Abstract
Study Objectives:
Sleep is known to enhance performance following physical practice (PP) of a new sequence of movements. Apart from a pilot study, it is still unknown whether a similar sleep-dependent consolidation effect can be observed following motor imagery (MI) and whether this mnemonic process is related to MI speed.
Design:
Counterbalanced within-subject design.
Setting:
The laboratory.
Participants:
Thirty-two participants.
Interventions:
PP, real-time MI, fast MI, and NoSleep (control) groups.
Measurements and Results:
Subjects practiced an explicitly known sequence of finger movements, and were assigned to PP, real-time MI, or fast MI, in which they intentionally imagined the sequence at a faster pace. A NoSleep group subjected to real-time MI, but without any intervening sleep, was also tested. Performance was evaluated before practice, as well as prior to, and after a night of sleep or a similar time interval during the daytime. Compared with the NoSleep group, the results revealed offline gains in performance after sleep in the PP, real-time MI, and fast MI groups. There was no correlation between a measure of underestimation of the time to imagine the motor sequence and the actual speed gains after sleep, neither between the ease/difficulty to form mental images and performance gains.
Conclusions:
These results provide evidence that sleep contributes to the consolidation of motor sequence learning acquired through MI and further suggests that offline delayed gains are not related to the MI content per se. They extend our previous findings and strongly confirm that performance enhancement following MI is sleep dependent.
Citation:
Debarnot U; Creveaux T; Collet C; Doyon J; Guillot A. Sleep contribution to motor memory consolidation: a motor imagery study. SLEEP 2009;32(12):1559-1565.
Keywords: Motor imagery, sleep, motor sequence learning, memory consolidation, mental chronometry
THERE IS NOW COMPELLING EVIDENCE THAT SLEEP CONTRIBUTES TO THE CONSOLIDATION PROCESS OF PROCEDURAL TYPES OF MEMORY,1–3 AND OF MOTOR-sequence learning in particular.4–9 The term memory consolidation refers to a poorly defined set of processes that convert an initial unstable memory representation into a more stable and effective form.10 More specifically, Stickgold and Walker10 also proposed that this term should refer to the automatic postencoding processing occurring without intent or awareness but not to those requiring conscious and behavioral rehearsal. Practically, researchers have reported the existence of delayed gains in performance using a sequential finger-tapping task after a night of sleep but not after a comparable time interval during daytime.6,11–13 These findings support the role of sleep in the offline processing or reprocessing of memories,10 and some authors have even suggested that physical practice (PP) should ideally be followed by sleep to ensure long-lasting storage of a newly acquired motor skill.5,8,14–16
By contrast, research investigating the possibility of sleep-related improvements in motor learning following mental practice with motor imagery (MI) has been almost nonexistent (see1, for an exception). Motor imagery is a dynamic state during which one mentally simulates an action without any concomitant body movement.16 Studies looking at this learning-enhancing technique have shown that MI and PP of motor tasks show several parallel outcome characteristics, as shown by their degree of similarity at the temporal, behavioral, and neural levels.18–22 Findings from these experiments have provided evidence that the time course of mentally simulated actions is correlated with that taken to execute the same movement. Second, the analysis of the autonomic nervous system activity shows similar responses during imagined and actual movements. Finally, brain-mapping techniques have shown that goal-directed actions, whether executed or imagined, recruit similar (albeit nonidentical) neural substrates. Practically, MI has been found to be effective in the rehabilitation of patients with neurologic disorders affecting the motor system.23–25 In an attempt to explore whether mental practice with MI can also elicit consolidation processes similar to those observed following PP, we have recently tested young healthy subjects who were required to imagine that they were performing a kinematic motor-adaptation task before and after a night of sleep.1 Interestingly, practice with MI produced a significant increase in the subjects' speed to complete the target-reaching task following sleep, suggesting that this physiologic state plays a role in the consolidation of newly learned adapted movements. Our interpretation of such findings was limited, however, by the fact that we could not exclude the possibility that performance gains were also partially due to the speed at which movements were performed during the MI condition. Indeed, all participants strongly underestimated the time needed to reach each target, i.e., they imagined doing the task faster than when they were physically performing the movements. Such a lack of temporal congruence between the actual and imagined movements is known to result in a significant decrease in movement time.26–27 Changing MI speed (voluntarily or not) is sufficient to elicit changes in the timing of actual movements, and it was therefore difficult to determine the influence of such effect, as compared with the contribution of sleep, to explain performance gains. Second, our previous study did not include any group that was trained and retested during the daytime, and, thus, it was not possible to determine whether the gain in performance following MI was sleep dependent or was also due to the simple passage of time.
The present study thus aimed to test further the hypothesis that mental practice of a motor sequence learning task with MI can elicit a consolidation process similar to that observed following PP of the same task. To do so, we used an adapted version of the sequential finger-tapping task first developed by Karni et al.28 This task was chosen because it is known that consolidation of this type of motor skill is sleep dependent,4,6,8 and because there is evidence of a temporal congruence between the physical and imagined conditions.29 Indeed, investigators have previously shown that the time necessary to imagine a sequence of finger movements does not differ from that needed to physically produce the same sequential movements29 and that MI is effective in improving the performance on this type of motor skill.30 In the present study, motor performance was evaluated before training, as well as before and after a night of sleep, or after a similar time interval without sleep during the daytime. Groups of young healthy participants were either asked to PP or to imagine an explicitly known motor sequence. Subjects in the MI condition were required to perform the sequence in real time (rtMI) or at a faster pace than usual (fMI) to control for possible effects of different mental representations on motor memory consolidation. Specifically, comparing 2 MI-speed conditions was a prerequisite to identify whether voluntary changes in MI speed play a specific and critical role in explaining performance gains. Last, a final NoSleep (control) group, in which subjects were trained and retested the same day (without any intervening sleep) after rtMI practice, was also included to test for the role of passage of time in consolidating this form of learning. We predicted that all groups would demonstrate a significant improvement in performance following the initial training session. Subjects in the PP, rtMI, and fMI conditions were also expected to show delayed gains in performance, whereas those in the NoSleep group were not. Finally, based on findings from our previous study,1 we hypothesized that greater offline delayed gains would be observed in the fMI group, compared with the rtMI group.
METHODS
Participants
A total of 32 healthy volunteers aged 20 to 35 years (mean age: 26.9 ± 3.5 years; 17 women) took part in this study. They were right handed, as assessed by the Edinburgh Handedness Inventory.31 They reported sleeping regularly between 7 and 9 hours per night, and extreme evening- and morning-type individuals, as well as regular nappers and smokers, were excluded. None had any prior history of drug or alcohol abuse or neurologic, psychiatric, or sleep disorders, and they were instructed to be drug, alcohol, and caffeine free for 24 hours prior to and during the experiment. Musicians and professional typists were excluded to avoid participants with previous experience on finger-tapping sequence tasks. This study was approved by the Research Ethics Committee of the University of Lyon, and all participants signed an informed consent form. The procedure used in this experiment was explained, and instructions regarding the motor task and questionnaires were given, although no information was provided about the objectives of the study or about the dependent variables of interest.
Design and Apparatus
Motor Tasks
A computerized version of the sequential finger-tapping task developed by Karni et al.28 was used to measure motor-sequence learning. This task was chosen because rapid changes in behavior performance are usually observed with practice and because it provides robust sleep-dependent consolidation effects.5,6,11 Moreover, Rodriguez and colleagues29 have shown that such types of physical and imagined motor sequences are, on average, performed at a similar pace. Participants were first asked to memorize a sequence of 8 moves using fingers 2 to 5 (2-4-3-5-3-4-2-5) until they were able to perform it explicitly from memory. The order of finger movements was pseudo-randomly selected such that each finger was used twice in a sequence. Subjects were requested to tap the sequence as fast and accurately as possible on a computer keyboard during periods lasting 30 seconds, while making as few errors as possible. Performance on each sequence task trial was validated by pushing the space bar of the computer to record the duration of each motor sequence. All key presses were recorded and averaged over the entire sequence using a home-made MATLAB-written routine. For each participant, this software compared the sequence of key presses produced by the participant with the correct sequence template to be performed, hence allowing detection of any discordance between the real and expected taps within the given sequence. Each 30-second period was then followed by a rest-period of 20 seconds. The number of correct sequences and the average speed used to perform each sequence constituted dependent variables of interest.
Sleep Characteristics and MI Abilities
All participants were first asked to fill out the Pittsburg Sleep Quality Index32 to assess sleep quality and quantity. This test was administered to exclude participants who were experiencing obvious disturbances during their sleep-wake cycles and to ascertain the participants' predisposition to benefit from the natural effects of sleep. Subjective measures of alertness and fatigue were also collected using the Stanford Sleepiness Score on the 2 days preceding the experiment. In regard to the individual imagery abilities, the revised version of the Movement Imagery Questionnaire33 was administered to measure the individual's ability to form kinesthetic and visual mental images. The latter consists of an 8-item self-report questionnaire in which subjects have to rate the vividness of their mental representation using two 7-point scales. The first series of items measures the individual's ability to form visual images (1 = very hard to see and 7 = very easy to visualize), whereas the second rates the ability to perceive the sensations usually elicited by the movement during kinesthetic imagery (1 = very hard to feel and 7 = very easy to feel). The subjects also filled out a recent revised version of the Vividness of Movement Imagery Questionnaire (VMIQ-234) to determine (on a 5-point scale) the clarity with which they were able to imagine movements and especially the difference between their capacity to use internal and external visual imagery.
Pretraining Session
The experiment was scheduled to begin at 20:00 (08:00 in the NoSleep group). As mentioned above, the participants were first asked to learn an explicitly known sequence of 8 finger movements using their nondominant hand. To familiarize themselves with the sequence, they were then given a few trials until they were able to physically perform 5 successive correct finger sequences. Following this introductory session, the pretraining session began and consisted of 4 practice blocks lasting 30 seconds each. Without visual feedback, participants had to repeat the sequence as fast and accurately as possible. To start each block, as well as after completing each MI and PP finger sequence, they were requested to push the space bar. At the end of the 30-second period, a 20-second countdown was automatically initiated on the computer screen before the next 30-second period. During this time lapse, the participants were explicitly asked not to imagine or physically execute the finger sequence. Hence, each 30-second period was systematically followed by a 20-second rest period, so that the pretraining session lasted 3 minutes altogether.
PP and MI Training
The NoSleep control group (n = 8) was subjected to MI training at approximately 08:00 and was retested on the same day at approximately 20:00. All of the other participants in the PP, rtMI, and fMI groups were first trained in the evening at approximately 20:00 and were retested the next morning at approximately 08:00. Subjects were randomly assigned to 1 of 3 experiment conditions (n = 8 in each group) that differed in the type of practice to be performed (PP, rtMI, or fMI). To ensure that the groups' performance during the learning and consolidation processes would not depend upon the individuals' imagery abilities, we verified that the individuals' Movement Imagery Questionnaire-revised and VMIQ-2 test scores did not significantly differ among the 4 groups. During practice, all participants were required to either actually perform or imagine the finger sequence during 12 blocks of 30 seconds each, which were separated by 20-second rest periods, for a total duration of 9 minutes. The NoSleep, as well as the rtMI and fMI groups, were required to imagine the motor sequence learning task using a combination of internal visual imagery and kinesthetic imagery, i.e., imagining movement from within one's body and perceiving the sensations of how it feels to perform the action. The participants rehearsed the finger sequence in a quiet room, without any environmental constraints, i.e., without distracting stimuli to facilitate focused attention on the formation of the mental images. An imagery script was read to the participants to ensure that they followed similar instructions throughout MI sessions (see Appendix). To prevent actual finger movements during MI, the participants were required to leave their left hand motionless on their right forearm and were asked to keep their eyes open to read the instructions on a computer screen. In both the rtMI and NoSleep groups, participants were requested to imagine producing the finger sequence at a pace that was similar to the duration of the motor performance during the pretraining session. To make sure that all subjects would follow such guidelines and to be able to record the duration of each sequence, they were asked to push the space bar with their right hand after imagining each motor sequence. By contrast, the fMI group was instructed to mentally increase the speed of their motor performance and, thus, to underestimate movement duration during mental practice. Finally, the PP group was required to physically perform the finger sequence for an equal amount of time, i.e., 12 periods of 30 seconds.
Posttraining and Retest Sessions
Two different posttraining test sessions were carried out to investigate the impact of MI and PP practice, as well as the sleep or wake-related effects on motor memory consolidation. The first posttraining test was conducted right after the training session and was similar to the pretraining session (i.e., four 30-second periods during which the participants were asked to actually perform the finger sequence as fast and accurately as possible). Individual debriefings were further scheduled in the NoSleep, rtMI, and fMI groups to investigate adherence to the MI instructions and to determine whether the participants encountered difficulty in forming mental images. Then, to control for MI accuracy, participants were asked to autoevaluate the quality of their mental images using a Likert-type scale (from 1 = poor mental representation to 6 = very accurate mental representation).
Finally, a second identical retest session was administered either following an 8-hour ( ± 1 h) night of sleep (the session began approximately 2 hours after waking up) or following a 12-hour daytime period in the NoSleep control group. Between the first and the second posttests, the participants were instructed to keep physical activity to a minimum.
Data Analysis
The dependent variables were the number of correct sequences and movement times. Imagined times were also considered to check whether the participants complied with the imagery guidelines, i.e., whether MI was adequately performed either in real time or at a faster pace. For the statistical analyses, we first checked that all of the data fit a normal distribution and that there was not any group difference during the pretraining test performance. Because no significant sex difference was found when comparing the number of correct sequences, as well as their average times, there was no need to take sex as an independent variable. Then, 2-way (Group × Session) analyses of variance for repeated measures were performed to compare the behavioral data in all groups. When the performance from 2 groups was compared directly, Student t tests followed by Bonferroni posthoc comparisons were carried out. Group scores on questionnaires were compared using Student paired t tests. Finally, the Pearson correlation test was used to assess a possible linear relationship between the temporal accuracy of MI during practice and the effect of sleep on behavior performance. The results are presented as mean (SEM), and a P level of less than 0.05 was considered critical for assigning statistical significance.
RESULTS
Questionnaires
The average sleep score, as measured by the Pittsburg Sleep Quality Index, was 2.8 (1.2), thus attesting to the “good quality” of sleep in all participants. There was no difference in the Stanford Sleepiness Score ratings between sessions or among groups. On the 7-point scale (1 = being most alert), mean values for the NoSleep group were 1.7 (0.7) during the first session and 1.6 (0.7) during the second session. The corresponding results in the PP groups were 1.6 (0.7) and 1.75 (0.8), 1.5 (0.53) and 1.75 (0.7) in the rtMI group, and 1.5 (0.53) and 1.3 (0.51) in the fMI group. With respect to sleep quality, the total sleep time was similar in all participants (8 h ± 1 h), and none of subjects had trouble sleeping. Mean (SEM) MIQ scores were 46 (2.3) in the PP group, 44.9 (4) in the rtMI group, 44 (2.5) in the fMI group, and 42.8 (2.4) in the NoSleep control group. As expected, visual scores were systematically higher than kinesthetic scores in all groups (t = 5.47, P < 0.001). No significant difference was found between the 3 MI groups, thus guaranteeing homogeneity in terms of individual ability to elicit motor mental images. Similarly, no difference was found when comparing the average VMIQ-2 scores in the 4 groups, i.e., 80.5 (3.4) in the PP group, 84 (7.2) in the rtMI group, 91 (5.13) in the MI group, and 79.9 (12.3) in NoSleep group. When considering the internal visual, external visual, and kinesthetic imagery scales, better external visual imagery scores were found, as compared with internal visual imagery (t = −2.56, P = 0.01) and kinesthetic imagery (t = −6.62, P < 0.001), whereas internal visual imagery scores were also better than kinesthetic imagery scores (t = −4.07, P < 0.001), in all participants (whole sample average scores [SEM] being 23.1 [1.38], 26.86 [1.61], and 36.45 [1.49] for the internal, visual, and kinesthetic imagery scales, respectively).
Behavior Data
There was no main effect of Group, or Group × Session interaction when comparing the mean number of correct finger sequences during the pretraining session, hence demonstrating that the 4 groups did not differ in their ability to learn the finger sequence. Similarly, there was no difference when considering the subjects' average movement time to produce the sequence. We also verified that all groups of participants reached an asymptotic level of performance during the initial training session, hence showing that performance was stabilized before moving to the posttraining session.
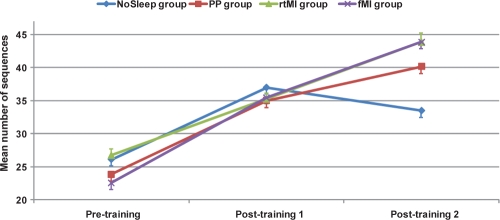
The average number of correct sequences during the pretraining session was 26.13 seconds (2.63) in the NoSleep group, 23.88 (4.86) in the PP group, 26.75 (1.87) in the rtMI group, and 22.63 (1.46) in fMI groups (Table 1), and they were 37 (4.31), 35 (6.05), 35.25 (1.73), and 35.5 (3.67) during the posttraining, and 33.5 (4.42), 40.13 (6.87), 43.88 (2.8), and 43.88 (5.38) during the retest sessions, respectively. A repeated-measure analysis of variance yielded a significant main effect of Session (F6,30 = 62.96, P < 0.001), as well as a significant Group x Session interaction (F6,30 = 2.9, P = 0.016). Accordingly, further Bonferroni posthoc analyses revealed that the PP group improved its performance from the pretraining to the posttraining session (t = 4.86, P = 0.002), as well as from the posttraining to the retest session (t = 5, P = 0.002) (see Figure 1). Similarly, the rtMI group showed a significant difference between the pretraining and the posttraining session (t = 5.4, P < 0.001), as well as between the posttraining and the retest session (t = 3.1, P = 0.02). Analyses of the fMI group showed that the subjects' performance was significantly different from that recorded during the posttraining (t = 4.2, P = 0.004) and retest sessions (t = 3.6, P < 0.01), providing further evidence of a significant effect of sleep. Finally, the NoSleep control group increased the number of correct sequences from the pretraining to the posttraining (t = 5.1, P < 0.001). By contrast, however, participants showed a slight tendency to make more errors during the retest session, although the difference did not reach significance (t = 1.15, P > 0.05).
Table 1.
The Number of Correct Sequences and Movement Speed in the 3 Groups During Pretraining, Posttraining, and Retesting Sessions
| Number of correct sequences |
Movement times |
|||||
|---|---|---|---|---|---|---|
| Pretraining | Posttraining | Retest | Pretraining | Posttraining | Retest | |
| PP group | 23.88 (4.86) | 35 (6.05)aa | 40.13 (6.87)a | 4.12 (.5) | 2.81 (.29)a | 2.5 (.29)a |
| rtMI group | 26.75 (1.87) | 35.25 (1.73)b | 43.88 (2.8)c | 3.3 (.25) | 2.47 (.15)b | 2.08 (.16)a |
| fMI group | 22.63 (1.46) | 35.5 (3.67)a | 43.88 (5.38)a | 3.78 (.22) | 2.66 (.22)b | 2.27 (.21)b |
| NoSleep group | 26.13 (2.63) | 37 (4.31)b | 33.5 (4.42) | 3.51 (.37) | 2.67 (.38)b | 3.07 (.39) |
Data are presented as mean (SEM). PP refers to physical practice; rtMI, real-time mental imagery; fMI, fast mental imagery.
P < 0.01
P < 0.001
P < 0.05
Figure 1.
Mean (SEM) number of correct sequences during the 3 experimental sessions.
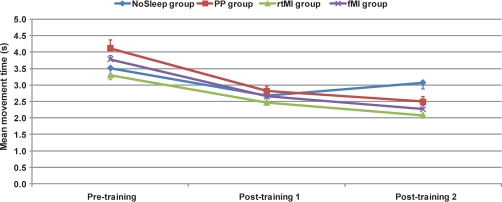
A similar pattern of results was observed when using average movement times as a dependent measure during the pretraining, mean times (SD) being 3.51 seconds (0.37) in the NoSleep group, 4.12 seconds (0.5) in the PP group, 3.3 seconds (0.25) in the rtMI group, and 3.78 seconds (0.22) in the fMI group (Table 1). An analysis of variance for repeated measures provided evidence of a significant Session effect (F6,30 = 79.46, P < 0.001), as well as a Group × Session interaction (F6,30 = 3.8, P = 0.003), but no main effect of Group. In the PP group, the participants decreased their movement times from the pretraining to the posttraining session (t = 4.16, P = 0.004), as well as from the posttraining to the retest session (t = 4.11, P = 0.004). Subjects in the rtMI group also increased their movement speed from the pretraining to the posttraining session (t = 5.96, P < 0.001), and from the posttraining to the retest session (t = 3.24, P = 0.014). Similar performance gains were observed in the fMI group (t = 6.46, P < 0.001 in pretraining vs posttraining, and t = 5.66, P < 0.001 in posttraining vs retest, respectively). Finally, and contrasting with the sleep-related effects in the other groups, the simple passage of time did not result in similar findings. Although the NoSleep control group (see Figure 2) logically decreased its average movement time from the pretraining to the posttraining following practice (t = 7.1, P < 0.001), the participants showed a slight tendency of being slower during the retest session (t = 1.85, P = 0.1).
Figure 2.
Mean (SEM) movement speed during the 3 experimental sessions.
Average MI times per sequence during the initial practice session were 4.07 seconds (0.3) in the NoSleep group, 3.44 seconds (0.3) in the rtMI group, and 2.99 seconds (0.25) in the fMI group. The results of an analysis of variance for repeated measures revealed a significant Group × Session interaction (F2,22 = 11.1, P < 0.001). Subsequent 2-by-2 comparisons demonstrated that the NoSleep and rtMI groups imagined doing sequences in a time that was not statistically different from the actual time to produce the sequence physically during the pretraining session. As expected, however, the difference reached significance in the fMI group (t = 4.05, P = 0.005), thus attesting that participants had increased their speed of movements in this MI condition.
Finally, there was no correlation between the subjects' extent of underestimation or overestimation of movement times during MI and both the number of correct finger sequences and the decrease in movement times following sleep. Likewise, the latter dependent variables were not correlated with the individual imagery ability, as measured by the imagery questionnaires.
Assessment of Imagery Use
First, no group difference was found when comparing the subjects' ratings in evaluating the vividness of their mental images during MI practice. The mean score of the NoSleep group was 4.3 (0.2), whereas it was 4.5 (0.3) in the rtMI group, and 4.3 (0.3) in the fMI group. Furthermore, during the debriefing following MI, all participants reported that they used the imagery type outlined in the scripts. They combined internal visual and kinesthetic imagery without switching to external visual imagery. None reported changing the imagery script to suit their individual needs, and all rehearsed the motor sequence as they were requested to do. Indeed, they were able to report each movement with an explicit knowledge of each key that they had to press in the PP condition.
DISCUSSION
The present study aimed to further investigate the sleep-related effects of mental practice with MI on memory consolidation of a newly learned sequence of movements. MI is known to impact the motor memory consolidation, in the same way that impairment in working memory might compromise the long-term retention of a skilled behavior with MI, hence hindering the ability to engage successfully in MI. Despite this, however, little was known in regard to the combined effect of sleep and MI on memory consolidation. In line with our previous study,1 the main finding is that a night of sleep following MI elicited delayed gains in performance on the finger sequence task, hence reflecting a significant offline consolidation process. By contrast, a comparable interval of time without intervening sleep did not result in any performance gains. Furthermore, data revealed that, except for the NoSleep group, participants in the 3 other groups significantly improved their accuracy and speed of finger movement during the retest session. Compared with the rtMI and the PP groups, however, offline performance gains were not greater in the fMI group.
As expected, and consistent with the motor learning literature, all participants improved their performance following the same amount of either physical or mental practice, i.e., during the posttraining session.35–36 When first looking at the offline motor memory consolidation following PP, the results of the present study thus revealed significant offline gains in performance after sleep. These findings are in line with those of other studies and provide further evidence that sleep contributes to the enhancement of motor performance, both in accuracy and speed.8,12–13,37 For instance, our results are consistent with those of Fischer et al.,5 who used a similar finger-sequence task and reported that the first night of sleep following training was crucial for delayed gains.
As well, and in keeping with our previous study,1 similar sleep-related effects were seen following MI practice, hence supporting the principle of functional equivalence between MI and motor performance.18 Furthermore, the simple passage of time was not sufficient to provide additional benefits in the NoSleep group, which stabilized its performance, albeit with a nonsignificant tendency to make more mistakes and to perform with lower velocity. This latter result reinforces the influence of sleep in offline motor memory consolidation, and suggests that the wake state per se is not sufficient to promote significant memory improvement. Indeed, the results of the NoSleep group, in which the participants engaged in rtMI during practice, revealed stabilization across periods of wake, as there was a slight, albeit not significant, decrease in performance. Though participants were instructed to avoid using MI during their wake state, it may not be totally excluded that explicit learning gave them the opportunity to mentally rehearse some or the whole sequence during the day.7 Despite this, however, the NoSleep group did not improve the performance during the retest session. Instead, the stabilization of their performance might suggest that a memory trace steadily became less susceptible to interference following rtMI practice, without the benefit of sleep.38 This type of time-dependent offline processing reduces the fragility of the explicit memory but does not support offline skill improvements. Overall, these results reinforce the idea that performance gains following MI are somewhat sleep dependent,1 thus assuming that a night of sleep after MI practice results in similar motor memory consolidation than following PP.
Compared with the PP and rtMI groups, the fMI group did not benefit to a greater extent from the sleep-related enhancement of motor performance. Despite the fact that subjects intentionally imagined the movement at a faster pace during training, the speed of actual performance was very similar to that of the rtMI group before and after the night of sleep. This finding was confirmed by the lack of correlation between the extent of the underestimation during MI and the actual speed gains after sleep. This pattern of results confirms that offline delayed gains are primarily due to sleep but not by the content of MI practice per se (and most especially, the speed of imagined movements). In other words, we may hypothesize that sleep-related effects on motor consolidation are a general process resulting in higher performance but that they do not impact 1 specific characteristic of the motor act, such as speed. Also, no correlation was found between the individual ability to perform accurate MI and delayed gains, hence suggesting that the moderate individual imagery vividness of the participants cannot be considered to be an influencing factor for motor-memory consolidation through sleep.
Interestingly, the results of the fMI group did not reveal greater performance, compared with the rtMI group, even though the subjects imagined the motor sequence at a significantly faster pace. This pattern of findings differs slightly from those reported by Boschker et al.26 and Louis et al.,27 who have shown that imagining performing the task faster than during PP might result in a significant decrease in movement time. Such a discrepancy, however, may be explained by the difference in the nature of the tasks, as our finger-sequence task was very different from the complex body sequences and sporting skills used in these 2 studies. Based on these findings, we postulate that the benefits in movement speed following acceleration of the MI speed are not systematic but, rather, task related. Further studies are therefore needed to investigate in greater detail whether intentional changes in MI speed do effectively result in different actual motor performance times and whether a change in speed using MI has any effect on the consolidation process.
From a neurophysiologic approach, sleep stages (slow wave sleep, REM sleep, Stage 2 sleep, etc.) are complex phenomena with only some of the underlying processes specifically linked to memory consolidation (e.g., spindles). Basically, neuroimaging experiments have revealed that fingers-tapping tasks are usually associated with increased time in Stage 2 sleep, and/or spindle density, these being observed in the prefrontal, frontal, central, and parietal regions but not in the occipital areas.37 Moreover, performance improvement in finger-sequence tasks following PP correlates with either an increased amount of Stage 2 sleep or spindle density across the night.16,39–40 Based on these findings, it is possible that sleep features following rtMI and/or fMI would demonstrate similar spindle generation, primarily located within the parietal region,3,37 which would result in offline consolidation of a new sequence of finger movements. Although interesting, this working hypothesis awaits further experimental investigation. The latter assumption is also in line with the great amount of research looking for clearer associations between sleep-dependent changes in the neuronal representation and behavior-output measures of memory consolidation.
To conclude, our findings confirm and expand on the sleep-related effects on motor memory consolidation following MI practice that we reported previously in Debarnot et al.1 These results further reinforce the principle of functional equivalence between MI and PP. They have strong theoretic and practical applications in both motor learning and (neuro)rehabilitation processes, in which performing MI is cost effective and easily feasible.19,41 Similar cerebral plasticity to that seen following PP of a motor task has been reported during MI.42 MI practice could therefore be incorporated during the classical course of physical therapy, and most especially before a period of sleep, to benefit from the offline motor consolidation during the recovery process. Second, because sleep-spindle activity is thought to play a role in motor memory consolidation by facilitating the neuronal plasticity, we think that further investigations that include recording polysmonographic data are needed to determine whether features of Stage 2 sleep are similarly modulated following MI practice. Likewise, consolidation might also be connected to NREM sleep and slow wave sleep. Hence, future research should determine the specific stages of sleep that are critical for discrete steps in motor-memory consolidation following MI. As for motor-skill consolidation,10 there may be more than a single phase of sleep-dependent consolidation.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
Appendix
The following guidelines were given in the imagery script: “attempt to imagine yourself doing the finger motor sequence with your eyes open by visualizing the different movements as if you had a camera on your head—you see and feel only what you would if you actually executed the sequence. Pay attention to each finger movement and make sure to respect the correct sequence by imagining it at the required speed (alternative for the fast imagery group: at a faster pace). Try to keep the same speed throughout the entire sequence. Just feel yourself going through the different steps of the sequential motor action, keeping in mind the correct sequence.”
REFERENCES
- 1.Debarnot U, Creveaux T, Collet C, et al. Sleep-related improvements in motor learning following mental practice. Brain Cogn. 2009;69:398–405. doi: 10.1016/j.bandc.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–82. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 3.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 4.Doyon J, Korman M, Morin A, et al. Contribution of night and day sleep vs. simple passage of time to the consolidation of motor sequence and visuomotor adaptation Learning. Exp Brain Res. 2009;195:15–26. doi: 10.1007/s00221-009-1748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc Natl Acad Sci USA. 2002;99:11987–91. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10:1206–13. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- 7.Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14:208–12. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 9.Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–84. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007:331–43. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci USA. 2003;100:12492–7. doi: 10.1073/pnas.2035019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer S, Nitschke M, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci. 2005;25:11248–55. doi: 10.1523/JNEUROSCI.1743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuriyama K, Stickgold R, Walker MP. Sleep-dependent learning and motor-skill complexity. Learn Mem. 2004;11:705–13. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karni A, Meyer G, Rey-Hipolito C, et al. The acquisition of skilled motor performance: fast and slow experience driven changes in primary motor cortex. Proc Natl Acad Sci USA. 1998;95:861–8. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stickgold R, LaTanya J, Hobson JA. Visual discrimination learning requires posttraining sleep. Nat Neurosci. 2000;2:1237–8. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 16.Walker MP, Stickgold R. It's practice, with sleep, that makes perfect: implications of sleep-dependent learning and plasticity for skill performance. Clin Sports Med. 2005;24:301–17. doi: 10.1016/j.csm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Jeannerod M. The representing brain: Neural correlates of motor intention and imagery. Behav Brain Sci. 1994;17:187–245. [Google Scholar]
- 18.Holmes PS, Collins DJ. The PETTLEP approach to motor imagery: A functional equivalence model for sport psychologists. J Appl Sport Psychol. 2001;13:60–83. [Google Scholar]
- 19.Guillot A, Collet C. Construction of the motor imagery integrative model in sport: A review and theoretical investigation of motor imagery use. Int Rev Sport Exerc Psychol. 2008;1:32–44. [Google Scholar]
- 20.Guillot A, Collet C, Nguyen VA, Malouin F, Richards C, Doyon J. Functional neuroanatomical networks associated with expertise in motor imagery ability. Neuroimage. 2008;41:1471–83. doi: 10.1016/j.neuroimage.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Murphy S, Nordin SM, Cumming J. Imagery in sport, exercise and dance. In: Horn TS, editor. Advances in sport psychology. Champagne, IL: Human Kinetics; 2008. pp. 306–15. [Google Scholar]
- 22.Munzert J, Lorey B, Zentgraf K. Cognitive motor processes: the role of motor imagery in the study of motor representations. Brain Res Rev. 2009;60:306–26. doi: 10.1016/j.brainresrev.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Sharma N, Pomeroy VN, Baron JC. Motor imagery: a backdoor to the motor system after stroke? Stroke. 2006;37:1941–52. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- 24.De Vries S, Mulder T. Motor imagery and stroke rehabilitation: a critical discussion. J Rehab Med. 2007;39:5–13. doi: 10.2340/16501977-0020. [DOI] [PubMed] [Google Scholar]
- 25.Dunsky A, Dickstein R, Markovitz E, Levy S, Deutsch JE. Home-based motor imagery training for gait rehabilitation of people with chronic poststroke hemiparesis. Arch Phys Med Rehab. 2008;89:1580–8. doi: 10.1016/j.apmr.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 26.Boschker MSJ, Baker FC, Rietberg MB. Retroactive interference effects of mentally imagined movement speed. J Sports Sci. 2000;18:593–603. doi: 10.1080/02640410050082305. [DOI] [PubMed] [Google Scholar]
- 27.Louis M, Guillot A, Maton S, Doyon J, Collet C. Effect of imagined movement speed on subsequent motor performance. J Mot Behav. 2008;40:117–32. doi: 10.3200/JMBR.40.2.117-132. [DOI] [PubMed] [Google Scholar]
- 28.Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez M, Llanos C, Gonzalez S, Sabate M. How similar are motor imagery and movement? Behav Neurosci. 2008;4:910–6. doi: 10.1037/0735-7044.122.4.910. [DOI] [PubMed] [Google Scholar]
- 30.Nyberg L, Eriksson J, Larsson A, Marklund P. Learning by doing versus learning by thinking: An fMRI study of motor and mental training. Neuropsychol. 2006;44:711–7. doi: 10.1016/j.neuropsychologia.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychol. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, Monk TH, Timothy H. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Hall C, Martin K. Measuring movement imagery abilities: A revision of the movement imagery questionnaire. J Ment Imagery. 1997;21:143–54. [Google Scholar]
- 34.Roberts R, Callow N, Hardy L, Markland D, Bringer J. Movement imagery ability: Development and assessment of a revised version of the vividness of movement imagery questionnaire. J Sport Exer Psychol. 2008;30:200–21. doi: 10.1123/jsep.30.2.200. [DOI] [PubMed] [Google Scholar]
- 35.Allami N, Paulignan Y, Brovelli A, Boussaoud D. Visuo-motor learning with combination of different rates of motor imagery and physical practice. Exp Brain Res. 2007;184:105–13. doi: 10.1007/s00221-007-1086-x. [DOI] [PubMed] [Google Scholar]
- 36.Song S. Consciousness and the consolidation of motor learning. Behav Brain Res. 2009;196:180–6. doi: 10.1016/j.bbr.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morin A, Doyon J, Dostie V, et al. Motor sequence learning increases sleep spindles and fast frequencies in posttraining sleep. Sleep. 2008;31:1149–56. [PMC free article] [PubMed] [Google Scholar]
- 38.Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–5. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- 39.Fogel SM, Smith C. Learning-dependent changes in sleep spindles and stage 2 sleep. J Sleep Res. 2006;15:250–5. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 40.Fogel SM, Smith C, Cote K. Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behav Brain Res. 2007;180:48–61. doi: 10.1016/j.bbr.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 41.Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke. 2007;38:1293–7. doi: 10.1161/01.STR.0000260205.67348.2b. [DOI] [PubMed] [Google Scholar]
- 42.Jackson PL, Doyon J, Richards CL, Malouin F. The efficacy of combined physical and mental practice in the learning of a footsequence task after stroke: a case report. Neurorehab Neur Rep. 2004;18:106–11. doi: 10.1177/0888439004265249. [DOI] [PubMed] [Google Scholar]