Abstract
There is a large body of evidence that Cognitive Behavioral Therapy for insomnia (CBT) is an effective treatment for persistent insomnia. However, despite two decades of research it is still not readily available, and there are no immediate signs that this situation is about to change. This paper proposes that a service delivery model, based on “stepped care” principles, would enable this relatively scarce healthcare expertise to be applied in a cost-effective way to achieve optimal development of CBT services and best clinical care. The research evidence on methods of delivering CBT, and the associated clinical leadership roles, is reviewed. On this basis, self-administered CBT is posited as the “entry level” treatment for stepped care, with manualized, small group, CBT delivered by nurses, at the next level. Overall, a hierarchy comprising five levels of CBT stepped care is suggested. Allocation to a particular level should reflect assessed need, which in turn represents increased resource requirement in terms of time, cost and expertise. Stepped care models must also be capable of “referring” people upstream where there is an incomplete therapeutic response to a lower level intervention. Ultimately, the challenge is for CBT to be delivered competently and effectively in diversified formats on a whole population basis. That is, it needs to become “scalable”. This will require a robust approach to clinical governance.
Citation:
Espie CA. “Stepped care”: A health technology solution for delivering Cognitive Behavioral Therapy as a first line insomnia treatment. SLEEP 2009;32(12):1549-1558.
Keywords: Insomnia, psychological treatment, cognitive behavior therapy, primary care, population
THERE IS COMPELLING EVIDENCE THAT COGNITIVE BEHAVIORAL THERAPY (CBT) IS A LASTINGLY EFFECTIVE TREATMENT FOR CHRONIC PRIMARY INSOMNIA, and mounting evidence that it is similarly effective for persistent insomnia associated with medical or psychiatric disorders.
The challenge for CBT is no longer to prove its credentials, but to punch its weight. For at least a decade, CBT should have been a contender as the treatment of first choice for insomnia. In reality, however, it has had very little impact on the high volume of insomnia patient care. Indeed, it has amounted to little more than a patchy cottage industry.
This is not a criticism of individual professionals, or of groups of practitioners, or of local service initiatives. People are doing their best; as indeed we have tried to do in Scotland. Rather, it is stark recognition that the challenge for CBT with respect to delivery is very much greater than so far envisaged.
The argument in this paper is that insomnia constitutes an international public health problem. As such, it needs to be addressed systemically not just clinically; that is at the level of care organisation. Prevalence and morbidity data alone demonstrate that CBT would need to be scaled up enormously if it were to address population need. This will not happen soon, or at all, unless new horizons are scanned. There needs to be serious engagement with models that are capable of supporting regional, national and international CBT service delivery.
The principal aim of this paper is to open debate on one such model, based upon the “stepped care” approach. Before doing so, a brief review of CBT is presented to reflect upon its evidence base and its suitability as a “health technology” for adaptation to stepped care.
The Efficacy of CBT
Nine systematic reviews or meta-analyses of CBT have been published in the past 15 years.1–9 To take two examples, the American Academy of Sleep Medicine [AASM (formerly the American Sleep Disorders Association of Sleep)] taskforce reports (1999 and 2006)4,8 comprised 85 clinical trials (4,194 participants), and indicated that CBT was associated with improvement in 70% of patients, that was sustained at least 6 months post-treatment. Importantly, there is growing evidence not only that sleep parameters improve, but also reports of daytime functioning. These clinical and generalized benefits reflect moderate to large standardised effect sizes (ES). Moreover, the 2006 review included 12 trials on insomnia associated with medical or psychiatric disorders, suggesting that CBT may be effective also in more complex populations.
Based on these data, AASM published practice parameters statements10,11 using standardized appraisal criteria12,13 endorsing the efficacy of stimulus control therapy, progressive muscular relaxation, biofeedback, paradoxical intention therapy, sleep restriction and two alternative multi-component CBT approaches. It is important then to note that CBT is a treatment modality, just as is sleep pharmacotherapy (PCT). The latter comprises a range of licensed medications, and the former a range of (seven) proven psychotherapeutic methods. Please also note that “sleep hygiene” is not one of these.
In routine practice, the overwhelming majority of insomnia patients is treated with PCT rather than CBT. In contrast to CBT, this is not evidence-based for chronic insomnia.5,9,14 There are no data to support the long-term resolution of sleep problems following either short-term or medium-term (up to 6 months) PCT, whereas the beneficial effects of CBT are known to persist for months or years after the treatment course is completed. For example, Morin et al. (1999) compared CBT, medication (temazapam), combined therapy, and a placebo control condition.15 All three active treatments produced short-term improvements in sleep, but the temazapam-only condition regressed to baseline during follow-up. By comparison, both groups treated with CBT exhibited good 12-month outcome, suggesting the durable efficacy of CBT relative to PCT. Recently, Sivertsen et al. (2006) reported that CBT was associated with greater benefit than zopiclone.16 CBT was associated with a 10% increase in polysomnographic sleep efficiency (SEFF) at post-treatment and 6-month follow-up, relative to no reliable change with zopiclone.
Based on the published evidence, the National Institutes of Health Consensus and State of the Science Statement (2005) concluded that CBT is “as effective as prescription medications are for short-term treatment of chronic insomnia. Moreover, there are indications that the beneficial effects of CBT, in contrast to those produced by medications, may last well beyond the termination of active treatment” (page 14).14
The Clinical Effectiveness of CBT
For any treatment to become a “gold standard” in routine care it is important to have effectiveness data as well as efficacy data. Effectiveness studies speak to the generalizability of beneficial effects to the population at large,17 and although research of this type is limited for CBT (as it is for PCT), there are indications that CBT may be effective across a range of presenting populations.
For example, a comparative meta-analysis, comparing CBT outcomes in middle-aged and older adults (55 years plus), reported moderate to large ES, regardless of age, in sleep onset latency (SOL) and wake time after sleep onset (WASO).7 Consistent with this, AASM also recommend CBT as a standard treatment for insomnia in older adults.11 Likewise, a recent randomized controlled trial has found that CBT is clinically helpful in depressed patients with co-morbid insomnia. Manber et al (2008) compared citalopram + CBT with citalopram + “sham” CBT, and reported significant benefits not only to sleep but to depression itself, reflected in an ES difference of 0.24 in favour of citalopram + CBT on the Hamilton Rating Scale for Depression.18
We have recently reported on the effectiveness of CBT in post-cancer care19; reinforcing the earlier findings of Savard et al.20 that CBT is effective in insomnia associated with cancer. Indeed, much of our work in Glasgow has been in the clinical effectiveness tradition, enrolling relatively unselected patients into our trials programme. We have randomized 490 patients across 3 clinical trials of CBT versus treatment as usual,19,21,22 and have not found any consistent pattern of demographic or clinical predictors of poor response to CBT. In other words, our findings support the effectiveness of CBT, obtaining an approximate 70% treatment response regardless of severity or chronicity of presenting characteristics.23 This is consistent with other UK data showing that insomnia in chronic hypnotic users also responds well to CBT.24
The Challenge for CBT
All treatment modalities evolve over time as active elements are identified and refined, as new interventions are evaluated, and as efficacy data are replicated in clinical effectiveness paradigms. CBT methods are no exception. There always will be questions on treatment outcome to answer – but this is not the main challenge. At this point, doubts about CBT do not reside in its efficacy, nor even in its effectiveness, but in its feasibility. Can CBT really become a first line treatment for insomnia in everyday practice?
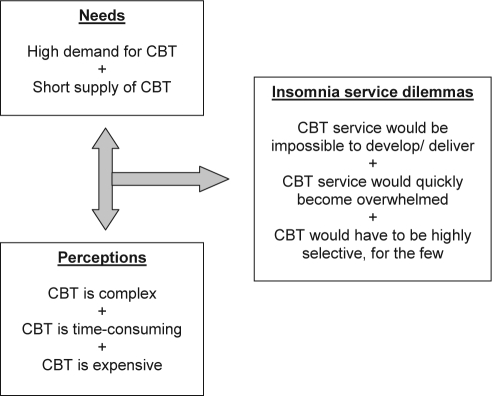
Figure 1 illustrates some of the dilemmas to be faced in considering how to offer CBT services to the population who may benefit from it. Can a CBT service delivery model be developed to cope with high volume of need, safely and effectively, whilst still being affordable?
Figure 1.
The CBT feasibility agenda.
Stepped Care as a Potential Vehicle for CBT Service Delivery
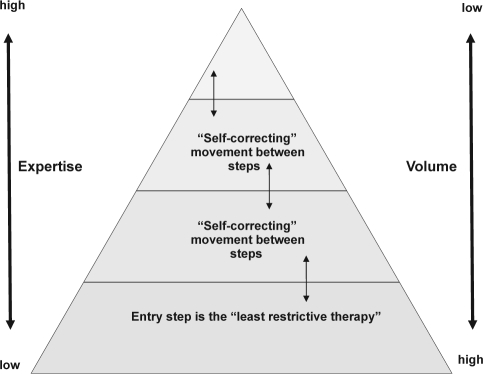
An “insomnia care pathway” is required if we are to prevent nascent CBT services from being strangled at birth. Such a pathway would fit well with a stepped care design solution. We have previously suggested how a CBT for insomnia stepped care “triage” system might work,22,64 as have others.65 Indeed, the potential of stepped care has been discussed at professional and scientific meetings for some time. Stepped care offers a generic approach to care management,25 and is often conceptualised as a pyramid. As can been seen in Figure 2, high patient volume is managed at the base of the pyramid using low intensity treatments, with progressively smaller volumes, and greater expertise in assessment and treatment, being concentrated towards the top step. Certainly in the UK, an important part of mental health service commissioning is focussing upon the urgent need to increase access to psychological therapies for common mental health problems, with stepped care being proferred as part of that solution.26
Figure 2.
A generic stepped care model illustrating incremental levels (steps) of intervention complexity. The most efficient service will ensure maximal throughput by stepping patients according to need, matching interventions to needs, and making best use of available expertise.
Crucial, to the successful and safe operation of stepped care, is that the level of intervention that people receive, either initially or subsequently, is not arbitrary; rather, it should reflect assessed need. Moreover, the number of steps in any stepped care model would be determined by the number of interventions or levels of intervention that are proven and available, and also by the upper limits of what within the healthcare system would be affordable.
Let us then consider criteria for selecting an “entry level” treatment within a stepped care system. At this level, represented by the base of the pyramid, the least restrictive therapy has to be identified (Figure 2). According to Bower & Gilbody (2005),25 this should be a:
readily accessible form of treatment,
provided at the lowest cost and
least personal inconvenience to patients, and
requiring the lowest treatment intensity and
the least specialist time.
Bower and Gilbody go on to suggest several important principles or assumptions underlying stepped care. These are summarized in Table 1.
Table 1.
Assumptions Underpinning the Stepped Care Model [adapted from Bower & Gilbody, 2005] 25
| 1. Equivalence assumption | Minimal interventions can provide “significant health gain” |
| 2. Efficiency assumption | Using minimal interventions reflects healthcare resource |
| 3. Acceptability assumption | Stepped care is acceptable to patients and to professionals |
First, this least restrictive therapy must satisfy what is known as the equivalence assumption. That is, despite being a minimal intervention, the entry level treatment must be evidence-based to provide significant health gain. That is, a deliverable and relatively inexpensive treatment that can benefit at least a substantial proportion of patients, but without risking adverse effects. So, for example, if a simple and easily administered benign treatment had been shown to lead to remission or clinical response, in say 30% of patients, it would be equivalent in outcome for these patients to any more complex, more time-consuming, or more expensive intervention that they might otherwise (unnecessarily) receive.
Second, and extending the above argument, the efficiency assumption supports the use of the minimal effective intervention at each step as a reality check upon the true (finite) availability of healthcare resources. That is, not only would it be effective to follow a stepped care approach, it would also be prudent. By investing resources across a spread of intervention steps, it is more likely that the maximum number of patients can be treated in an optimal way, within budget. Again, using the above example, the minimal intervention might meet the needs of say 30% of the patient population. Thus, it satisfies what would otherwise be a substantial demand on resources whilst actually consuming relatively little resource, and without detriment to care outcomes. At the whole population level, this helps to deliver population health gain because it provides effective treatment quickly to many, and conserves resources (time, expertise, funds) for those patients whose effective treatment will require greater resource.
Third, a stepped care service must be acceptable both to professionals and to patients. The acceptability assumption is addressed by capacity in the system both for an initial evaluation of need and for subsequent “self-correcting movement,” so that patients can shift between levels when this is deemed appropriate. Supposing an individual starts off at a given level, a decision might be made later about discharge or about stepping up to the next level, based upon their progress. This might be appraised through review of validated data collected on their condition (e.g Insomnia Severity Index27,28 scores against threshold criteria), or through clinician judgement that outcomes are or are not (yet) satisfactory. But also, the patient would be permitted the perspective that s/he may prefer to see someone with a high level of expertise and/or for longer, even though this might not appear to be necessary from an evidence-based perspective.
Stepped care models have proven useful for a wide range of disorders. It is beyond the scope of this paper to go into this in detail, but, for example, a good review of stepped care for chronic illness was published recently29 and stepped care for primary care patients with persistent depression has been encouraged for at least the past 10 years.30 It is noteworthy in this latter respect that three meta-analyses of brief behavioral interventions for depression have been published within the past couple of years.31–33
Is Insomnia a Suitable Disorder for Stepped Care?
There are four main reasons for considering insomnia to be a suitable condition for stepped care.
First, insomnia is very common.34,35 A problem that affects 20-25% of the adult population, and up to 10% on a chronic basis, requires a care pathway to be developed to manage it effectively. All practitioners and healthcare services operate on finite resources, hence the need to manage common problems both soundly and equitably.
Second, and related to the first, insomnia presents with varying levels of severity and complexity, from short-term troublesome symptom, through persistent primary disorder, to co-morbid disorder.36 The fact that persistent insomnia is an established risk factor for mental ill-health37,38 also suggests that judgement needs to be exercised to determine when, and at what level, a patient's insomnia should be addressed.
Third, there are pragmatic reasons to develop stepped care for the behavioral management of insomnia, not least of which is the shortage of skilled practitioners. There are relatively few people with sufficient expertise to treat the high volume of insomnia patients who might benefit from CBT. Some useful suggestions have been made about how numbers of behavioral sleep medicine specialists might be increased.39 However, the base number remains very low, even in the US where sleep services are better established.
Fourth, insomnia is typically a chronic disorder that does not necessarily remit.40 Certainly, there are everyday sequelae of insomnia, but these are seldom novel, acute, or inherently dangerous. Rather, the morbidities associated with persistent insomnia are longer term risks to health and well-being. Consequently, insomnia is a suitable disorder for stepped care, even at the entry level, because there may be few adverse consequences to providing a minimal intervention, even if a proportion of patients are initially non-responders. Besides, they would then be stepped up to a higher intensity treatment.
Finally, it should be noted that pharmacotherapy for insomnia also can be readily accommodated within a stepped care approach, ranging from herbal and other “medicinal” products that people purchase for themselves “over the counter” at one extreme, through primary care management, to expert prescription and review of licensed and “off label” medications. It is beyond the scope of this paper to discuss PCT in any detail, but it is important to acknowledge that the evidence-based treatment of insomnia at population level requires both CBT and PCT to be available and that, in practice, CBT and PCT may be offered to individuals in combination. Consideration of an insomnia stepped care pathway, therefore, does not intrinsically prejudice clinical practice or research study in one direction or the other.
Is CBT a Suitable Intervention for Stepped Care?
There are several compelling reasons for believing that CBT is an inherently suitable treatment modality for stepped care.
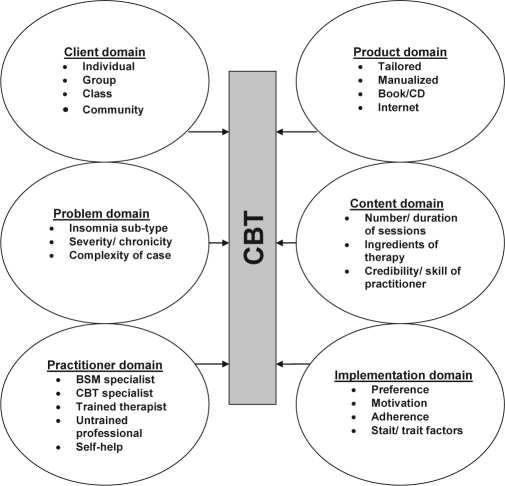
First, as psychotherapies go, CBT methods have proven to be remarkably adaptable, across a wide range of disorders.26 CBT is no exception. Indeed, Figure 3 illustrates that there are six domains of resource intensity that might be (co-) varied to yield treatment solutions. So, for example, one-to-one therapy that is tailored specifically to a patient's complex sleep needs over numerous sessions delivered by an expert psychologist trained in behavioural sleep medicine would represent the most resource intensive approach. This may be necessary for some patients, but probably not for very many. This is a crucial point. The great majority of insomnia patients need not, should not, and could not (possibly) be treated by such highly qualified individuals.
Figure 3.
Methods of delivery of CBT: Interacting domains reflect levels of resource intensity in CBT.
At the other extreme, a sizeable proportion of patients might derive significant benefit from a guided self-help book, from participation in a CBT class in a community college or sports centre, or through a web-based CBT portal. There is an insufficient evidence base at present to differentiate these precisely, or indeed other resource combinations that might be constructed, but the point is that CBT is intrinsically an adaptable health technology.
Second, although a limited analogy, it may be helpful to think of stepped care as a “dosing schedule,” whereby any given patient will require the necessary amount of intervention to respond (not less because it will not work, and not more because it will not add anything). “Dose” might be determined by the number of elements in the treatment, the number of sessions, the amount of personal tailoring of treatment, the qualifications and experience of the therapist, ….. and so on, as per the content of the domains in Figure 3. Consequently, entry level CBT should be the lowest dose proven to be associated with clinical improvement. Traditionally, a dose response is the relationship between the amount of exposure (dose) to a substance, and the resulting changes in body function or health (response). It has been suggested that the therapeutic session is the natural quantitative unit of psychotherapy. That is, the number of sessions is stochastically related to exposure to the active ingredients in any psychotherapy.41 Following this rationale, Edinger et al. (2007) have shown that a brief CBT (4 session) intervention is clinically effective in primary insomnia, but less so in co-morbid insomnia,42 implying that dosing may need to reflect complexity of presentation. Whatever the input is chosen as the dosing unit, and whatever the limitations of the analogy in general, it does seem that CBT would be a suitable intervention for stepped care also because there are numerous escalating characteristics that might be applied to its delivery (cf. Figure 3).
Third, CBT is also suitable for stepped care because, in the real world, people do make choices about treatment. In one elegant study, behavioral and pharmacological treatment scenarios were offered to insomnia patients who rated these in terms of their acceptability.43 Results showed that typical patient preference was for a behavioral treatment approach. We have recently replicated this preference for CBT in patients with psychophysiological insomnia and idiopathic insomnia. We have also demonstrated that some patients with idiopathic insomnia would be open to a (non-curative) acceptance-based approach to therapy.44 Importantly, people make choices not only about the type of treatment that they would prefer, but they also make behavioural choices about home implementation of treatments. Such motivational processes are cognitive-behavioral in nature, again indicating the suitability of CBT for insomnia. So, whereas the number of sessions offered or attended reflects an administration (exposure) dose of CBT provided to the patient, the “absorbed” dose taken by the patient reflects their home implementation/ adherence to the therapeutic instructions. We know, for example, that patients who achieve more consistent bedtimes and rising times have improved sleep efficiency 45 suggesting that application of advice is an important predictor of outcome. Likewise, it has been found in one study that only adherence explained variance in patients' post-treatment outcome.46 Indeed, the importance of choice is evident in that not all patients with insomnia even seek help through traditional clinical routes.47,48 Stepped care offers greater engagement with such issues by explicitly recognizing that acceptability is central to the way people behave (Table 1).
What would be the Entry Step for CBT that is Evidence Based?
In order to construct a stepped care model for CBT, it is of first importance to consider what would be the minimal intervention, known to provide significant health gain (Figure 2), that satisfies the assumptions summarized in Table 1.
Sleep Hygiene
In practice, the most common non-pharmacological approach is probably provision of sleep hygiene education, either verbally during consultation or by clinic leaflet. In terms of Figure 3, this represents self-help material, BUT with very limited CBT content either conceptually or therapeutically. Certainly it is unrestrictive (cf. Table 1), however, there is no evidence from trials data or from meta-analyses that sleep hygiene is an effective treatment. Indeed, sleep hygiene is not even mentioned in either the 2005 US NIH insomnia consensus statements, 14 or in the 2004 UK NICE technology appraisal on the management of insomnia.49 Furthermore, current AASM insomnia practice parameters state that “insufficient evidence is available for sleep hygiene education to be an option as a single therapy. Whether this therapy is effective when added to other specific approaches could not be determined from the available data” (p. 1417).11 Thus, the apparent proliferation of sleep hygiene advice as if it were a stand alone treatment, or even as an adjunct to PCT, is a practice that is independent of supporting data. Sleep hygiene does not meet the essential criteria for a minimally effective intervention offering significant health gain and so cannot be the first step within a stepped care CBT approach.
Manualized CBT
In contrast to sleep hygiene, an intervention “product” that does appear to be well evidenced is manualized CBT.4,8,36 This is where the practitioner follows a highly structured treatment protocol based upon therapeutically indicated CBT elements (content domain in Figure 3). The manual helps to ensure treatment fidelity, and also consistency in clinical practice. Manualized CBT has been found to be effective also across a range of client groups from primary insomnia to insomnia associated with medical or psychiatric disorder. The majority of published studies has utilized individual therapy, typically provided by a psychologist, but a substantial minority has adopted a group treatment approach.1–8
Our own approach involves CBT practitioners delivering manualized CBT over five, weekly, small group sessions.19,21,22 We have used nurses as therapists, for professional (e.g. familiar with protocols) and pragmatic (largest group of healthcare professionals) reasons. However, we believe that other staff (e.g. PSG staff, pharmacists) could deliver manualized CBT equally faithfully and effectively. This has yet to be formally evaluated. Taking our most recent trial as an example,19 standardised relative ES at post-treatment and at 6-month follow up for CBT were medium to large for SOL, WASO, and SEFF, with a small ES for total sleep time. Medium ES were also found for reductions in anxiety, depressive and fatigue symptoms, and for improvements in health-related quality of life, indicating that the benefits of CBT may go beyond sleep pattern per se.
There is then substantial evidence for manualized CBT1–8 and that it may be delivered in cost-effective ways.19,21,22,50 Manualized CBT could be the entry level treatment in the stepped care model, but there may be a step below this.
Self-administered CBT
“Self-help” is a rapidly growing area within mental health, and consistent with this, bibliotherapy, CD-ROM, televised media and internet-delivered CBT methods have now been reported.51 Whereas the first published paper on self-administered behavioral treatment for insomnia appeared 30 years ago,52 it is only relatively recently that such approaches have become a focus of systematic research investigation.
A meta-analysis of 10 studies enrolling a total of 1,000 participants concludes that minimal contact (e.g., telephone support, brief appointments) or entirely self-directed (e.g., books/ booklets, audiovisual, internet) CBT yields small to medium ES.53 Thus, there appears to be significant population benefit, but the effects are weaker than in individual or group treatment. Consistent with this conclusion are data from formal comparisons of self-administered vs. “face to face” CBT, where the latter has generally shown superior outcomes, although self-help was also beneficial.54–56 Internet-delivered CBT is relatively novel. Three randomized trials have been reported to date57,58,61 and results so far are encouraging, particularly given the potential of the web to reach extremely large populations
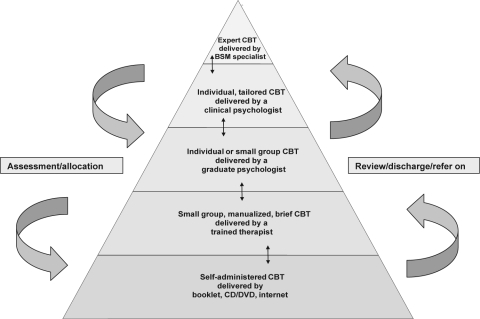
Therefore, taking all the evidence on CBT into account, it seems reasonable to conclude that self-administered CBT offers the least restrictive entry based treatment that has satisfactory outcome data, with nurse-delivered small group CBT providing the second level (Figure 4).
Figure 4.
An evidence-based stepped care model for CBT (c. 2009) illustrating how patients might be allocated to resources in relation to assessed need, to achieve optimal service provision. Arrows represent self-correcting referral movements.
What Might Constitute the CBT Stepped Care Hierarchy?
The domains in Figure 3 should assist researchers to consider how resource intensity of CBT might be modelled in healthcare services and in future outcome studies. From the present literature, we know that nurses, graduate psychologists, experienced clinical psychologists, and expert clinicians have all been involved in CBT trials, and that there is an emerging range of delivery methods. Figure 4 is proposed as an exemplar of what evidence-based stepped care might look like, circa 2009. It also parallels contemporary pathways for other common mental disorders.26,59
Working on this occasion from top down, at the top of the hierarchy, we might have the most experienced specialists, with the relatively rare combination of CBT and Behavioral Sleep Medicine skills, working with individual complex cases, perhaps on a tertiary referral basis at a sleep centre. At the next level, clinical psychologists might take on individual CBT patients. Indeed, there may be considerable untapped potential for qualified psychologists who already have extensive generic training in CBT methods to extend their expertise to include insomnia disorders. Below this, a less experienced graduate student might provide individual therapy or a flexible version of group CBT where the manual could be tailored in parts to the presenting needs and problem formulation. A strictly manualized group CBT programme led by a nurse (or other professional) would then sit at the level below, and immediately above the proposed entry level of a self-administered form of CBT. The three lowest tiers might best be provided in primary care, although there is an argument also for the next again (fourth) level also being in primary care. This may depend on the nature of local services.
How would people be allocated and move between levels of the stepped care CBT system?
In the general description of stepped care, presented earlier, the equivalence assumption would suggest that people should be allocated initially to an entry level treatment, providing they are content to accept that, and that there is no specific evidence that they would be unlikely to respond and no contraindication in terms of risk. This approach places emphasis upon skilled assessment, and herein lies a potential problem — of creating a bottle-neck of (more) people waiting to have their needs evaluated, and so waiting (even longer) for treatment.
Therefore, for a stepped care system to work on a large scale it would be necessary for the healthcare system to settle upon a range of common, validated, and simple tools for initial assessment of the insomnia complaint, and of associated complaints. There is a number of such self-report scales available, as reported earlier. Indeed, consensus opinion on these is available. Likewise, a standard post-intervention assessment should be used to determine what, if any, next steps are required, coupled of course with clinical judgement.
In circumstances where a patient is subsequently “stepped up” to a more intensive intervention, particular attention would need to be paid to their expectations (which may be negative if they have already failed to respond), and to their prior knowledge (which on the other hand could be built upon). Crucially, limited or no response may indicate that the previous assessment missed something important (e.g., associated disorders), underestimated the severity of the insomnia and the “level” of CBT required to address it, misjudged the acceptability of a CBT approach, or that the primary therapeutic challenge now is that of motivation and adherence to CBT rather than CBT per se.
All of these factors indicate that iterative assessment and review is the key to the success of the stepped care system (Figure 4) and that the standardisation of these assessment processes would benefit not only consistent clinical practice but also the pooling on a large scale of research data to evaluate benefit.
The Evidence Base for Clinical Governance in CBT
All services offered to patients must be safe, effective and ethical. It is normal practice to have policies and procedures on how referrals are made, how cases are allocated, how and when to review progress, and how to end episodes of care or to commence new episodes. Likewise, it is incumbent on all professional groups to know, and to work within, the boundaries of their training and their clinical competence, and to maintain their skills for (re-)accreditation purposes. In a stepped care system it is particularly important to raise issues of clinical governance because of the relatively fluid nature of the boundaries between the steps.
At the time of writing, from an evidence-based perspective, about all we can say is that clinical leadership in CBT services can be safely and effectively provided by clinical psychology professionals. This conclusion is underpinned by the summary of points provided in Table 2, and by the available data.
Table 2.
Reasons for Suggesting that Clinical Leadership in CBT Should Normally Lie with Psychology
| 1. CBT is essentially psychological practice |
| 2. CBT is based on psychological theory – principles |
| 3. Psychologists have developed CBT – will continue to develop new CBT interventions |
| 4. Psychologists are trained to use advanced CBT with complex cases |
| 5. Psychologists are qualified to deliver CBT methods that are not yet invented |
| 6. Psychologists have extensive mental health expertise |
| 7. Psychologists are trained behavioural scientists |
| 8. The published literature on CBT is almost exclusively from psychology research groups |
| 9. Psychologists are trained as psychometricians and are trained in systemic working |
| 10. Psychologists are developing competency frameworks for CBT methods generally |
In brief, CBT is undeniably a psychological theory and therapy, the published evidence for which has been delivered almost exclusively by psychology-led research.1–8,53 In this respect, it may be important to clarify that, in Glasgow, our nurse therapists work entirely under the training, direction and supervision of the psychology team. Indeed, this actually demonstrates how a stepped care system can work, because the role of the psychologist is in assessment, triage, case management and review, and clinical service development, and not only in direct patient care. This is a fairly direct parallel with the “medical model”; clinical responsibility in our setting rests with the supervisor of the CBT programme, the clinical psychologist. Table 2 also highlights that clinical psychology professionals are trained mental health professionals, with generic skills in CBT methods (and other psychosocial interventions). Importantly, therefore, they are qualified to administer psychological therapies that are not yet invented, just as physicians are (already) licensed to prescribe new medicines that come on the market. Psychologists are also the people most likely to develop, instrument and operationalize novel psychological interventions because they are doctorally qualified behavioral scientist-practitioners.
None of this is to say that other professionals do not have generic psychological competencies or specific CBT competencies (and certainly they could develop them), but the only evidence that we have currently available is that CBT is effective in contexts where it is practised by psychologists or under close psychology supervision. It is noteworthy here that there are ongoing efforts being made to develop generic competency frameworks for different levels of CBT practice, that would be open to any practitioner.59,60
In consequence of these matters relating to clinical governance, it seems appropriate to assert that professional leadership in behavioral aspects of sleep is essential to safe, effective, and comprehensive clinical practice in sleep medicine. It is recommended, therefore:
that the appointment of a suitably qualified and experienced clinical psychologist with behavioral sleep medicine credentials should become an essential criterion for the accreditation of sleep centres;
that the roles of this lead person should comprise a) advanced clinical practice, b) staff training and clinical supervision, and c) service development;
that these roles are not restricted to insomnia, but extend to other sleep disorders where a cognitive-behavioral approach to care is appropriate and is evidence-based (e.g. adherence to treatments, parasomnias)
It has to be acknowledged that there are some tricky inter-professional issues that will have to be faced here; perhaps even intra-professional ones. This has been recognised both within the sleep field,39 and more generally.62 It is important to understand that, quite apart from whatever extant, or future, evidence tells us about who can deliver and who can supervise CBT, there will be other sources of influence. Clinical governance arrangements, line management structures, issues relating to pay and reimbursement, may all vary nationally and locally. It is wise to accept that there will be vested professional interests and that some of these will have no genuine evidence base. Consequently, consultation, negotiation, and above all, pragmatism, will be necessary to find a workable way ahead. Further research is certainly needed to inform such debate, particularly in relation to cost-effectiveness for the purposes of manpower planning.
Consequently a further recommendation is:
4. that work should be completed urgently to consider the advantages to patient care of developing an inter-disciplinary approach to behavioral sleep medicine practice. The knowledge-base, the theoretical orientations, and the competencies of the the various professional groups who might be involved should be seen as complementary, rather than competing, bearing in mind that cost-effective use of people's time and of other health care resource is of the utmost importance
Stepped Care and the Health Care System
The stepped care model helps to ensure that the right level of effective care can be provided to the many, not just to the few, and that in so doing, relatively scarce expertise is appropriately targeted. As we have seen, this economy of resources applies not only to direct treatment but also to clinical governance of the service. All of this seems both necessary and timely for CBT.63
Of course, each health care system will need to consider how it could implement a stepped care model. The application of stepped care is perhaps easiest to envisage in a publicly funded health care system, like the UK National Health Service, where services are funded entirely through tax revenue (from those eligible to pay income tax) and where ability to pay has no influence over the nature or quality of care received. Nevertheless, principles such as equitability of health care provision are central to most health philosophies. Coupled with the CBT growing evidence base for remission and recovery from sleep disorder, health services, whether publically funded or insurance-based, would do well to consider the population morbidity that is normally associated with persistent insomnia. Stepped care CBT may offer a feasible service model, enabling not only insomnia patients to be treated at the right level, but having sufficient “reach” that associated population health might improve. Indeed, the potential for such “down-stream” benefits may be important, persuasive arguments for encouraging some health care organisations (such as HMOs in the US) to invest in stepped care for insomnia. The development and persistence of insomnia is not good for people, who are then more likely to go on to develop (other) costly conditions that potentially reduce the profits of the health care industry. The early and effective treatment of insomnia may improve health and save money. Moreover, there is likely to be some cost saving associated with treating at the assessed level of need within stepped care, because the majority of patients may not need to be seen beyond the primary care level of intervention.
Conclusions and Future Directions
There is compelling evidence that CBT is effective in the treatment of chronic insomnia. Moreover it appears to be popular, safe and lastingly beneficial. In short, it has an excellent product profile, but it cannot be bottled. Consequently, CBT has made little impact on the numbers of people who might need it, want it, and benefit from it.
In this paper, a stepped care model has been proposed as a health technology solution for delivering CBT as a first line insomnia treatment. The advantages of this approach are a) that it helps to ensure that patients will receive the least complex and most accessible intervention from which they are likely to benefit; b) that the greatest number of people who need treatment may receive it; c) that scarce expertise and expensive resource is available to those in greatest need; and c) that an insomnia treatment pathway can ensure seamless transition from one level of care to another, through active clinical collaboration (e.g. amongst professionals, between primary and secondary care).
At the time of writing, self-administered CBT has accumulated sufficient evidence to suggest that it can provide the least restrictive minimal intervention for the stepped care platform. Where face to face therapy is concerned, small group manualized CBT offers the potential for trained health care staff to become critical to a new CBT workforce. The clinical leadership role of psychologists is clearly best evidenced, and crucially the stepped care model would see such expertise being spread across assessment, training, supervision and service management roles, as well as direct clinical care of complex cases at an advanced level.
A hierarchy comprising five levels of CBT stepped care has been proposed. It has to be acknowledged that this represents a blend of evidence and pragmatism. In particular, the second and third levels have some similarities. These levels are differentiated by the generic nature of the healthcare workforce (in primary care) who potentially might be recruited to deliver absolutely standardized CBT for insomnia as per manual (second level); versus the graduate psychologist (third level), who are less available and more highly trained (in psychology) and so better equipped to provide basic tailoring of standard CBT, thus raising the introduction of one-on-one therapy.
In looking ahead it will be important also to consider whether a distinction needs to be made between self-help (however effective) and publically funded/ reimbursed healthcare. What an individual chooses to do privately by way of self-help and self-improvement ultimately may, or may not, breach the threshold of organized healthcare delivery through professionally staffed services. Either way, the stepped care approach appears to be sufficiently flexible as a clinical triage system and as a resource allocation model to deliver appropriate care to people at their point of need. What ultimately will drive health care professionals to participate in a stepped care approach will be the interplay between a) much greater public health recognition of the medical, psychological and socio-economic imperatives relating to insomnia; b) the potential benefits to the patient group of taking a population based approach (access and effectiveness); and c) the professional advantages of participation (role clarification and reward).
Finally, it is clear that there is a considerable research agenda. This has to focus more on “phase four,” clinical effectiveness studies, capable of evaluating the insomnia health care system. This means not merely a simple allocation to group model, with analysis of comparative outcomes against “usual care,” but recognition that the assessment and review process is iterative. People will move (appropriately) between levels, so we will need to know the sensitivity/ specificity of the original, and subsequent, decisions about placement within the hierarchy, in terms of treatment response and recovery. Moreover, mediator and moderator variables (e.g. patient characteristics, treatment preference, format, setting, therapist factors) that we usually try to control for, or at best try to evaluate post hoc, need to become the primary focus of research endeavour if we are to know what best works for whom. Above all, if we were to reach the dizzy heights, that the availability of CBT were no longer an issue, the single and combined use of CBT and PCT would need to be evaluated pragmatically, in real world settings, in respect of both short and long-term outcomes at each level of the stepped care model.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Espie has participated in speaking engagements for Takeda, Lundbeck, and GlaxoSmithKline and is on the advisory board of Sanofi-Aventis, GlaxoSmithKline, Lundbeck, and Actelion.
ACKNOWLEDGMENTS
Research supported by Chief Scientist Office, Scottish Government Health Department, The Wellcome Trust, National Health Service Research & Development, Cancer Research UK, Dr Mortimer and Theresa Sackler Foundation.
This paper was first presented as an Invited Lecture at the 22nd Annual Meeting of the Associated Professional Sleep Societies, Baltimore, June 2008.
REFERENCES
- 1.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological Interventions for Insomnia - A Metaanalysis of Treatment Efficacy. American Journal of Psychiatry. 1994;151:1172–80. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 2.Murtagh DRR, Greenwood KM. Identifying Effective Psychological Treatments for Insomnia - A Metaanalysis. Journal of Consulting and Clinical Psychology. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 3.Pallesen S, Nordhus IH, Kvale G. Nonpharmacological interventions for insomnia in older adults: A meta-analysis of treatment efficacy. Psychotherapy. 1998;35:472–82. [Google Scholar]
- 4.Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. Sleep. 1999;22:1134–56. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- 5.Smith MT, Perlis ML, Park A, Smith MS, Pennington J, Giles DE, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. American Journal of Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+ Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003161. CD003161. [DOI] [PubMed] [Google Scholar]
- 7.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+years of age. Health Psychology. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 8.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 9.Riemann D, Perlis ML. The treatments of chronic insomnia: A review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Medicine Reviews. 2009;13:205–214. doi: 10.1016/j.smrv.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Chesson AL, Anderson WM, Littner M, Davila D, Hartse K, Johnson S, et al. Practice parameters for the nonpharmacologic treatment of chronic insomnia. Sleep. 1999;22:1128–33. doi: 10.1093/sleep/22.8.1128. [DOI] [PubMed] [Google Scholar]
- 11.Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine Report. Sleep. 2006;29:1415–19. [PubMed] [Google Scholar]
- 12.Sackett DL. Rules of Evidence and Clinical Recommendations. Canadian Journal of Cardiology. 1993;9:487–89. [PubMed] [Google Scholar]
- 13.Eddy D. A manual for assessing health practices and designing practice policies: the explicit approach. Philadelphia: American College of Physicians; 1992. [Google Scholar]
- 14.NIH Consens Sci Statements; NIH State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults; Jun 13-15; 2005. pp. 1–30. [PubMed] [Google Scholar]
- 15.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia - A randomized controlled trial. Jama-Journal of the American Medical Association. 1999;281:991–99. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 16.Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults - A randomized controlled trial. Journal of the American Medical Association. 2006;295:2851–58. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 17.Clarke GN. Improving the Transition from Basic Efficacy Research to Effectiveness Studies - Methodological Issues and Procedures. Journal of Consulting and Clinical Psychology. 1995;63:718–25. doi: 10.1037//0022-006x.63.5.718. [DOI] [PubMed] [Google Scholar]
- 18.Manber R, Edinger JD, Gress JL, Pedro-Salcedo MGS, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. Journal of Clinical Oncology. 2008;26:4651–58. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 20.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-bahavioural therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. Journal of Clinical Oncology. 2005;23:6083–96. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 21.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behaviour Research and Therapy. 2001;39:45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 22.Espie CA, MacMahon KMA, Kelly HL, Broomfield NM, Douglas NJ, Engleman HM, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30:574–84. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 23.Espie CA, Inglis SJ, Harvey L. Predicting clinically significant response to cognitive behavior therapy for chronic insomnia in general medical practice: Analyses of outcome data at 12 months posttreatment. Journal of Consulting and Clinical Psychology. 2001;69:58–66. doi: 10.1037//0022-006x.69.1.58. [DOI] [PubMed] [Google Scholar]
- 24.Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M. Psychological treatment for insomnia in the management of long-term hypnotic drug use: a pragmatic randomised controlled trial. British Journal of General Practice. 2003;53:923–28. [PMC free article] [PubMed] [Google Scholar]
- 25.Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency - Narrative literature review. British Journal of Psychiatry. 2005;186:11–17. doi: 10.1192/bjp.186.1.11. [DOI] [PubMed] [Google Scholar]
- 26.Department of Health. Commissioning a Brighter Future: Improving access to Psychological Therapies. Positive Practice Guide. Department of Health; 2007. [Google Scholar]
- 27.Morin CM. Insomnia: Psychological Assessment and Management. New York: The Guildford Press; 1993. [Google Scholar]
- 28.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Von Korff M, Glasgow RE, Sharpe M. ABC of psychological medicine - Organising care for chronic illness. British Medical Journal. 2002;325:92–94. doi: 10.1136/bmj.325.7355.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katon W, Von Korff M, Lin E, Simon G, Walker E, Unutzer J, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression - A randomized trial. Archives of General Psychiatry. 1999;56:1109–15. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 31.Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychological Medicine. 2008;38:611–23. doi: 10.1017/S0033291707001614. [DOI] [PubMed] [Google Scholar]
- 32.Gellatly J, Bower P, Hennessy S, Richards D, Gilbody S, Lovell K. What makes self-help interventions effective in the management of depressive symptoms? Meta-analysis and meta-regression. Psychological Medicine. 2007;37:1217–28. doi: 10.1017/S0033291707000062. [DOI] [PubMed] [Google Scholar]
- 33.Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: A meta-analysis. Clinical Psychology Review. 2007;27:318–26. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 35.Lichstein KL, Durrence HH RB, Taylor D, Bush A. The Epidemiology of Sleep: Age, gender, and ethnicity. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. [Google Scholar]
- 36.Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum publishers; 2003. [Google Scholar]
- 37.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? Journal of Affective Disorders. 2003;76:255–59. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 38.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: A systematic review and meta-analysis. American Journal of Psychiatry. 2003;160:1147–56. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 39.Perlis ML, Smith MS. How can we make CBT-I and other BSM services widely available? Journal of Clinical Sleep Medicine. 2008;4:11–13. [PMC free article] [PubMed] [Google Scholar]
- 40.Morin CM, Belanger L, LeBlanc M, Ivers H, Savard J, Espie CA, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–53. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 41.Howard KI, Kopta SM, Krause MS, Orlinsky DE. The Dose-Effect Relationship in Psychotherapy. American Psychologist. 1986;41:159–64. [PubMed] [Google Scholar]
- 42.Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: A randomized clinical trial. Sleep. 2007;30:203–12. doi: 10.1093/sleep/30.2.203. [DOI] [PubMed] [Google Scholar]
- 43.Morin CM, Gaulier B, Barry T, Kowatch RA. Patients Acceptance of Psychological and Pharmacological Therapies for Insomnia. Sleep. 1992;15:302–5. doi: 10.1093/sleep/15.4.302. [DOI] [PubMed] [Google Scholar]
- 44.Espie C. A., Barrie L. Does ‘acceptance’ have a role to play in managing Idiopathic Insomnia? A group comparison of beliefs, coping style and treatment acceptance in idiopathic insomnia and psychophysiological insomnia. Sleep. 2009;32(Abstract Supplement):A278. [Google Scholar]
- 45.Riedel BW, Lichstein KL. Strategies for evaluating adherence to sleep restriction treatment for insomnia. Behaviour Research and Therapy. 2001;39:201–12. doi: 10.1016/s0005-7967(00)00002-4. [DOI] [PubMed] [Google Scholar]
- 46.Vincent NK, Hameed H. Relation between adherence and outcome in the group treatment of insomnia. Behav Sleep Med. 2003;1:125–39. doi: 10.1207/S15402010BSM0103_1. [DOI] [PubMed] [Google Scholar]
- 47.Stinson K, Tang NKY, Harvey AG. Barriers to treatment seeking in primary insomnia in the United Kingdom: A cross-sectional perspective. Sleep. 2006;29:1643–46. doi: 10.1093/sleep/29.12.1643. [DOI] [PubMed] [Google Scholar]
- 48.Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Medicine. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 49.National Institute for Clinical Excellence (NICE) Guidance on the use of zaleplon, zolpidem and zopiclone for short-term management of insomnia. Vol. 77. London: National Institute for Clinical Excellence (NICE); 2004. p. 27p. [Google Scholar]
- 50.Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M. Psychological treatment for insomnia in the regulation of long-term hypnotic drug use. Health Technology Assessment. 2004;8:1–68. doi: 10.3310/hta8080. [DOI] [PubMed] [Google Scholar]
- 51.Currie S. Self-help therapies for insomnia. In: Watkins P, Clum G, editors. Handbook of self-help therapies. Routledge: Taylor & Francis Group, LLC; 2008. pp. 215–41. [Google Scholar]
- 52.Alperson J, Biglan A. Self-administered treatment of sleep onset insomnia and the importance of age. Behavior Therapy. 1979;10:347–56. [Google Scholar]
- 53.van Straten A, Cuijpers P. Self-help therapy for insomnia: A meta-analysis. Sleep Medicine Reviews. 2009;13:61–71. doi: 10.1016/j.smrv.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Bastien CH, Morin CM, Ouellet MC, Blais FC, Bouchard S. Cognitive-Behavioral Therapy for Insomnia: Comparison of Individual Therapy, Group Therapy, and Telephone Consultations. Journal of Consulting and Clinical Psychology. 2004;72:653–59. doi: 10.1037/0022-006X.72.4.653. [DOI] [PubMed] [Google Scholar]
- 55.Mimeault V, Morin CM. Self-help treatment for insomnia: Bibliotherapy with and without professional guidance. Journal of Consulting and Clinical Psychology. 1999;67:511–19. doi: 10.1037//0022-006x.67.4.511. [DOI] [PubMed] [Google Scholar]
- 56.Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of Two Behavioral Treatment Programs for Comorbid Geriatric Insomnia. Psychology and Aging. 2002;17:288–98. [PubMed] [Google Scholar]
- 57.Strom L, Pettersson R, Andersson G. Internet-Based Treatment for Insomnia: A Controlled Evaluation. Journal of Consulting and Clinical Psychology. 2004;72:113–20. doi: 10.1037/0022-006X.72.1.113. [DOI] [PubMed] [Google Scholar]
- 58.Vincent N, Lewycky S. Logging on for better sleep: RCT of the effectiveness of online treatment for insomnia. Sleep. 2009;32:807–815. doi: 10.1093/sleep/32.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Royal College of Psychiatrists & Royal College of General Practitioners. Psychological therapies in Psychiatry and Primary Care. London: Royal College of Psychiatrists; 2008. [Google Scholar]
- 60.Roth A. D., Pilling S. The competencies required to deliver effective cognitive and behavioural therapy for people with depression and with anxiety disorders. Department of Health. 2007 http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_078537. [Google Scholar]
- 61.Ritterband LM, Thorndike FP, Gonder-Frederick LA, Magee JC, Bailey ET, Saylor DK, Morin CM. Efficacy of an internet-based behavioral intervention for adults with insomnia. Archives of General Psychiatry. 2009;66:692–698. doi: 10.1001/archgenpsychiatry.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turpin G, Hope H, Duffy R, Fossey M, Seward J. Journal of Mental Health Workforce Development . 2006. Improving access to psychological therapies: implications for the mental health workforce; pp. 12–21. [Google Scholar]
- 63.Lamberg L. Despite Effectiveness, Behavioral Therapy for Chronic Insomnia Still Underused. Jama-Journal of the American Medical Association. 2008;300:2474–75. doi: 10.1001/jama.2008.719. [DOI] [PubMed] [Google Scholar]
- 64.Espie CA, MacMahon KMA, Kelly H-L, et al. Manualised CBT for insomnia delivered by nurse practitioners: results from studies conducted in primary care and in oncology. Sleep Med. 2005;6(Suppl 2):S29. [Google Scholar]
- 65.Edinger JD, Carney C. Behavioral treatment of insomnia. In: Kushida C, editor. Handbook of Sleep Disorders. 2nd edition. Taylor & Francis Inc.: New York; 2008. pp. 71–90. [Google Scholar]