Abstract
Background
Hemodialysis (HD) patients have a greatly increased risk of cardiovascular morbidity and mortality. For this reason, attempts are often made to normalize hyperhomocysteinemia. This randomized prospective study sought to determine which risk factors are predictors of mortality and whether high doses of folates or 5-methyltetrahydrofolate (5-MTHF) could improve hyperhomocysteinemia and survival in HD patients.
Methods
341 patients were divided into two groups: group A was treated with 50 mg i.v. 5-MTHF, and group B was treated with 5 mg/day oral folic acid. Both groups received i.v. vitamin B6 and B12. By dividing patients into C-reactive protein (CRP) quartiles, group A had the highest survival for CRP <12 mg/l, whereas no survival difference was found for group B. CRP was the only predictive risk factor for death (RR 1.17, range 1.04–1.30, p = 0.02). Dialysis age, hyperhomocysteinemia, methylenetetrahydrofolate reductase polymorphism, albumin, lipoprotein (a) and folate did not influence mortality risk. Survival in group A was higher than that in group B, namely 36.2 ± 20.9 vs. 26.1 ± 22.2 months (p = 0.003).
Results
Our results suggest that CRP, but not hyperhomocysteinemia, is the main risk factor for mortality in HD patients receiving vitamin supplements. Intravenous 5-MTHF seems to improve survival in HD patients independent from homocysteine lowering.
Key Words: ESRD patients; ESRD, survival; C-reactive protein; Homocysteine; 5-Methyltetrahydrofolate
Introduction
Hemodialysis (HD) patients show a 20-fold increase in cardiovascular disease (CVD) mortality in comparison to the general population [1]. The high CVD mortality is thought to be due to a combination of different risk factors including: ‘traditional risk factors’ such as those evidenced in the Framingham Study as well as ‘non-traditional risk factors’ such as anemia, albuminuria, altered calcium-phosphate metabolism, hypervolemia, malnutrition, oxidative stress, lipoprotein (a) (Lp(a)), prothrombotic factors, acute phase response, growth factors release and hyperhomocysteinemia [2].
Although hyperhomocysteinemia has been implicated as an important independent risk factor in both the general population [3], as well as for end-stage renal disease (ESRD) patients [4,5,6], several studies have questioned the benefit of lowering homocysteine in ESRD patients. Paradoxically, two recent studies showed that patients with very low homocysteine plasma levels had worse outcomes including a higher incidence of hospitalization and mortality [7, 8]. This raises the question as to whether elevated homocysteine in uremic patients is consequential rather than causal in the role of cardiovascular complications [9,10,11].
Despite this uncertainty, many ESRD and pre-ESRD patients receive treatments to lower homocysteine. Elevated homocysteine is frequently reported for ESRD patients with a prevalence ranging from 85 to 100% [12,13,14].
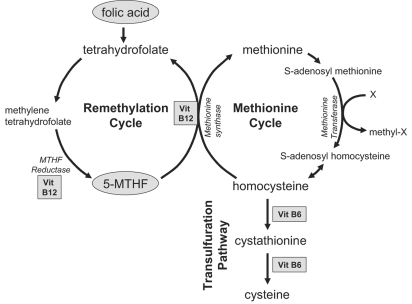
There are two basic strategies that can be used to lower homocysteine. Both attempt to increase levels of biologically active folate which is essential in the remethylation pathway of homocysteine metabolism via its active metabolite 5-methyltetrahydrofolate (5-MTHF), thus lowering homocysteine efflux from tissues into the plasma compartment. The first, and most common approach, is by oral administration of folic acid. Folic acid is not biologically active; however, it is more stable than folate, and is often used in tablets and food fortification. The second approach is to supplement 5-MTHF, the natural circulating form of folate. In addition to folate, both vitamin B6 and vitamin B12 are necessary cofactors in homocysteine metabolism. The main homocysteine metabolic pathways are illustrated in figure 1.
Fig. 1.
Homocysteine metabolic pathways. Biologically active folate (derived from folic acid or 5-MTHF) and vitamin B12 are necessary to convert homocysteine to methionine.
ESRD patients are often resistant to homocysteine lowering by administration of both folic acid and 5-MTHF. This might be due to either genetic or acquired pathogenetic mechanisms [15]. For instance, there are several methylenetetrahydrofolate reductase (MTHFR) polymorphisms that can affect MTHFR activity. MTHFR is a key regulatory enzyme involved in folate-dependent homocysteine remethylation. It catalyzes the reduction of 5,10-methylenetetrahydrofolate to 5-MTHF.
Although supplementation with folic acid, B6 and B12 usually decreases homocysteine in patients with vascular disease [16, 17], it often remains elevated in ESRD patients despite supplementation of folic acid, B6 and B12. Several studies have reported only moderate effects, even with very high doses of folic acid (up to 15 mg/daily) [18].
The aim of this study was to investigate whether supplementation with 5-MTHF versus folic acid treatment could affect patient survival. In particular, we detected homocysteine blood levels and MTHFR genetic polymorphisms to evaluate if they can be considered as independent cardiovascular risk factors. 5-MTHF doses considered were higher than those previously reported [19] which failed to induce significant homocysteine lowering. Folic acid therapy was administered in accordance with the standard dosage reported from other authors for homocysteine lowering [9,20,21,22].
Methods
Patients
341 stable HD patients were randomized into two groups: group A consisted of 174 patients (87 F, 87 M) treated with intravenous 5-MTHF (Prefolic®, Knoll, Milan, Italy) 50 mg at the end of each HD session; group B consisted of 167 patients (77 F, 90 M) treated with 5 mg/day of oral folic acid (Folina®, Schwarz Pharma, Milan, Italy). Both groups received supplementation with vitamin B6 300 mg (Benadon®, Roche, Milan, Italy) and vitamin B12 1,000 μg (Dobetin®, ACRAF, Rome, Italy) administered by intravenous injection at the end of the HD session three times per week.
For at least 3 months prior to the study, all patients received the vitamin B6 and B12 i.v. supplementation and three times weekly 0.35 mg i.v. folic acid (Epargriseovit®, Pharmacia & Upjohn, Milan, Italy). In addition, a therapy of 150 ± 60 U/kg/week epoetin was administered to all patients included in our study.
A baseline screening was done for homocysteine, albumin, Lp(a), C-reactive protein (CRP), hematocrit, hemoglobin, folates, vitamin B6, vitamin B12 and MTHFR enzyme gene polymorphisms. Normal serological homocysteine levels were considered between 5 and 12 μmol/l. Other samples were collected at 6, 12, 24 and 55 months from the baseline to check homocysteine, Lp(a), CRP, vitamin B6 and vitamin B12 levels for a total follow-up of 55 months. All the samples were obtained before starting the midweek HD session.
Glomerular filtration rate, obtained by means of Cockroft Gault's formula, was <5 ml/min for each patient enrolled.
Patients’ characteristics: in group A (174), 87 were females (50%) and 87 males (50%), 83 patients died (48%), 71 (41%) were on HD treatment and 19 (11%) underwent renal transplantation; in group B (167), 77 (46%) were females and 90 (54%) males, 112 (67%) patients died, 48 (29%) were on HD and 7 (4%) underwent renal transplantation.
All patients were on regular HD or hemodiafiltration (HDF) treatment three times a week, HD treatment duration ranged between 3 and 312 months at the entry in the study for both groups. Ultrafiltration changes were based on clinical need, blood flow of 310 ± 30 ml/min and dialysate flow of 500 ml/min. 198 patients (58%) were on bicarbonate dialysis standard with polysulfone membrane (F7 HPS, Fresenius, Bad Homburg, Germany), 143 (42%) patients were on HDF with polyetheresulfone (Diapes BLS 814, Bellco, Mirandola, Italy). Adequate dialysis dose was 1.3 ± 0.3, according to Daugirdas’ Kt/V.
CVD was assessed as presence/absence of hypertension, ischemic cardiac disease, cerebral and peripheral vascular disease, and diabetes. Clinical history, laboratory analysis, instrumental examinations, specific evaluations suspected and confirm the diagnosis. Coronary artery disease was investigated by determination of at least one of the following parameters: (1) previous documentation of acute myocardial infarction (laboratory or ECG modifications); (2) symptomatic CVD events in the clinical history confirmed by a positive treadmill test, and (3) coronary artery stenosis more than 50% in one of the three major coronary vessels documented by an angiographic study. All patients with coronary artery disease were examined by a treadmill test (thallium scan) or coronary angiographic examination before entering the study. Cerebrovascular disease was investigated by one of the following criteria: (1) a previous ictus (ongoing clinical evidence of neurological deficit in the 3 months before beginning the study, confirmed by a TC scan, a nuclear magnetic resonance or a physician's record of clinical history), and (2) carotid vessel stenosis more than 50% documented by a Doppler examination.
Peripheral vascular disease was assessed by the evidence of claudicatio intermittens, previous vascular surgical procedure (including amputation for ischemic limb or by angiographic/Doppler documentation of atherosclerotic plaques in abdominal, iliac and femoral vessels). The vascular surgical procedure was conducted at least 3 months before the study started.
The study was approved by our institutional review board and all patients gave informed consent.
Laboratory Methods
Plasma homocysteine (after reduction of the protein-bound fraction) was determined by HPLC [23]. Folates (normal range 6.1–38.5 nmol/l) and vitamin B12 (normal range 162–632 pmol/l) were evaluated by a radioimmunoassay method according to Ciba-Corning Diagnostic Corp. Vitamin B6 values were determined by HPLC [24]. Plasma levels of CRP were assessed by an immunonephelometric assay (Behring, Marburg, Germany) with a quantization limit of 3.2 mg/l. Lp(a) was evaluated by an immunometric assay (Bouty Inc., Sesto S. Giovanni, Italy). Albumin, hemoglobin and hematocrit were determined by standard laboratory methods.
The genotyping protocol for the detection of the MTHFR 677 C>T polymorphism was adapted from Schneider et al. [25]. The presence of the MTHFR 1298 A>C polymorphism was analyzed by using a modification of a method from Skibola et al. [26].
Statistical Analysis
Statistical analysis was performed by the Statistical Package for the Social Sciences (SPSS for Windows Software Package, Version 9.0.1). Data are presented as means ± SD. The Student t test was used for group comparison of continuous variables with normal population distribution (Kolmogorov-Smirnov test, Z = 0.77). The χ2 test was used for group comparison of no continuous variables, in particular in relation to gene study. p < 0.05 was considered statistical significant. A linear regression analysis was performed to evaluate the presence of a statistically significant relationship between the baseline homocysteine levels and other parameters like risk factors for hyperhomocysteine: CRP, albumin, folates, and Lp(a). A multivariate Cox analysis risk was conducted to determine an independent association between the baseline characteristics and cardiovascular risk of death during follow-up. The following parameters were considered: patients’ age, HD age, basal homocysteine, albumin, Lp(a) and CRP values, MTHFR gene polymorphism, and folate levels.
Results
We did not observe any statistically significant differences at baseline between the groups concerning age, dialysis age, CRP, albumin, and Lp(a) levels (table 1). Hemoglobin levels were similar between the two groups at each time interval checked: at 6 months 100 ± 12 vs. 105 ± 9 g/l, p = NS; at 12 months 104 ± 13 vs. 102 ± 8 g/l, p = NS; at 24 months 105 ± 14 vs. 103 ± 19 g/l, p = NS; at 24 months 104 ± 16 vs. 106 ± 17 g/l, p = NS.
Table 1.
Baseline characteristics of the patients
| Group A | Group B | p | |
|---|---|---|---|
| Patients | 174 | 167 | NS |
| Males/females | 87/87 | 90/77 | NS |
| Age, years | 60.1±15.5 | 63.8±12.0 | NS |
| Dialysis age, months | 57.2±73 | 39.7±59.7 | NS |
| Peripheral vasculopathy, % | 67.8 | 70.8 | NS |
| Cardiac ischemic disease, % | 63.3 | 69.7 | NS |
| Cerebral vasculopathy, % | 25.6 | 27.0 | NS |
| Diabetes, % | 22.2 | 30.3 | NS |
| Hypertension, % | 57.8 | 66.3 | NS |
| Homocysteinemia, μmol/l | 51.1±53.1 | 43.0±37.9 | NS |
| Lp(a), μmol/l | 1.1±1.0 | 0.8±0.8 | NS |
| C-reactive protein, mg/l | 20.6±23.5 | 21.7±37.4 | NS |
| Albuminemia, g/l | 36±4 | 34±5 | NS |
| Folates, nmol/l | 16.8±11.8 | 17.4±14.0 | NS |
| Vitamin B6, nmol/l | 42.6±61.2 | 56.7±87.0 | NS |
| Vitamin B12, pmol/l | 1,220.6±3,059.2 | 772.2±1,424.8 | NS |
| Hemoglobin, g/l | 110±11 | 107±9 | NS |
Group A = Patients treated with i.v. 5-MTHF; group B = patients treated with oral folate.
Homocysteine basal values, folate levels, B6 baseline levels, B12 values were similar in both groups (table 1). Hyperhomocysteine prevalence (>12 μmol/l) was 93%. With regard to the kind of dialysis techniques, no difference was found between patients undergoing HD versus those undergoing HDF at each time interval checked (table 2). Gene polymorphism analysis on C677T and A1298C loci and baseline homocysteine concentrations are shown in table 3. Higher homocysteine plasma levels were identified in patients with TT/AA homozygous polymorphism in C677T position (92.1 ± 82.9%), although no statistical significant relevance was determined in comparison with the other polymorphisms. On the other hand, there were no differences in polymorphisms distribution in both groups.
Table 2.
Dialysis techniques and homocysteine levels: HD vs. HDF
| Homocysteine, μmol/l | HD | HDF | p |
|---|---|---|---|
| Basal | 48.1±52.1 | 47.0±35.8 | NS |
| 6 months | 35.7±19.5 | 34.1±25.4 | NS |
| 12 months | 25.8±12.0 | 26.3±18.1 | NS |
| 24 months | 21.2±8.3 | 21.7±10.1 | NS |
| 55 months | 20.4±9.3 | 21.2±7.3 | NS |
Table 3.
MTHFR gene polymorphism prevalence and homocysteine levels after 6 months' treatment with i.v. 5-MTHF or oral folates
| Polymorphisms | Whole study population, baseline |
5-MTHF (group A), 6 months |
Folate (group B), 6 months |
|||
|---|---|---|---|---|---|---|
| prevalence % | homocysteine μmol/l | homocysteine reduction, % | patients n | homocysteine reduction, % | patients n | |
| CT/CA | 29.3 | 34.6±20.0 | 37.4±31.2 | 51 | 20.4±47.2 | 49 |
| CT/AA | 25.6 | 46.3±37.3 | 30.4±45.0 | 43 | 19.6±43.0 | 45 |
| TT/AA | 20.7 | 92.1±82.9 | 53.6±31.1 | 38 | 23.6±41.1 | 33 |
| CC/CA | 12.2 | 27.6±12.5 | 3.2±31.0 | 19 | 4.2±22.0 | 22 |
| CC/AA | 7.4 | 33.9±26.7 | 41.3±21.2 | 13 | 23.7±41.2 | 12 |
| CC/CC | 2.4 | 62.3±12.8 | 57.8±12.4 | 6 | 21.8±42.4 | 2 |
| CT/CC | 1.2 | 30.5 | 40.9 | 2 | 20.9 | 2 |
| TT/CA | 1.2 | 212.3 | 94.3 | 2 | 34.4 | 2 |
During follow-up, 194 patients died, while 24 underwent kidney transplantation. Causes of death included: myocardial infarction in 46.4% (90 patients), cachexia in 15.5% (30 patients), sudden death in 12.4% (24 patients), sepsis in 8.7% (17 patients), malignancy in 5.7% (11 patients), stroke in 4.1% (8 patients), gastrointestinal bleeding in 3.6% (7 patients), and unknown in 3.6% (7 patients). The deaths were equally distributed over the study period in each group.
At 6 months from the beginning of the study, homocysteine levels were more greatly reduced in group A than in group B (20.7 ± 9.5 vs. 35.0 ± 26.4 μmol/l, p < 0.01) with a percentage reduction of −36.9 ± 37% in group A and −11.2 ± 31.6% in group B. At 12, 24 and 55 months’ follow-up, homocysteine levels were similar in both groups (p = NS). There was a greater reduction of homocysteine in group A patients with TT/AA polymorphism; however, this was not statistically significant versus the other gene polymorphisms (table 3).
We also did not detect any statistically significant difference in homocysteine levels between surviving versus deceased patients: 44.4 ± 30.6 vs. 49.6 ± 29.3 μmol/l, nor after division into homocysteine quartiles (≤20, 21–30, 31–55, >55 μmol/l). Patients with the lowest homocysteine quartile (≤20 μmol/l) had a 23.3% patient survival compared to 22% deceased, a homocysteine level between 20 and 30 μmol/l had 22.4% survival versus 28% deceased, homocysteine levels between 30 and 55 μmol/l were detected in 25.8% of surviving patients and 27% of deceased, homocysteine values >50 μmol/l were observed in 22.4% of surviving patients and in 23% of deceased.
Linear regression analysis to evaluate a possible correlation between baseline homocysteine and other risk factors like CRP, albumin, folates and Lp(a), showed a significant correlation between homocysteine and folates at baseline (r = −0.04, p = 0.03).
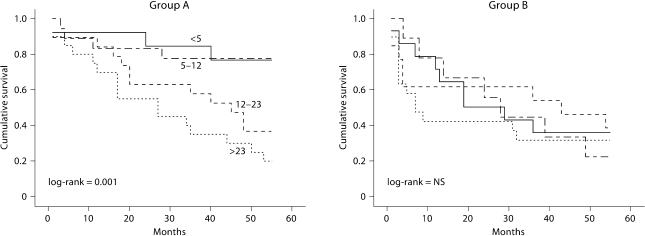
5-MTHF therapy seemed to attenuate the patients’ inflammatory state. Although CRP values were initially similar in both groups at baseline, 6 and 12 months; at 24 months the CRP value was 9.0 ± 5.0 mg/l in the treated group versus 21.1 ± 19.3 mg/l in the control group (p = 0.02); at 55 months it was 8.1 ± 4.2 vs. 18.4 ± 13.3 mg/l, respectively (p < 0.05). By dividing patients into CRP quartiles (<5, 5–12, 12–23, >23 mg/l), we observed a statistically significant higher survival rate in patients with a CRP value located in the two lower quartiles, log-rank = 0.005 (fig. 2). Hence we investigated CRP separately in both groups. In group A (5-MTHF), the highest survival was determined for those with a CRP value <12 mg/l, whereas no significant difference in survival related to CRP levels was observed in group B.
Fig. 2.
Kaplan-Meier survival analysis for CRP (mg/l) quartiles for each group considered. Group A = Patients treated with i.v. 5-MTHF, log-rank = 0.001; group B = patients treated with oral folate, log-rank = NS.
A Cox regression analysis, adjusted by age, demonstrated that only CRP was a predictive risk factor for death (RR 1.17, range 1.04–1.30, p = 0.02). Dialysis age, homocysteine plasma level, gene polymorphism detection, albumin, Lp(a) levels and folate should not be considered as mortality risk factors (table 4). Cox analysis, corrected by age, in patients treated with 5-MTHF confirmed this result, emphasizing that CRP is the only predictive mortality factor (RR 1.15, range 1.02–1.28, p = 0.01; table 4).
Table 4.
Cox regression analysis of risk factors for mortality adjusted by age
| Whole study population |
5-MTHF (group A) |
Folate (group B) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RR | range | p | RR | range | p | RR | range | p | |
| Dialysis age | 1.00 | 0.99–1.00 | NS | 1.00 | 0.99–1.00 | NS | 0.92 | 0.83–1.02 | NS |
| Lipoprotein (a) | 0.99 | 0.96–1.01 | NS | 0.99 | 0.98–1.01 | NS | 1.00 | 0.99–1.01 | NS |
| CRP | 1.17 | 1.04–1.30 | 0.02 | 1.15 | 1.02–1.28 | 0.01 | 1.01 | 0.89–1.14 | NS |
| Homocysteine | 1.00 | 0.99–1.01 | NS | 0.99 | 0.99–1.00 | NS | 1.01 | 0.98–1.04 | NS |
| MTHFR polymorphism | 0.84 | 0.64–1.10 | NS | 0.83 | 0.58–1.20 | NS | 0.48 | 0.17–1.34 | NS |
| Albumin | 0.78 | 0.20–3.00 | NS | 0.39 | 0.08–1.69 | NS | 6.96 | 0.16–303 | NS |
| Folates | 1.00 | 0.90–1.10 | NS | 0.97 | 0.90–1.05 | NS | 1.00 | 0.91–1.10 | NS |
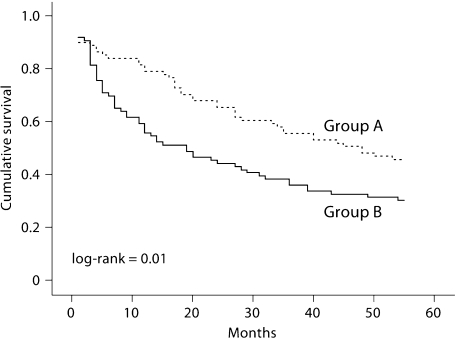
In particular, it should be noted that the mean survival of the 5-MTHF-treated patients (group A) from the beginning of therapy was higher than that of the folate-treated group (group B): 36.2 ± 20.9 vs. 26.1 ± 22.2 months (p = 0.003; fig. 2). Kaplan-Meier survival analysis confirmed a higher survival in patients treated with 5-MTHF (log-rank = 0.01; fig. 3).
Fig. 3.
Kaplan-Meier survival analysis for patients treated with i.v. 5-MTHF (group A) vs. patients treated with oral folate (group B) (log-rank = 0.01).
Discussion
Our study confirmed the high incidence (>90%) of hyperhomocysteinemia in HD patients; however, it failed to assess any role for baseline hyperhomocysteinemia and MTHFR polymorphism as independent risk factors for overall and cardiovascular mortality. The Cox regression analysis, adjusted by age, showed that only CRP was an independent risk factor for mortality in our cohort of HD patients.
As expected, both folic acid and 5-MTHF resulted in lower levels of homocysteine, but less than a quarter of our patients were within the normal range after 6 months of treatment. This is similar to many other studies that showed that homocysteine could be lowered by administration of either folic acid or 5-MTHF, but rarely can be brought within a normal range [20, 21, 27]. In our study, the patients had an overall drop of nearly 50% within the first 6 months, then these values remained relatively stable throughout the rest of the follow-up period.
However, none of the MTHFR polymorphisms considered were able to influence baseline homocysteine values, nor homocysteine response to 5-MTHF therapy. This is similar to the findings of Bostom et al. [19] in a study that compared administration of the equimolar equivalent of oral 15 mg/day folic acid to oral 17 mg/day 5-MTHF to HD patients. Although they observed a decrease of homocysteine for both groups, neither group achieved significant normalization.
We also observed that MTHFR polymorphisms were not identified as an independent risk factor for cardiovascular mortality by means of Cox analysis. This is similar to observations from a large cross-sectional study of Japanese HD patients that showed that genotype and serum total homocysteine concentration were not independent risk factors for vascular disease [28].
Recently, several large randomized studies showed that although homocysteine can be lowered, there is not necessarily a reduced risk of major cardiovascular events [16, 17, 29]. This raises the questions whether any benefit can be gained from lowering homocysteine and what role homocysteine actually plays in contributing towards cardiovascular events. In particular, HD patients often have rather skewed distributions of risk factors compared to the general population. Similar to the reverse epidemiology related to higher mortality in HD patients with low cholesterol, a few recent studies showed that lower levels of homocysteine were inversely correlated with mortality [7, 29]. In the study by Kalantar-Zadeh et al. [7], they did not observe any correlation between homocysteine and body mass index or other inflammatory markers; however, they did observe a direct correlation between albumin and homocysteine. In contrast with that study, we did not observe any correlation between albumin and homocysteine.
The unexpected result of the present study was an improvement in survival rate of patients treated with 5-MTHF, particularly since there was no difference in homocysteine levels between the two groups of therapy. This raises the question as to whether 5-MTHF may have other unique properties not directly related to homocysteine lowering.
The pharmacokinetic profile of 5-MTHF results in a higher bioavailability of folic acid irrespective of the patient's genotype [30]. In a recent study comparing a single daily oral dose of 5 mg folic acid versus 5 mg of 5-MTHF, the peak concentration of 5-MTHF was nearly sevenfold higher than folic acid [30]. The authors also detected high levels of the non-natural isomer 6[R] 5-MTHF that lasted over a prolonged period of time; however, the significance of this isomer is not known. Several studies suggest that high concentrations of 5-MTHF may be beneficial in CVD. In vitro, 5-MTHF has important antioxidant properties that appear to be related to its structural similarity to tetrahydrobiopterin (BH4) [31, 32]. BH4 is a critical cofactor that is necessary to maintain endothelial nitric oxide synthase (eNOS) with a net production balance towards nitric oxide rather than superoxide. In addition it is a potent peroxynitrite scavenger [33]. Hyndman et al. [31] provided evidence indicating that 5-MTHF is capable of binding the pterin site in the eNOS. Furthermore, this direct binding to eNOS mimics the orientation and interactions of the natural cofactor BH4. 5-MTHF supplementation restored NO-dependent endothelial function in BH4-deficient fructose-fed rats [31]. In addition, 5-MTHF was shown to reduce superoxide production and also improve endothelial function in aortae isolated from BH4-deficient rats [32]. Antoniades et al. [33] also showed a benefit of 5-MTHF administration on endothelial function and vascular superoxide production in patients undergoing coronary artery bypass grafting. Recent studies suggested that 5-MTHF was associated with improved endothelial function as measured by B-mode ultrasonography on the brachial artery in HD and peritoneal dialysis patients [34, 35].
Interestingly, the only risk factor that influenced survival rate in our study, by Cox regression analysis, was CRP. During the follow-up, a significant reduction in CRP values was observed in patients treated with 5-MTHF at 24 and 55 months. In relation to the CRP quartiles distribution, it should be noted that the highest survival rate was in patients with low CRP levels at the beginning of the study (<12 mg/l) and only in patients treated with 5-MTHF. Conversely, in patients who were not treated with 5-MTHF, no difference in survival rate was detected among quartiles.
To summarize, our study demonstrated that hyperhomocysteinemia is not a mortality predictive risk factor in HD patients. It also supported the hypothesis that a reduction in homocysteine plasma levels was not associated with decreased mortality risk.
Our results suggest that treatment with 5-MTHF seems to induce an increase in survival rate probably by inducing a lower inflammatory state in HD patients, supported by the fact that the inflammatory state was recognized as a predictive mortality risk factor in our study. Further studies are needed to better define 5-MTHF mechanisms in decreasing CRP values and in benefiting overall survival in the HD population.
Acknowledgements
The study was supported in part by the Fondazione Cassa di Risparmio in Bologna, Project No. 2007/0234 ‘Innovazioni diagnostiche e terapeutiche nell’ approccio alle criticità immunologiche del paziente nel percorso Dialisi – Trapianto di Rene', year 2007.
References
- 1.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65:2380–2389. doi: 10.1111/j.1523-1755.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 2.Bayés B, Pastor MC, Bonal J, Romero R. ‘New’ cardiovascular risk factors in patients with chronic kidney disease: role of folic acid treatment. Kidney Int Suppl. 2005:S39–S43. doi: 10.1111/j.1523-1755.2005.09309.x. [DOI] [PubMed] [Google Scholar]
- 3.Nygard O, Vollset SE, Refsum H, Stensvold I, Tverdal A, Nordrehaug JE, Ueland M, Kvale G. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995;274:1526–1533. doi: 10.1001/jama.1995.03530190040032. [DOI] [PubMed] [Google Scholar]
- 4.Buccianti G, Baragetti I, Bamonti F, Furiani S, Dorighet V, Patrosso C. Plasma homocysteine levels and cardiovascular mortality in patients with end-stage renal disease. J Nephrol. 2004;17:405–410. [PubMed] [Google Scholar]
- 5.Mallamaci F, Bonanno G, Seminara G, Rapisarda F, Fatuzzo P, Candela V, Scudo P, Spoto B, Testa A, Tripepi G, Tech S, Zoccali C. Hyperhomocysteinemia and arteriovenous fistula thrombosis in hemodialysis patients. Am J Kidney Dis. 2005;45:702–707. doi: 10.1053/j.ajkd.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Perna AF, De Santo NG, Ingrosso D. Adverse effects of hyperhomocysteinemia and their management by folic acid. Miner Electrolyte Metab. 1997;23:174–178. [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Block G, Humphreys MH, McAllister CJ, Kopple JD. A low, rather than a high, total plasma homocysteine is an indicator of poor outcome in hemodialysis patients. J Am Soc Nephrol. 2004;15:442–453. doi: 10.1097/01.asn.0000107564.60018.51. [DOI] [PubMed] [Google Scholar]
- 8.Nair AP, Nemirovsky D, Kim M, Geer EB, Farkouh ME, Winston J, Halperin JL, Robbins MJ. Elevated homocysteine levels in patients with end-stage renal disease. Mt Sinai J Med. 2005;72:365–373. [PubMed] [Google Scholar]
- 9.Bayes B, Pastor MC, Bonal J, Junca J, Hernandez JM, Riutort N, Foraster A, Romero R. Homocysteine, C-reactive protein, lipid peroxidation and mortality in haemodialysis patients. Nephrol Dial Transplant. 2003;18:106–112. doi: 10.1093/ndt/18.1.106. [DOI] [PubMed] [Google Scholar]
- 10.Bowden RG, Wyatt FB, Wilson R, Wilborn C, Gentile M. Homocysteine and vascular access thrombosis in a cohort of end-stage renal disease patients. Ren Fail. 2004;26:709–714. doi: 10.1081/jdi-200037117. [DOI] [PubMed] [Google Scholar]
- 11.Chen TC, Wang IK, Lee CH, Chang HW, Chiou TT, Lee CT, Fang JT, Wu MS, Hsu KT, Yang CC, Wang PH, Chiang FR. Hyperhomocysteinaemia and vascular access thrombosis among chronic hemodialysis patients in Taiwan: a retrospective study. Int J Clin Pract. 2006;60:1596–1599. doi: 10.1111/j.1742-1241.2006.00848.x. [DOI] [PubMed] [Google Scholar]
- 12.Chuang FR, Fang JT, Chen JB, Lin CL, Chen HY, Lee CN, Wang PH, Lee CH. Hyperhomocysteinemia and the prevalence of symptomatic atherosclerotic vascular disease in Taiwanese chronic hemodialysis patients: a retrospective study. Ren Fail. 2003;25:765–774. doi: 10.1081/jdi-120024292. [DOI] [PubMed] [Google Scholar]
- 13.Dennis VW, Robinson K. Homocysteinemia and vascular disease in end-stage renal disease. Kidney Int Suppl. 1996;57:S11–S17. [PubMed] [Google Scholar]
- 14.Suliman ME, Stenvinkel P, Heimburger O, Barany P, Lindholm B, Bergstrom J. Plasma sulfur amino acids in relation to cardiovascular disease, nutritional status, and diabetes mellitus in patients with chronic renal failure at start of dialysis therapy. Am J Kidney Dis. 2002;40:480–488. doi: 10.1053/ajkd.2002.34887. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, Stampfer MJ, Hennekens CH, Frosst P, Selhub J, Horsford J, Malinow MR, Willett WC, Rozen R. Methylenetetrahydrofolate reductase polymorphism, plasma folate, homocysteine, and risk of myocardial infarction in US physicians. Circulation. 1996;94:2410–2416. doi: 10.1161/01.cir.94.10.2410. [DOI] [PubMed] [Google Scholar]
- 16.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K, NORVIT Trial Investigators Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 17.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J, Jr, Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 18.Touam M, Zingraff J, Jungers P, Chadefaux-Vekemans B, Drüeke T, Massy ZA. Effective correction of hyperhomocysteinemia in hemodialysis patients by intravenous folinic acid and pyridoxine therapy. Kidney Int. 1999;56:2292–2296. doi: 10.1046/j.1523-1755.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 19.Bostom AG, Shemin D, Bagley P, Massy ZA, Zanabli A, Christopher K, Spiegel P, Jacques PF, Dworkin L, Selhub J. Controlled comparison of L-5-methyltetrahydrofolate versus folic acid for the treatment of hyperhomocysteinemia in hemodialysis patients. Circulation. 2000;101:2829–2832. doi: 10.1161/01.cir.101.24.2829. [DOI] [PubMed] [Google Scholar]
- 20.Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004;15:420–426. doi: 10.1097/01.asn.0000110181.64655.6c. [DOI] [PubMed] [Google Scholar]
- 21.Van Guldener C, Janssen MJ, Lambert J, ter Wee PM, Jakobs C, Donker AJ, Stehouwer CD. No change in impaired endothelial function after long-term folic acid therapy of hyperhomocysteinaemia in haemodialysis patients. Nephrol Dial Transplant. 1998;13:106–112. doi: 10.1093/ndt/13.1.106. [DOI] [PubMed] [Google Scholar]
- 22.Manns B, Hyndman E, Burgess E, Parsons H, Schaefer J, Snyder F, Scott-Douglas N. Oral vitamin B12 and high-dose folic acid in hemodialysis patients with hyper-homocys- t(e)inemia. Kidney Int. 2001;59:1103–1109. doi: 10.1046/j.1523-1755.2001.0590031103.x. [DOI] [PubMed] [Google Scholar]
- 23.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 24.Sassi S, Grossi G, Bozza D, Mirighi R: Plasma pyridoxal-5′-phosphate determination by HPLC after pre-column derivatization. 21st International Symposium on High-Performance Liquid Phase Separations and Related Techniques, Birmingham/UK, 1997.
- 25.Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet. 1998;62:1258–1260. doi: 10.1086/301836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skibola CF, Smith MT, Kane E, Roman E, Rollinson S, Cartwright RA, Morgan G. Polymorphisms in the methylenetetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc Natl Acad Sci USA. 1999;96:12810–12815. doi: 10.1073/pnas.96.22.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunder-Plassmann G, Fodinger M, Buchmayer H, Papagiannopoulos M, Wojcik J, Kletzmayr J, Enzenberger B, Janata O, Winkerlmayer WC, Paul G, Auinger M, Barnas U, Hörl WH. Effect of high dose folic acid therapy on hyperhomocysteinemia in hemodialysis patients: results of the Vienna multicenter study. J Am Soc Nephrol. 2000;11:1106–1116. doi: 10.1681/ASN.V1161106. [DOI] [PubMed] [Google Scholar]
- 28.Kimura H, Gejyo F, Suzuki S, Miyazaki R. The C677T methylenetetrahydrofolate reductase gene mutation in hemodialysis patients. J Am Soc Nephrol. 2000;11:885–893. doi: 10.1681/ASN.V115885. [DOI] [PubMed] [Google Scholar]
- 29.Toole JF, Malinow MR, Chambless LE, Spence JD, Pettigrew LG, Howard VJ, Sides EG, Wang CH, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 30.Willems FF, Boers GH, Blom HJ, Aengevaeren WR, Verheugt FW. Pharmacokinetic study on the utilisation of 5-methyltetrahydrofolate and folic acid in patients with coronary artery disease. Br J Pharmacol. 2004;141:825–830. doi: 10.1038/sj.bjp.0705446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyndman ME, Verma S, Rosenfeld RJ, Anderson TJ, Parsons HG. Interaction of 5-methyltetrahydrofolate and tetrahydrobiopterin on endothelial function. Am J Physiol. 2002;282:H2167–H2172. doi: 10.1152/ajpheart.00935.2001. [DOI] [PubMed] [Google Scholar]
- 32.Stroes ES, van Faassen EE, Yo M, Martasek P, Boer P, Govers R, Rabelink TJ. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ Res. 2000;86:1129–1134. doi: 10.1161/01.res.86.11.1129. [DOI] [PubMed] [Google Scholar]
- 33.Antoniades C, Shirodaria C, Warrick N, Cai S, de Bono J, Lee J, Leeson P, Neubauer S, Ratnatunga C, Pillai R, Refsum H, Channon KM. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 34.Baragetti I, Raselli S, Stucchi A, Terraneo V, Furiani S, Buzzi L, Garlaschelli K, Alberghini E, Catapano AL, Buccianti G. Improvement of endothelial function in uraemic patients on peritoneal dialysis: a possible role for 5-MTHF administration. Nephrol Dial Transplant. 2007;22:3292–3297. doi: 10.1093/ndt/gfm402. [DOI] [PubMed] [Google Scholar]
- 35.Buccianti G, Raselli S, Baragetti I, Bamonti F, Corghi E, Novembrino C, Patrosso C, Maggi FM, Catapano AL. 5-Methyltetrahydrofolate restores endothelial function in uraemic patients on convective haemodialysis. Nephrol Dial Transplant. 2002;17:857–864. doi: 10.1093/ndt/17.5.857. [DOI] [PubMed] [Google Scholar]