Abstract
To induce mixed chimerism and renal allograft tolerance in cynomolgus monkeys, cyclophosphamide (CP) and total body irradiation (TBI) were compared as part of a nonmyeloablative conditioning regimen. CP induced dose-dependent neutropenia and lymphopenia, but hematopoietic recovery was more rapid than that observed in the TBI group. Absolute B cell counts after CP were significantly higher (P<0.01) than those in the TBI group. With CP, a total dose of 200 mg/kg with CD154 blockade regularly induced multilineage chimerism. Nevertheless, the recipients failed to achieve long-term survival because of rejection (3 of 5), posttransplantation B cell lymphoma (1 of 5), and toxicities of CP (1 of 5). As previously reported, 3Gy of TBI with either splenectomy or CD154 blockade induced mixed chimerism and renal allograft tolerance, with significantly less morbidity and mortality than that produced by CP. Thus, TBI is more effective and less toxic than CP as part of a nonmyeloablative regimen for the induction of mixed chimerism and renal allograft tolerance in cynomolgus monkeys.
Keywords: Mixed chimerism, Kidney transplantation, Tolerance, Monkeys, Cyclophosphamide
Although numerous protocols for the induction of allograft tolerance have been successfully applied in rodent studies, only a limited number of these have been translated to nonhuman primates. We have previously reported a nonyeloablative treatment regimen for the induction of mixed chimerism and renal allograft tolerance in nonhuman primates (1–3). The regimen included total body irradiation (TBI), local thymic irradiation (TI), antithymocyte globulin (ATG), either splenectomy or a short course of CD154 blockade (4) and a 1-month postoperative course of cyclosporine. With this protocol, the recipients of MHC mismatched kidney allografts developed transient multilineage mixed chimerism and approximately 50% to 60% of them acquired renal allograft tolerance. More recently, we extended this approach to HLA matched (5–7) or mismatched (8) human kidney transplant recipients. In the clinical trials, we have substituted cyclophosphamide (CP), in place of TBI, because of concern for secondary malignancy after TBI. In our HLA-mismatched kidney transplant recipients, all developed transient mixed chimerism, and four of the five have been off all immnosuppression with a follow-up of 2 to 5 years (8).
Encouraged by these clinical studies and prompted by the desire to develop a more similar preclinical model, we evaluated CP in place of TBI in our monkey conditioning regimen. As reported here, we found that cynomolgus monkeys were significantly more resistant to the modulatory effects of CP, requiring larger and more toxic doses to induce chimerism than what was observed in humans. The TBI-based regimen therefore has continued to be essential for the induction of multilineage chimerism and renal allograft tolerance in our nonhuman primate studies.
MATERIALS AND METHODS
A total of 66 male cynomolgous monkeys weighing 3 to 6 kg were used (Charles River Primates, Wilmington, MA). Recipients and donor pairs were selected for compatible ABO blood types and mismatched class I and II cynomolgus leukocyte (CyLA)-MHC antigens.
Kidney and Bone Marrow Transplantation
Monkeys underwent heterotopic kidney transplantation under general anesthesia as previously reported (9). Bone marrow was harvested from donor iliac bones by multiple percutaneous aspirations. If the donor animal was killed, bone marrow cells were also harvested from the vertebral bones after the euthanasia. These cells (1.0 –3.0×108 mononuclear cells/kg) were infused intravenously.
Statistical Analysis
Values from the treatment groups were compared by the nonparametric Mann-Whitney U statistic, using Prism 4.0 (Graphpad Software, San Diego, CA).Atwo-tailed P value of <0.05 was considered significant.
Host Conditioning
The conditioning regimen consisted of either nonlethal TBI (on Days – 6 and –5) or CP (Day –5 to –2), local TI (7 Gy) on day –1, intravenous equine antithymocyte globulin (ATGAM, the Upjohn Co., Kalamazoo, MI) (50 mg/kg/day) on days –2, –1, and 0. The recipients also received either splenectomy (day 0) or a short course of mouse anti-CD154 mAb (5c8, 20 mg/kg on day 0 and 2). The recipients underwent combined kidney and bone marrow transplantation on day 0 followed by a 1-month course of intramuscular injection of cyclosporine (Novartis, Basel, Switzerland) (tapered from an initial dose of 15 mg/kg/day) to maintain therapeutic serum levels (>300 ng/mL). Cyclosporine was discontinued during the fourth postoperative week after which the serum levels typically became undetectable by day 60 to 70 (Table 1).
TABLE 1.
Conditioning regimen and results
| CP/TBI dose | N | TIa | ATG | Anti-CD154 | Spxb | Multilineage chimerism | Kidney graft survival (d) | Outcome (no. animals) | |
|---|---|---|---|---|---|---|---|---|---|
| A | CP 40 mg/kg×3 | 1 | + | + | + | − | 0/1 | 59 | ACRc (1) |
| B | CP 150 mg/kg×3 | 2 | + | + | + | − | 0/2 | 76, 1 | ACR (1), bleeding (1) |
| C | CP 50 mg/kg×4 | 5 | + | + | + | − | 4/5 | 175, 118, 82, 70, 5 | ACR (1), ACR+pyelonephritis (1), CHRd (1), PTLDe (1) Hemorrahagic cystitis (1) |
| D | CP 50 mg/kg×4 | 2 | + | + | − | − | 0/2 | 51, 11 | PTLD (1), infection (1) |
| E | TBI 1.5 Gy×1 | 1 | + | + | − | + | 0/1 | 56 | ACR |
| F | TBI 2.3 Gy×1 | 3 | + | + | − | + | 0/3 | 69, 59, 43 | ACR (3) |
| G | TBI 1.25 Gy×2 | 9 | + | + | − | + | 2/9 | 155, 145, 93, 93, 92, 90, 61, 50, 37 | ACR (7), urolithiasis (2) |
| Hf | TBI 1.5 Gy×2 | 13 | + | + | − | + | 11/13 | >3478, >2569, 834, 771, 405, 260, 198, 196, 137, 72, 44, 40, 37 | No rejection (7), ACR (3) |
| Ig | TBI 1.5 Gy×2 | 8 | + | + | + | − | 8/8 | >1710, >1167, 837, 755, 401, 373, 206, 58 | No rejection (4), ACR (1), CR (3) |
Thymic irradiation
Splenectomy
acute cellular rejection
chronic humoral rejection
Posttransplant lymphoproliferative disease
Kawai T et al. Transplantation 1999; 68: 1767–1775
Kawai T et al. Am J Transplant 2004; 4: 1391.
The CP recipients were divided into four groups: group A (n=1); CP 40 mg/kg×3 on days –5, –4, and –3 with 5c8 on days 0 and 2, group B (n=2); CP 50 mg/kg×3 on days –5, –4, and –3 with 5c8 on days 0 and 2. Group C (n=5); CP 50 mg/kg×4 on days –5, –4, –3, and –2 with 5c8 on days 0 and 2. Group D (n=2); CP 50 mg/kg×4 on days –5, –4, –3, and –2, no 5c8.
The TBI treated recipients were divided into five groups: group E (n=1); 1.5 Gy of TBI on day –6 with splenectomy on day 0. Group F (n=3); 2.3 Gy of TBI on day –6 and splenectomy on day 0. Group G (n=9); 1.25 Gy of TBI on days –6 and –5 and splenectomy on day 0. Group H (n=13); 1.5 Gy×2 of TBI on days –6 and –5 with splenectomy on day 0, group I (n=8); 1.5 Gy×2 of TBI on days –6 and –5 with 5c8 on days 0 and 2, but no splenectomy. Some of the results of groups H and I have been previously published (1, 3, 4).
RESULTS
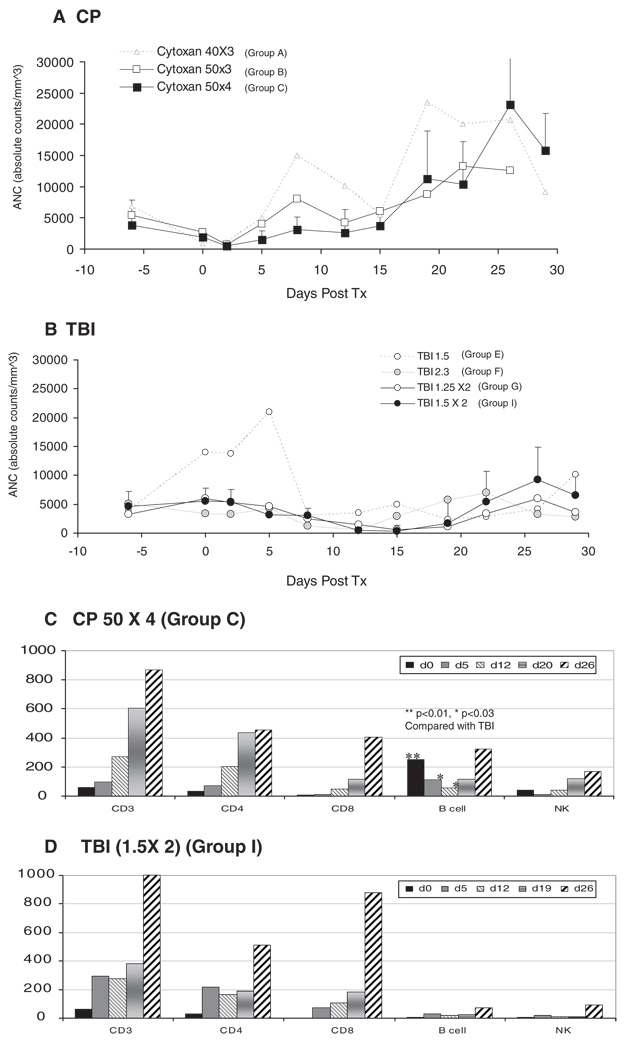
Absolute neutrophil counts (ANC) of the CP versus TBI treated recipients are depicted in Figure 1(A and B), respectively. In recipients treated with CP, neutropenia was observed in dose-dependent fashion, with the nadir of 500/mm3, occurring on posttransplantation day 2 (7–8 days after administration of CP). This was earlier than the nadir observed in the TBI recipients (18–21 days after irradiation). Neutrophils then recovered to low normal levels(>3000/mm3) by day 5 in groups A and B (<200 mg/kg), and by day 8 in group C. The ANC then rebounded and remained higher than 10,000/ mm3 until day 30 (Fig. 1A), after which they returned to pretransplant levels by day 50.
FIGURE 1.
Absolute neutrophil counts (ANC) in CP group (A) and TBI group (B). In CP-treated recipients, neutropenia was observed in dose-dependent fashion and the nadir of ANC was around 500/mm3 on post-transplantation day 2. ANC then recovered to normal (>3000/mm3) by day 5 in groups A (CP 40 mg/kg×3) and B (50 mg/kg×3), and by day 8 in group C(CP 50 mg/kg×4). In TBI treated recipients, neutropenia was not observed with 1.5×1 Gy of TBI. With TBI 2.3 Gy, the recipients showed earlier recovery of ANC by day 15. Similar levels of neutropenia were observed in the recipients treated with TBI 1.25×2 and 1.5×2 Gy between days 8 and 18. After day 18, ANC was higher in group I, because of higher levels of donor chimeric neutrophils. Lymphocyte subsets in group C (C) and in group I (D). There was no significant difference in absolute counts of CD3, CD4, CD8 and NK cells between groups C and I. B cell absolute counts were significantly higher in group C (CP 50×4 mg/kg) on days 0, 5 and 12 than those in group I (TBI 1.5 Gy×2).
In the TBI groups, neutropenia was not observed with 1.5×1 Gy of TBI. With TBI 2.3 Gy, the recipients developed transient neutropenia between days 8 and 12, but recovered by day 15. Neutropenia was more severe and protracted in the recipients treated with TBI 1.25×2 or 1.5×2 Gy, the nadir occurring between days 12 and 15 after DBM transplantation. After day 18, the ANC were highest in group I because of improved DBM engraftment leading to higher levels of donor hematopoietic cells (Fig. 1B).
In Figure 1(C and D), lymphocyte subsets of the most intensively treated recipients were compared: group C (CP 50 mg/kg×4) and group I (TBI 1.5 Gy×2). Although CD3+, CD4+, CD8+ and NK (CD3-CD16+) cells were similarly depleted in both groups, B cell (CD20+) counts were significantly higher on days 0 (P<0.01), 5 (P<0.01) and 12 (P<0.03) in group C than those observed in group I.
None of the recipients treated with either 40×3 or 50×3 mg/kg of CP developed chimerism (groups A and B, Table 1). One monkey died due to bleeding on day 1 because of thrombocytopenia and two other recipients rejected their kidney allografts soon after discontinuation of cyclosporine. In group C, four of the five recipients developed mixed chimerism. CD 154 blockade was also essential for this regimen as emphasized by failure of DBM engraftment in group D (Table 1, group D).
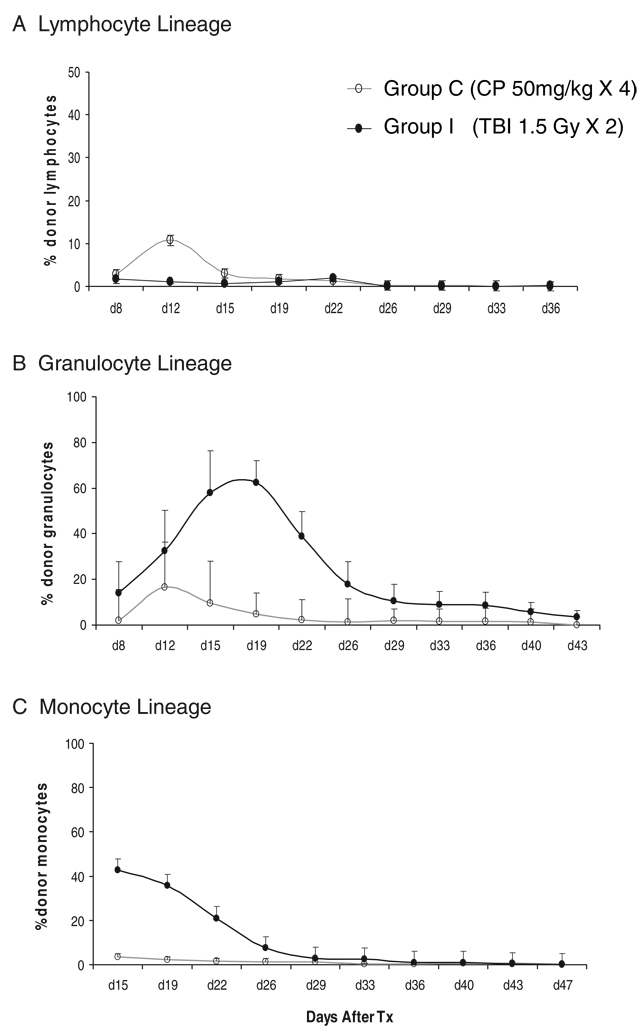
Figure 2 compares granulocyte, monocyte, and lymphocyte chimerism in groups C and I. Lymphoid chimerism in group C peaked at more than 10% on day 12, which was even higher than that detected in group I (Fig. 2A). In contrast, chimerism in myeloid lineages was significantly higher after TBI (Fig. 2B, C).
FIGURE 2.
Chimerism in groups C and I. Chimerism in the lymphocyte lineage (A), granulocyte lineage (B), and monocyte lineage (C). The levels of chimerism in the granulocyte, monocyte and lymphocyte lineages were compared between groups C and I. Lymphoid chimerism was higher in group C than those detected in group I (A). In contrast, chimerism in the myeloid lineage was significantly higher in group I than those in group C. The levels of granulocytic and monocytic chimerism were significantly higher in group I on day 19 and 22 than those in group C (B and C).
Two recipients that failed to develop chimerism in groups A and B rejected their kidney allograft soon after discontinuation of cyclosporine. Three of 5 recipients in group C developed acute rejection after discontinuation of cyclosporine. One recipient died on day 5 because of hemorrhagic cystitis despite prophylactic treatment with Mesna, and the fifth recipient died because of posttransplantation lymphoproliferative disease (PTLD) on day 70. Another recipient in group D also developed PTLD. Immunochemical staining of these lymphoma cells revealed CD19+ B cell lymphoma (data not shown). In contrast, approximately half of recipients in groups H and I acquired renal allograft tolerance, with the longest survival exceeding 13 years.
DISCUSSION
In our model for the induction of hematopoietic chimerism, we have suggested that myelosuppressive conditioning is required not only for T cell depletion, but also to make the bone marrow environment permissive for engraftment of allogeneic bone marrow cells. Such conditioning has been shown to be necessary even in syngeneic bone marrow transplantation, leading to the conclusion that TBI or CP to create physical “space” for engraftment of bone marrow cells (10). We initially applied TBI in our monkey regimen based upon the previous successful studies in mice (11). Subsequently, CP has been used in the clinical trials because of concerns regarding secondary malignancies after TBI. The hematopoietic recovery in human recipients treated with CP was similar to what was observed in monkeys treated with TBI, and the results of ongoing clinical trials for HLA mismatched kidney transplantation have been very encouraging (8). However, we have observed a shorter duration of chimerism in the human recipients treated with the CP-based regimen, in contrast to what was observed in monkeys treated with TBI (groups H and I). The current study was therefore undertaken in an attempt to develop a preclinical model with which the CP regimen could be more systematically evaluated. The initially treated nonhuman primates revealed significant resistance to CP treatment in monkeys. Although a total of 120 mg/kg (60×2 mg/kg) of CP consistently permitted chimerism in humans (8), 200 mg/kg (50×4 mg/kg) of CP with additional CD154 blockade was necessary to allow chimerism in cynomolgus monkeys. In the current study, CP was administered on days –5, –4, –3, and –2 (group C). It may be argued that DBM engraftment can be improved by altering the timing of CP administration. However, if the timing of CP administration is altered earlier, recipient cells would fully recover by the time of DBM injection. Conversely, because the half life of CP is 12 hr, the timing cannot be delayed until day –1 to avoid the toxicity of CP on DBM cells. In addition, none of the monkey recipients treated with CP achieved long-term renal allograft survival, in striking contrast to the TBI-treated animals. The leading causes of failure after CP treatment of the monkeys were posttransplantation B-cell lymphoma and acute rejection. Among various parameters observed after conditioning, we noted a significant difference in B cell depletion between the CP- and TBI-treated groups. In recipients treated with CP, significantly higher levels of CD20+ B cells remained in the peripheral blood. Concomitant B-cell depletion may be especially important in these regimens to prevent both PTLD and acute humoral responses. Failure to deplete B cells in the presence of severe T cell suppression could increase the risk of PTLD. Since CD8+ cytolytic T cells play an essential role in the killing of EBV-infected lymphoblastoid cells and CD4+ effector T cells are required to prevent early-phase EBV-induced B-cell proliferation (12), EBV-infected B cells divide rapidly in the absence of T cells. The human protocol has been modified by adding rituximab to suppress humoral rejection (8), but deleting B cells may also be a logical approach to prevent the development of B cell lymphoma. Although one of three recipients developed antibody-mediated rejection, the significance of the residual B cells for humoral responses in these monkeys was not conclusive from this study.
The exact mechanism of tolerance induction after the disappearance of donor chimerism in our monkey model and initially treated patients has been under active investigation. Our current hypothesis is that T-regulatory cells are induced after transient mixed chimerism. Evidence supporting this mechanism includes the observation of increased number of T regulatory cells in the circulation after conditioning and increased infiltration of Foxp3+ T cells in the renal allograft (manuscript in preparation). It is possible that immature dendritic cells derived from donor hematopoietic stem cells could induce such T regulatory cells (13, 14) during periods of mixed chimerism. The lower levels of myeloid chimerism in CP-treated monkeys, especially in the monocyte or dendritic cell lineage, may explain the failure of this regimen to induce tolerance. Unfortunately, the unacceptable toxicity of CP in the monkey model despite the resistance of these animals to its myelosuppressive effects prevented our pursuing this approach any further.
The current studies revealed the advantages of TBI over CP in both efficacy and safety for the induction of mixed chimerism and renal allograft tolerance in cynomolgus monkeys.
REFERENCES
- 1.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256. [PubMed] [Google Scholar]
- 2.Kimikawa M, Sachs DH, Colvin RB, Bartholomew A, Kawai T, Cosimi AB. Modifications of the conditioning regimen for achieving mixed chimerism and donor-specific tolerance in cynomolgus monkeys. Transplantation. 1997;64:709. doi: 10.1097/00007890-199709150-00008. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Poncelet A, Sachs DH, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68:1767. doi: 10.1097/00007890-199912150-00022. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 5.Spitzer TR, Delmonico F, Tolkoff-Rubin N, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: The induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68:480. doi: 10.1097/00007890-199908270-00006. [DOI] [PubMed] [Google Scholar]
- 6.Buhler LH, Spitzer TR, Sykes M, et al. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74:1405. doi: 10.1097/00007890-200211270-00011. [DOI] [PubMed] [Google Scholar]
- 7.Fudaba Y, Spitzer TR, Shaffer J, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: In vivo and in vitro analyses. Am J Transplant. 2006;6:2121. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosimi AB, Delmonico FL, Wright JK, et al. Prolonged survival of nonhuman primate renal allograft recipients treated only with anti-CD4 monoclonal antibody. Surgery. 1990;108:406. [PubMed] [Google Scholar]
- 10.Tomita Y, Sachs DH, Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939. [PubMed] [Google Scholar]
- 11.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikiforow S, Bottomly K, Miller G. CD4+ T-cell effectors inhibit Epstein-Barr virus-induced B-cell proliferation. J Virol. 2001;75:3740. doi: 10.1128/JVI.75.8.3740-3752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coenen JJ, Koenen HJ, Emmer PM, van Rijssen E, Hilbrands LB, Joosten I. Allogeneic stimulation of naturally occurringCD4+CD25+T cells induces strong regulatory capacity with increased donor-reactivity. Transpl Immunol. 2007;17:237. doi: 10.1016/j.trim.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]