Abstract
Background
Hyperglycemia may potentiate the adverse renal effects of angiotensin II (AII). In the kidney, the major target of AII action is the glomerular mesangial cell, where its hemodynamic and proinflammatory action contributes to renal injury. AII action is mediated by several types of cell receptors. Among those, the AT1 receptor has been best studied using specific AII receptor blockers (ARBs). These agents have emerged as major new modalities in the prevention and amelioration of renal disease where the ARB renoprotective anti-inflammatory properties could be more important than previously appreciated. Like the ARBs, statins may also modulate inflammatory responses that are renoprotective and complement their cholesterol-lowering effects.
Aim
The aim of this project was to (i) identify a repertoire of proinflammatory mesangial cell AII-inducible mRNAs; (ii) determine if the AII-induced proinflammatory mRNA responses depend on ambient glucose, and (iii) test the anti-inflammatory effectiveness of an ARB, valsartan, either alone or in combination with a statin, simvastatin.
Results/Conclusions
Using high-density microarrays and real-time PCR we identified several AII-inducible proinflammatory mesangial genes that exhibited augmented mRNA responses in high-glucose milieu. Valsartan blocked the AII-induced mRNA expression of proinflammatory genes (i.e. MCP-1, LIF and COX-2) maintained in normal and high glucose. These observations add to the mounting evidence that ARBs have anti-inflammatory effects in the kidney, a beneficial effect that may be more important in protecting renal function in diabetic patients. While simvastatin inhibited expression of some mRNAs encoding chemokines/cytokines, it enhanced expression of mRNA encoding COX-2, a key mediator of inflammation. Thus, the non-cholesterol effects of statins on inflammatory responses appear complex.
Key Words: Angiotensin II, Hyperglycemia, Glomerular mesangial cell, Proinflammatory action
Introduction
Angiotensin II (AII) is a multifunctional peptide found in organisms as evolutionarily distant as invertebrates and man [1, 2]. In invertebrates the renin-angiotensin system (RAS) regulates not only osmoregulation but also inflammatory responses [1,2,3]. Thus the immunoregulatory role of RAS is as ancient as its vasoconstrictive action. Still, it is only recently that the proinflammatory action of AII has been more widely appreciated [4,5,6,7,8]. Many of the AII inflammatory responses are mediated by reactive oxygen species (ROS) [9] resulting in the activation of NF-κB, a transcriptional factor central to the inflammatory response [8, 10].
Angiotensin type 1 (AT1) and type 2 (AT2) are the two major classes of receptors that mediate the action of AII [11, 12]. The AT1 receptor is expressed ubiquitously, but the tissue expression of AT2 is more restricted [11,12,13]. In mesangial cells the number of AT1 receptors expressed on the cell surface far exceeds that of AT2[14]. Although signaling through AT1 is thought to be the major contributor to the glomerular inflammatory lesion [15], signaling through AT2 may also play a role in renal damage [16, 17].
Although the etiology of micro- and macrovascular complications of diabetes is multifactorial, low grade inflammation is increasingly being recognized as one of the systemic hallmarks not only associated with increased cardiovascular complications but also with diabetic nephropathy [18]. In diabetic nephropathy the dysfunctional mesangial cell is one of the major effectors of glomerular injury. When exposed to the diabetic metabolic milieu, mesangial cells display growth abnormalities, increased expression and synthesis of prosclerotic growth factors and cytokines, accumulation of normal components of extracellular matrix, as well as increased synthesis of proinflammatory ligands [19,20,21].
Based on the abundant evidence spanning from in vitro studies in various renal cell types to large clinical trials, RAS and its major effector AII have been implicated in the pathophysiology of diabetic nephropathy. There is increasing evidence that the proinflammatory action of AII is directly involved where the mesangial cell plays a key role in the pathogenesis of diabetic nephropathy. Inhibition of RAS with angiotensin-converting enzyme inhibitors or AII receptor blockers (ARBs) plays a pivotal role in the treatment of this disorder, and these beneficial effects in the diabetic kidney are at least in part attributable to amelioration of ROS generation and proinflammatory signal transduction [22].
Thus, studies in a variety of experimental settings, including studies in patients with diabetes, have suggested synergistic actions of hyperglycemia and AII in diabetic renal pathophysiology [15, 23, 24]. On the molecular level, these synergistic actions involve stimulation of proinflammatory signal transduction pathways and genes. High ambient glucose by itself activates some of the same proinflammatory signaling mediators as those triggered through the AT1 receptor, such as ROS, NF-κB, JAK/STAT, and others [16, 17, 23, 25, 26]. These observations suggest a possibility that the glucose milieu could modulate AII-mediated expression of genes that drive progression of diabetic nephropathy, such as the proinflammatory cytokines, enzymes and transcription factors.
The 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors, or statins, are potent inhibitors of cholesterol biosynthesis that are used extensively to treat hypercholesterolemia [27]. Although traditionally the beneficial effects of statins were thought to result from the competitive inhibition of cholesterol synthesis [28], it has become increasingly apparent that statins exert additional cellular effects [29]. These studies indicate that statins exert cholesterol-independent effects by modulating signal transduction pathways and suggest that, like the ARBs, statins modulate inflammatory responses independent of HMG-CoA reductase inhibition [30]. Indeed, evidence is accumulating that statins confer renoprotection in a variety of glomerular diseases, including diabetic nephropathy [31,32,33], and in models of AII-induced injury, statins have been shown to be beneficial [34,35,36,37].
Until now a comprehensive study of the mesangial cell repertoire of inflammatory genes activated by AII, and the modulatory effects of high glucose on these AII-induced mesangial cell responses has not been done. To address this issue, we used high-density microarrays and real-time PCR to identify several AII-inducible proinflammatory mesangial gene mRNAs that exhibit augmented responses in a high-glucose milieu. Furthermore, we evaluated whether these proinflammatory responses were influenced by the AT1 receptor blocker, valsartan, or the HMG-CoA inhibitor, simvastatin, common treatments for diabetic nephropathy.
Material and Methods
Cells
Primary human glomerular mesangial cells (6–9 passages) were grown in Dulbecco's modified Eagle's medium (Hyclone, Logan, Utah, USA). Culture medium was supplemented with 16% (vol/vol) fetal calf serum, 2 mML-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 8 μg/ml insulin, 1 mM sodium pyruvate and 10 mM HEPES buffer. Cells were incubated at 37°C in humidified 5% CO2–95% air.
Study Design
AII enhances renal production of a host of proinflammatory polypeptides including TNF-α, MCP-1 and IL-6 [38, 39]. The increased synthesis of these cytokines/chemokines, at least in part, reflects NF-κB-mediated transcription observed as early as 30–60 min following AII stimulation [39]. Thus to identify inducible proinflammatory genes in human mesangial cells we have chosen to assess transcript levels at 0 and 60 min following treatment with AII. A wide range of AII levels in renal interstitial fluid has been reported [40,41,42,43]. For example, in rats the intrarenal AII concentration was reported to be in the range of 1–4 nM[42], while in the dogs the levels have been reported to be as high as 740 nM[41]. The concentration of AII in the human mesangium is not known. We have chosen to use 100 nM AII [44, 45].
For microarray profiling, serum-deprived (24 h) cells maintained in normal glucose (100 mg/ml) and preincubated with or without Val (100 nM, 30 min) were treated with or without AII (100 nM, 60 min).
For real-time PCR, cells maintained in either normal (NG; 100 mg/dl) or high (HG; 450 mg/dl) glucose (72 h) were serum deprived for 24 h and then stimulated with AII (100 nM) for 60 min in normoglycemic or hyperglycemic conditions in the absence or presence of ARB valsartan (100 nM) or the HMG-CoA inhibitor simvastatin (1 μM). The study conditions are summarized in table 1.
Table 1.
Design of the real-time PCR experiment
| Glucose | Untreated | AII | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal (100 mg/ml) | UNT | VAL | SMV | VAL+SMV | UNT | VAL | SMV | VAL+SMV |
| High (450 mg/ml) | UNT | VAL | SMV | VAL+SMV | UNT | VAL | SMV | VAL+SMV |
AII = Angiotensin II (100 nM); UNT = untreated; VAL = valsartan (100 nM); SMV = simvastatin (1 μM).
Reagents
AII purchased from AnaSpec (San Jose, Calif., USA) was dissolved in water 10–4M. Valsartan (a gift from Novartis) was dissolved in ethanol to a concentration of 10–4M and aliquots were stored frozen (–20°C). Simvastatin, carboxylate-activated (Cat. No. 567020, Calbiochem, San Diego, Calif., USA), was dissolved in ethanol to a concentration of 10–3M and aliquots were stored frozen (–20°C).
RNA Extraction
TRIzol was used to purify RNA according to the manufacturer's protocol (Invitrogen Corp., Carlsbad, Calif., USA). Total extracted RNA was dissolved in 20 μl sterile water and stored at −80°C. The RNA concentration was measured with a spectrophotometer (Bio Mate3, Thermo Fisher Scientific) and the 260/280 ratio of RNA was >1.7.
Microarrays
Mesangial cell transcripts were profiled using the Affymetrix Human Genome U133 Plus 2.0 gene array (HG-U133 Plus). A single GeneChip® array contains probe sets representing more than 47,000 transcripts derived from approximately 33,000 well-substantiated human genes. The probes were made by one round of linear amplification by the Eberwine method used at the Center for Expression Arrays (CEA), University of Washington [46]. Labeled probes were hybridized using the Affymetrix protocol. Four separate experiments were done using 14 Affymetrix chips. GeneTraffic software (http://www.stratagene.com) was used for microarray data management and statistical analysis (ANOVA).
Reverse Transcription
First strand cDNA was synthesized by priming 1 μg total RNA with 10 μM random hexamers (Promega Co, Madison, Wisc., USA) then by heating at 65°C for 10 min, and snap-cooling on ice. Reverse transcription (37°C for 1 h) was performed in the presence of 10 mM each of dATP, dCTP, dTTP and dGTP (Invitrogen Corp.), 4 μl 5× first strand buffer (Invitrogen Corp.), 0.1 M DTT (Invitrogen Corp.), 200 units of Moloney-murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen Corp.), and 20 units of RNase inhibitor (Invitrogen Corp.). Following the reaction the sample was heated at 94°C for 5 min, cooled on ice and, after adding 180 μl water, samples were stored −80°C.
Real-Time PCR
PCR primers were designed using the Primer3 software (http://frodo.wi.mit.edu/). The reaction mixture contained 5 μl 2× SYBR Green PCR Master Mix (ABI), 2.5 μl DNA template and 0.3 μM primers (10 μl final volume) in 384-Well Optical Reaction Plate (ABI). Amplification (three steps, 40 cycles), data acquisition and analysis were done using the 7900HT Real-Time PCR system and SDS Enterprise Database (ABI). Standard curves were generated for each primer set by serial dilution of human genomic DNA. Primer sequences are shown in table 2. mRNA levels were calculated relative to untreated cells grown in normal glucose and all results were corrected to the β-actin transcript.
Table 2.
PCR primer list
| MCP-1 (CCL2) | fwd | 5′-TGTTGATGTGAAACATTATGCC-3′ | |||||||
| Gene ID 6347 | rev | 5′-AATGATTCTTGCAAAGACCCTC-3′ | |||||||
| LIF | fwd | 5′-ATTCAGTGATGCTGTGCAGG-3′ | |||||||
| Gene ID 3976 | rev | 5′-ATCACCTCATCTCCCTGTGG-3′ | |||||||
| FGF5 | fwd | 5′-TCCTAAACCTTTGGTGGCTG-3′ | |||||||
| Gene ID 2250 | rev | 5′-GTTCAAGAATGAGGGCAAGG-3′ | |||||||
| COX-2 (PTGS2) | fwd | 5′-TCCCTGAGCATCTACGGTTT-3′ | |||||||
| Gene ID 5743 | rev | 5′-TACTCTGTTGTGTTCCCGCA-3′ | |||||||
| FOSL1 | fwd | 5′-ATGTGGGATACTGTCCAGGC-3′ | |||||||
| Gene ID 8061 | rev | 5′-CATCGCAAGAGTAGCAGCAG-3′ | |||||||
| TIEG | wd | 5′-ATCCTGGGTGGCTACAGATG-3′ | |||||||
| Gene ID 7071 | rev | 5′-GTGCAGAGTTCAAAGCCTCC-3′ | |||||||
| IER3 | fwd | 5′-CTCCTACTTTGCCGCAGTTC-3′ | |||||||
| Gene ID 8870 | rev | 5′-CGTCCTCCTAGGTGATGGAG-3′ | |||||||
| EGR-1 | fwd | 5′-TGAACAACGAGAAGGTGCTG-3′ | |||||||
| Gene ID 1958 | rev | 5′-GGTCATGCTCACTAGGCCAC-3′ | |||||||
| β-Actin | fwd | 5′-AGAGCTACGAGCTGCCTGAC-3′ | |||||||
| Gene ID 60 | rev | 5′-AAGGTAGTTTCGTGGATGCC-3′ | |||||||
Statistics
Real-time PCR mRNA results were compared by four-way ANOVA for each gene [47]. The model included main effects for normal versus high glucose, presence or absence of AII, presence or absence of valsartan and presence or absence of simvastatin along with all two-way interactions. mRNA levels were log transformed due to skewness and robust standard error estimates were used to protect against departures from the constant variance assumption. The level of significance was taken to be 0.05, and p values were not adjusted for multiple comparisons due to the exploratory nature of the analysis. The statistical analysis is summarized in table 3.
Table 3.
Summary of statistical analysis
| Comparison | PTGS2 (COX-2) | CCL2 (MCP-1) | EGR-1 | FOSL1 | LIF | TIEG (KLF10) | FGF5 | IER3 |
|---|---|---|---|---|---|---|---|---|
| HG vs. LG control | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | NS | <0.01 | NS |
| NG AII vs. NG control | <0.001 | 0.001 | <0.001 | NS | <0.001 | <0.001 | NS | <0.001 |
| HG AII vs. HG control | <0.001 | 0.001 | <0.001 | 0.1 | <0.001 | <0.001 | NS | <0.001 |
| HG AII vs. NG AII | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.02 | 0.01 |
| NG AII Val vs. NG AII | <0.001 | <0.001 | <0.001 | NS | <0.001 | 0.001 | NS | 0.01 |
| HG AII Val vs. HG AII | <0.001 | <0.001 | <0.001 | NS | <0.001 | 0.001 | NS | 0.02 |
| NG AII Val Sim vs. NG AII Val | <0.001 | <0.001 | NS | NS | NS | <0.001 | <0.001 | NS |
| HG AII Val Sim vs. HG AII Val | <0.001 | <0.001 | NS | <0.001 | <0.001 | <0.001 | <0.001 | NS |
| ΔHG AII vs. cont–ΔLG AII vs. cont | NS | NS | NS | NS | <0.05 | NS | NS | NS |
| NG vs. NG Sim | <0.05 | <0.001 | NS | NS | <0.001 | <0.001 | <0.001 | <0.05 |
NG = Normal glucose; HG = high glucose; AII = angiotensin II; Val = valsartan; Sim = simvastatin; NS = statistically not significant; cont = control.
Results
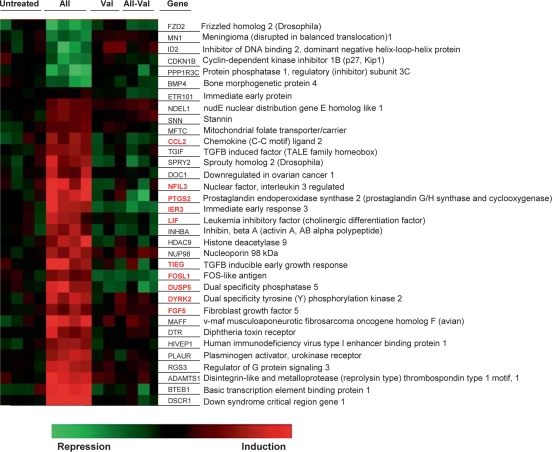
Expression microarray profiling of the RNA identified 27 genes that were induced by AII and 7 that were suppressed (100 nM, 60 min treatment; ANOVA p < 0.05; fig. 1). In all cases the AII-induced effects were blocked by pretreatment of cells with the AT1 receptor blocker valsartan (100 nM). In agreement with reported dominant expression of AT1[12], our results suggest that in primary human mesangial cell culture AII-responsive gene expression is largely mediated by the AT1 receptor. Several of the AII-responsive genes identified by microarrays encode proteins, such as cytokines, enzymes and transcription factors that could be involved in inflammatory responses. Among the candidate genes, mRNAs encoding proteins potentially involved in inflammatory responses, including MCP-1 [8, 48], LIF [49], PTGS2 (COX-2) [9], FOSL1 [50] and NFIL3 [51] were previously shown to be induced in response to AII treatment. The list of the genes and link to iHOP (Information Hyperlinked over Proteins) is http://www.ihop-net.org.
Fig. 1.
Heat map showing AII-induced transcripts in untreated and valsartan-pretreated (Val) primary human mesangial cells. Serum-deprived (24 h) cells maintained in normal glucose (100 mg/ml) and preincubated with or without Val (100 nM, 30 min) were treated with or without AII (100 nM, 60 min). Total RNA extracted was labeled for hybridization of high-density oligonucleotide microarrays (Material and Methods). Data were acquired and analyzed using the Gene Traffic program. The list of genes and link to iHOP (Information Hyperlinked over Proteins) can be found at http://www.ihop-net.org. The AII-inducible genes encoding putative mediators of inflammatory responses are labeled in red in the online version.
Next we used real-time PCR (i) to confirm microarray data, (ii) to test if ambient glucose modulates these mRNA responses, and (iii) to compare the effectiveness of AT1 blockade with or without pretreatment with a statin, simvastatin. The outline of the treatment groups is shown in table 1. The AII-induced inflammatory gene mRNAs can be grouped into three classes, cytokines/chemokines, enzymes, and transcription factors. Each functional class of genes will be presented as a group.
A summary of the statistical analyses is shown in table 3 and to the right of 2, 3, 4.
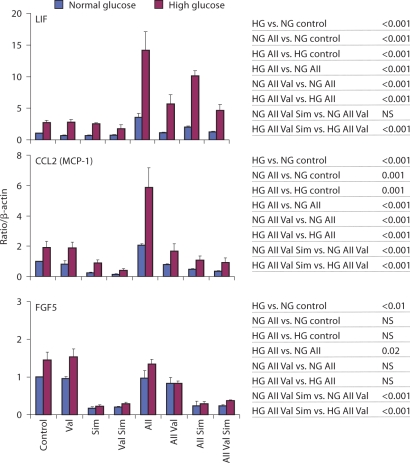
Fig. 2.
AII effects on cytokine mRNAs expressed in primary human mesangial cells maintained in normal and high glucose: effects of valsartan and/or simvastatin. Cells were grown to 75–85% confluence in NG (100 mg/dl) or HG (450 mg/ml), following serum deprivation (24 h) cells were incubated with or without 1 μM simvastatin (24 h). After pretreatment with or without valsartan (100 nM, 30 min) cells were stimulated with or without AII (100 nM, 60 min; table 1). Total RNA was extracted, reverse transcribed and transcript levels were assessed by real-time PCR done in triplicates using specific primers. Results are reported as relative expression normalized to β-actin mRNA. Results shown as mean ± SE from the data of three separate cell experiments. Statistical analysis is shown on the right and in table 3. NS = Statistically not significant.
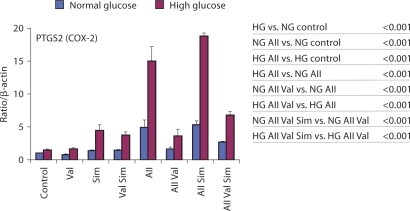
Fig. 3.
AII effects on mRNAs encoding enzymes expressed in primary human mesangial cells maintained in normal and high glucose: effects of valsartan and/or simvastatin. Results are reported as relative expression normalized to β-actin mRNA. Results shown as mean ± SE from the data of three separate cell experiments. Statistical analysis is shown on the right and in table 3. NS = Statistically not significant.
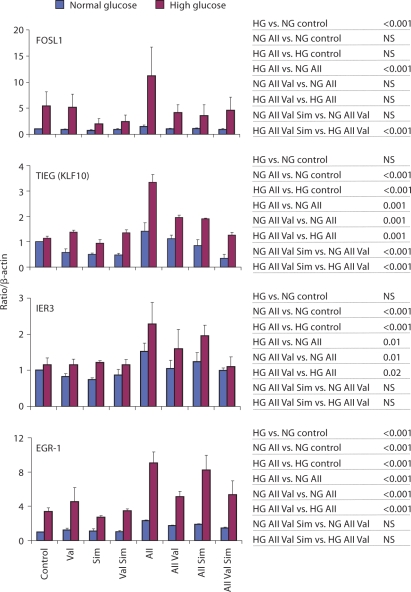
Fig. 4.
AII effects on mRNAs encoding transcription factors expressed in primary human mesangial cells maintained in normal and high glucose: effects of valsartan and/or simvastatin. Results are reported as relative expression normalized to β-actin mRNA. Results shown as mean ± SE from the data of three separate cell experiments. Statistical analysis is shown on the right and in table 3. NS = Statistically not significant.
Cytokines/Chemokines
Leukemia inhibitory factor (LIF) belongs to the IL-6 family of cytokines that share the common co-receptor gp130 and activate similar cellular responses [49]. Several studies reported AII-induced LIF and IL-6 gene expression in different cell types [49,52,53,54]. Importantly, it has been shown that increased systemic [18] and renal [55] expression of IL-6 is associated with diabetic nephropathy. Thus, the involvement of the IL-6 family of cytokines in mediating adverse cardiovascular [49] and renal [18, 55] outcomes appears well documented. HG by itself increased LIF mRNA levels 2.71 ± 0.35-fold over NG (p < 0.001; fig. 2). AII-increased LIF mRNA levels 3.56 ± 0.63-fold in NG (p < 0.001) and 14.15 ± 2.93-fold in HG (p < 0.001), and the AII-induced mRNA level was greater in HG (HG AII vs. NG AII, p < 0.001; fig. 2). The AII-induced increase in high ambient glucose was greater than the sum of AII and HG alone (ΔHG AII vs. control – ΔLG AII vs. control, p < 0.05; table 3), suggesting synergism. Valsartan inhibited the AII-induced LIF mRNA response in both NG (p < 0.001) and HG (p < 0.001) to 1.12 ± 0.05 and 5.66 ± 1.46 levels, respectively. Valsartan was a slightly more effective inhibitor of AII-induced LIF mRNA response compared to when used in combination with simvastatin in HG (p < 0.001) but not NG (p = 0.071, NS).
The chemokine MCP-1 (CCL2) is considered key mediator of AII-induced inflammation in the kidney and other organs [8, 15, 25, 48, 52, 53]. Production of MCP-1 by mesangial cells has been postulated to be part of an early inflammatory process that causes renal injury in diabetes. Transcription of MCP-1 in mesangial and other cell types is primarily regulated by NF-κB [15]. HG by itself increased MCP-1 mRNA levels by 1.91 ± 0.72-fold over NG (p < 0.001; fig. 2). AII increased MCP-1 mRNA levels, 2.07 ± 0.08-fold in NG (p = 0.001) and 5.86 ± 1.30-fold in HG (p = 0.001; fig. 2). The AII-induced levels were greater in HG (p < 0.001). Valsartan totally blocked the AII-induced MCP-1 mRNA response in both NG (p < 0.001) and HG (p < 0.001) to 0.79 ± 0.02 and 1.67 ± 0.48 levels, respectively. Pretreatment with simvastatin decreased the expression of MCP-1 mRNA (p < 0.001; table 3) and prevented the AII-induced response. Addition of simvastatin to valsartan lowered AII-induced responses in both NG (p < 0.001) and HG (p < 0.001).
Fibroblast growth factors (FGFs), are a family of factors involved in many processes including wound healing and embryonic development. In the glomerulus they appear to regulate mesangial cell proliferation [56, 57]. Real-time PCR did not confirm the AII-induced FGF5 mRNA levels (p = 0.357 in NG, p = 0.317 in HG).
The lack of real-time PCR confirmation of microarray data is not uncommon [58], underscoring the need to validate microarray data. Nonetheless, the real-time PCR revealed that simvastatin reduced FGF5 levels by more than 80%, an effect that was equally potent in NG and HG conditions (p < 0.001; table 3).
Enzymes
Cyclooxygenase-2 (COX-2) encoded by the PTGS2 (prostaglandin-endoperoxide synthase 2) gene catalyzes the conversion of arachidonic acids to prostaglandins. In agreement with published reports in monocytes in vitro [59], and in the diabetic kidney in vivo [60, 61], HG increased COX-2 mRNA levels (1.46 ± 0.17-fold over NG, p < 0.001; fig. 3). Prostaglandins are synthesized in large amounts in the glomerulus in response to a host of ligands, including AII. Like AII, COX-2-derived metabolites have hemodynamic and mitogenic effects. AII potently induced COX-2 mRNA levels, 4.90 ± 1.15-fold in NG (p < 0.001) and 14.99 ± 2.20-fold in HG (p < 0.001; fig. 3). The AII-induced COX-2 mRNA levels were higher in HG compared to NG (p < 0.001). Valsartan inhibited the AII-induced COX-2 mRNA response in both NG (p < 0.001) and HG (p < 0.001) to 1.61 ± 0.33 and 3.60 ± 1.03 levels, respectively. In contrast to the cytokine/chemokine effects, addition of simvastatin to valsartan increased AII-mediated COX-2 mRNA levels in both NG (p < 0.001) and HG (p < 0.001). In fact, simvastatin alone increased COX-2 mRNA levels, both in NG (1.36 ± 0.09-fold, p < 0.05) and in HG (4.43 ± 0.88-fold, p < 0.001).
Transcription Factors
Fos-related antigen 1, FOSL1 or FRA1, is a member of the FOS family of transcription factors [62, 63] that are responsive to mitogens including AII [50] and insulin [62]. Increased expression of the FOS family of proteins has been reported in response to high glucose [64] and in diabetic nephropathy [65]. In agreement with these studies, ambient HG increased FOSL1 mRNA levels 5.38 ± 2.72-fold over NG (p < 0.001; fig. 4). In AII-treated cells, FOSL1 mRNA levels averaged 1.46 ± 0.33 in NG and 11.16 ± 5.56 in HG (fig. 4), levels that were not statistically different from the untreated control. Nonetheless, statistical analysis of AII effects in HG versus NG showed that in the AII-treated cells the levels of FOSL1 mRNA were higher in HG (p < 0.001). This difference was not present in valsartan-pretreated cells. Thus, there is an interaction between AII and HG to increase FOSL1 mRNA levels.
TGF-β activates expression of genes that encode factors promoting renal cell hypertrophy and stimulates extracellular matrix accumulation, lesions typical of diabetic nephropathy [66,67,68]. TGF-β-inducible early growth response protein 1, TIEG (KLF10), is a Sp-1-like transcription factor. TIEG-1 has not previously been reported to respond to AII treatment. Unlike some of the other genes, HG by itself did not increase TIEG mRNA levels (HG vs. NG; p = NS; fig. 4). AII increased TIEG mRNA levels 1.41 ± 0.33-fold in NG (p < 0.001) and the AII-induced level was greater in HG, 3.35 ± 0.30 (HG AII vs. NG AII p < 0.001; fig. 4). Valsartan inhibited the AII-induced TIEG mRNA response in both NG (p = 0.001) and HG (p = 0.001), 1.12 ± 0.15 and 1.95 ± 0.09, respectively. With the combination of valsartan and simvastatin the levels of AII-induced TIEG mRNA in NG and HG were lower than when each of the agents was used alone (p < 0.001 in NG and HG).
The immediate early response 3 [IER3 (IEX1)] protein belongs to the NF-κB family of transcription factors. Expression of this gene is enhanced with cell proliferation and it may regulate apoptosis [69]. HG by itself did not increase IER3 mRNA levels (HG vs. NG; p = NS; fig. 4). AII increased IER3 mRNA levels to 1.52 ± 0.52 in NG (p < 0.001) and in HG to 2.28 ± 0.61 (p < 0.001; fig. 4). In the presence of valsartan the AII-induced IER3 mRNA responses in both NG (p = 0.01) and HG (p = 0.02) were decreased. Addition of simvastatin did not appear to have an effect.
The EGR-1, early growth response-1, gene is a transcription factor of the immediate early response class [51]. Expression of EGR-1 is induced by a host of growth factors including insulin [70], AII [71] and high glucose [72]. Increased expression of EGR-1 has been linked to cardiovascular and renal disease [73, 74]. High ambient glucose increased EGR-1 mRNA levels 3.38 ± 0.44-fold compared to cells maintained in NG (p < 0.001). AII increased EGR-1 mRNA levels, 2.31 ± 0.06-fold in NG (p < 0.001) and 9.06 ± 1.28-fold in HG (p < 0.001; fig. 4). The AII-induced levels were higher in HG (HG vs. NG, p < 0.001). Valsartan inhibited the AII-induced EGR-1 mRNA response in both NG (p < 0.001) and HG (p < 0.001), 1.72 ± 0.10 and 5.11 ± 0.64, respectively. Addition of simvastatin to valsartan had no effect on AII-induced responses.
Discussion
Our study not only adds to the mounting evidence that AII activates expression of diverse classes of proinflammatory mediators [75, 76], but also provides new data that in human mesangial cells AII induction of proinflammatory gene mRNA expression is augmented in high ambient glucose. The AII effects are inhibited by valsartan and can be modulated by simvastatin.
We found that high ambient glucose (HG) altered constitutive (COX-2, LIF, MCP-1, FGF5, FOSL1, and EGR-1) and AII-inducible (COX-2, LIF, MCP-1, FGF5, FOSL1, EGR-1, IER3 and TIEG) levels of mRNAs encoding proinflammatory mediators.
In addition to intracellular glucose-specific pathways, HG levels may alter cell functions by increasing ambient osmolarity, an effect that is not unique to glucose. In some studies, osmotically active substances, such as mannitol, induced the same genes as HG, effects that may or may not represent shared hyperosmolarity-triggered pathways [77,78,79,80,81,82]. Regardless whether or not glucose and mannitol pathways are common, the hyperosmolarity is likely to play a role in gene induction in a variety of cell types because the postprandial osmolarity in diabetic patients is abnormally elevated [81,82,83,84].
Previous studies and our in vitro observations about levels of those mRNAs that encode factors involved in inflammatory responses suggest a possible model of how hyperglycemia could be aggravating the AII role in the development of diabetic nephropathy. The NF-κB/Rel family of transcription factors are among the most important intracellular mediators of inflammatory responses [16, 85, 86]. Hyperglycemia increases ROS production which in turn activates NF-κB [10, 59, 87]. Like high ambient glucose, AII also increases ROS production and activates NF-κB [10, 59, 87]. Both HG and AII have been shown to increase ROS production via NADPH oxidase [9, 77,88,89,90]. There is evidence that HG- and AII-mediated activation of NADPH oxidase is mediated through the activation of protein kinase C (PKC) where the PKC-β isoform may play a particularly important role [90]. Several of the AII- and HG-induced genes that we identified (2, 3, 4) including MCP-1 [8, 15, 25, 38], LIF/IL-6 [38], COX-2 [91], EGR-1 [92] and FOSL1 (Fra-1) [93] are activated by NF-κB. Thus, the greater AII-induced responses seen under high ambient glucose conditions may reflect augmented NF-κB responses. There are several potential NF-κB-dependent mechanisms that could be explain the synergistic (e.g. LIF) and additive (e.g. MCP-1) effects of AII and HG effects. (i) Both AII and HG activate NF-κB and together the level of NF-κB activity is greater. Indeed, in addition to ROS, HG and AII synergistically activate signaling molecules acting upstream of NF-κB, such as PKCβ [94] and p38 MAP kinase [95]. Since PKCβ activates NADPH oxidase [90] this enzyme could be mediating HG-AII synergistic activation of ROS production. (ii) AII and HG activate other transcription factors that cooperate with NF-κB (e.g. EGR-1, KFL4 and FOSL-1). (iii) AII and HG increase synthesis of chemokines/cytokines that augment activation of NF-κB and other transcription factors via autocrine mechanism.
The ARB valsartan inhibited all AII-inducible genes studied here, suggesting that this agent has broad anti-inflammatory properties that appear as effective in high as in normal ambient glucose. The anti-inflammatory effects of ARBs likely reflect an indirect effect through the blockade of AT1R-mediated production of ROS intermediaries [88] and block of NF-κB induction which could result, in part, from the increased ROS levels [8, 87]. Because high ambient glucose renders the mesangial cells more sensitive to the AII proinflammatory action (2, 3, 4), the renoprotective RAS inhibition is particularly important in sparing the kidney from diabetes.
There is strong evidence that statins have organ-protective properties independent of their cholesterol-lowering effects [30, 36, 37, 96]. In experimental models of glomerulonephritis, lipid-induced nephritis and AII-induced injury, simvastatin suppressed mesangial cell proliferation, matrix expansion and inflammatory markers [37,97,98,99], a process associated with decreased expression of cyclin-dependent kinase 2 (Cdk2), a key regulator of cell proliferation [100].
In our cultured human mesangial cell system, addition of simvastatin to valsartan further lowered some (e.g. MCP-1, LIF and TIEG) but not all (e.g. COX-2) AII-induced mRNAs. In addition, simvastatin by itself inhibited both HG-induced (FGF5, and MCP-1) and AII-inducible (FOSL1) gene expression. Taken together, these results suggest that some of the statin's renoprotective mechanisms are synergistic with RAS inhibitors, whereas others are mediated by pathways different from those of ARBs. One specific mechanism whereby statins ameliorate renal inflammation could be mediated by inhibition of small GTPases, such as RhoA [99, 101, 102]. However, we also found that simvastatin increased COX-2 mRNA expression, an effect that was greater in the HG environment (fig. 3). Statins have also been reported to increase COX-2 expression in macrophages, an effect mediated by ERK1/2 pathways [103]. Because hyperglycemia activates ERK1/2 [10], activation of this pathway thus may explain the greater effect of simvastatin in HG. Taken together, these observations suggest that statins modulate inflammatory responses but, depending on the context, statins may have either anti- or proinflammatory-like effects.
It seems plausible that acting through both the same and different pathways the combined effects of ARBs and statins could be more renoprotective than using only one of the agents. Indeed, observations in some models of renal injury, such as Heymann nephritis, the combination of lisinopril with simvastatin was significantly more nephroprotective than monotherapies, including the inflammatory markers [104, 105]. Nonetheless, it should be noted that available evidence in experimental diabetic nephropathy about the potential superiority of combination of statins with RAS inhibitors is less clear. Although in streptozotocin-diabetic rats, this drug combination conferred superiority over monotherapies on renal function (prevention of albuminuria and rise in plasma BUN and creatinine), no advantage of combination therapy was seen with respect to attenuating renal structural injury and renal expression of TGF-β and VEGF in experimental diabetes [106].
In summary, our observations add to the growing evidence that ARBs and statins modulate renal cell inflammatory responses [4,5,6, 76]. However, in vivo studies measuring activities and protein levels encoded by the mRNAs identified in this and other studies are needed to fully explore the interaction between ARBs and statins in the diabetic kidney.
Acknowledgement
This work was supported by a Novartis IIRP Research Grant and NIH (DK-R37-45978 to K.B.), and Juvenile Diabetes Research Foundation grants (K.B. 1-2002-80 and R.K. 12-008-314).
References
- 1.Salzet M, Wattez C, Baert JL, Malecha J. Biochemical evidence of angiotensin II-like peptides and proteins in the brain of the rhynchobdellid leech Theromyzon tessulatum. Brain Res. 1993;631:247–255. doi: 10.1016/0006-8993(93)91542-z. [DOI] [PubMed] [Google Scholar]
- 2.Salzet M, Deloffre L, Breton C, Vieau D, Schoofs L. The angiotensin system elements in invertebrates. Brain Res Brain Res Rev. 2001;36:35–45. doi: 10.1016/s0165-0173(01)00063-7. [DOI] [PubMed] [Google Scholar]
- 3.Salzet M, Verger-Bocquet M. Elements of angiotensin system are involved in leeches and mollusks immune response modulation. Brain Res Mol Brain Res. 2001;94:137–147. doi: 10.1016/s0169-328x(01)00229-7. [DOI] [PubMed] [Google Scholar]
- 4.Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21:20–27. doi: 10.1038/sj.jhh.1002101. [DOI] [PubMed] [Google Scholar]
- 5.Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- 6.Dagenais NJ, Jamali F. Protective effects of angiotensin II interruption: evidence for antiinflammatory actions. Pharmacotherapy. 2005;25:1213–1229. doi: 10.1592/phco.2005.25.9.1213. [DOI] [PubMed] [Google Scholar]
- 7.Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, Milford E, Abdi R. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18:1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 8.Theuer J, Dechend R, Muller DN, Park JK, Fiebeler A, Barta P, Ganten D, Haller H, Dietz R, Luft FC. Angiotensin II induced inflammation in the kidney and in the heart of double transgenic rats. BMC Cardiovasc Disord. 2002;2:3. doi: 10.1186/1471-2261-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int. 2005;68:2143–2153. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen YW, Liu F, Tran S, Zhu Y, Hebert MJ, Ingelfinger JR, Zhang SL. Reactive oxygen species and nuclear factor-kappa B pathway mediate high glucose-induced Pax-2 gene expression in mouse embryonic mesenchymal epithelial cells and kidney explants. Kidney Int. 2006;70:1607–1615. doi: 10.1038/sj.ki.5001871. [DOI] [PubMed] [Google Scholar]
- 11.Siragy HM, El-Kersh MA, De Gasparo M, Webb RL, Carey RM. Differences in AT2-receptor stimulation between AT1 receptor blockers valsartan and losartan quantified by renal interstitial fluid cGMP. J Hypertens. 2002;20:1157–1163. doi: 10.1097/00004872-200206000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Xoriuchi M, Hamai M, Cui TX, Iwai M, Minokoshi Y. Cross talk between angiotensin II type 1 and type 2 receptors: cellular mechanism of angiotensin type 2 receptor-mediated cell growth inhibition. Hypertens Res. 1999;22:67–74. doi: 10.1291/hypres.22.67. [DOI] [PubMed] [Google Scholar]
- 13.Li XC, Carretero OA, Zhuo JL. Cross-talk between angiotensin II and glucagon receptor signaling mediates phosphorylation of mitogen-activated protein kinases ERK 1/2 in rat glomerular mesangial cells. Biochem Pharmacol. 2006;71:1711–1719. doi: 10.1016/j.bcp.2006.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrass CK, Berfield AK, Ryan MC, Carter WG, Hansen KM. Abnormal development of glomerular endothelial and mesangial cells in mice with targeted disruption of the lama3 gene. Kidney Int. 2006;70:1062–1071. doi: 10.1038/sj.ki.5001706. [DOI] [PubMed] [Google Scholar]
- 15.Lee FT, Cao Z, Long DM, Panagiotopoulos S, Jerums G, Cooper ME, Forbes JM. Interactions between angiotensin II and NF-kappaB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am Soc Nephrol. 2004;15:2139–2151. doi: 10.1097/01.ASN.0000135055.61833.A8. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21:16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Ortega M, Lorenzo O, Ruperez M, Konig S, Wittig B, Egido J. Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: molecular mechanisms. Circ Res. 2000;86:1266–1272. doi: 10.1161/01.res.86.12.1266. [DOI] [PubMed] [Google Scholar]
- 18.Dalla Vestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, Plebani M, Fioretto P. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(suppl 1):S78–S82. doi: 10.1681/asn.2004110961. [DOI] [PubMed] [Google Scholar]
- 19.Phillips A, Janssen U, Floege J. Progression of diabetic nephropathy. Insights from cell culture studies and animal models. Kidney Blood Press Res. 1999;22:81–97. doi: 10.1159/000025912. [DOI] [PubMed] [Google Scholar]
- 20.Abrass CK, Spicer D, Raugi GJ. Induction of nodular sclerosis by insulin in rat mesangial cells in vitro: studies of collagen. Kidney Int. 1995;47:25–37. doi: 10.1038/ki.1995.3. [DOI] [PubMed] [Google Scholar]
- 21.Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int. 1999;56:393–405. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 22.Coughlan MT, Forbes JM, Cooper ME. Role of the AGE crosslink breaker, alagebrium, as a renoprotective agent in diabetes. Kidney Int Suppl. 2007;(106):S54–S60. doi: 10.1038/sj.ki.5002387. [DOI] [PubMed] [Google Scholar]
- 23.Amiri F, Shaw S, Wang X, Tang J, Waller JL, Eaton DC, Marrero MB. Angiotensin II activation of the JAK/STAT pathway in mesangial cells is altered by high glucose. Kidney Int. 2002;61:1605–1616. doi: 10.1046/j.1523-1755.2002.00311.x. [DOI] [PubMed] [Google Scholar]
- 24.Lansang MC, Price DA, Laffel LM, Osei SY, Fisher ND, Erani D, Hollenberg NK. Renal vascular responses to captopril and to candesartan in patients with type 1 diabetes mellitus. Kidney Int. 2001;59:1432–1438. doi: 10.1046/j.1523-1755.2001.0590041432.x. [DOI] [PubMed] [Google Scholar]
- 25.Ha H, Yu MR, Choi YJ, Kitamura M, Lee HB. Role of high glucose-induced nuclear factor-kappaB activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am Soc Nephrol. 2002;13:894–902. doi: 10.1681/ASN.V134894. [DOI] [PubMed] [Google Scholar]
- 26.Marrero MB, Banes-Berceli AK, Stern DM, Eaton DC. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F762–F768. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 27.LIPID Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 28.Brown MS, Goldstein JL. Atherosclerosis. Scavenging for receptors. Nature. 1990;343:508–509. doi: 10.1038/343508a0. [DOI] [PubMed] [Google Scholar]
- 29.Heusinger-Ribeiro J, Fischer B, Goppelt-Struebe M. Differential effects of simvastatin on mesangial cells. Kidney Int. 2004;66:187–195. doi: 10.1111/j.1523-1755.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 30.Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007;28:88–98. doi: 10.1016/j.it.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sander GE, Giles TD. Diabetes mellitus and heart failure. Am Heart Hosp J. 2003;1:273–280. doi: 10.1111/j.1541-9215.2003.02085.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim SI, Kim HJ, Han DC, Lee HB. Effect of lovastatin on small GTP binding proteins and on TGF-beta1 and fibronectin expression. Kidney Int Suppl. 2000;77:S88–S92. doi: 10.1046/j.1523-1755.2000.07714.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim SI, Han DC, Lee HB. Lovastatin inhibits transforming growth factor-beta1 expression in diabetic rat glomeruli and cultured rat mesangial cells. J Am Soc Nephrol. 2000;11:80–87. doi: 10.1681/ASN.V11180. [DOI] [PubMed] [Google Scholar]
- 34.Dechend R, Fiebler A, Lindschau C, Bischoff H, Muller D, Park JK, Dietz R, Haller H, Luft FC. Modulating angiotensin II-induced inflammation by HMG Co-A reductase inhibition. Am J Hypertens. 2001;14:55S–61S. doi: 10.1016/s0895-7061(01)02070-2. [DOI] [PubMed] [Google Scholar]
- 35.Dechend R, Fiebeler A, Park JK, Muller DN, Theuer J, Mervaala E, Bieringer M, Gulba D, Dietz R, Luft FC, et al. Amelioration of angiotensin II-induced cardiac injury by a 3-hydroxy-3methylglutaryl coenzyme a reductase inhibitor. Circulation. 2001;104:576–581. doi: 10.1161/hc3001.092039. [DOI] [PubMed] [Google Scholar]
- 36.Dechen R, Muller D, Park JK, Fiebeler A, Haller H, Luft FC. Statins and angiotensin II-induced vascular injury. Nephrol Dial Transplant. 2002;17:349–353. doi: 10.1093/ndt/17.3.349. [DOI] [PubMed] [Google Scholar]
- 37.Park JK, Muller DN, Mervaala EM, Dechend R, Fiebeler A, Schmidt F, Bieringer M, Schafer O, Lindschau C, Schneider W, et al. Cerivastatin prevents angiotensin II-induced renal injury independent of blood pressure- and cholesterol-lowering effects. Kidney Int. 2000;58:1420–1430. doi: 10.1046/j.1523-1755.2000.00304.x. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002;(82):S12–S22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Ortega M, Bustos C, Hernandez-Presa MA, Lorenzo O, Plaza JJ, Egido J. Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 synthesis. J Immunol. 1998;161:430–439. [PubMed] [Google Scholar]
- 40.Awad AS, Webb RL, Carey RM, Siragy HM. Increased renal production of angiotensin II and thromboxane B2 in conscious diabetic rats. Am J Hypertens. 2005;18:544–548. doi: 10.1016/j.amjhyper.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Siragy HM, Howell NL, Ragsdale NV, Carey RM. Renal interstitial fluid angiotensin. Modulation by anesthesia, epinephrine, sodium depletion, and renin inhibition. Hypertension. 1995;25:1021–1024. doi: 10.1161/01.hyp.25.5.1021. [DOI] [PubMed] [Google Scholar]
- 42.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–134. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 43.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng L, Xu H, Chew TL, Chisholm R, Sadeghi MM, Kanwar YS, Danesh FR. Simvastatin modulates angiotensin II signaling pathway by preventing Rac1-mediated upregulation of p27. J Am Soc Nephrol. 2004;15:1711–1720. doi: 10.1097/01.asn.0000129839.91567.68. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi Y, Yamauchi K, Nakamura J, Shigematsu S, Hashizume K. Angiotensin II regulates migration in mouse cultured mesangial cells: evidence for the presence of receptor subtype-specific regulation. J Endocrinol. 2006;191:361–367. doi: 10.1677/joe.1.06860. [DOI] [PubMed] [Google Scholar]
- 46.Kacharmina JE, Crino PB, Eberwine J. Preparation of cDNA from single cells and subcellular regions. Methods Enzymol. 1999;303:3–18. doi: 10.1016/s0076-6879(99)03003-7. [DOI] [PubMed] [Google Scholar]
- 47.Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied Linear Statistical Models. Boston: WCB/McGraw-Hill; 1996. [Google Scholar]
- 48.Hisada Y, Sugaya T, Yamanouchi M, Uchida H, Fujimura H, Sakurai H, Fukamizu A, Murakami K. Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest. 1999;103:627–635. doi: 10.1172/JCI2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sano M, Fukuda K, Kodama H, Pan J, Saito M, Matsuzaki J, Takahashi T, Makino S, Kato T, Ogawa S. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem. 2000;275:29717–29723. doi: 10.1074/jbc.M003128200. [DOI] [PubMed] [Google Scholar]
- 50.Campos AH, Zhao Y, Pollman MJ, Gibbons GH. DNA microarray profiling to identify angiotensin-responsive genes in vascular smooth muscle cells: potential mediators of vascular disease. Circ Res. 2003;92:111–118. doi: 10.1161/01.res.0000049100.22673.f6. [DOI] [PubMed] [Google Scholar]
- 51.Romero DG, Rilli S, Plonczynski MW, Yanes LL, Zhou MY, Gomez-Sanchez EP, Gomez-Sanchez CE. Adrenal transcription regulatory genes modulated by angiotensin II and their role in steroidogenesis. Physiol Genomics. 2007;30:26–34. doi: 10.1152/physiolgenomics.00187.2006. [DOI] [PubMed] [Google Scholar]
- 52.Chang LT, Sun CK, Chiang CH, Wu CJ, Chua S, Yip HK. Impact of simvastatin and losartan on antiinflammatory effect: in vitro study. J Cardiovasc Pharmacol. 2007;49:20–26. doi: 10.1097/FJC.0b013e31802ba4ec. [DOI] [PubMed] [Google Scholar]
- 53.Ni W, Kitamoto S, Ishibashi M, Usui M, Inoue S, Hiasa K, Zhao Q, Nishida K, Takeshita A, Egashira K. Monocyte chemoattractant protein-1 is an essential inflammatory mediator in angiotensin II-induced progression of established atherosclerosis in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2004;24:534–539. doi: 10.1161/01.ATV.0000118275.60121.2b. [DOI] [PubMed] [Google Scholar]
- 54.Manabe S, Okura T, Watanabe S, Fukuoka T, Higaki J. Effects of angiotensin II receptor blockade with valsartan on proinflammatory cytokines in patients with essential hypertension. J Cardiovasc Pharmacol. 2005;46:735–739. doi: 10.1097/01.fjc.0000185783.00391.60. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki D, Miyazaki M, Naka R, Koji T, Yagame M, Jinde K, Endoh M, Nomoto Y, Sakai H. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes. 1995;44:1233–1238. doi: 10.2337/diab.44.10.1233. [DOI] [PubMed] [Google Scholar]
- 56.Floege J, Burg M, Hugo C, Gordon KL, Van Goor H, Reidy M, Couser WG, Koch KM, Johnson RJ. Endogenous fibroblast growth factor-2 mediates cytotoxicity in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol. 1998;9:792–801. doi: 10.1681/ASN.V95792. [DOI] [PubMed] [Google Scholar]
- 57.Floege J, Eng E, Lindner V, Alpers CE, Young BA, Reidy MA, Johnson RJ. Rat glomerular mesangial cells synthesize basic fibroblast growth factor. Release, upregulated synthesis, and mitogenicity in mesangial proliferative glomerulonephritis. J Clin Invest. 1992;90:2362–2369. doi: 10.1172/JCI116126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morey JS, Ryan JC, Van Dolah FM. Microarray validation: factors influencing correlation between oligonucleotide microarrays and real-time PCR. Biol Proced Online. 2006;8:175–193. doi: 10.1251/bpo126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shanmugam N, Gaw Gonzalo IT, Natarajan R. Molecular mechanisms of high glucose-induced cyclooxygenase-2 expression in monocytes. Diabetes. 2004;53:795–802. doi: 10.2337/diabetes.53.3.795. [DOI] [PubMed] [Google Scholar]
- 60.Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest. 2001;107:889–898. doi: 10.1172/JCI10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng HF, Wang CJ, Moeckel GW, Zhang MZ, McKanna JA, Harris RC. Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int. 2002;62:929–939. doi: 10.1046/j.1523-1755.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- 62.Mohn KL, Laz TM, Melby AE, Taub R. Immediate-early gene expression differs between regenerating liver, insulin-stimulated H-35 cells, and mitogen-stimulated Balb/c 3T3 cells. Liver-specific induction patterns of gene 33, phosphoenolpyruvate carboxykinase, and the jun, fos, and egr families. J Biol Chem. 1990;265:21914–21921. [PubMed] [Google Scholar]
- 63.Adiseshaiah P, Peddakama S, Zhang Q, Kalvakolanu DV, Reddy SP. Mitogen regulated induction of FRA-1 proto-oncogene is controlled by the transcription factors binding to both serum and TPA response elements. Oncogene. 2005;24:4193–4205. doi: 10.1038/sj.onc.1208583. [DOI] [PubMed] [Google Scholar]
- 64.Kreisberg JI, Radnik RA, Ayo SH, Garoni J, Saikumar P. High glucose elevates c-fos and c-jun transcripts and proteins in mesangial cell culture. Kidney Int. 1994;46:105–112. doi: 10.1038/ki.1994.249. [DOI] [PubMed] [Google Scholar]
- 65.Ingram AJ, Scholey JW. Protooncogene expression and diabetic kidney injury. Kidney Int Suppl. 1997;60:S70–S76. [PubMed] [Google Scholar]
- 66.Schnaper HW, Hayashida T, Poncelet AC. It's a Smad world: regulation of TGF-beta signaling in the kidney. J Am Soc Nephrol. 2002;13:1126–1128. doi: 10.1681/ASN.V1341126. [DOI] [PubMed] [Google Scholar]
- 67.Choi ME. Mechanism of transforming growth factor-beta1 signaling. Kidney Int Suppl. 2000;77:S53–S58. [PubMed] [Google Scholar]
- 68.Border WA, Noble NA, Ketteler M. TGF-β: a cytokine mediator of glomerulosclerosis and a target for therapeutic intervention. Kidney Int Suppl. 1995;49:S59–S61. [PubMed] [Google Scholar]
- 69.Kumar R, Lutz W, Frank E, Im HJ. Immediate early gene X-1 interacts with proteins that modulate apoptosis. Biochem Biophys Res Commun. 2004;323:1293–1298. doi: 10.1016/j.bbrc.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jhun BH, Haruta T, Meinkoth JL, Leitner W, Draznin B, Saltiel AR, Pang L, Sasaoka T, Olefsky JM. Signal transduction pathways leading to insulin-induced early gene induction. Biochemistry. 1995;34:7996–8004. doi: 10.1021/bi00025a005. [DOI] [PubMed] [Google Scholar]
- 71.Rupprecht HD, Dann P, Sukhatme VP, Sterzel RB, Coleman DL. Effect of vasoactive agents on induction of Egr-1 in rat mesangial cells: correlation with mitogenicity. Am J Physiol. 1992;263:F623–F636. doi: 10.1152/ajprenal.1992.263.4.F623. [DOI] [PubMed] [Google Scholar]
- 72.Eto K, Kaur V, Thomas MK. Regulation of insulin gene transcription by the immediate-early growth response gene Egr-1. Endocrinology. 2006;147:2923–2935. doi: 10.1210/en.2005-1336. [DOI] [PubMed] [Google Scholar]
- 73.Solow BT, Derrien A, Smith JA, Jarett L, Harada S. Angiotensin II inhibits insulin-induced egr-1 expression in mesangial cells. Arch Biochem Biophys. 1999;370:308–313. doi: 10.1006/abbi.1999.1389. [DOI] [PubMed] [Google Scholar]
- 74.Gousseva N, Kugathasan K, Chesterman CN, Khachigian LM. Early growth response factor-1 mediates insulin-inducible vascular endothelial cell proliferation and regrowth after injury. J Cell Biochem. 2001;81:523–534. doi: 10.1002/1097-4644(20010601)81:3<523::aid-jcb1066>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 75.Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368:581–588. doi: 10.1016/S0140-6736(06)69201-5. [DOI] [PubMed] [Google Scholar]
- 76.Volpe M, Ruilope LM, McInnes GT, Waeber B, Weber MA. Angiotensin-II receptor blockers: benefits beyond blood pressure reduction? J Hum Hypertens. 2005;19:331–339. doi: 10.1038/sj.jhh.1001831. [DOI] [PubMed] [Google Scholar]
- 77.Leehey DJ, Isreb MA, Marcic S, Singh AK, Singh R. Effect of high glucose on superoxide in human mesangial cells: role of angiotensin II. Nephron Exp Nephrol. 2005;100:e46–e53. doi: 10.1159/000084348. [DOI] [PubMed] [Google Scholar]
- 78.Iglesias-de la Cruz MC, Ziyadeh FN, Isono M, Kouahou M, Han DC, Kalluri R, Mundel P, Chen S. Effects of high glucose and TGF-beta1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62:901–913. doi: 10.1046/j.1523-1755.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 79.Nahman NS, Jr, Leonhart KL, Cosio FG, Hebert CL. Effects of high glucose on cellular proliferation and fibronectin production by cultured human mesangial cells. Kidney Int. 1992;41:396–402. doi: 10.1038/ki.1992.55. [DOI] [PubMed] [Google Scholar]
- 80.Wilmer WA, Dixon CL, Hebert C. Chronic exposure of human mesangial cells to high glucose environments activates the p38 MAPK pathway. Kidney Int. 2001;60:858–871. doi: 10.1046/j.1523-1755.2001.060003858.x. [DOI] [PubMed] [Google Scholar]
- 81.Murphy M, Godson C, Cannon S, Kato S, Mackenzie HS, Martin F, Brady HR. Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth factor and other genes in human mesangial cells. J Biol Chem. 1999;274:5830–5834. doi: 10.1074/jbc.274.9.5830. [DOI] [PubMed] [Google Scholar]
- 82.Clarkson MR, Murphy M, Gupta S, Lambe T, Mackenzie HS, Godson C, Martin F, Brady HR. High glucose-altered gene expression in mesangial cells. Actin-regulatory protein gene expression is triggered by oxidative stress and cytoskeletal disassembly. J Biol Chem. 2002;277:9707–9712. doi: 10.1074/jbc.M109172200. [DOI] [PubMed] [Google Scholar]
- 83.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 84.Lappin DW, Doran P, Godson C, Brady HR. Gene responses to hyperglycaemia. Exp Nephrol. 2002;10:120–129. doi: 10.1159/000049907. [DOI] [PubMed] [Google Scholar]
- 85.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 86.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48:855–864. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 88.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30:860–866. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- 89.Yu HY, Inoguchi T, Nakayama M, Tsubouchi H, Sato N, Sonoda N, Sasaki S, Kobayashi K, Nawata H. Statin attenuates high glucose-induced and angiotensin II-induced MAP kinase activity through inhibition of NAD(P)H oxidase activity in cultured mesangial cells. Med Chem. 2005;1:461–466. doi: 10.2174/1573406054864052. [DOI] [PubMed] [Google Scholar]
- 90.Shaw S, Wang X, Redd H, Alexander GD, Isales CM, Marrero MB. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. J Biol Chem. 2003;278:30634–30641. doi: 10.1074/jbc.M305008200. [DOI] [PubMed] [Google Scholar]
- 91.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–608. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 92.Hasan RN, Schafer AI. Hemin upregulates Egr-1 expression in vascular smooth muscle cells via reactive oxygen species ERK-1/2-Elk-1 and NF-kappaB. Circ Res. 2008;102:42–50. doi: 10.1161/CIRCRESAHA.107.155143. [DOI] [PubMed] [Google Scholar]
- 93.Fujioka S, Niu J, Schmidt C, Sclabas GM, Peng B, Uwagawa T, Li Z, Evans DB, Abbruzzese JL, Chiao PJ. NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol. 2004;24:7806–7819. doi: 10.1128/MCB.24.17.7806-7819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amiri F, Garcia R. Regulation of angiotensin II receptors and PKC isoforms by glucose in rat mesangial cells. Am J Physiol. 1999;276:F691–F699. doi: 10.1152/ajprenal.1999.276.5.F691. [DOI] [PubMed] [Google Scholar]
- 95.Tsiani E, Lekas P, Fantus IG, Dlugosz J, Whiteside C. High glucose-enhanced activation of mesangial cell p38 MAPK by ET-1, ANG II, and platelet-derived growth factor. Am J Physiol Endocrinol Metab. 2002;282:E161–E169. doi: 10.1152/ajpendo.2002.282.1.E161. [DOI] [PubMed] [Google Scholar]
- 96.Tsubouchi H, Inoguchi T, Sonta T, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Statin attenuates high glucose-induced and diabetes-induced oxidative stress in vitro and in vivo evaluated by electron spin resonance measurement. Free Radic Biol Med. 2005;39:444–452. doi: 10.1016/j.freeradbiomed.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 97.Yoshimura A, Inui K, Nemoto T, Uda S, Sugenoya Y, Watanabe S, Yokota N, Taira T, Iwasaki S, Ideura T. Simvastatin suppresses glomerular cell proliferation and macrophage infiltration in rats with mesangial proliferative nephritis. J Am Soc Nephrol. 1998;9:2027–2039. doi: 10.1681/ASN.V9112027. [DOI] [PubMed] [Google Scholar]
- 98.Hattori M, Nikolic-Paterson DJ, Miyazaki K, Isbel NM, Lan HY, Atkins RC, Kawaguchi H, Ito K. Mechanisms of glomerular macrophage infiltration in lipid-induced renal injury. Kidney Int Suppl. 1999;71:S47–S50. doi: 10.1046/j.1523-1755.1999.07112.x. [DOI] [PubMed] [Google Scholar]
- 99.Kolavennu V, Zeng L, Peng H, Wang Y, Danesh FR. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes. 2008;57:714–723. doi: 10.2337/db07-1241. [DOI] [PubMed] [Google Scholar]
- 100.Yoshimura A, Nemoto T, Sugenoya Y, Inui K, Watanabe S, Inoue Y, Sharif S, Yokota N, Uda S, Morita H, et al. Effect of simvastatin on proliferative nephritis and cell-cycle protein expression. Kidney Int Suppl. 1999;71:S84–S87. doi: 10.1046/j.1523-1755.1999.07121.x. [DOI] [PubMed] [Google Scholar]
- 101.Ni W, Egashira K, Kataoka C, Kitamoto S, Koyanagi M, Inoue S, Takeshita A. Antiinflammatory and antiarteriosclerotic actions of HMG-CoA reductase inhibitors in a rat model of chronic inhibition of nitric oxide synthesis. Circ Res. 2001;89:415–421. doi: 10.1161/hh1701.096614. [DOI] [PubMed] [Google Scholar]
- 102.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39:245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 103.Yano M, Matsumura T, Senokuchi T, Ishii N, Murata Y, Taketa K, Motoshima H, Taguchi T, Sonoda K, Kukidome D, et al. Statins activate peroxisome proliferator-activated receptor gamma through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circ Res. 2007;100:1442–1451. doi: 10.1161/01.RES.0000268411.49545.9c. [DOI] [PubMed] [Google Scholar]
- 104.Zoja C, Corna D, Rottoli D, Cattaneo D, Zanchi C, Tomasoni S, Abbate M, Remuzzi G. Effect of combining ACE inhibitor and statin in severe experimental nephropathy. Kidney Int. 2002;61:1635–1645. doi: 10.1046/j.1523-1755.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- 105.Zoja C, Corna D, Camozzi D, Cattaneo D, Rottoli D, Batani C, Zanchi C, Abbate M, Remuzzi G. How to fully protect the kidney in a severe model of progressive nephropathy: a multidrug approach. J Am Soc Nephrol. 2002;13:2898–2908. doi: 10.1097/01.asn.0000034912.55186.ec. [DOI] [PubMed] [Google Scholar]
- 106.Qin J, Zhang Z, Liu J, Sun L, Hu L, Cooper ME, Cao Z. Effects of the combination of an angiotensin II antagonist with an HMG-CoA reductase inhibitor in experimental diabetes. Kidney Int. 2003;64:565–571. doi: 10.1046/j.1523-1755.2003.00127.x. [DOI] [PubMed] [Google Scholar]