Abstract
Background
Continuous increase in the number of patients with end-stage renal disease demands early detection of chronic kidney disease (CKD). The aim of the present study was to diagnose CKD in its earliest stages in a randomly selected population using a diagnostic algorithm developed by the working group.
Methods
An algorithm for the diagnostic procedure was created to identify patients with CKD requiring further nephrological care. Randomly chosen adult inhabitants of a city with a population of 60,000 were invited to participate in this study. Screening procedures included a microalbuminuria dipstick test accompanied by blood pressure measurement and medical questionnaire. In further diagnosis of CKD, estimated glomerular filtration rate (eGFR), albumin concentration in urine, urinalysis and ultrasound examination were used according to the algorithm. Multivariate logistic regression was performed to identify associations between participants’ characteristics and albuminuria.
Results
Out of 9,700 invited subjects, 2,471 individuals participated in the PolNef study. Albuminuria was detected in 15.6% of the investigated population using the dipstick test and thereafter confirmed in 11.9% by the turbidimetric method. The modeling of multivariate logistic regression indicated the following independent predictors of albuminuria: male sex, diabetes, nocturia and hypertension. For people without diabetes and without hypertension, nocturia independently predicted detection of albuminuria. 481 people received a consultation with a nephrologist, and 96% of them were recognized as having CKD. At least 9% of patients with CKD had eGFR by MDRD <60 ml/min/1.73 m2. Six persons were referred for further treatment because of newly diagnosed kidney tumor.
Conclusions
CKD in early stages occurs frequently in the studied population. The proposed diagnostic algorithm seems to be a powerful tool to identify subjects at risk of CKD. The role of nocturia as an independent predictor of albuminuria, both in the general population and in people without diabetes or hypertension, should be further examined.
Key Words: Albuminuria, Chronic kidney disease, Diagnostic algorithm, Nocturia
Introduction
Chronic kidney disease (CKD) is a worldwide underdiagnosed public health problem with increasing incidence and prevalence that has high costs and poor outcome [1,2,3,4,5]. The number of patients on renal replacement therapy has doubled every decade since 1980, and prevalence of CKD in the early stages is also markedly increased [6,7,8,9,10,11]. Unfortunately, CKD in its earliest stages is usually an asymptomatic condition which progresses to its end stage over a period of several years and is diagnosed late in its course. Therefore, strategies to reduce the incidence of end-stage renal disease require effective methods of screening early in the disease process. Intervention in the early stages of CKD seems to be more effective to prevent or delay the progression of CKD. Moreover, reduced kidney function was found to be an independent risk factor for cardiovascular events and/or all-cause mortality [12,13,14]. Therefore, early detection of CKD and treatment of its complications seems to be important to improve outcome in cardiovascular diseases. However, the screening methods most suitable to identify for further diagnosis individuals with CKD remain to be settled. Albuminuria was used as a screening tool but by itself is not sufficient. Garg et al. [15] found that albuminuria and renal insufficiency measured on a single occasion identified different segments of the population. More than one third of people with an eGFR below 30 ml/min/1.73 m2 demonstrated no albuminuria. Moreover, one third of diabetic patients and almost two thirds of nondiabetic hypertensive patients demonstrated no albuminuria. There is no simple correlation between the progress of glomerular disease and kidney function [16]. Both glomerular and tubulointerstitial damage can mediate impairment of renal function [17]. Furthermore, inulin clearance is better correlated with tubulointerstitial damage than glomerular disease [18]. In many forms of renal diseases due to primary glomerular lesions, there is a significant inverse correlation between the extent of tubulointerstitial damage and the GFR [19]. Therefore, supplementing screening methods such as albuminuria and eGFR with other diagnostic tools seems to be necessary for early detection of kidney disease.
It is worth mentioning at this point that both albuminuria and decrease in eGFR were used as 2 basic markers for the new classification of CKD proposed by the Kidney Disease Outcomes Quality Initiative of the National Kidney Foundation [20] and introduced widely after slight modification by the KDIGO (Kidney Disease: Improving Global Outcomes) international community [1].
Damage to the interstitial compartment, especially to more distal segments of tubules, is accompanied by an inability to maximally concentrate the urine, and therefore may result in nocturia. We believe that asking simple questions about nocturia could help distinguish the missing segment of the population with CKD who demonstrate no albuminuria (after exclusion of the main reasons for nocturia, such as urinary tract infection, poorly controlled diabetes, severe heart failure and evening dosing of diuretics). The definition of nocturia was accepted as the necessity to urinate more than once during the night, which disturbs sleep and which had lasted at least 3 months.
Detection of CKD in its early stages has never been studied in central or eastern Europe. Our attempt to diagnose CKD in the early stages in a randomly selected population of a Polish city and its district was the first such study performed in this region.
Subjects and Methods
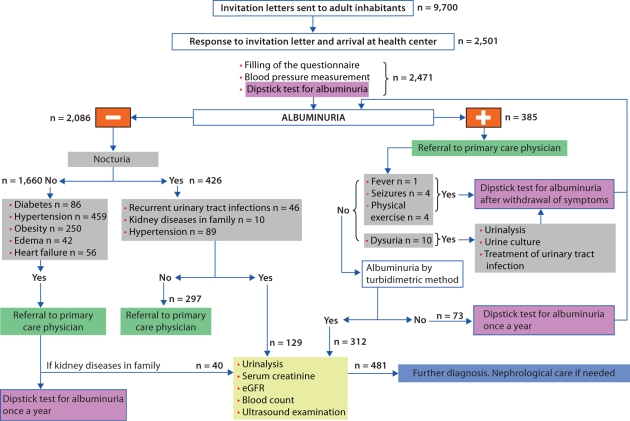
Starogard Gdański, a city district in north Poland with a population of 60,000, was randomly chosen from the 2 districts with a population between 50,000 and 100,000 in the Pomeranian administrative region. With the permission of the Mayor of Starogard Gdański, we received from the local administration the address list of all adult inhabitants. We assumed that the Polish population was similar to a previously studied Dutch one [21] and would have a microalbuminuria prevalence of 7%. Therefore, to keep the confidence interval (CI) in the range of 2%, 2,500 people were required for the study. We considered a likely response rate of 25–30% and so estimated that between 8,333 and 10,000 invitations would need to be sent to adult inhabitants of the chosen district [22, 23]. In total, 9,700 invitations to take part in the PolNef study were sent to adult inhabitants randomly chosen from the address list. Of these, 2,501 (25.5%) responded, brought a morning urine sample, gave their consent for blood pressure measurement. They also filled in a questionnaire on their demographic characteristics, weight and height, symptoms of kidney diseases, medications taken and coexistence of other diseases, especially hypertension, diabetes and cardiovascular diseases. The algorithm for further evaluation of participants is shown in figure 1. A dipstick test for microalbuminuria (Micral Test II, Roche Diagnostics Ltd., UK) was performed to detect albumin in the first morning urine samples. The cut-off point for this test is an albumin concentration in urine equal to 20 mg/l. Moreover, the albumin concentration in urine was additionally measured in the laboratory before renal consultation from a separate urine sample by the chemical turbidimetric method (multigent microalbumin assay; Architect ci 8200 system, Abbott Laboratories Inc.). Any amount of albumin in urine can be detected using the turbidimetric method, and an albumin concentration in urine equivalent 20 mg/l or more was assumed to indicate albuminuria. Serum creatinine was measured using the modified method of Jaffe's reaction with kits from Abbott Laboratories Inc. on the automated Architect ci 8200 analyzer. All laboratory analyses, except for the dipstick test (which was completed next to the participant immediately after the urine sample was brought), were performed in the laboratory of the Medical University Hospital in Gdańsk. Daily intralaboratory control (Randox sets) and international control of quality of laboratory tests (Labquality, Finland) were carried out. The Cockcroft-Gault formula adjusted for body surface area and the abbreviated MDRD formula as well were used to estimate GFR in all individuals qualified for renal consultation. When calculating an eGFR, serum creatinine values as mg/dl to 2 decimal places were used. We assumed that a participant had diabetes mellitus if he was on hypoglycemic medication or his primary care physician answered positively questions concerning this disease. Diagnosis of hypertension was made on the basis of actual hypotensive treatment or on the basis of a median value ≥140/90 of 3 separate measurements of blood pressure with adequate cuff. Nocturia, which was self reported, was defined as the need to urinate more than once during night, which interrupted sleep, which occurred on most of the days during a week (at least 4), and which had been present for at least the last 3 months. Ultrasound examinations (US) were performed by a nephrologist experienced in diagnosis with US using a phased-array transducer, 2–5 MHz (Panther 2002, B&K). The length of kidneys, renal parenchymal thickness and echogenicity were assessed in the lateral decubitus position, after the patient had fasted for 8 h. Liver echogenicity was used as the standard reference, and when the liver parenchyma was hyperechoic, spleen echogenicity was also used.
Fig. 1.
Diagnostic algorithm.
A participant was enrolled into the study if, in responding to the invitation, he brought a sample of his morning urine for microalbuminuria testing, filled in the questionnaire, had blood pressure measurements taken and authorized laboratory and US examinations according to the given algorithm. If, on the questionnaire, the participant answered positively to having fever, seizures, pain during urination or having undertaken excessive physical activity (such as exercise for at least 45 min) on the day before the urine test for albuminuria he was temporarily excluded. Such participants were invited again after resolution of those symptoms. Body mass index (BMI) was calculated as the ratio of weight (in kg) to height (in m2) and the definition of obesity was made on the basis of BMI. A BMI <25 was considered as normal, ≥25 and <30 was overweight, and ≥30 was obese.
The initial diagnoses of underlying nephropathies were made during renal consultation on the basis of general clinical information, physical examination and laboratory tests, without any proof in kidney biopsy. General criteria for initial diagnoses of underlying renal diseases were as follows. For chronic glomerular disease: proteinuria accompanying hematuria, especially with dysmorphic red blood cells, high blood pressure or increased echogenicity of renal cortex on US. For tubulointerstitial chronic nephropathy: recurrent urinary tract infections and/or analgesic abuse in the past medical history, with proteinuria in the presence of echogenic kidney with irregular outlines on US, or optionally leukocyturia, without urinary tract infection, together with any chronic changes on US. For hypertensive nephropathy: long-term past medical history of hypertension, especially when treated ineffectively, without evidence of other kidney disease, in the presence of signs of chronic kidney damage on US. For diabetic nephropathy: long-term diabetes accompanied by proteinuria and retinopathy in medical history.
Polycystic kidney disease, nephrolithiasis and renal tumors were diagnosed according to US criteria. If none of the above initial diagnoses was probable but some abnormalities on US were present (e.g. solitary kidney, duplicated pyelocaliceal system, hydronephrosis, or horseshoe, hypoplastic or small scarred kidney), the patient was classified as having a diagnosis of ‘other’. No initial diagnosis was made if isolated abnormalities such as albuminuria, erythrocyturia or only decreased eGFR without any other anomalies in laboratory tests or on US were found.
Statistical Analysis
A multivariate logistic-regression model was applied in order to investigate the relation between clinical characteristics, such as sex, diabetes, age (divided in groups), nocturia, hypertension, smoking, BMI and the odds to detect albuminuria. A backward-selection procedure, with a p value of <0.1 used for retention in the model, was performed in order to identify important factors at the 0.05 level of statistical significance. The Hosmer-Lemeshow test was used to check the goodness-of-fit of the model. The initial list of covariates included female and male sex, diabetes, 5 age groups (18–39, 40–49, 50–59, 60–69 and >70 years), nocturia, hypertension, smoking and 3 groups divided according to BMI (normal weight, overweight and obese). For the subpopulation of participants with no diabetes and no hypertension, similar modeling of a multivariate logistic-regression was performed.
All the tests performed as a part of multivariate analyses were 2-sided. p < 0.05 was considered statistically significant. The analyses were computed using StataCorp statistical software, version 8.
Results
A total of 2,501 adults responded to an invitation to participate in the PolNef study. 30 persons did not come for the blood tests and US, and therefore did not satisfy the inclusion criteria. As a result, 2,471 participants were included in the analysis. The general characteristics of the total population of participants and groups without albuminuria and with nocturia, without albuminuria and without nocturia, and with albuminuria are shown in table 1.
Table 1.
General characteristics of PolNef participants
| Total | Albuminuria– nocturia– | Nocturia+ albuminuria– | Albuminuria+ | Consulted | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Female | 1,533 (62.0) | 1,019 (61.4) | 302 (70.9) | 171 (54.8) | 284 (59) |
| Male | 938 (38.0) | 641 (38.6) | 124 (29.1) | 141 (45.2) | 197 (41) |
| Age, years | |||||
| Range | 18-98 | 18-98 | 18-83 | 18-83 | 18-83 |
| Mean±SD | 50±14.5 | 48.4±14.5 | 56.43±12.1 | 52.7±14.6 | 55.3±13.5 |
| BMI | |||||
| Range | 15.8-56.7 | 14.2-49.6 | 17.2-56.7 | 15.9-48.4 | 15.9-49.5 |
| Mean±SD | 26.6±5.0 | 25.7±5 | 28.1±5.7 | 27.3±5.4 | 28±5.6 |
| Systolic BP, mm Hg | |||||
| Range | 80-250 | 80-200 | 90-250 | 80-220 | 80-250 |
| Mean±SD | 133.5±21 | 132.1±19.3 | 135.1±22.4 | 138.2±24.2 | 140.6±24.3 |
| Diastolic BP, mm Hg | |||||
| Range | 40-160 | 50-130 | 50-120 | 40-120 | 40-120 |
| Mean±SD | 82.9±11 | 82±10.5 | 84.1±11.5 | 85.6±12.8 | 86.8±12.5 |
| Hypertension, n (%) | 726 (29.4) | 589 (35.5) | 225 (52.8) | 173 (55.4) | 319 (66.3) |
| Smoking, n (%) | 595 (24.1) | 427 (25.7) | 92 (21.6) | 85 (27.2) | 114 (23.7) |
| Diabetes, n (%) | 163 (6.6) | 67 (4) | 48 (11.3) | 38 (12.2) | 69 (14.3) |
| Race, n (%) | |||||
| Non-Hispanic white | 2,471 (100) | 1,660 (100) | 426 (100) | 312 (100) | 481 (100) |
Circumstances preceding morning urine collection which could possibly influence a result of the dipstick test, such as fever (0.65% of all participants), physical exercise (2.22%), pain during urination (1.25%) and seizures (0.25%), were quite rarely present in the examined population. Those participants were excluded temporarily and the dipstick test was repeated after the circumstances given above had withdrawn. In females, the dipstick test was performed in the period between menstruations.
In the overall population of a 2,471 subjects, the dipstick test detected any kind of albuminuria in the first morning urine sample in 15.6% of all participants (n = 385, 212 female, 173 male). 11.9% of all participants demonstrated albuminuria according to both methods. A concentration of albumin in the urine sample in the range 10–20 mg/l was present in 47 participants, and in 43 it was <10 mg/l. Albuminuria >200 mg/l was found in 10 people.
256 participants collected 24-hour urine for measurement of the protein excretion rate. In 176 subjects the protein in daily urine was found to be <150 mg, in 65 people it was 150–500 mg, in 12 it was 500–1,000 mg, and it was >1,000 mg in 3 participants. Among those 3 participants only 1 demonstrated nephrotic range proteinuria, with 4.98 g per 24 h.
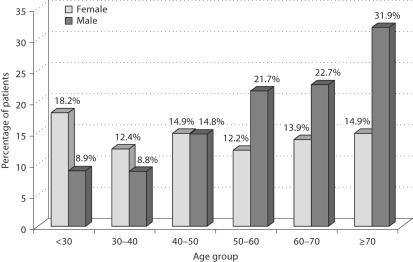
The frequency of albuminuria (fig. 2) rises gradually among the age groups in men, from 8.8% in the youngest group to 21.7% among 50–59-year-olds, and up to 32% in the oldest group. In women, the frequency of albuminuria fluctuated from 18% in the youngest age group to 15% in the oldest women. 10.7% of people with albuminuria showed abnormalities in urinalysis. 114 (36.5%) patients with albuminuria demonstrated simultaneously presence of nocturia.
Fig. 2.
Albuminuria in women and men by age group.
The number of persons needed to be screened to identify 1 individual with albuminuria in the whole studied population was 7, the number of diabetics needed to be screened to identify 1 with albuminuria was 4, and for hypertensives it was 5.
A multivariate logistic-regression model was applied in order to investigate the relation between clinical factors and the odds to detect albuminuria. The modeling for all participants in the PolNef study included the following initial list of covariates: female and male sex, diabetes, 5 age groups (18–39, 40–49, 50–59, 60–69 and >70), nocturia, hypertension, smoking and 3 groups divided according to BMI (<25, 25–30 and ≥30). The independent predictors and estimated odds ratios for detecting albuminuria are given in table 2.
Table 2.
Association between patient characteristics and albu-minuria
| Covariate in the model for detectionof albuminuria | OR (95% CI) | p value |
|---|---|---|
| Sex (male to female) | 1.41 (1.13–1.77) | <0.005 |
| Diabetes | 1.66 (1.14–2.44) | <0.01 |
| Nocturia | 1.97 (1.54–2.52) | <0.001 |
| Hypertension | 1.81 (1.41–2.31) | <0.001 |
The Hosmer-Lemeshow test confirmed the goodness-of-fit of the model, with a p value of 0.37. A p value >0.05 was considered to indicate no significant lack of fit.
Additional modeling by multivariate logistic-regression was conducted to investigate possible associations between the characteristics of participants who received a consultation with a nephrologist and the odds of detecting albuminuria at the concentration ≥20 mg/l measured in laboratory. The modeling indicated hypertension (OD ratio 1.93; confidence interval 1.16–3.20; p < 0.02) and age >60 years (OD ratio 1.84; confidence interval 1.08–3.14; p < 0.05) as the independent predictors of laboratory-measured albuminuria. The Hosmer-Lemeshow test confirmed the goodness-of-fit of the model with a p value of 0.98.
For the subpopulation without diabetes and without hypertension, independent predictors of paticipants’ characteristics and the odds of detecting any albuminuria were nocturia (OD ratio 2.15; confidence interval 1.55–2.99; p < 0.001) and being in the sixth decade of life (OD ratio 0.62; confidence interval 0.43–0.89; p < 0.001). The p value was 0.99 for the Hosmer-Lemeshow test, which confirmed the goodness-of-fit of the model.
The main symptoms and initial diagnosis established by a nephrologist are shown in table 3. Participants with suspected chronic nephropathy or any other kidney abnormalities were subjected to further tests. The nephrologist decided that almost 16% of all participants in the PolNef study will require nephrological care, either at the next consultation in 6–12 months or permanent care.
Table 3.
Results of renal consultations
| Main signs and symptoms, n (%) | |
| Hypertension | 319 (66.32) |
| Diabetes mellitus | 69 (14.34) |
| Recurrent urinary tract infections | 93 (19.33) |
| Analgesics frequently used | 89 (18.5) |
| Nocturia | 239 (49.69) |
| Pain in the kidney area | 10 (2.1) |
| Initial diagnosis of renal disease, n (%) | |
| Chronic glomerulopathy | 11 (2.28) |
| Tubulointerstitial nephropathy | 119 (24.74) |
| Polycystic kidney disease | 1 (0.2) |
| Nephrolithiasis | 42 (8.73) |
| Kidney tumors | 6 (1.25) |
| Probable hypertensive nephropathy | 50 (10.39) |
| Diabetic nephropathy | 18 (3.74) |
| Others | 76 (15.8) |
| No initial diagnosis | 158 (32.85) |
More than half of participants referred to the nephrologist had an eGFR <90 ml/min/1.73 m2 with both Cockcroft-Gault and MDRD formulas, 11% were <60 ml/min/1.73 m2 according to Cockcroft-Gault and 8.8% were <60 ml/min/1.73 m2 according to the abbreviated MDRD formula. The average eGFR was lower in females than in males in these same age groups and it dropped gradually from 96 to 62 ml/min/1.73 m2 in females and from 108 to 71 ml/min/1.73 m2 in males, when calculated using MDRD. It dropped from 107 to 70 ml/min/1.73 m2 in females and 118 to 70 ml/min/1.73 m2 in males when the Cockcroft-Gault formula, adjusted for body surface area, was used.
US examination performed in patients referred to nephrologists will be analyzed in detail in a separate paper, but preliminary results are shown below. The average length ± SD of right kidney was 111.8 ± 10.2 mm, and the left was 113.2 ± 9.7 mm. Eight persons had 1 of their kidneys below the normal length (<90 mm): 3 in the group with arterial hypertension, 5 in the nonhypertensive group. The prevalence of kidneys with abnormal echogenicity of the cortex (above or equal to the echostructure of the liver and spleen) was 18.7% on the right side and 15.1% on left. In the hypertensive group, the echogenicity of the cortex and the prevalence of renal cysts were significantly increased compared to the normotensive group (echogenicity 22 vs. 14%, p < 0.05; cysts 15 vs. 7%, p < 0.02). One new case of autosomal dominant polycystic kidney disease was found on US examination. In 6 participants (2 women, 4 men) kidney tumors were newly diagnosed and those people were referred for verification and further treatment. In 3 of those people albuminuria was detected, 1 had proteinuria on urinalysis and none presented erythrocyturia. The age range was 50–74 years. All of them declared nocturia on the questionnaire, 5 were hypertensive and 2 smoked.
Discussion
Using the dipstick test, albuminuria was detected in the first morning urine samples of 15.6% of the participants selected randomly from the general population of Starogard Gdański, a city with 60,000 residents. Such a high prevalence of albuminuria in the general population would be the highest in Europe and one of the highest in the world. Nevertheless, when 2 methods of detecting albumin in urine were combined (the dipstick test and albumin urine concentration limited to ≥20 mg/l), then the percentage of participants who demonstrated albuminuria dropped to 11.9%. This latter figure is still higher than those seen in the general populations of the United States (9.2%) [5, 15], Australia (6.7%) [24, 25] or the Netherlands (7%) [21, 26], and would be closer to those of specific high-risk populations, such as nondiabetics with cardiovascular disease (14.8%) [27], those with high risk of glomerulonephritis (12%) [28] or hypertensives (10%), even after exclusion of renal insufficiency [29]. One of the reasons for the discrepancy in the proportion of people demonstrated to have albuminuria might be the different methods used in other studies. In the NHANES (National Health and Nutrition Examination Survey) and AusDiab (Australian Diabetes, Obesity and Lifestyle) studies, the urinary albumin-to-creatinine ratio was calculated, which is in contrast to our study where the dipstick test for albuminuria confirmed by albumin concentration measurement were used. Unfortunately, the concentration of urine creatinine was not measured and therefore we were not able to give the albumin-to-creatinine ratio, which could of course minimize an influence of urine volume on urine albumin concentration. It is worth mentioning that estimation of urinary albumin greatly depends on laboratory methods: the prevalence of albuminuria increased by 2–4 times when high-performance liquid chromatography was used in the AusDiab study [25]. Usually in an overall population, 5–9% of people demonstrate albuminuria [15, 21, 24, 25, 30, 31]. Among Korean normoglycemic and normotensive subpopulations, the percentage of albuminuric people could be as low as 2.8% [32]. In the targeted screening programs mentioned above, some specific populations demonstrated much higher prevalence of albuminuria than the general population. These included ethnic minorities, and individuals with diabetes, hypertension or who were close relatives of patients with CKD. Because of differences in ethnicity, gender, age and concomitant diseases (especially hypertension and diabetes), it is difficult to compare the proportion of albuminuric people in these diverse populations. Even if mean age was similar in many studies [21, 24, 25, 30,33,34,35], there are still many differences, such as the proportion of males to females, hypertension, diabetes or ethnic background. In our study, the examined population was homogenous – 100% of participants were non-Hispanic white, while in the NHANES III study black and Mexican Americans were included and around 80% of examined population was non-Hispanic white. Overall prevalence of albuminuria (micro + macro) was 9.2%, but if we look into the age groups, it raised dramatically: from 5.9 in the 40–49-year-olds to 10.7% in the 50–59 year-olds to 14.6% in the 60–69-year-olds and further to 21.2% in the 70–79-year-olds [15]. In our study, there was an increase in the prevalence of albuminuria in men between the 30–39 years group and 40–49 years group from 8.8 to 14.8%, and further to 21.7% in the age group 50–59 years. Besides ethnic diversity, the earlier increase of the prevalence of albuminuria in Polish men may result from socioeconomic reasons, which unfortunately were not investigated in the present study. Interestingly, no such a trend was observed in women. The highest prevalence of albuminuria in women was seen in the youngest age group. More than 18% of women <30 years had albuminuria compared with 15% in the oldest female group (>70 years). This phenomenon should be studied more carefully in future. Possibly there is some association with the activity of the reproductive organs in young sexually active women. It is worth mentioning at this point that in a performed in parallel study in 15-year-old students we detected albuminuria in 13.2% of young females, compared with 6% of young males [36]. The prevalence of albuminuria (micro + macro) in the second stadium of CKD (eGFR by MDRD 60–89 ml/min/1.73 m2) in NHANES III was 12.9%, and for the total population including all stages of CKD it was 11.6% [5], which is quite close to our results. Moreover, when in the PREVEND study subjects with newly diagnosed impaired kidney function were examined [18], 11.6% of them demonstrated albuminuria of >30 mg/day.
The evaluation of albuminuria using the first morning urine sample seems to be an acceptable alternative to the more complex 24-hour albumin excretion and it is recommended as its surrogate [37]. Moreover, the simpler urinary albumin concentration measurement may be preferable to albumin-to-creatinine ratio as an initial screening test [26, 38]. The Micral II dipstick test done immediately after the participant brought a urine sample was used as a first-line diagnostic tool for detecting albuminuria and potentially CKD, then it was confirmed in the laboratory by the measurement of albumin concentration in urine by the turbidimetric method. Such a sequential approach in screening procedures was chosen by creating our algorithm. The Micral II dipstick test is less expensive and more convenient and was performed as a first-line screening test. Those participants who screened positively were subsequently retested using the quantitative turbidimetric method. Such a sequential approach in the process of early detection of a disease was also recommended by Jaar et al. [39].
In recent years, a number of screening strategies have been implemented for the detection of CKD. Numerous screening methods included albuminuria alone [21, 24, 25, 28, 30,32,33,34, 40], whereas others used urinalysis [31, 35, 41] or albuminuria together with GFR [5, 15, 24, 25, 42, 43]. None of them seems to be sufficient in detecting populations with CKD. In the NHANES III study 37% of people with an eGFR <30 ml/min/1.73 m2 demonstrated no albuminuria [15]. The screening program KEEP, which was conducted by the National Kidney Foundation of the United States, included both albuminuria and GFR, and was performed in a population at high risk of CKD. Very high (up to 50%) prevalence of CKD was identified among individuals at high risk: diabetics, hypertensives, and first-degree relatives of patients with CKD [42]. No more than half of them presented albuminuria. It seems to confirm that complementary methods of screening are needed for the early detection of CKD. We proposed nocturia, one of the symptoms associated with tubulointerstitial damage, as a simple marker of CKD. After ruling out urinary tract infection, diabetes with high glucose level, severe circulatory insufficiency and evening use of diuretics, nocturia seems to have clinical importance for detecting CKD. Especially if we realize that in many forms of renal diseases, even due to primary glomerular diseases, there is an inverse correlation between the degree of tubulointerstitial damage and the GFR. There are so far no data to support nocturia as a risk factor of CKD. On the basis of previous investigations, we might postulate that only nocturia, after the above-mentioned exclusions, could help to indicate patients with CKD. Even if one third of albuminuric patients also demonstrated nocturia, still one fourth of nonalbuminuric patients with CKD would be detected because of nocturia.
Similarly to the PREVEND, AusDiab, Takahata, and NHANES screening programs [5, 15, 21, 24, 34, 43] the PolNef study identified male gender, diabetes and hypertension as being significantly associated with albuminuria. In our study, nocturia next to male gender, diabetes and hypertension were independent predictors of detecting albuminuria. In participants without diabetes and without hypertension, besides being in the sixth decade of life, nocturia was the independent risk factor for detecting albuminuria. On the other hand, additional modeling of the multivariate logistic-regression between these same characteristics and the odds of detecting albuminuria using the turbidimetric method indicated hypertension and age >60 years old as the independent predictors. Those results could imply that further investigation of the role of nocturia in detecting CKD is required.
It is also worth noting that only 10% of all participants presented some abnormality on urinalysis, including proteinuria, leukocyturia or erythrocyturia. Although measurement of dipstick proteinuria in single urine samples was shown to be a strong predictor of end-stage renal disease [35], it seems to not be a sufficient tool for the detection of CKD in its early stages. The low number of abnormalities in urinalysis seems to have implications for implementing albuminuria together with nocturia in a questionnaire and US as a tool in the early detection of renal disease.
The percentage of patients who received tubulointerstitial nephropathy as an initial diagnosis from the nephrologist seems to be quite high. Nevertheless, it corresponds with the data from the Polish Renal Registry [44]. Chronic interstitial nephropathies as primary renal disease accounted for 13.9% in incidence and 16.5% in prevalence of dialysis patients, which is the fourth most common reason for end-stage renal disease, after diabetic nephropathy, chronic glomerulonephritis and hypertensive nephropathy.
It is worth noting how important the identification of 6 kidney tumors was. Those patients were referred for further diagnosis and treatment, which has so far been successful. Not one of those patients has erythrocyturia and the only abnormality detected in urinalysis was proteinuria in 1 of them. The dipstick test for microalbuminuria was positive in 3 cases. Therefore, we suggest US to be a part of CKD screening.
Patients with hypertension demonstrated increased echogenicity of the renal cortex and a higher prevalence of renal cysts compared to normotensives. Those findings could be either cause or effect of hypertension, maybe as an equivalent of organ damage in hypertension. We believe that optimal screening of hypertensive people should be complemented by US examination. The cost effectiveness of implementing US should be verified, but from a long-term perspective for CKD patients it could be commendable.
The proposed algorithm represents an integrated approach based on cooperation between the primary care physician and nephrologist. The simplicity of this algorithm allows practitioners – included specialists other than nephrologists – to effectively detect patients in the early stages of CKD. Our study proves that appropriate and good cooperation between the primary care physician and nephrologist is possible. Moreover, our pilot study on early detection of CKD extends now into a long-term prevention program in the Pomeranian district, and hopefully in the whole country in the future.
We believe that such a strategy of early detection of kidney diseases, started in the PolNef study [22, 23], should bring a better quality of life for every patient through adequate treatment that slows progression of kidney damage. We suppose that identification of an early stage of kidney disease, especially important from the point of view of the individual patient, may also bring substantial economic benefits in future for the health protection system.
Acknowledgements
The authors are grateful to all who responded to the questionnaires and to Roche for the scientific grant covering laboratory measurements.
References
- 1.Levey AS, Eckhardt K-U, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 2.Schieppati A, Remuzzi G. Chronic renal diseases as a public health problem: epidemiology, social, and economic implications. Kidney Int. 2005;68(suppl 98):S7–S10. doi: 10.1111/j.1523-1755.2005.09801.x. [DOI] [PubMed] [Google Scholar]
- 3.Bello A, Nwankwo E, El Nahas AM. Prevention of chronic kidney disease: a global challenge. Kidney Int. 2005;68(suppl 98):S11–S17. doi: 10.1111/j.1523-1755.2005.09802.x. [DOI] [PubMed] [Google Scholar]
- 4.El Nahas AM, Bello A. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Astor B, Greene T, Eknoyan G, Levey A. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 6.Rutkowski B. Changing pattern of end-stage renal disease in Central and Eastern Europe. Nephrol Dial Transplant. 2000;15:156–160. doi: 10.1093/ndt/15.2.156. [DOI] [PubMed] [Google Scholar]
- 7.Rutkowski B. Changing epidemiology of end-stage renal disease in Poland. Ann Acad Med Gedan. 2003;33(suppl 1):19–36. [Google Scholar]
- 8.Rutkowski B. Highlights of the epidemiology of renal replacement therapy in Central and Eastern Europe. Nephrol Dial Transplant. 2006;21:4–10. doi: 10.1093/ndt/gfi251. [DOI] [PubMed] [Google Scholar]
- 9.Atkins R. The epidemiology of chronic kidney disease. Kidney Int. 2005;67(suppl 94):S14–S18. doi: 10.1111/j.1523-1755.2005.09403.x. [DOI] [PubMed] [Google Scholar]
- 10.Xue J, Ma J, Louis T, Collins A. Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol. 2001;12:2753–2758. doi: 10.1681/ASN.V12122753. [DOI] [PubMed] [Google Scholar]
- 11.Gilbertson D, Liu J, Xue J, Louis T, Solid C, Ebben J, Collins A. Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol. 2005;16:3736–3741. doi: 10.1681/ASN.2005010112. [DOI] [PubMed] [Google Scholar]
- 12.Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Doi Y, Okubo K, Wakugawa Y, Hata J, Oishi Y, Shikata K, Yonemoto K, Hirakata H, Iida M. Chronic kidney disease and cardiovascular disease in a general Japanese population: the Hisayama Study. Kidney Int. 2005;68:228–236. doi: 10.1111/j.1523-1755.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 13.Muntner P, He J, Hamm L. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 14.Yuyun M, Adler A, Wareham N. What is the evidence that microalbuminuria is a predictor of cardiovascular disease events? Curr Opin Nephrol Hypertens. 2005;14:271–276. doi: 10.1097/01.mnh.0000165895.90748.3b. [DOI] [PubMed] [Google Scholar]
- 15.Garg A, Kiberd B, Clark W, Haynes RB, Clase C. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int. 2002;61:2165–2175. doi: 10.1046/j.1523-1755.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 16.Brenner B. Nephron adaptation to renal injury or ablation. Am J Physiol. 1985;249:F334–F337. doi: 10.1152/ajprenal.1985.249.3.F324. [DOI] [PubMed] [Google Scholar]
- 17.Spühler O, Zollinger H. Die chronische interstitielle Nephritis (in German) Z Klin Med. 1953;131:1–50. [PubMed] [Google Scholar]
- 18.Schainuck U, Striker G, Cutler R, Benditt E. Structural-functional correlations in renal disease. Hum Pathol. 1970;1:631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- 19.Eddy A. Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol. 1994;5:1273–1287. doi: 10.1681/ASN.V561273. [DOI] [PubMed] [Google Scholar]
- 20.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 21.de Jong P, Hillege H, Pinto-Sietsma SJ, de Zeeuw D. Screening for microalbuminuria in the general population: a tool to detect subjects at risk for progressive renal failure in an early phase? Nephrol Dial Transplant. 2003;18:10–13. doi: 10.1093/ndt/18.1.10. [DOI] [PubMed] [Google Scholar]
- 22.Rutkowski B, Czekalski S, Sułowicz W, Więcek A, Król E, Szubert R, Kraszewska E. Epidemiology of kidney disease in Poland – pilot study PolNef. Przegl Lek. 2004;61:22–24. [PubMed] [Google Scholar]
- 23.Król E, Rutkowski B, Czekalski S, Sułowicz W, Więcej A, Lizakowski S, Szubert R, Karczewska-Maksymienko Ł, Orlikowska M, Kraszewski E, Magdoń R. Early diagnosis of renal diseases – preliminary report from the pilot study PolNef. Przegl Lek. 2005;62:690–693. [PubMed] [Google Scholar]
- 24.Atkins R, Polkinghorne K, Briganti E, Shaw J, Zimmet P, Chadban S. Prevalence of albuminuria in Australia: The AusDiab Kidney Study. Kidney Int. 2004;66(suppl 92):S22–S24. doi: 10.1111/j.1523-1755.2004.09206.x. [DOI] [PubMed] [Google Scholar]
- 25.Polkinghorne K, Su Q, Chadban S, et al. Population prevalence of albuminuria in the Australian Diabetes, Obesity, and lifestyle (AusDiab) study: immunonephelometry compared with high-performance liquid chromatography. Am J Kidney Dis. 2006;47:604–613. doi: 10.1053/j.ajkd.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Gansevoort R, Verhave J, Hillege H, et al. The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int. 2005;94(suppl):S28–S35. doi: 10.1111/j.1523-1755.2005.09408.x. [DOI] [PubMed] [Google Scholar]
- 27.Gerstein H, Mann J, Yi Q, Zinman B, Dinneen S, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. Albuminuria and risk of cardiovascular events, death, and hearth failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 28.Stidley C, Shah V, Narva A. A population-based, cross-sectional survey of the Zuni Pueblo: a collaborative approach to an epidemic of kidney disease. Am J Kidney Dis. 2002;39:358–368. doi: 10.1053/ajkd.2002.30557. [DOI] [PubMed] [Google Scholar]
- 29.Wachtell K, Ibsen H, Olsen M. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann Intern Med. 2003;139:901–906. doi: 10.7326/0003-4819-139-11-200312020-00008. [DOI] [PubMed] [Google Scholar]
- 30.Romundstad S, Holmen J, Kvenild K, Hallan H, Ellekjær H. Microalbuminuria and all-cause mortality in 2,089 apparently healthy individuals: a 4.4-year follow-up study. The Nord-Tr⊘ndelag Health Study (HUNT), Norway. Am J Kidney Dis. 2003;42:466–473. doi: 10.1016/s0272-6386(03)00742-x. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez S, Hsu S, McClellan W. Taking a public health approach to the prevention of end-stage renal disease: the NKF Singapore Program. Kidney Int. 2003;66(suppl 83):S61–S65. doi: 10.1046/j.1523-1755.63.s83.13.x. [DOI] [PubMed] [Google Scholar]
- 32.Choi HS, Sung KC, Lee KB. The prevalence and risk factors of microalbuminuria in normoglycemic, normotensive adults. Clin Nephrol. 2006;65:256–261. doi: 10.5414/cnp65256. [DOI] [PubMed] [Google Scholar]
- 33.de Zeeuw D, Hillege H, de Jong P. The kidney, a cardiovascular risk marker, and a new target for therapy. Kidney Int. 2005;68(suppl 98):S25–S29. doi: 10.1111/j.1523-1755.2005.09805.x. [DOI] [PubMed] [Google Scholar]
- 34.Verhave J, Gansevoort R, Hillege H, Bakker S, De Zeeuw D, de Jong P. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int. 2004;66(suppl 92):S18–S21. doi: 10.1111/j.1523-1755.2004.09205.x. [DOI] [PubMed] [Google Scholar]
- 35.Ishani A, Grandits G, Grimm R, Svendsen K, Collins A, Prineas R, Neaton J. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the Multiple Risk Factor Intervention Trial. J Am Soc Nephrol. 2006;17:1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 36.Czarniak P, Król E, Zdrojewski T, et al. Program of early diagnosis of chronic kidney disease in children – SopKard 15 nephrological project. Pol Merkuriusz Lek. 2008;24(suppl 4):108–110. [PubMed] [Google Scholar]
- 37.de Jong P, Gansevoort R. Screening techniques for detecting chronic kidney disease. Curr Opin Nephrol Hypertens. 2005;14:567–572. doi: 10.1097/01.mnh.0000183948.13739.ee. [DOI] [PubMed] [Google Scholar]
- 38.Dyer A, Greenland P, Elliott P, et al. Evaluation of measures of urinary albumin excretion in epidemiological studies. Am J Epidemiol. 2004;160:1122–1131. doi: 10.1093/aje/kwh326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaar B, Khatib R, Platinga L, Boulware L, Powe N. Principles of screening for chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:601–609. doi: 10.2215/CJN.02540607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonalds S, Maguire G, Hoy W. Renal function and cardiovascular risk markers in a remote Australian Aboriginal community. Nephrol Dial Transplant. 2003;18:1555–1561. doi: 10.1093/ndt/gfg199. [DOI] [PubMed] [Google Scholar]
- 41.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63:1468–1474. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 42.Brown W, Peters R, Ohmit S. Early detection of kidney disease in community settings. The Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2003;42:22–35. doi: 10.1016/s0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 43.Konta T, Hao Z, Abiko H, Ishikawa M, Takahashi T, Ikeda A, Ichikawa K, Takasaki S, Kubota I. Prevalence and risk factor analysis of microalbuminuria in Japanese general population: the Takahata study. Kidney Int. 2006;70:751–756. doi: 10.1038/sj.ki.5001504. [DOI] [PubMed] [Google Scholar]
- 44.Rutkowski B, Lichodziejewska-Niemierko M, Grenda R, et al. Report on the Renal Replacement Therapy in Poland – 2006. Gdańsk: Drukonsul; 2008. [Google Scholar]