measured and a paired comparison made with the noninduced (mock-treated) half-leaf. This was done by subtracting the leaf areas, giving a value of leaf area differential for each leaf, with positive values indicating a greater area on the induced half-leaf and negative values indicating a greater area on the noninduced half-leaf. A value of zero would indicate that both halves of a leaf had exactly the same area at the end of the treatment.
Considering the Tet∷EXP leaves (Fig. 3, A–D), for leaves induced during the early phase of growth (Fig. 3A) there was no preferential growth on either side of the mid vein (Wilcoxen rank test, P > 0.4). However, for leaves induced during the mid growth phase the induced side of the leaf grew more than the control noninduced side of the leaf (Fig. 3B, Wilcoxon rank test [P < 0.05]). When the leaves were allowed to grow to maturity (Fig. 3C) a similar result was obtained (Wilcoxon rank test, P < 0.05), with analysis of combined data from Figure 3, B and C, indicating that the induced Tet∷EXP half-leaves had an increased area relative to the noninduced side at a confidence limit of 0.01% (Wilcoxon rank test, P < 0.01). When similar inductions were performed on late growth phase leaves (Fig. 3D), there was no statistically significant difference in growth between the two half-leaves (Wilcoxon rank test, P > 0.4). Analysis of Tet∷GUS leaves treated in a comparable fashion (Fig. 3, E–H) indicated that there was no increase in leaf area in the induced half-leaves, irrespective of the growth phase when induction was performed or the time at which the analysis was performed (Wilcoxon rank test, P > 0.4). Indeed, the induced half-leaves showed a decrease in growth relative to the noninduced halves, suggesting that the Ahtet inducer might have some inhibitory affect on growth. However, these differences were not significant at the 0.05% confidence limit. For all experiments, leaf area differentials for the untreated leaf 10 on each plant were also calculated (Supplemental Fig. S3) and these indicated that there was no significant difference in growth between the two halves of these nontreated control leaves, neither for Tet∷EXP nor Tet∷GUS plants (Wilcoxon rank test, P > 0.4 in all cases other than for Tet∷EXP untreated leaf 10 data, P < 0.2). Supplemental Table S1 shows the absolute leaf areas measured in all of the above experiments.
Figure 3.
Analysis of leaf area after half-leaf induction of expansin gene expression. A to D, Leaf area differentials for individual leaves of Tet∷EXP (leaf 9) induced on one half with Ahtet (I) and mock induced on the other half (NI) either at the beginning of early growth phase (A), mid growth phase (B and C), or late growth phase (D). Area differentials were measured at 48 h after induction (A, B, and D) or at flowering after induction (C). E to H, Leaf area differentials for individual leaves of Tet∷GUS (leaf 9) induced on one half with Ahtet and mock induced on the other half either at the beginning of early growth phase (E), mid growth phase (F and G), or late growth phase (H). Area differentials were measured at 48 h after induction (E, F, and H) or at flowering after induction (G). Leaf area differential = area I − area NI (cm2), so that a positive value equates to more growth in the induced leaf half.
To investigate the cellular outcome of increased leaf area in the mid growth phase Tet∷EXP half-leaves after Ahtet induction, we performed a histological analysis of the tissue and a quantitative analysis of epidermal cell size and shape. Representative images are shown in Supplemental Figure S4 and the data are shown in Supplemental Table S2. Essentially, there was no observable change in histology in the regions after induction of the Tet∷EXP half-leaves. Although there was an increase in mean cell size in the induced half-leaves, these differences were not statistically significant at the 0.05% confidence level (t test). The cell size analysis did indicate that cells at the distal tip of the mid growth phase leaves were larger than cells in the middle portion of the leaf, which were themselves larger than cells at the proximal base of the leaf.
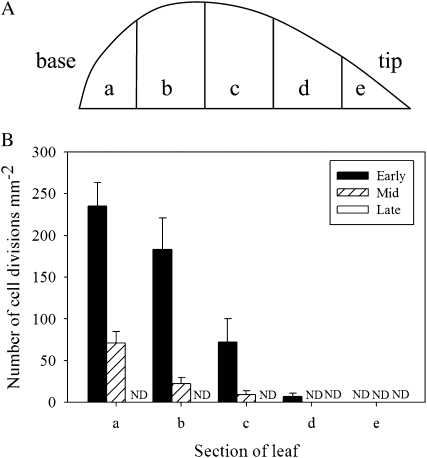
During the very earliest stages of normal leaf development essentially all cells within the organ undergo proliferation. However, as development proceeds there is a characteristic termination of division that initiates at the distal tip of the leaf and proceeds in a wave toward the proximal base (Poethig and Sussex, 1985; Donnelly et al., 1999). To characterize this pattern of cell division termination in tobacco, we performed an analysis of cell division pattern at various stages of development of untreated leaves using an aniline blue staining technique (Kuwabara and Nagata, 2006). This technique allows visualization of the cell plate forming at the phragmoplast during the later stages of cell division (Supplemental Fig. S5). Each leaf was divided into five sections along the proximal-distal axis (Fig. 4A) and cell division counts made in each section. As shown in Figure 4B, at the early phase of leaf growth cell division frequency was highest at the base of the leaf, with over 200 divisions mm−2 in the basal fifth, and decreased gradually toward the tip, with no divisions in the upper fifth of the leaf. There was also a gradient of cell division frequency during the mid growth phase, but this gradient was shifted toward the base with approximately 70 divisions mm−2 in the basal fifth of the leaf and no divisions in the distal two-fifths of the leaf. No cell divisions were detectable in any regions of the late growth phase leaves.
Figure 4.
Pattern of cell division frequency during leaf development. A, Schematic showing division of leaf into five sections (a–e) from base to tip. B, Cell divisions were visualized by aniline blue staining and counted in the various sections defined in A. Analysis was performed on leaves at early, mid, and late growth phases, with a total of at least six fields of view being analyzed per sample. Divisions were not detectable (ND) in any region of late growth phase leaves or in the upper 20% region (e) at any growth phase.
Induction of Expansin in Half-Leaves Leads to Leaf Curvature at All Phases of Leaf Growth
Although differential induction of the Tet∷EXP leaves during the early growth phase did not reveal any significant difference in area between induced and noninduced half-leaves, visually the two leaf halves were distinguishable. There was an apparent bending of the leaf mid vein so that the mid vein was convex in relation to the induced leaf half and concave in relation to the mock-treated, noninduced half-leaf (Fig. 5A), i.e. the mid vein curved away from the induced region. This bending was not apparent in Tet∷GUS leaves similarly treated during the early growth phase. Indeed, occasionally a slight curving of the mid rib toward the induced area was observed in these plants (Fig. 5B). An untreated early growth phase Tet∷EXP leaf 10 in which no overt curvature of the mid vein is apparent is shown in Figure 5C for comparison.
Figure 5.
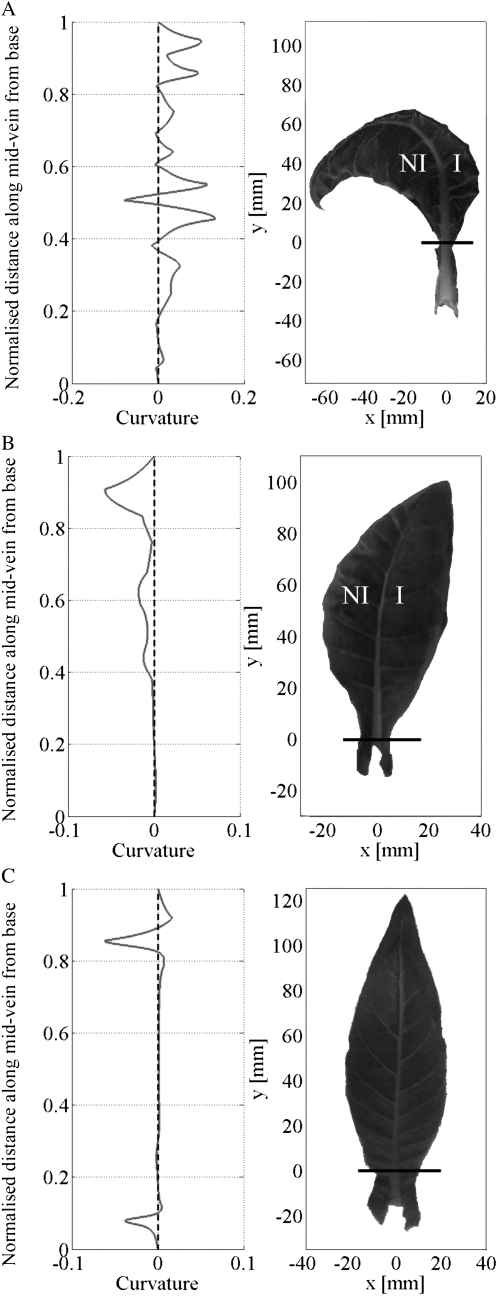
Leaf curvature analysis. A, Curvature profile and leaf image of an early growth phase Tet∷EXP leaf; the mid vein bends away from the induced (I) side of the leaf toward the noninduced (NI) side. The curvature profile (m 10−3) shows that most of the mid vein curvature energy is positive, i.e. bending is to the left. B, Curvature profile and leaf image of an early growth phase Tet∷GUS leaf; the mid vein bends toward the induced side and away from the noninduced side. Most of the mid vein curvature energy (m 10−3) is negative, i.e. bending is to the right. C, Curvature profile and leaf image of an early growth phase Tet∷EXP untreated control leaf 10. The mid vein is essentially straight. The curvature profile (m 10−3) shows some deviation along its length, but the curvature energy is essentially zero.
To facilitate a quantitative analysis of the observed curvature phenomenon, we developed a computational image processing tool that allowed us to measure the curvature at each point along the leaf mid vein (see “Materials and Methods”). The curvature quantifies the bending or deformation of a plane curve with a straight line having a curvature of zero. With increasing bending the curvature increases in value and indicates in which direction the curves bend. A negative curvature indicates a right-hand bending; a positive curvature indicates a left-hand side bending. Curvature plots for the mid veins of each of the leaves shown in Figure 5, A to C, are shown to the left of the leaf images. By integrating the area below the curvature plots, an average curvature can be calculated and statistical comparisons made between samples. Figure 6 shows a comparison of average curvatures for the leaves analyzed in Figure 3.
Figure 6.
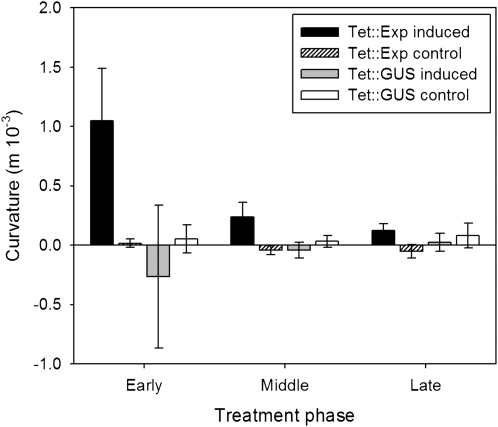
Curvature is increased after induction of expansin gene expression on one side of a leaf. Normalized, mean mid vein curvature values for induced and control Tet∷EXP and Tet∷GUS leaves. For induced leaves, Ahtet was applied to one side of the leaf, the other treated with a control solution. For control leaves, no treatment was applied. Positive curvature values indicate bending of the mid vein away from the induced side. Tet∷EXP values represent the mean calculated for 10 leaves; Tet∷GUS values represent the mean calculated for five leaves. Error bars = se.
At all growth phases, treatment (one half of leaf induced, the other half noninduced) of Tet∷EXP leaf 9 led to an increased curvature energy relative to nontreated control Tet∷EXP leaves (leaf 10), consistent with curvature of the mid vein away from the induced side of the leaf. This difference was significant at the 0.05% confidence limit (t test). No such alteration in curvature was calculated for similarly treated Tet∷GUS leaves. The degree of curvature was higher for Tet∷EXP leaves induced at an early growth phase rather than at later growth phases, although the induced Tet∷EXP leaves showed an increased curvature energy at the 0.05% confidence level for all three growth phases investigated (Fig. 6).
Induction of Expansin Gene Expression Leads to an Elevated Level of Expansin Activity Only during the Mid Phase of Leaf Growth
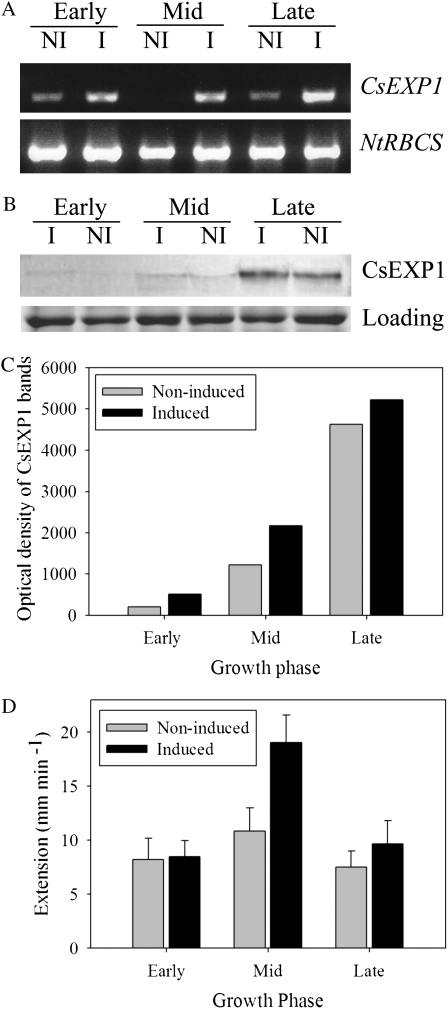
Although the transgenic material used in this work has been previously described (Pien et al., 2001), we performed a number of molecular analyses to confirm that induction of Tet∷EXP plants with Ahtet led to an increase in expansin gene expression. Reverse transcription (RT)-PCR analysis of induced tissue confirmed that an increase in CsEXP1 transcript level occurred in half-leaves at all three growth phases, although there was some background signal in noninduced tissue (Fig. 7A). This might reflect leakiness of the promoter or some diffusion of the Ahtet inducer from the half-leaf where Ahtet was applied. Second, we analyzed expansin protein levels in half-leaves (induced and noninduced) from early, mid, and late growth phases. Although the antibody used for this analysis was raised against CsEXP1, it does not discriminate between endogenous expansins from various species, and thus provides an indication of total expansin protein level (Rochange et al., 2001). Our data (Fig. 7B) indicated that in noninduced tissue expansin protein was detectable at all three stages of leaf development but that there was a significant accumulation of expansin protein during leaf development, with the late growth phase having the highest level of expansin protein. After induction of Tet∷EXP leaves there was a slight increase in total detectable expansin protein level at all three different phases, but these differences were not large (Fig. 7C). To test whether any changes in expansin activity occurred in the three growth phases, both with and without induction of expansin gene expression, we extracted cell wall protein and measured expansin activity using an in vitro assay of an artificial cellulose/hemicellulose matrix. These data (shown in Fig. 7D) indicate that during normal leaf growth there is a slight elevation of endogenous expansin activity per mass during the mid growth phase. Following induction of expansin gene expression an increase of extractable expansin activity was recorded, but only in mid growth phase tissue. Since the artificial substrate was similar in all assays, this difference in activity must reflect differences in the expansin activity in the cell wall extract taken from leaves at different phases of growth.
Figure 7.
Induction at the mid growth phase leads to an increased expansin activity. A, RT-PCR of CsEXPA1 and NtRBCS from induced (I) and noninduced (NI) halves of early, mid, and late growth phase leaves. B, Western-blot analysis of CSEXP1 protein level in induced and noninduced halves of early, mid, and late phase leaves. A loading control shows equivalent levels in all lanes. This experiment was repeated twice with similar results. C, Optical density measurements of western-blot data shown in B. D, Expansin activity measured in extracts from early, mid, or late growth phase leaves, either induced or noninduced with Ahtet. Activity values represent the mean calculated for three samples assayed at least in triplicate. Error bars = se of the mean.
DISCUSSION
Our results indicate that the effectiveness of expansin on the promotion of leaf growth is context dependent, with different outcomes depending on the growth phase of the leaf. The identification of this window of sensitivity to expansin action required a careful and standardized growth regime and focus of the analysis on leaves of defined growth phases, without which the outcome would have been difficult to both detect and interpret. This context-specific dependent outcome of altered expansin gene expression both fits with previous investigations (Cho and Cosgrove, 2000; Pien et al., 2001) and may account for the often confusing or unclear phenotypes observed after constitutive manipulation of expansin gene expression (e.g. Rochange et al., 2001).
Although transcriptional induction of expansin was detected at all growth stages, increases in expansin protein level were very limited. Such a poor correlation of expansin transcript and protein levels has been previously reported and has led to the suggestion that possibly only a fraction of the expansin protein detectable in a tissue is actually active at any point in time (Caderas et al., 2000). Thus, relatively minor changes in total expansin protein level might lead to relatively large changes in activity. However, if this is the case, our data indicate an added layer of complexity. Induction of expansin gene expression only led to a promotion of extractable expansin activity during the mid phase of leaf growth, with induction at earlier and later phases having no discernable outcome. Since the substrate used for these activity assays is artificially synthesized, these data must reflect a difference in the leaf tissue at these different growth phases. One possibility is that there is a modifier or modulator of expansin activity whose expression is developmentally controlled, with a peak of expression occurring during the mid growth phase. In this scenario, induction of expansin gene expression would only lead to actual change in expansin activity when the level of the putative expansin cofactor was not limiting. We speculate that this might be the case during the mid growth phase, i.e. levels of the cofactor are low during early and late growth phases. The nature of such a modulator of expansin activity is unknown but clearly warrants future investigation.
It is interesting to note that the phase when the level of this proposed modulator increases coincides with the developmental phase during which cell division termination is occurring within the leaf in a wave from the distal tip to the proximal base (Fig. 4). Previous authors have suggested that this wave of division termination is a key event in leaf development (Donnelly et al., 1999) and analysis of mutants indicates that alteration of the pattern of cell division termination is associated with altered leaf morphogenesis (Nath et al., 2003; White, 2006). However, the mechanism relating cell division termination to morphogenesis is obscure. A model in which a particular cell wall-loosening facilitator (expansin cofactor) is expressed at a particular cellular development phase (transition from dividing to nondividing state) could account for the importance of this wave of cell division termination for leaf morphogenesis, and account for the endogenous control of response to an artificially induced increase in expansin gene expression. The molecular nature of the factors controlling cell division termination within the leaf is unknown, although various hints are available in the literature (e.g. Beemster et al., 2003; Wyrzykowska et al., 2006; Anastasiou and Lenhard, 2007). Identification and characterization of the events associated with cell division termination might provide novel tools to modify and increase leaf growth, as well as providing insights into the endogenous mechanism for the modulation of expansin activity.
Irrespective of the mechanism by which endogenous expansin activity is controlled, consequent to the induction of expansin activity during the mid phase of leaf growth there was an increase in final leaf size compared to noninduced leaves, supporting the idea that expansin acts to promote tissue growth (Cosgrove, 2000). Analysis of constituent leaf cell size did not reveal any significant change in mean cell size, suggesting that cell division within the induced tissue adapted to the increase in tissue growth. However, this analysis is complicated by the inherent variability in cell size within a leaf and by the relatively small absolute changes in total leaf area observed following expansin gene induction, i.e. the integration of a small change in mean cell size not detectable by our statistical analysis would be sufficient to lead to the observed change in leaf size.
The mid phase of leaf growth coincides with a phase of declining leaf RGR, thus increased expansin activity at this stage acts to halt the decline in RGR, thus promoting overall growth. A previous article by Cookson et al. (2005) identified a correlation of maximum absolute rate (Emax) of leaf growth and final leaf size and the inflection point of the RGR curve correlates with this phase of Emax. Delaying the decline of RGR by promoting expansin activity would have the outcome of increasing Emax, i.e. our data support the correlation of Emax and final leaf size. However, since we were unable to promote expansin activity during the early phase of leaf growth, we could not test whether delaying the decline of RGR at other time points would have the same effect on leaf size.
During normal leaf growth total expansin protein amount per tissue mass increased significantly while the extractable expansin activity per tissue mass was reasonably constant over time, i.e. neither correlated with the calculated decline in leaf RGR over the same time period. These data are again consistent with the idea that there is an endogenous system that controls the efficacy of expansin action. Separating out the different aspects of the control of expansin function remains a challenge for the future.
Although our manipulations of expansin gene expression did not lead to a change in leaf area at the early and late phase of leaf growth, they did lead to altered curvature of the mid vein at all stages investigated. No significant curvature was observed in treated Tet∷GUS leaves. These observations suggest that the developing mid vein has a differential sensitivity to expansin compared to other tissues of the leaf. Alternatively (and not exclusively), it could be that lamina tissue is more flexible and provides less resistance to bending imposed on it via the mid vein. In the absence of tools to measure the in vivo extensibility of tissue, it is very difficult to distinguish these alternatives, but it is interesting to note that a previous report on altered leaf shape via altered expansin expression utilized a promoter that targeted gene expression to the early stages of petiole development (Cho and Cosgrove, 2000). A histological analysis of induced half-leaves of Tet∷EXP plants did not reveal any significant difference between the two halves of the mid vein (data not shown) with respect to cell size. However, if any increase in cellular expansion was accompanied by cell division, then no overt change in mean cell size would occur. In this scenario, there should be an increase in the number of cells on the induced side of the mid vein relative to the noninduced side. However, due to the size of the tobacco leaves being studied and the number of cells that comprise the mid vein, it is difficult to obtain accurate quantitative values for these parameters.
In conclusion, our data indicate that although increased expression of expansin can be used to promote leaf growth, there is an endogenous mechanism that limits the efficacy of the protein to a defined window that coincides with the phase of maximum leaf growth. The molecular nature of this endogenous facilitator of expansin activity is unknown but clearly is a target for future research, both to provide further understanding of the mechanism of plant growth and to provide future tools to promote leaf growth.
MATERIALS AND METHODS
Plant Material and Growth Analysis
Seeds were as described by Pien et al. (2001). Briefly, R7 tobacco (Nicotiana tabacum) seedlings (a gift of Alan Jones, University of North Carolina) were transformed with pBinHyg-Tx-CsExp1 or pBinHyg-Tx-b GUS to create a line of plants inducible for CsEXP1 (Tet∷EXP) or GUS (Tet∷GUS), respectively, by external Ahtet application. Seeds were germinated on Whatman filter paper in 9-mm petri dishes with 4 mL of reverse osmosis water in a SANYO growth chamber (24°C 16-h day, 20°C 8-h night). Dishes were foil wrapped for 5 d, then exposed to the light for 3 d. Seedlings were then transplanted onto Levington M3 compost and transferred to a Conviron CMP4030 chamber (25°C 14-h day, 20°C 10-h night, relative humidity 60%). Leaf width was measured using a ruler; measurements were taken at the widest part of the leaf, in a line perpendicular to the mid vein. Leaf initiation was taken as the point when the leaf reached 10 mm in length. Measurements were made every 2 or 3 d from day of leaf 1 initiation to flowering time.
Final leaf area measurements were taken using a Hancocks gel irradiation box and Baxall DeltaT area meter. Leaves were cut into sections that lay flat on the light box to get a true measure of leaf area.
Curvature Analysis
An image was taken of each leaf. Ten points were distributed by hand along the mid vein from base to tip on the leaf image. An algorithm was then used to calculate a plane curve through these points by means of spline interpolation. This spline curve was then sampled to generate 100 points with equal curve length in between, with an identical number of points for each leaf mid vein. The points were labeled ranging from zero to one, thereby making the curve representation scale invariant and allowing comparison between different lengths of mid vein. The sampling led from the base to the tip of the curve. The curvature of the plane curve was calculated at each of these points using the first and second derivative of the plane curve. Finally the curvature was integrated along the contour. This value is zero for an entirely straight line, negative for a line bent to the right, and positive for a line bent to the left in an upright positioned leaf. This program is available on request.
Half-Leaf Induction
Ahtet solution was made up at 200 μg/mL, 2.3% (w/v) Tween 20 in reverse osmosis water. Control solution was 2.3% Tween 20 in reverse osmosis water. Control solution was painted onto the left side of the adaxial surface of the leaf at the relevant developmental phase. Ahtet solution was painted onto the right side of the adaxial surface of the leaf 2 h later to avoid seepage. The mid vein was taken as the division between the two halves. Induction (or mock induction) was repeated twice, at 24-h intervals.
Histology and GUS Staining
To determine epidermal cell number, leaf samples from specified regions along the leaf axis were taken and fixed in 1:7 (v/v) acetic acid:ethanol then bleached and hydrated in an ethanol series (Kuwabara and Nagata, 2006). A BX51 Olympus microscope and Olympus DP71 camera were used for Nomarski viewing of cells. For each sample, four fields of view were imaged; cells were counted using a mark and record method in Photoshop (CS3 Extended version 10.0.1, Adobe), and a mean cell number for the four images was calculated.
To determine the distribution of cell division frequency, leaves were divided into five even sections lengthways (Fig. 4A) and a 5- × 5-mm sample was taken from the center of each section. Cell divisions were measured using aniline blue staining to visualize newly formed cell walls (Kuwabara and Nagata, 2006). Staining was visualized on a fluorescence microscope (Olympus BX51) under UV excitation with a standard 4′,6-diamino-phenylindole filter set. From each sample, three fields of view were analyzed using Photoshop (CS3 Extended version 10.0.1, Adobe); the number of newly formed cell walls (bright fluorescence, Supplemental Fig. S4) was counted using a mark and record method.
GUS staining was carried out as described by Jefferson et al. (1986). Leaf material was vacuum infiltrated with GUS assay solution for 3 × 5 min before incubation at 37°C overnight. Leaf material was destained in an ethanol dilution series to 100%.
Molecular Analysis
For RT-PCR, RNA was extracted using Sigma's Spectrum total plant RNA kit, as described in the manufacturer's protocol. Protocol A was used and purified RNA was eluted in 30 μL of elution solution. Ambion's DNA-free kit was used to remove contaminating DNA, then cDNA synthesized using 5 μg of RNA as a substrate using Promega reverse transcriptase in a 20-μL reaction volume. PCR was carried out using Bioline Taq polymerase with 4 μL of cDNA substrate in a 50-μL reaction.
NtRBCS primer sequences: FP, TGGCTTCCTCTGTTCTTTCC; RP, CCTTCAGGCTTGTAGGCAAT. CsEXP1 primer sequences: FP, TTTGTCTTCACCTTCGCTGA; RP, GCCTTGCCATTGAGATAGT.
The initial denaturation step of 94°C for 5 min was followed by 30 cycles of 94°C for 30 s, 61°C annealing for 30 s, and 72°C for 1 min. A final 10 min 72°C extension was used. PCR product was run down a 1% agarose gel with ethidium bromide at 65 V for 30 min.
For protein analysis and expansin activity measurements, cell wall proteins were extracted from complete half-leaves (1–5 g fresh weight) according to McQueen-Mason et al. (1992). Basically, after extraction in NaCl and elution in NaAc, proteins were desalted and concentrated using MICROCON YM-10 (Millipore) filter devices, then 50 μg of protein loaded per well for immunodetection on western blots. Proteins were separated in 12% polyacrylamide standard SDS gels then blotted onto polyvinylidene difluoride membrane (Bio-Rad). Blocking was with 5% (w/v) bovine serum albumin in Tris-buffered saline (pH 7.3) containing 0.05% (v/v) Tween 20 for 1 h at RT. For immunodetection, 1:2,000 dilution of primary antibody was used and hybridization was carried out at 4°C overnight. Membranes were washed five times (5 min) with Tris-buffered saline, 0.05% (v/v) Tween 20, then used for hybridization with 1:10,000 dilution of secondary antibody (Alkaline phosphatase conjugated anti-rabbit IgG [Sigma]) for 1 h at RT. After washing, protein detection was with SIGMAFAST Fast Red TR/Naphthol AS-MX alkaline phosphatase substrate.
Extensibility assays were carried out on 100- × 5-mm sections of artificial cellulose-xyloglucan matrix (Whitney et al., 2000) using a constant load extensometer, as described by McQueen-Mason et al. (1992). Matrix was suspended between two clamps under constant tension of 20g. Plastic cuvettes were fitted around one end of the matrix and filled with 100 μL of 50 mm sodium acetate, pH 4.5 for 10 min. Movement of the upper clamp was detected with an electronic position transducer and recorded on a microcomputer. This allowed mean background extensibility to be measured over 8 min, then the bathing solution was replaced with 100 μL of 1 mg/mL cell wall protein extract (before desalting and concentration) and the mean protein extensibility activity measured over a period of 8 min. Extensibility measurements were calculated by subtracting the background extensibility rate from the protein extensibility activity rate. All assays were performed at least in triplicate.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Growth curve analysis.
Supplemental Figure S2. Growth curve comparison for leaf 9 of untreated R7, Tet∷EXP, and Tet∷GUS plants.
Supplemental Figure S3. Growth differentials in control leaves.
Supplemental Figure S4. Upper epidermal cells of leaf 9 from a Tet∷EXP plant.
Supplemental Figure S5. Aniline blue staining to reveal cell division.
Supplemental Table S1. Leaf area values.
Supplemental Table S2. Epidermal cell numbers.
Supplementary Material
Acknowledgments
Claire Percival and Stephanie Holland (University of Sheffield) helped with cell size analysis. We thank Steve Coates (Advanced Technologies Cambridge) for both financial and intellectual support during this project. The R7 seeds were a gift of Alan Jones (University of North Carolina). Thanks go also to all members of the Fleming lab for useful discussions and advice, especially Asuka Kuwabara for advice on histology.
This work was supported by the Biotechnology and Biological Sciences Research Council (Collaborative Awards in Science and Engineering award to J.S.). R.W. and A.B. were supported by the European Union Transfer of Knowledge (grant no. GIPS MTKD-CT 2006–042236).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Andrew J. Fleming (a.fleming@sheffield.ac.uk).
The online version of this article contains Web-only data.
References
- Anastasiou E, Lenhard M (2007) Growing up to one's standard. Curr Opin Plant Biol 10 63–69 [DOI] [PubMed] [Google Scholar]
- Beemster GT, Fiorani F, Inzé D (2003) Cell cycle: the key to plant growth control? Trends Plant Sci 8 154–158 [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P (1999. a) Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell 11 2203–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH, Dunsmuir P (1999. b) Differential expression of expansin gene family members during growth and ripening of tomato fruit. Plant Mol Biol 39 161–169 [DOI] [PubMed] [Google Scholar]
- Caderas D, Muster M, Vogler H, Mandel T, Rose JK, McQueen-Mason S, Kuhlemeier C (2000) Limited correlation between expansin gene expression and elongation growth rate. Plant Physiol 123 1399–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Bradford KJ (2000) Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol 124 1265–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Cosgrove DJ (2000) Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana. Proc Natl Acad Sci USA 97 9783–9788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HT, Kende H (1997) Expansins and internodal growth of deepwater rice. Plant Physiol 113 1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Lee Y, Cho HT, Kende H (2003) Regulation of expansin gene expression affects growth and development in transgenic rice plants. Plant Cell 15 1386–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson SJ, Van Lijsebettens M, Granier C (2005) Correlation between leaf growth variables suggest intrinsic and early controls of leaf size in Arabidopsis thaliana. Plant Cell Environ 28 1355–1366 [Google Scholar]
- Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407 321–326 [DOI] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215 407–419 [DOI] [PubMed] [Google Scholar]
- Hutchison KW, Singer PB, McInnis S, Diaz-Sala C, Greenwood MS (1999) Expansins are conserved in conifers and expressed in hypocotyls in response to exogenous auxin. Plant Physiol 120 827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Burgess SM, Hirsch D (1986) b-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci USA 83 8447–8451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H, Bradford KJ, Brummell DA, Cho HT, Cosgrove DJ, Fleming AJ, Gehring C, Lee Y, McQueen-Mason S, Rose JKC, et al (2004) Nomenclature for members of the expansin superfamily of genes and proteins. Plant Mol Biol 55 311–314 [DOI] [PubMed] [Google Scholar]
- Kim JH, Cho HT, Kende H (2000) α-Expansins in the semiaquatic ferns Marsilea quadrifolia and Regnellidium diphyllum: evolutionary aspects and physiological role in rachis elongation. Planta 212 85–92 [DOI] [PubMed] [Google Scholar]
- Kuwabara A, Nagata T (2006) Cellular basis of developmental plasticity observed in heterophyllous leaf formation of Ludwigia arcuata (Onagraceae). Planta 224 761–770 [DOI] [PubMed] [Google Scholar]
- Lee Y, Choi D, Kende H (2001) Expansins: ever-expanding numbers and functions. Curr Opin Plant Biol 4 527–532 [DOI] [PubMed] [Google Scholar]
- Li LC, Cosgrove DJ (2001) Grass group I pollen allergens (β-expansins) lack proteinase activity and do not cause wall loosening via proteolysis. Eur J Biochem 268 4217–4226 [DOI] [PubMed] [Google Scholar]
- Li Y, Jones L, McQueen-Mason S (2003) Expansins and cell growth. Curr Opin Plant Biol 6 603–610 [DOI] [PubMed] [Google Scholar]
- McQueen-Mason S, Cosgrove DJ (1994) Disruption of hydrogen bonding between plant cell wall polymers by proteins that induce wall extension. Proc Natl Acad Sci USA 91 6574–6578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ (1995) Expansin mode of action on cell walls—analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol 107 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4 1425–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Bourdais G, Reidy B, Bencivenni C, Massonneau A, Condamine P, Rolland G, Conéjéro G, Rogowsky P, Tardieu F (2007) Association of specific expansins with growth in maize leaves is maintained under environmental, genetic, and developmental sources of variation. Plant Physiol 143 278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath U, Crawford BCW, Carpenter R, Coen E (2003) Genetic control of surface curvature. Science 299 1404–1407 [DOI] [PubMed] [Google Scholar]
- Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A (2001) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98 11812–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig RS, Sussex IM (1985) The developmental morphology and growth dynamics of the tobacco leaf. Planta 165 158–169 [DOI] [PubMed] [Google Scholar]
- Qin L, Kudla U, Roze EH, Goverse A, Popeijus H, Nieuwland J, Overmars H, Jones JT, Schots A, Smant G, et al (2004) Plant degradation: a nematode expansin acting on plants. Nature 427 30. [DOI] [PubMed] [Google Scholar]
- Reidy B, McQueen-Mason S, Nösberger J, Fleming A (2001) Differential expression of a- and β-expansin genes in the elongating leaf of Festuca pratensis. Plant Mol Biol 46 491–504 [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Wittwer F, Mandel T, Kuhlemeier C (1998) Localized upregulation of a new expansin gene predicts the site of leaf formation in the tomato meristem. Plant Cell 10 1427–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochange SF, Wenzel CL, McQueen-Mason SJ (2001) Impaired growth in transgenic plants over-expressing an expansin isoform. Plant Mol Biol 46 581–589 [DOI] [PubMed] [Google Scholar]
- Rose JK, Lee HH, Bennett AB (1997) Expression of a divergent expansin gene is fruit-specific and ripening-regulated. Proc Natl Acad Sci USA 94 5955–5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL, Llewellyn DJ, Furbank RT (2001) The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin. Plant Cell 13 47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper O, Schaefer D, Reski R, Fleming A (2002) Expansins in the bryophyte Physcomitrella patens. Plant Mol Biol 50 789–802 [DOI] [PubMed] [Google Scholar]
- White DW (2006) PEAPOD regulates lamina size and curvature in Arabidopsis. Proc Natl Acad Sci USA 103 13238–13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SEC, Gidley MJ, McQueen-Mason SJ (2000) Probing expansin action using cellulose/hemicellulose composites. Plant J 22 327–334 [DOI] [PubMed] [Google Scholar]
- Wu Y, Sharp RE, Durachko DM, Cosgrove DJ (1996) Growth maintenance of the maize primary root at low water potentials involves increases in cell-wall extension properties, expansin activity, and wall susceptibility to expansins. Plant Physiol 111 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrzykowska J, Schorderet M, Pien S, Gruissem W, Fleming AJ (2006) Induction of differentiation in the shoot apical meristem by transient overexpression of a retinoblastoma-related protein. Plant Physiol 141 1338–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.