Abstract
Study Objectives:
(1) Compare sleep behaviors of children with autism spectrum disorders (ASD) with sleep behaviors of typically developing (TD) children using the Children's Sleep Habits Questionnaire (CSHQ); (2) compare sleep quality—defined as mean activity, sleep latency, number of awakenings, sleep efficiency and total sleep time—of the cohort of children with ASD and TD, as measured by 10 nights of actigraphy; and (3) estimate the prevalence of sleep disturbances in the ASD and TD cohorts.
Design:
Descriptive cross-sectional study.
Setting:
The Children's Hospital of Philadelphia.
Participants:
Randomly selected children from the Regional Autism Center. The ASD cohort of 59 children, aged 4 to 10 years, (26 with autism, 21 with pervasive developmental disorder-not otherwise specified [PDD-NOS], and 12 with Asperger disorder) were compared with 40 TD control subjects.
Measurements and Results:
The CSHQ, sleep diaries, and 10 nights of actigraphy using the Sadeh algorithm of children with ASD and TD control subjects were compared. CSHQ showed 66.1% of parents of children with ASD (62.5% autism, 76.2% PDD-NOS, 58.3% Asperger disorder) and 45% of parents of the control subjects reported that their children had sleep problems. Actigraphic data showed that 66.7% of children with ASD (75% autism, 52.4% PDD-NOS, 75% Asperger disorder) and 45.9% of the control subjects had disturbed sleep.
Conclusions:
The prevalence estimate of 45% for mild sleep disturbances in the TD cohort highlights pediatric sleep debt as a public health problem of concern. The prevalence estimate of 66% for moderate sleep disturbances in the ASD cohort underscores the significant sleep problems that the families of these children face. The predominant sleep disorders in the ASD cohort were behavioral insomnia sleep-onset type and insomnia due to PDD.
Citation:
Souders MC; Mason TBA; Valladares O; Bucan M; Levy SE; Mandell DS; Weaver TE; Pinto-Martin D. Sleep behaviors and sleep quality in children with autism spectrum disorders. SLEEP 2009;32(12):1566-1578.
Keywords: Autism spectrum disorder, insomnia, prevalence of sleep disturbances
THE AUTISM SPECTRUM DISORDER (ASD) IS ONE OF THE MOST DEVASTATING NEUROBIOLOGIC DISORDERS OF PRENATAL AND POSTNATAL BRAIN development.1 The triad of difficulties or core deficits of ASD include significant impairments in social interaction, limited communication, and a restricted repertoire of behaviors, interests, and activities.2 Sleep disturbances are endemic among children with ASD; studies have estimated the prevalence of sleep disturbance as 40% to 80% in children with ASD,3–6 compared with 20% to 40% in typically developing (TD) children.7–9 Sleep disturbances can have detrimental effects on children's cognitive development and daily functioning in areas such as attention, learning, memory, mood regulation, and behavior.10,11 These areas of cognition are among those impacted by the presence of an ASD, suggesting that sleep disturbances could further impede learning and general daily functioning in children with ASD. In addition, poor sleep in children with ASD has been shown to severely alter parents' sleep quality and add great stress.5,12,13
In a recent consensus statement, the National Sleep Foundation, in collaboration with Best Practice Project Management, Inc., identified children with ASD as one of the highest priority populations for sleep research.14 The core deficits of ASD, and their underlying neurophysiology and neurochemistry, may predispose children with ASD to intrinsic stressors that threaten sleep. However, basic epidemiologic data on sleep behaviors and sleep quality in this vulnerable population are insufficient. Previous studies often had multiple methodologic limitations, including lack of control groups, inclusion of subjects without confirmed ASD diagnoses, small sample sizes, use of non-validated questionnaires, and few objective measures. Because of the paucity of data, management options for these complex sleep disturbances in children with ASD are often limited. Consequently, health care providers and parents may struggle to manage sleep disturbances on a trial-and-error basis. Therefore, the purpose of this descriptive cross-sectional study was to characterize the sleep behaviors and sleep quality in a well-defined ASD cohort from a regional autism center and to estimate the prevalence of sleep disturbances in children with ASD, as compared with TD children.
The first aim of this study was to compare the subjective assessment of sleep behaviors of children with ASD (including autism, pervasive developmental disorder- not otherwise specified [PDD-NOS] and Asperger disorder) with TD children, as measured by the Children's Sleep Habits Questionnaire (CSHQ). We hypothesized that children with ASD would have more sleep problems, as indicated by higher scores on the total and subscale scores on the CSHQ. Our second aim was to objectively assess sleep quality in children with ASD and TD through 10 nights of actigraphy. We hypothesized that children with ASD would have lower sleep efficiency, increased sleep latency, greater number of awakenings, greater mean activity, and shorter total sleep time than TD children, as measured by actigraphy. Finally, our third aim was to estimate the prevalence of sleep disturbances in a cohort of children with ASD (including autism, PDD-NOS, and Asperger disorder) and a cohort of TD children. We hypothesized that the prevalence of sleep disturbances in the ASD cohort would be at least twice that of the TD cohort.
METHODS
Subject Recruitment and Selection
This study evaluates sleep behaviors and sleep quality in a cohort of children with ASD who receive care at the Regional Autism Center (RAC) at The Children's Hospital of Philadelphia (CHOP). The RAC provides a unique opportunity to enroll a population-based sample. There are approximately 5510 individuals with ASD (all ages) in Philadelphia and the 4 surrounding suburban counties, Delaware, Montgomery, Chester, and Bucks (2005 Census Bureau). These 5 counties are located at the tip of the southeastern region of Pennsylvania and contain approximately 25% of the total ASD population in the state. The RAC team has diagnosed ASD in more than 3000 children and adolescents, about half of whom reside in the southeastern region of the state. By randomly selecting the children from the RAC registry, we strove to obtain a representative sample of the Greater Philadelphia Area.
Children, aged 4 to 10 years, were randomly selected from the CHOP registry and divided into 3 subgroups: autism, PDD-NOS, and Asperger disorder. Selecting a random sample from the RAC enabled us to obtain an estimate of the prevalence of sleep disturbances. The TD children (aged 4-10 years) from the Greater Philadelphia Area who had no immediate family member with ASD were compared with the ASD cohort. The TD cohort was obtained from 3 referral sources: (1) neighbors of RAC families participating in the sleep study, (2) families who attended a health fair in a Delaware county community with a CHOP Kid's First Practice, and (3) friends of parents involved in Cure Autism Now, Philadelphia Chapter.
This study was approved by the Institutional Review Boards of CHOP and the University of Pennsylvania. During the first of 3 home visits, parent permission and child assent (when developmentally appropriate) was obtained. Children with ASD randomly selected from the RAC were matched by age, sex, and ethnicity to subjects from the TD cohort. A goal was to have a 25% minority representation in both cohorts, which is characteristic of the Greater Philadelphia Area. The target enrollment for the study was 16 children in each ASD subgroup (autism, PDD-NOS, and Asperger disorder) and 16 TD controls derived from a sample-size calculation using an α = 0.05, power of 0.80, 1:1 ratio, and an estimate of the expected prevalence of sleep disturbances of 20% to 40% in the TD cohort and 40% to 80% in the ASD cohort.
Inclusion Criteria
Children enrolled as cases and control subjects in the study met the following criteria: age between 4 and 10 years, stable medical and behavior conditions, and no change in medication or health status in the past 3 months. ASD diagnosis was made by a developmental pediatrician and confirmed by the Autism Diagnostic Observation Schedule (ADOS) or Asperger Syndrome Diagnostic Scale (ASDS) and DSM-IV-TR checklist. Children enrolled as control subjects were screened for typical development with the Social Communication Questionnaire (SCQ) and developmental history.
Exclusion Criteria
Exclusion criteria included parents or guardians who were unable or unwilling to provide consent, those with no telephone access, children with significant hearing or vision loss, and children with a psychiatric disorder established by DSM-IV-TR criteria. The co-morbid psychiatric disorders were identified during chart review by the nurse investigator and further explored during the interview with the parent at the first home visit. All children in the study were evaluated for their diagnosis by a developmental pediatrician. Children with co-morbid psychiatric disorders were excluded from the study. In addition, children with a complex neurologic disorder (cerebral palsy, phenylketonuria, tuberous sclerosis, neurofibromatosis, unstable seizure disorder [a seizure within 6 months], Rett disorder) and children with an unstable medical condition (eg, asthma, diabetes, cystic fibrosis, cardiac disease) were excluded.
Data Collection and Timeline
Data were collected during 3 home visits over 17 days; this included 1 week of sleep-diary completion followed by 10 nights of actigraphy and sleep-diary completion. Sleep data were collected for children who had a consistent daytime schedule (school, daycare).
Diagnostic and Screening Measures
The ADOS
The ADOS is a standardized assessment of communication, social interaction, and play designed to identify children who have the communicative and social deficits of autism or ASD.15 The ADOS has 3 diagnostic categories: autism, ASD, and non-ASD. The ADOS includes 4 modules that are based on the patient's functional expressive-language age. Interrater reliability for each module has been found to be high (91.5% for Module 1, 89% for Module 2, 88.2 % for Module 3, and 88.25% for Module 4).15
The ASDS
The ASDS is a parent- or professional-completed report questionnaire that explores 5 specific areas of behavior (cognitive, maladaptive, language, social, and sensorimotor) and is designed to identify Asperger syndrome in children aged 5 to 18 years. Studies have documented the efficiency of the instrument in discriminating Asperger syndrome from a sample without Asperger syndrome.16
The SCQ: Lifetime Version
The SCQ is a parent-report screening instrument that has been validated in children aged 4 and older to screen for ASD.17 The SCQ has 40 items; a cutoff score of 15 or more provides the best differentiation of ASDs from other diagnoses.17
The CSHQ
The CSHQ is a parent-report sleep screening instrument validated in children aged 4 to 10 years.18 A total score (48 items) and 8 subscale scores (33 items) reflect key sleep domains that encompass the major medical and behavior sleep disorders in this age group. Higher scores indicate more problems. The subscales include bedtime resistance, sleep-onset delay, sleep duration, sleep anxiety, night waking, parasomnias, sleep disordered breathing, and daytime sleepiness. In 1 study, a cutoff total score of 41 generated by analysis of the receiver operator characteristic curve yielded a sensitivity of 0.80 and a specificity of 0.72.18
Medical and Sleep History
A comprehensive medical and sleep-history questionnaire was completed during a semistructured interview in the course of the first home visit. The questionnaire included demographic information, medical and sleep concerns and past management, sleep and nap schedule, and family medical and sleep history.
Sleep Diaries
A daily report (diary) of sleep parameters, including the time the child went to bed (lights-out time), night wakings, morning wake time, naps, and health status for each participant was documented by a parent each morning for 17 days. The first week's sleep diary was collected and reviewed during the second home visit prior to starting actigraphy. The second sleep diary was completed by the parent each morning in conjunction with 10 nights of actigraphy.
Actigraphy
Actigraphy is a miniaturized wristwatch-like microcomputer that records motion. After motion is transduced into an analog electric form, it is digitized and stored. Each child was monitored with a MicroMini-Motionlogger actigraph (AMA-32) in the 0-crossing mode from Ambulatory Monitoring, Inc. (Ardsley, NY). The actigraph was activated with the Act Millennium 3.10.49 (ACTME) software program in Mode 18, with 1-minute epoch intervals, and was able to collect data for 22 days, 16 hours, and 0 minutes. This actigraph and zero-crossing mode of analysis were chosen because they can be used with the Sadeh sleep algorithm, the only sleep algorithm that has normative data on the pediatric population.8,19 Actigraphic raw data were translated into sleep measures with the Action-W software version 2.5.30 and Actigraphic Scoring Analysis program for an IBM-compatible PC. Long Wake Episodes was set for 5 minutes. If the parents noted on the sleep diary that their child was sick or had an unusual night (sleepover with friends), the night's data were discarded. Additionally, if diary data and actigraph recording for a night were discrepant and could not be clarified, the night's data were discarded.
Actigraphic data appear to be reliable when obtained from different locations, including the trunk, wrist, and ankles.20 However, Acebo21 found that a minimum of 7 nights was necessary to obtain reliable data. Children with ASD often have sensory challenges, including tactile defensiveness,22,23 and parents in the RAC voiced concerns about their children wearing and keeping on a wrist actigraph. Therefore, an innovative approach was piloted to address these concerns and obtain a minimum of 7 days of actigraphic data in children with autism and significant maladaptive behaviors.
Actigraphic Pilot Data
The actigraph was placed in a small, 6-cm × 6-cm soft cotton pocket hidden in the upper sleeve–arm area of a snug-fitting pajama top. The pocket was sewn in just below the shoulder seam, on the inside of the pajama top, and on the side of the nondominant arm. The opening was secured with Velcro for easy access to the actigraph, and each child had 2 or 3 customized pajama tops. Pilot data for the first 15 subjects in the TD cohort were collected with 2 simultaneous actigraphs for 10 nights. One actigraph was in the customized pajama-sleeve pocket, and the second was on the nondominant wrist. Parents completed a sleep diary for the 10 days. Analyses showed that there were no significant differences between the data from the nondominant wrist site and the nondominant upper-arm site, respectively: sleep efficiency 81.06% (SD 6.53%) versus 82.69% (SD 6.18%) (P < 0.576); sleep latency 21.42 minutes (SD 17.15) versus 23.70 minutes (SD 19.05) (P < 0.522); mean activity 24.95 counts per epoch (SD 8.02) versus 24.24 counts per epoch (SD 6.65) (P < 0.809); total sleep time, in minutes, 460.89 (SD 51.05) versus 465.83 (SD 49.622) (P < 0.779); and number of long wake episodes ( ≥ 5 minutes) 7.19 (SD 1.93) versus 6.84 (SD 2.13) (P < 0.677). As a result, the nondominant upper-arm site was utilized for all the children enrolled in this study employing the protocol described above.
Actigraph Recordings
Actigraphy recordings for each subject were reviewed by 3 authors (MCS, TAM, MB) from CHOP's Sleep Center and the University of Pennsylvania. Each subject's actigraphic recording data were evaluated for the overall activity pattern and 4 sleep parameters (sleep efficiency, sleep latency, total sleep time in minutes, and number of night wakings) generated from analysis using the Sadeh algorithm. CHOP's Sleep Center uses a sleep efficiency of 85% and a sleep latency of greater than 30 minutes as a cutoff for defining poor sleep quality. These parameters were chosen for the actigraphy recording score algorithm along with the TD cohort mean values for sleep in minutes, long wake episodes ( ≥ 5 minutes) per night, and activity mean. A consensus score was agreed upon by the 3 evaluators using a scoring system ranging from 0 to 3: Normal (0.0), Normal-Mild (0.5), Mild (1.0), Mild-Moderate (1.5), Moderate (2.0), Moderate-Severe (2.5), and Severe (3.0). The actigraph pattern was considered abnormal if the consensus score was 1.0 or greater.
Data Analyses
All analyses were performed using SPSS, version 11.5 for Windows (SPSS, Inc, Chicago, IL). All statistical significance was set at P value < 0.05. T tests and analysis of variance (ANOVA) were used for continuous data generated from the CSHQ scores and actigraphy. A χ2 analysis was used for categorical data generated from the CSHQ, comprehensive sleep history questionnaire, and actigraphic data. The prevalence of sleep disturbances in the ASD and TD cohorts was analyzed using a t test for continuous data and a χ2 test for categorical data. A comparison between the subgroups of the ASD and TD cohorts was accomplished using ANOVA. Logistic regression was used to analyze the effect of medication on the prevalence of sleep disturbances in the ASD and TD cohorts using the CSHQ and the actigraphic recording scores.
RESULTS
Sample
The TD Cohort
From April 2006 to September 2007, while school was in session, 40 TD children aged 4 to 10 years with no immediate family members with an ASD were recruited for the control group. All TD participants scored below the cutoff for being “at risk” for ASD (cutoff of 15) on the SCQ; scores ranged from 0 to 9, with a mean score of 2. The TD cohort was a convenience sample generated from neighbors and friends of children with ASD from the Greater Philadelphia Area and is a limitation to the generalizability of the TD prevalence results.
The ASD Cohort
From September 2006 to September 2007, children were randomly selected from the RAC Registry for contact regarding the study. In total, 440 charts were reviewed, and 250 children met inclusion criteria and were invited to participate. Sixty-two families responded positively (25.7%). Sixty families agreed to the first home visit, and all of these families consented to participate in the study. One child dropped out because his family relocated. In total, 59 children with ASD and their families completed the study: 26 (44%) children with autism, 21 (35.6%) children with PDD-NOS, and 12 (20.4%) children with Asperger disorder. The purpose of randomly selecting the ASD cohort was to generate a representative sample of the RAC to estimate the prevalence of sleep disturbances. Prevalence is often estimated with smaller representative samples of a population and can be viewed as a snapshot in time, indicating who has an illness or disorder and who does not (Gordis24). However, we identified 3 limitations to the prevalence estimates derived from our data.
First, the 25.7% response rate for this study generated concerns of sample bias. As a result, we conducted a chart review of the 250 families invited to participate. As expected, a greater number of families (57.6 %) who participated in the study had concerns about their child's sleep, as compared with invited families that did not participate (45.3%). However, the difference between the groups (P = 0.099) was not statistically significant. This finding supported our belief that the sample was representative of the RAC population with respect to sleep concerns.
Second, the sample of 59 children with ASD, derived from the RAC registry, is approximately 1% (59/5510) of the southeastern region of Pennsylvania ASD population (Census, 2005). Though the sample is small, we do feel it is representative of the RAC. In addition, the representation of ASD diagnoses (44% autism, 35.6% PDD, and 20.4% Asperger disorder) in this study is similar to the recent findings of Interactive Autism Network (IAN) (www.IANproject.org). IAN, launched in April, 2007, collects data on the ASD population in the United States. IAN Research is a study at the Kennedy Krieger Institute overseen by the Johns Hopkins Medicine Institutional Review Board. Families participating in the network must live in the US and have a child under the age of 18 with an ASD diagnosis. In December, 2008 IAN shared their data collected on 6000 individuals who had an ASD. Included in the IAN database is information on children and adolescents with the following diagnoses: 44%, autism; 29%, PDD-NOS; 15%, Asperger disorder; and 12%, other diagnosis such as ASD or PDD. Because of the similarity in distribution in our sample, we believe it to be representative of the distribution in the national autism community.
Lastly, CHOP is a world leader in the diagnosis and treatment of pediatric illness. Families with children and adolescents who have severe and complicated ASD would be motivated to receive care and treatment guidance from CHOP. Sampling from this population would, theoretically, bias the results toward a higher prevalence of sleep disturbances. To address this concern, we excluded children with complicated medical conditions and other comorbid psychiatric diagnoses. In addition, the results from this study showed that the ASD and TD cohorts did not differ in terms of mild common health conditions such as asthma and allergies. In summary, the prevalence estimates derived from this study are generalizable only to similar populations of children who are receiving care at tertiary diagnostic and treatment centers for children with ASD.
Children with the diagnosis of autism in this study met the criteria for autism on the ADOS. Developmental skills were assessed by receptive-language age, academic history, and individual education plans. IQ testing was not available for all subjects. Developmental skills in the autism subgroup included severe disability 19.2% (5 children), moderate disability 42.3% (11), borderline-mild disability 34.6% (9), and typical ability (1). Twenty-three children (88.5%) were in an autistic support classroom, 2 children with borderline skills were in a learning support classroom, and 1 child was mainstreamed. Children with PDD-NOS met the criteria for ASD on the ADOS. Developmental skills in the PDD-NOS subgroup included typical ability 52.3% (11 children), moderate disability 9.5% (2), and borderline-mild disability 38.2 % (8). Thirteen of these children were in a typical classroom with support, 4 children were in a learning disability classroom, 3 children were in autistic support classroom, and 1 child was home schooled. Children with the diagnosis of Asperger disorder met the criteria for Asperger disorder on the ASDS. All children in the Asperger subgroup had typical to above-average academic ability. One child was in a learning-disabled classroom, and 11 were in a typical classroom.
Demographics
Age, race, and sex did not differ between the TD and ASD cohorts (Table 1). Minority representation of 22.5% for the TD cohort and 25% for the ASD cohort reflects the demographics of minority populations in the Greater Philadelphia Area (25.8%). Maternal and paternal age, marital status, socioeconomic status, work status, and paternal education showed no statistical differences between the TD cohort and the ASD cohort (Table 1). However, maternal education was significantly different (P = 0.028), with the TD cohort having 45% of mothers with graduate-level education, compared with 25% of the ASD cohort.
Table 1.
Demographics
| Variables | TD Controls | ASD Cohort | P Value |
|---|---|---|---|
| n = 40 | n = 59 | ||
| Race | 0.74 | ||
| White | 31 (77.5) | 44 (74.6) | |
| Nonwhite | 9 (22.5) | 15 (25.4) | |
| Sex | 0.07 | ||
| Female | 14 (35) | 11 (18.6) | |
| Male | 26 (65) | 48 (81.4) | |
| Age, y | |||
| Participant | 7.089 ± 2.087 | 7.532 ± 1.923 | 0.29 |
| Mother | 39.97 ± 4.503 | 40.71 ± 5.308 | 0.51 |
| Father | 42.06 ± 4.885 | 41.89 ± 6.079 | 0.89 |
| Parents' marital status | 0.10 | ||
| Married | 29 (87.9) | 48 (85.7) | |
| Single | 1 (3.0) | 7 (12.5) | |
| Divorced | 3 (9.1) | 1 (1.8) | |
| Mother's work status | 0.08 | ||
| Home | 7 (22.6) | 21 (37.5) | |
| Part-time | 3 (9.7) | 11 (19.6) | |
| Full-time | 23 (67.7) | 24 (42.9) | |
| Father's work status | 0.13 | ||
| Home | 0 | 4 (7.3) | |
| Part-time | 0 | 0 (0) | |
| Full-time | 31 (100) | 51 (92.7) | |
| Mother's education | 0.03 | ||
| Some high school | 0 (0) | 2 (3.6) | |
| High school | 3 (9.1) | 19 (33.9) | |
| College | 15 (45.5) | 21 (37.5) | |
| Graduate school | 15 (45.5) | 14 (25) | |
| Father's education | 0.08 | ||
| Some high school | 1 (3) | 2 (3.6) | |
| High school | 3 (9.1) | 14 (25) | |
| College | 10 (33.3) | 24 (42.9) | |
| Graduate school | 18 (54.3) | 16 (28.6) | |
| Family income, $ | 0.76 | ||
| 0-50,000 | 5 (12.5) | 9 (15.3) | |
| 50,000-100,000 | 6 (17.5) | 12 (2.0) | |
| > 100,000 | 22 (70) | 35 (62.7) |
Data are presented as number (%) except age, which is presented as mean ± SD. TD refers to typically developing children; ASD, children with autism spectrum disorder.
Sleep Environment and Sleep Hygiene
Most families in the study lived in a single-family house in a Philadelphia suburb (Table 2). Most children slept in their own bedroom. The parents of the ASD cohort often described a consistent bedtime routine. There were no statistical differences between the TD and ASD cohorts for the following variables: home environment, single versus shared bedroom, bedtime routine, paternal versus maternal involvement in the bedtime routine, parental presence in the bedroom with sleep onset, naps, and bedtime on the weeknights and weekends. However, there was a significant difference between parental sleep concerns (P < 0.001) and wake time during the weekdays (P = 0.010) between the ASD and TD cohorts. More than half of the families of the children with ASD (57.6%) voiced sleep concerns, including long sleep latencies despite a bedtime routine (n = 12), frequent night wakings (n = 15), sleep terrors (n = 3), and early risings at 0400 or 0500 (n = 6) (Table 2). Only 12.5% families of the TD cohort reported sleep concerns, which included inadequate sleep duration (n = 2), difficulties falling asleep (n = 1), and night wakings (n = 2).
Table 2.
Sleep Environment and Bedtime Routine
| TD cohort | ASD cohort | P Value | |
|---|---|---|---|
| n = 40 | n = 59 | ||
| Home Environment | 0.14 | ||
| Apartment | 2 (5) | 3 (5.1) | |
| Row house | 2 (5) | 11 (18.6) | |
| Unattached house | 36 (90) | 45 (76.3) | |
| Siblings | |||
| No | 4 (10) | 17 (28.8) | 0.02 |
| Yes | 36 (90) | 42 (71.2) | |
| Child has own room | |||
| No | 13 (32.5) | 15 (25.4) | 0.44 |
| Yes | 27 (67.5) | 44 (74.6) | |
| Falls asleep in parent's bed | 4 (10.0) | 4 (6.8 ) | 0.65 |
| Parent has concerns about child's sleep | 0.001 | ||
| No | 35 (87.5) | 25 (42.4) | |
| Yes | 5 (12.5) | 34 (57.6) | |
| Bedtime Routine | 0.89 | ||
| No | 3 (7.5) | 4 (6.8) | |
| Yes | 37 (92.5) | 55 (93.2) | |
| Naps during week | 0.18 | ||
| No | 34 (85.0) | 55 (93.2) | |
| Yes | 6 (15) | 4 (6.8) | |
| Puts child to bed | 0.57 | ||
| Mother | 13 (32.5) | 22 (37.3) | |
| Father | 5 (12.5) | 11 (18.6) | |
| Both parents | 13 (32.5) | 18 (30.5) | |
| Self | 6 (15) | 7 (11.9) | |
| Other | 3 (7.5) | 1 (1.7) | |
| Parent present when child is falling asleep | 0.71 | ||
| No | 31 (77.5) | 45 (76.3) | |
| Yes | 9 (22.5) | 14 (23.7) | |
| Bedtime | |||
| Weekday | 20:48 ± 42 | 20:43 ± 55 | 0.68 |
| Weekend | 21:24 ± 55 | 21:14 ± 61 | 0.42 |
| Wake time | |||
| Weekday | 07:08 ± 35 | 06:45 ± 46 | 0.01 |
| Weekend | 07:47 ± 62 | 07:19 ± 74 | 0.05 |
Data are presented as number (%) or mean ± SD. TD refers to typically developing children; ASD, children with autism spectrum disorder.
Medications
Twenty-two percent of the TD cohort and 56.7% of the ASD cohort were taking daily medication (Table 3). However, no children in the TD cohort and 37.3% of the ASD cohort (autism 38.5%, PDD-NOS 38.15%, Asperger disorder 33.35) were taking medication to aid sleep (P < 001). The types of medications used by the TD and ASD cohorts are listed in Table 3.
Table 3.
Medications
| Medication | TD cohort | ASD cohort |
|---|---|---|
| n = 40 | n = 59 | |
| Daily | 9 (22.5) | 34 (57.6) |
| To treat allergies | ||
| Total | 5 (12.5) | 4 (6.7) |
| Loratadine (Claritin) | 3 | 0 |
| Cetirizine (Zyrtec) | 1 | 2 |
| Diphenhydramine(Benadryl) | 1 | 0 |
| Hydroxyzine (Vistaril) | 0 | 1 |
| Fluticasone nasal (Flonase) | 0 | 1 |
| To treat asthma | ||
| Total* | 5 (12.5) | 5 (8.4) |
| Albuterol | 3 | 1 |
| Montelukast sodium (Singular) | 2 | 3 |
| Fluticasone propionate (Flovent) | 1 | 0 |
| Pulmicort | 0 | 1 |
| Stimulants | ||
| Total | 0 (0) | 8 (13.5) |
| Methylphenidate | 0 | 5 |
| Dexamphetamine | 0 | 1 |
| Dextroamphetamine mixed salts (Adderall) | 0 | 2 |
| SSRI | ||
| Total | 0 (0) | 7 (11.8) |
| Fluoxetine (Prozac) | 0 | 5 |
| Paroxetine (Paxil) | 0 | 1 |
| Fluvoxamine (Luvox) | 0 | 1 |
| Atypical neuroleptics | 0 (0) | 6 (10.16) |
| Risperidone (Risperdal) | 0 | 4 |
| Aripiprazole (Abilify) | 0 | 2 |
| Anticonvulsants | 0 (0) | 2 (3.4) |
| Oxcarbazepine (Trileptal) | 0 | 1 |
| (Topiramate) Topamax | 0 | 1 |
| Gastrointestinal | 0 (0) | 2 (3.4) |
| Polyethylene glycol(Miralax) | 0 | 1 |
| Lansoprazole (Prevacid) | 0 | 1 |
| Medication to aid sleep** | 0 (0) | 22 (37.3) |
| Melatonin | 0 | 15 |
| Catapres (Clonidine)*** | 0 | 3 |
| Risperidone (Risperdal)*** | 0 | 4 |
| Aripiprazole (Abilify)*** | 0 | 2 |
| Hydroxyzine (Vistaril)*** | 0 | 1 |
| Fluoxetine (Prozac)*** | 0 | 1 |
Data are presented as number (%). TD refers to typically developing children; ASD, children with autism spectrum disorder; SSRI, selective serotonin reuptake inhibitor.
One child with asthma was taking 2 medications.
Four of the 22 children were taking two medications to promote sleep.
Children received these medications in the evening prior to bedtime in order to facilitate sleep. These medications are included in the above total percentiles and again in the sleep aid section.
The CSHQ
We hypothesized that children with ASD would have more sleep problems, as indicated by higher scores on the total and subscale scores on the CSHQ. The CSHQ subscales total mean score and the total questionnaire mean score were higher and significantly different when comparing the ASD and TD cohorts (Table 4). The ASD cohort had mean scores for total questionnaire items that are consistent with the mean scores published in the CSHQ instrument paper for children diagnosed with sleep disorders (mean = 68.4 [SD 13.7]) followed at a sleep clinic.25 The TD cohort had a mean score for the total questionnaire consistent with the mean score of school-aged children (mean 56.2 [SD 8.9]) from the community sample.25
Table 4.
Children's Sleep Habits Questionnaire
| TD Cohort | ASD Cohort | P Value | |
|---|---|---|---|
| n = 40 | n = 59 | ||
| Total score | 59.96 (5.259) | 67.36 (9.558) | 0.001 |
| Subscale | 41.77 (4.560) | 47.39 (8.211) | 0.001 |
| Bedtime resistance | 7.202 (0.839) | 8.15 (2.163) | 0.076 |
| Sleep-onset delay | 1.25 (0.494) | 1.61 (0.766) | 0.010 |
| Sleep duration | 3.67 (0.997) | 4.67 (1.833) | 0.002 |
| Sleep anxiety | 4.95 (1.131) | 5.81 (1.978) | 0.014 |
| Night wakings | 3.92 (1.141) | 4.74 (1.635) | 0.007 |
| Parasomnias | 8.22 (1.250) | 9.59 (1.848) | 0.001 |
| Disordered breathing | 3.25 (0.588) | 3.32 (0.567) | 0.588 |
| Daytime sleepiness | 11.70 (2.564) | 12.3 (3.217) | 0.323 |
Data are presented as mean (SD). TD refers to typically developing children; ASD, children with autism spectrum disorder.
Parents of the ASD cohort reported longer sleep latencies, shorter sleep duration, more-frequent night wakings, more parasomnias, and greater sleep anxiety in their children than did parents of the TD cohort. The sleep anxiety subscale addresses nighttime fears. Subjects with ASD were more often afraid of the dark, being alone, and sleeping away from home than were TD children. The parasomnias subscale showed no significant difference in sleep talking (P = 0.374), sleep walking (P = 0.678), nightmares (P = 0.320), and restlessness (P = 0.131); however, sleep terrors (P = 0.009), bed wetting (P = 0.035), and bruxism (P = 0.002) were significantly different. The bedtime resistance (P = 0.076) and sleep disordered breathing (P = 0.567) subscales scores for the ASD and TD cohorts were not significantly different. Furthermore, there was no significant difference between the TD and ASD cohort scores on the daytime sleepiness scale (P = 0.323), suggesting that both TD and ASD parents report that their children are sleepy during the day.
Prevalence of Sleep Disturbances Using the CSHQ
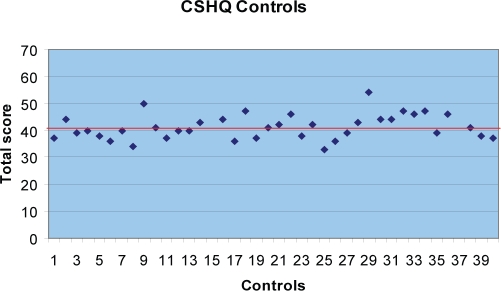
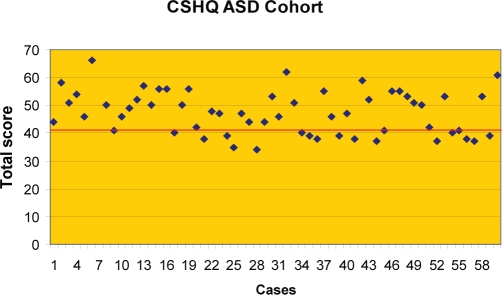
We hypothesized that the prevalence of sleep disturbances in the ASD cohort would be at least twice the rate of the TD cohort utilizing a cutoff score of 41 on the CSHQ. In the TD cohort, 45% (18/40) of the participants scored greater than 41 on the subscales total score. The mean score for the TD cohort was 41.7 with a SD of 4.56. The predominant reason for the elevated subscale total score in the TD cohort was, on average, a high score on the daytime sleepiness scale. The prevalence of sleep disturbances in the TD cohort can be estimated at 45%. Most children scoring at or just above the cutoff of 41, shown as a bold line in Figure 1, illustrated that most sleep difficulties in the TD cohort are mild. In contrast, 66.1% (39/59) of the ASD cohort (autism 62.5%, PDD-NOS 76.2%, asperger disorder 58.3%) scored greater than the cutoff of 41 on the subscales total score, with a mean score of 47.39 and a SD of 8.211 (Figure 2). Figure 2 highlights that the CSHQ scores for the ASD cohort are greater, representing more moderate to severe sleep problems. (See Table 5.)
Figure 1.
The Children's Sleep Habits Questionnaire (CSHQ) cutoff subscale total score is 41, shown as a bold line. Forty-five percent of the typically developing (TD) control subjects scored greater than 41 (mean score 41.7, SD 4.56). Most children scored at or just above the cutoff, illustrating that most sleep difficulties in the TD cohort are mild.
Figure 2.
The Children's Sleep Habits Questionnaire (CSHQ) cutoff subscale total score is 41, shown as a bold line. In contrast to the typically developing children (data shown in Figure 1), 66.1% of the cohort with an autism spectrum disorder (ASD) scored greater than the cutoff (mean score 47.39, SD of 8.21), representing more moderate to severe sleep problems.
Table 5.
Prevalence of Sleep Disturbances: CSHQ and Actigraphy
| CSHQ | TD Cohort | ASD Cohort | P Value |
|---|---|---|---|
| Cutoff score | 0.037 | ||
| < 41 | 22 (55) | 20 (33.9) | |
| > 41 | 18 (45) | 39 (66.1) | |
| Actigraphy: Linear Score | |||
| ≥ 1.0 | 17 (45.9) | 38 (66.7) | 0.046 |
| < 1.0 | 20 (54.1) | 19 (33.3) | |
| Mean ± SD | 0.608 ± 0.619 | 1.18 ± 1.020 | 0.003 |
Data are presented as number (%) or mean ± SD. The numbers in the typically developing (TD) and autism spectrum disorder (ASD) groups vary for those who completed the Children's Sleep Health Questionnaire (CSHQ) and actigraphy.
The CSHQ results were analyzed to determine the effect of medications on the mean scores of the CSHQ for the ASD and TD cohorts. There was no significant difference in the CSHQ scores of TD children taking medication (41.60, SD5.56) or not taking medication (42.66, SD 4.64; P = 0.602). However, the mean score of 49.26 (SD 8.87) on the CSHQ was higher for children with ASD taking medication than for those not taking medication (44.84, SD 6.50; P = 0.042). In addition, the mean score for children with ASD taking a medication to aid sleep was higher (50.45, SD 8.94) and significantly different (P < 0.001) than the mean CSHQ score of children with ASD not taking a sleep medication (44.84, SD 6.50). Thus, children with ASD continue to have disturbed sleep, as reported by parents, despite the use of sleep medication.
Actigraphy Adherence
Actigraphy was well tolerated in this study. Three participants, 2 with severe autism and 1 TD control, were unable to adhere to the actigraphy protocol. On average, the TD cohort contributed 10.4 nights, and the ASD cohort contributed 12 nights of actigraphy to be analyzed. Ninety-seven percent of participants had at least 8 nights of analyzable actigraphic data.
Actigraphic Data
Sleep latency (P = 0.002), the activity mean (P = 0.003), and the longest wake episodes (P = 0.002) were significantly different between the TD and ASD cohorts (Table 6). Children with ASD, on average, took 34 minutes to fall asleep. In addition, children with ASD had longer wake episodes (49 minutes, SD 21.90), compared with the TD cohort (34 minutes, SD 20.11; P = 0.002). However, sleep efficiency, long wake episodes, and total sleep time were not statistically different between the ASD and TD cohorts.
Table 6.
Actigraphy: TD Cohort Compared with ASD Cohort
| TD | ASD | P Value | |||
|---|---|---|---|---|---|
| n = 37 |
n = 57 |
||||
| Mean | SD | Mean | SD | ||
| Starting time | 21:16 | 0.031 | 21:17 | 0.031 | 0.869 |
| Ending time | 7:06 | 0.026 | 7:03 | 0.031 | 0.734 |
| Duration, min | 591.40 | 37.932 | 586.61 | 46.872 | 0.611 |
| Activity | |||||
| Mean | 21.53 | 5.864 | 27.76 | 11.182 | 0.003 |
| SD | 42.82 | 7.543 | 50.13 | 11.331 | 0.001 |
| Wake time, min | 121.83 | 43.819 | 134.61 | 54.415 | 0.243 |
| Sleep time, min | 469.57 | 52.626 | 452.00 | 65.070 | 0.181 |
| % Sleep | 79.44 | 7.275 | 77.16 | 9.078 | 0.212 |
| SE, % | 84.21 | 6.232 | 83.63 | 7.927 | 0.716 |
| SOL | 21.71 | 8.966 | 34.42 | 21.943 | 0.002 |
| WASO | 87.46 | 32.989 | 88.03 | 41.860 | 0.946 |
| Wake episodes | |||||
| > 1–min | 20.44 | 4.847 | 17.93 | 5.379 | 0.027 |
| > 5 min | 6.56 | 2.046 | 5.96 | 2.102 | 0.185 |
| Mean duration, min | 9.39 | 18.775 | 8.97 | 4.935 | 0.872 |
| Longest, min | 34.70 | 20.112 | 49.00 | 21.901 | 0.002 |
| Total, no. | 19.91 | 4.726 | 17.45 | 5.3843 | 0.029 |
T test for equality of means. Data are presented as mean and SD for typically developing (TD) children and children with autism spectrum disorders (ASD. TST refers to total sleep time; SE, sleep efficiency; SOL, sleep-onset latency; WASO, wake time after sleep onset.
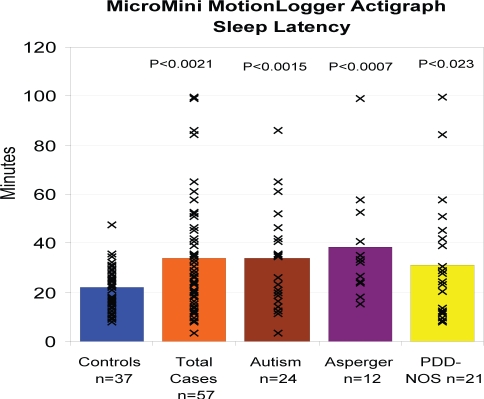
The actigraphic sleep parameters of the subgroups of ASD were also compared with those of the TD cohort (Table 7). The sleep latency for all 3 subgroups was statistically different from the TD cohort, showing that children across the autism spectrum had difficulties falling asleep (Figure 3). The Asperger disorder subgroup had the longest sleep latency (38.5 minutes, on average; P = 0.0007). The Autism subgroup showed a shorter total sleep time (437 minutes, on average; P = 0.023) compared with the TD Cohort (Table 7). The Asperger disorder subgroup had a greater total wake time, in minutes (P = 0.048), compared with the TD cohort. In addition, the Autism (P = 0.002) and Asperger disorder (P = 0.050) subgroups had the longest wake episodes, on average, compared to the TD cohort.
Table 7.
Actigraphy: TD Cohort Compared with ASD Subgroups
| TD | Autism | PDD | AD | Autism | PDD | AD | |
|---|---|---|---|---|---|---|---|
| P Value | P Value | P Value | |||||
| Starting time | 21:16 | 21:21 | 21:06 | 21:31 | 0.705 | 0.442 | 0.297 |
| Ending time | 07:06 | 06:50 | 07:02 | 07:31 | 0.154 | 0.684 | 0.074 |
| Duration, min | 591.40 | 570.63 | 595.10 | 603.713 | 0.077 | 0.738 | 0.339 |
| Activity | |||||||
| Mean | 21.53 | 29.96 | 23.96 | 29.992 | 0.0002 | 0.210 | 0.008 |
| SD | 42.82 | 53.44 | 46.38 | 50.088 | 0.0000 | 0.105 | 0.030 |
| Wake time, min | 121.83 | 133.25 | 122.11 | 159.236 | 0.309 | 0.982 | 0.048 |
| TST, min | 469.57 | 437.39 | 472.99 | 444.477 | 0.023 | 0.831 | 0.231 |
| % Sleep | 79.44 | 76.81 | 79.51 | 73.770 | 0.155 | 0.976 | 0.072 |
| SE, % | 84.21 | 84.01 | 85.30 | 79.960 | 0.897 | 0.551 | 0.124 |
| SOL, min | 21.71 | 34.09 | 32.54 | 38.353 | 0.0015 | 0.023 | 0.0007 |
| WASO, min | 87.46 | 83.58 | 80.02 | 110.945 | 0.637 | 0.439 | 0.107 |
| Wake episodes | |||||||
| > 1 min, no. | 20.44 | 17.39 | 17.31 | 20.101 | 0.022 | 0.033 | 0.841 |
| > 5 min, no. | 6.56 | 5.77 | 5.61 | 6.958 | 0.123 | 0.101 | 0.598 |
| Mean duration, min | 9.39 | 9.46 | 8.57 | 8.672 | 0.985 | 0.845 | 0.897 |
| Longest, min | 34.70 | 51.96 | 45.78 | 48.713 | 0.002 | 0.063 | 0.050 |
| Sleep episodes, no. | 19.91 | 16.94 | 16.82 | 19.584 | 0.023 | 0.034 | 0.846 |
Data are presented as mean and p values for typically developing (TD) children and children with autism spectrum disorders (ASD), including autism, pervasive developmental disorders (PDD), and Asperger disorder (AD). TST refers to total sleep time; SE, sleep efficiency; SOL, sleep-onset latency; WASO, wake time after sleep onset.
Figure 3.
The sleep latency for all three subgroups was statistically different from the TD cohort (see P values at top), showing that children across the autism spectrum had difficulties falling asleep. The Asperger Disorder subgroup had the longest sleep latency 38.5 minutes.
Prevalence of Sleep Disturbances Using Actigraphy
The prevalence of sleep disturbances in the ASD and TD cohorts was estimated using the actigraphy recording score for each participant (Figure 4). Sleep efficiency of 85%, sleep latency of 30 minutes, and TD cohort mean values for total sleep time, in minutes (469); long wake episodes per night (6); and activity mean of 21.5 counts per epoch were used as cutoff scores for the 5 parameters evaluated to establish an actigraphy recording score. The prevalence of sleep disturbances in the ASD and TD cohorts were estimated at 66.7% and 45.9%, respectively (Table 5), and the difference between the groups was statistically significant (P = 0.046). The prevalence of sleep disturbances was 75% for the Autism and Asperger disorder subgroups and 52.4% in the PDD-NOS subgroup. In addition, the analysis of the actigraphy recording mean scores showed significant differences between the TD cohort mean score (0.608, SD 0.619) and ASD subgroups: Autism mean score (1.375, SD 1.065), PDD-NOS mean score (1.00, SD 1.036) and Asperger disorder mean score (1.125, SD 0.907; P = 0.012).
Figure 4.
Data from 10 consecutive nights of actigraphy, obtained from an actigraph that was contained in a specially designed upper-sleeve pocket.
Medications
There were no statistical differences when comparing actigraphy recording scores between children taking medication and children not taking medications in the TD cohort (P = 0.476) and in the ASD cohort (P = 0.156). In addition, there were no statistical differences in the actigraphy recording scores between children in the ASD cohort taking medication to aid sleep and children not taking medication to aid sleep (P = 0.115) when comparing the actigraphy recording scores. The use of sleep medication, on average, did not “normalize” the actigraphy recording scores, and the overall scores were higher.
Effect of Sleep Medication on the Prevalence of Sleep Disturbances
Although 37.3% of children in the ASD cohort were taking medication to improve sleep, no children in the TD cohort were taking sleep medication. Therefore, a logistic-regression model was used to determine the effect of any medication on sleep disturbances in the ASD and TD cohorts using the CSHQ and the actigraphy recording scores. The CSHQ subscale total cutoff (yes/no) was the dependent variable, and medication use (yes/no) and ASD group (yes/no) were the covariates. Results for the ASD group variable was P = 0.054 and EXP (B) 2.362, and the results for the medication variable was P = 0.951 and Exp (B) 1.028. The actigraphy recording score (normal/abnormal) was replaced as the dependent variable in the logistic-regression model. Results for the ASD variable was P = 0.101 and Exp (B) 2.130, and results for the medication variable was P = 0.539 and Exp (B) 1.328. The results from the logistic-regression model provide evidence that medication use had no significant effect on the prevalence of sleep disturbances in the ASD and TD cohorts, using the CSHQ and the actigraphy recording scores.
Average Activity Counts per Epoch per Hour of the ASD and TD Cohorts
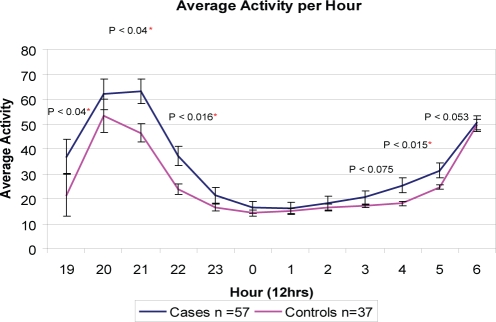
Further analysis of the actigraphy data was conducted to examine the activity counts in 1-minute epochs for the TD and ASD cohorts across a 12-hour continuum. The average activity counts per epoch per hour of the ASD and TD cohorts at hourly intervals with P values are shown in Figure 5. The figure illustrates the statistically different activity counts between the TD and ASD groups at 2100 (P = 0.04) and 2200 (P = 0.016) and again in the early morning at 0400 (P = 0.015) and 0500 (P = 0.053).
Figure 5.
The x axis shows the time of day, 1900 to 0700. The y axis is the average activity count per hour of the typically developing (TD) and autism spectrum disorder (ASD) cohorts. On average, the TD and ASD cohorts went to bed wearing the actigraph at 2115 and woke at 0705, as reported by parents on the sleep diary.
Sleep Diagnoses
An important component of pediatric sleep evaluation, in addition to describing the sleep behaviors and sleep quality of children, is to identify sleep disorders.5 There are more than 80 different sleep disorders listed in the International Classification of Sleep Disorders, 2nd edition (ICSD-2) that could be the cause of an underlying sleep problem. Identifying the cause of insomnia in a child is a complex task and is often multifactorial. Moreover, clear definitions of childhood sleep disorders are just starting to emerge from the rapidly evolving field of pediatric sleep. In this study, each child's sleep was evaluated from multiple sources of data: home observations; a comprehensive medical, developmental, and sleep history, including medication history; chart review; sleep questionnaire total and subscale results; 2 weeks of sleep diary data; and 10 nights of actigraphy.
The ICSD-2 defines insomnia as adequate sleep opportunity with a persistent sleep difficulty and associated daytime dysfunction. Children in this study met criteria for an insomnia if they (1) had difficulties falling asleep, defined by a sleep latency of greater than 30 minutes obtained with actigraphy; (2) had difficulties maintaining sleep, as documented on the CSHQ, sleep diary and/or actigraphy; and (3) experienced daytime sleepiness or dysfunction, as reported by parents on the CSHQ or during the comprehensive sleep interview. Thirty-nine children with ASD (66%) were identified with an insomnia based on the subjective and objective measures. Insomnia is a symptom that often arises from other sleep disorders, primary medical conditions, and mental disorders. Each child was assessed for possible causes of the insomnia on a case-by-case basis (Table 8).
Table 8.
Sleep Diagnoses in 59 Children with Autism Spectrum Disorders
| Sleep diagnoses | No. (%) |
|---|---|
| None | 9 (15.2) |
| No sleep problems at present, on medication that improves sleepa | 11 (18.6) |
| Insomnia possibly due to a medical condition | 6 (10.2) |
| Behavioral insomnia of childhood—sleep association type | 12 (20.3) |
| Behavioral insomnia of childhood—limit-setting type | 6 (10.2) |
| Parasomnias (associated with night waking) | 3 (5) |
| Symptoms of sleep disordered breathing | 1 (2) |
| Insomnia due to pervasive developmental disorders | 11 (18) |
Medications used to improve sleep include melatonin n = 4, atypical neuroleptic n = 6, and Catapres n = 1.
Other Sleep Disorders
Ten percent (4/39) of the children with ASD and insomnia were identified with another sleep disorder most likely causing the insomnia symptoms: sleep disordered breathing (1) and night terrors (3).
Medical Conditions
Fifteen percent (6/39) of the children with ASD and insomnia were identified with medical conditions that may be related to the insomnia symptoms: seizures (1), asthma (3), and gastroesophageal reflux (2). All 6 children also met criteria for a behavioral insomnia of childhood, and their insomnia may be multifactorial.
Medications
Twenty-three percent (9/39) of children with ASD and insomnia were taking medications that have disturbed sleep as a potential side effect (stimulants n = 4; selective serotonin reuptake inhibitors n = 5). All parents reported that their child's sleep disturbances were present prior to starting the medication and that the medications were not making the child's sleep problems more severe. All the parents of children taking selective serotonin reuptake inhibitors thought that their child's bedtime anxiety had improved since starting the medication.
Behavioral Insomnia of Childhood-Sleep Association Type
Thirty one percent (12/39) of children with ASD and insomnia were diagnosed with behavioral insomnia (sleep-association type). Parents were able to establish a strong bedtime routine, but they needed to be touching their child (rocking, patting, lying next to them) as the child fell asleep. These children had frequent night wakenings and early risings and required interventions from their parents to return to sleep. Most parents reported that they tried extinction protocols but that they were too exhaustive to implement.
Behavioral Insomnia of Childhood—Limit-setting Type
Fifteen percent (6/39) of the children with ASD and insomnia were diagnosed with behavioral insomnia (limit-setting type). These families had tried to establish a routine but were unable to do so and made no further attempts to establish a routine. During the study, these children had no consistent bedtime routines.
Inadequate Sleep Hygiene
Two children with behavioral insomnia limit—setting-type also had inadequate sleep hygiene. One child frequently drank cola soda at bedtime and slept on the couch in the living room. One child fell asleep in his older brother's room on the floor in front of the TV.
Insomnia Due to a Mental Disorder: PDD
The ICSD −2 guidelines note that insomnia due to a mental disorder is diagnosed only when insomnia is a predominant compliant, warrants independent clinical attention, and is not better explained by another sleep disorder. The ICSD-2, Appendix B, lists the diagnostic category of PDD—a group of disorders including autistic disorder, Asperger disorder, and Rett disorder. The essential feature of insomnia due to mental disorder is the occurrence of insomnia that is caused by an underlying mental disorder. In this study, 11 (28%) of the children with ASD with an insomnia had adequate sleep hygiene, strong bedtime routines, fell asleep by themselves, and had no identifiable medical condition that might cause sleep disturbances. Parents reported that, despite robust behavior techniques and, in some cases, medication to aid sleep, their child continued to have difficulties with initiating and/or maintaining sleep and had daytime sleepiness. These cases were not better explained by another sleep disorder and might be best described as an insomnia due to PDD (Code 327.15).
DISCUSSION
Sleep behaviors and sleep quality in children represent a complex exhibition of biologic, developmental, psychological, environmental, and cultural influences.27 Therefore, careful assessment of a child's sleep behaviors and sleep quality in the context of their family, home, school, and community is paramount. This descriptive cross-sectional study presents the characterization of sleep in an ASD cohort, compared with a TD cohort, utilizing home observations; a comprehensive medical, developmental, and sleep history, including medication history; chart review; validated sleep questionnaire; sleep diary; and 10 nights of actigraphy.
The TD cohort was recruited from the same neighborhoods and counties as those of the children in the ASD cohort to help control for variables not collected in the study. The prevalence of sleep disturbances was estimated at 45% and 66% for the TD and ASD cohorts, respectively. Analysis of the sleep environment, sleep-hygiene practices, medical concerns, bedtime routines, and subjective and objective measures of sleep provides evidence that the difference in prevalence and severity of sleep disturbances between the 2 cohorts may be a function of the underlying neurobiologic disorder, namely, ASD.
It is important to identify the underlying sleep disorders in children with ASD, since the choice of treatment and future research directions should be based on the child's sleep disorder and not on the presenting symptoms.5 Potential contributors to insomnia, including obstructive sleep apnea, periodic limb movements, epilepsy, inadequate sleep hygiene, behavior disorders (limit-setting and sleep association disorders), medications, parasomnias. circadian rhythm abnormalities, and arousal dysfunction5 were considered for each participant.26 Sleep disordered breathing was not identified as a predominant sleep disorder in this study based on parent interviews and questionnaire data; only 1 child had a history of snoring and was referred to a sleep center. A single subject had epilepsy, which was controlled on medication; no other subjects were reported to have seizures, features of periodic leg movements in sleep, or restless legs syndrome. In addition, parents reported adequate sleep-hygiene practices, and these findings were substantiated by low bedtime resistance subscale scores. The two predominant sleep disorders identified in the ASD cohort were behavioral insomnia of childhood (sleep-onset type) and insomnia due to PDD (Code 327.15) (ICSD-2).27
The findings from our study are consistent with those from Wiggs and Stores' landmark study utilizing actigraphy in children with ASD.5 They identified behavioral insomnia as a predominant sleep disorder in children with ASD. However, they also noted that some children with ASD woke at night and did not alert their parents, which they termed “contented sleeplessness.” This “contented” behavior is not often described in TD children with sleep association disorder. These children often alerted their parents by coming into their bedrooms or crying out at night. This finding highlights the need to investigate all possible causes of insomnia in children with ASD. The contented sleeplessness group supports the possibility that insomnia experienced by children with ASD may not be due to behavior causes alone. In 2006, Malow and colleagues also documented that the predominant sleep disorder in children with ASD could be best described as insomnia, difficulty falling and staying asleep.26
Insomnia Due to PDD—ICSD-2
There are multiple hypotheses for intrinsic causes of sleep disturbances in children with ASD, including abnormal melatonin rates and rhythms, Clock/Clock-related gene anomalies, and synaptic pathway and gene anomalies.28,29 The most robust evidence is related to abnormal melatonin rhythms and peaks.28 Abnormal melatonin levels have been identified in individuals with ASD by multiple investigators.30–34 Melatonin, produced in the pineal gland in the brain, is sensitive to light and plays a role in phase setting the circadian pacemaker.35 Sleep-initiation problems may be due to a late peaking melatonin rhythm. Night wakings and early rising may be a result of reduced melatonin-rhythm amplitudes. In addition, there is emerging evidence of the efficacy of exogenous melatonin in children with ASD.36–38 However, further systematic research is urgently needed to understand the role melatonin plays in sleep disturbances among children with ASD and the safety and efficacy of melatonin as a sleep aid.
In 2002, Clock/Clock-related gene anomalies were suggested as possible contributory factors in the etiology of core deficits of autism involving temporal synchrony and social timing deficits.39 Results from genetic linkage studies from multiplex families implicated chromosome 2q as a possible autism site, and region 2q37 has been found to code for circadian rhythm in humans.39 This hypothesis was further tested in 2007, when 2 Clock gene variants of Per1 and Npas2 were found to be associated with a 110 member autism cohort from Autism Genetic Resource Exchange.40 However, the sample size and lack of significance after corrections for multiple analyses merits further large-scale investigations.28
Another hypothesis is that synaptic pathway and gene anomalies associated with ASD alter levels of monoaminergic neurotransmitters associated with the wake state.28 This arousal dysregulation may be the underlying mechanism of the high levels of agitation, anxiety, and fears experienced by children with ASD and may contribute to their difficulties in initiating and maintaining sleep.28 In this study, parents reported that their children's high levels of anxiety, repetitive thoughts, fears, difficulty with transitions, and hypersensitivity to environmental stimuli may have contributed to prolonged sleep latency, night wakings, and early rising. These findings suggest that children with ASD may be experiencing a “hyperaroused” state similar to that of primary insomnia described in adults.41 Converging areas of recent inquiry suggest that a hyperaroused state may impair cognition and produce behavior symptoms, including inattention, hyperactivity, anxiety, and panic, and may result in insomnia.42 Arousal dysfunction may play a significant role in the high prevalence of sleep disturbances in this population.43,44
Prevalence Data: TD Cohort
The 45% prevalence of mild sleep disturbances in the TD cohort is consistent with previously published studies and highlights pediatric sleep debt as a public health problem of concern.9 Although only 12.5% of the TD cohort parents were worried about their child's sleep, the prevalence of disturbed sleep in the TD cohort was demonstrated by standardized measures. The National Sleep Foundation recommends 10 to 11 hours of sleep per night for children aged 4 to 10 years. However, in this study, sleep-diary data showed a mean sleep duration of 9.8 hours (591 minutes), and actigraphy revealed, on average, 7.8 hours (469 minutes) of sleep. The Sadeh algorithm has a low activity threshold and defines a small number of activity counts in a 1-minute epoch as an awake epoch.44 As a result, the algorithm may be interpreting the child's movement during sleep as wakefulness, thereby underestimating total sleep time. The algorithm has a high sensitivity for sleep and consequently has the best predictive value for sleep.
Despite the limitations of actigraphy as a proxy for sleep, these objective findings are consistent with results from the validated sleep questionnaire, CSHQ, which showed a disturbed sleep prevalence estimate of 45% and elevated sleepiness subscale scores. The high prevalence of mild sleep disturbances in this study suggests that all children should be screened for sleep disturbances during routine pediatric primary care with a validated sleep questionnaire, and parents should be educated on strategies to promote strong sleep behaviors.
Prevalence Data: ASD Cohort
The prevalence of sleep disturbances in our sample of children with ASD was 66%, is within the range of previously published studies, and confirms the endemic sleep problems these families face.3–6 The subjective and objective data showed that, in addition to occurring more frequently, the sleep disturbances in the ASD cohort were more severe than in the TD cohort. The sleep latency for all 3 subgroups was statistically different from the TD cohort, showing that children across the autism spectrum have difficulties falling asleep (Figure 3). The results of this study are consistent with the previously published results of studies that found no correlation between cognitive ability and sleep disturbances.3,46 It is noteworthy that the subjective and objective measures in this study had very similar estimates of the rate of sleep disturbances in the ASD cohort, further supporting the validity of these measures in the ASD population. Future research investigating the options for actigraphy placement should also be pursued.
Medications and Severity of Sleep Disturbances
Children with ASD taking sleep medication had, on average, more disturbed sleep, higher scores on the CSHQ subscales, and higher actigraphy recording scores than did children with ASD not taking sleep medication. These findings suggest that the use of sleep medication is not, on average, restoring sleep to “normal” and is more a predicator of sleep disturbances. However, parents voiced concern that, even though the sleep medications did not resolve the sleep problems, they decreased the severity of their child's sleep problems and improved the quality of their lives.
Conclusions
The prevalence of mild sleep disturbances (45%) in the TD cohort and moderate sleep disturbances (66%) in the ASD cohort were estimated. Analysis of the sleep environment, sleep-hygiene practices, and subjective and objective measures of sleep for each child, in the context of their family, home, and community, provided evidence that the difference in prevalence and severity of sleep disturbances between the 2 cohorts may be a function of the underlying neurobiologic disorder, namely, ASD. The 2 predominant sleep disorders identified in the ASD cohort were behavioral insomnia of childhood—sleep-onset type and insomnia due to PDD (ICSD-2).27 Research for the etiology and treatment of sleep disturbances in children with ASD is urgently needed. Further studies exploring the causal mechanisms of insomnia in children with ASD, as well as intervention studies aimed at improving their sleep through behavior, pharmacologic, and integrative modalities, is warranted. Ultimately, future studies may elucidate the likely complex contributions of genes from multiple loci toward the ASD phenotypes, including sleep patterns and insomnia.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Weaver has received research support from Respironics, Protech, and Cephalon; has consulted for Apnex Medical and Cephalon; and has Functional Outcomes of Sleep Questionnaire (FOSQ) license agreements with Sanofi-Aventis, Merck, Sleep Solutions, N.V. Organon, Apnex Medical, Ventus Medical, GlaxoSmithKline, and Johnson – Johnson Pharmaceutical Research and Development, LLC. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The research was conducted at: The Children's Hospital of Philadelphia, Philadelphia PA.
This manuscript was generated from the dissertation work of the corresponding author, Dr. Margaret C. Souders. There was no financial support.
We would like to offer a heartfelt thanks to all the families that participated in this research study. The commitment these families have for the optimal development of all children is outstanding. Witnessing the tremendous daily effort parents of children with Autism Spectrum Disorders make on their child's behalf continues to inspire us and renews our spirit.
REFERENCES
- 1.DiCicco-Bloom E, Lord C, Zwaigenbaum L, et al. The development neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rapin I, Tuchman RF. Autism: definition, neurobiology, screening, diagnosis. Pediatr Clin North Am. 2008;55:1129–46, viii. doi: 10.1016/j.pcl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Patzold LM, Richdale AL, Tonge BJ. An investigation into sleep characteristics of children with autism and Asperger's Disorder. J Paediatr Child Health. 1998;34:528–33. doi: 10.1046/j.1440-1754.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- 4.Richdale AL. Sleep problems in autism: prevalence, cause, and intervention. Dev Med Child Neurol. 1999;41:60–6. doi: 10.1017/s0012162299000122. [DOI] [PubMed] [Google Scholar]
- 5.Wiggs L, Stores G. Sleep patterns and sleep disorders in children with autistic spectrum disorders: insights using parent report and actigraphy. Dev Med Child Neurol. 2004;46:372–80. doi: 10.1017/s0012162204000611. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Hubbard JA, Fabes RA, Adam JB. Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry Hum Dev. 2006;37:179–91. doi: 10.1007/s10578-006-0028-3. [DOI] [PubMed] [Google Scholar]
- 7.Mindell JA. Empirically supported treatments in pediatric psychology: bedtime refusal and night wakings in young children. J Pediatr Psychol. 1999;24:465–81. doi: 10.1093/jpepsy/24.6.465. [DOI] [PubMed] [Google Scholar]
- 8.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73:405–17. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- 9.Fricke-Oerkermann L, Pluck J, Schredl M, et al. Prevalence and course of sleep problems in childhood. Sleep. 2007;30:1371–7. doi: 10.1093/sleep/30.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–20. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 11.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–52. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 12.Meltzer LJ. Brief report: sleep in parents of children with autism spectrum disorders. J Pediatr Psychol. 2008;33:380–6. doi: 10.1093/jpepsy/jsn005. [DOI] [PubMed] [Google Scholar]
- 13.Honomichl RD, Goodlin-Jones BL, Burnham MM, Hansen RL, Anders TF. Secretin and sleep in children with autism. Child Psychiatry Hum Dev. 2002;33:107–23. doi: 10.1023/a:1020778108068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mindell JA, Emslie G, Blumer J, et al. Pharmacologic management of insomnia in children and adolescents: consensus statement. Pediatrics. 2006;117:e1223–32. doi: 10.1542/peds.2005-1693. [DOI] [PubMed] [Google Scholar]
- 15.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- 16.Myles B, Bock S, Simpson R, editors. Austin, TX: Pro-Ed; 2000. Asperger Syndrome Diagnostic Scale. [Google Scholar]
- 17.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–51. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 18.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- 19.Sadeh A. Assessment of intervention for infant night waking: parental reports and activity-based home monitoring. J Consult Clin Psychol. 1994;62:63–8. doi: 10.1037//0022-006x.62.1.63. [DOI] [PubMed] [Google Scholar]
- 20.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 21.Acebo C, Sadeh A, Seifer R, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22:95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- 22.Rapin I, Katzman R. Neurobiology of autism. Ann Neurol. 1998;43:7–14. doi: 10.1002/ana.410430106. [DOI] [PubMed] [Google Scholar]
- 23.Souders MC, Freeman KG, DePaul D, Levy SE. Caring for children and adolescents with autism who require challenging procedures. Pediatr Nurs. 2002;28:555–62. [PubMed] [Google Scholar]
- 24.Gordis L. Epidemiology. Second Edition. Philadelphia: W.B. Saunders Company, ; 2000. [Google Scholar]
- 25.Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. J Dev Behav Pediatr. 2000;21:27–36. doi: 10.1097/00004703-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep. 2006;29:1563–71. doi: 10.1093/sleep/29.12.1563. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Sleep Medicine. Westchester IL: 2005. The International Classification of Sleep Disorders. [Google Scholar]
- 28.Bourgeron T. The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb Symp Quant Biol. 2007;72:645–54. doi: 10.1101/sqb.2007.72.020. [DOI] [PubMed] [Google Scholar]
- 29.Limoges RJ. Prescriptions denied: pharmacy clauses have become the latest battleground in the provision of safe and legal medical services. Conscience. 2005;26:36–8. [PubMed] [Google Scholar]
- 30.Nir I. Biorhythms and the biological clock involvement of melatonin and the pineal gland in life and disease. Biomed Environ Sci. 1995;8:90–105. [PubMed] [Google Scholar]
- 31.Kulman G, Lissoni P, Rovelli F, Roselli MG, Brivio F, Sequeri P. Evidence of pineal endocrine hypofunction in autistic children. Neuro Endocrinol Lett. 2000;21:31–4. [PubMed] [Google Scholar]
- 32.Tordjman S, Anderson GM, Pichard N, Charbuy H, Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol Psychiatry. 2005;57:134–8. doi: 10.1016/j.biopsych.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Melke J, Goubran Botros H, Chaste P, et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol Psychiatry. 2008;13:90–8. doi: 10.1038/sj.mp.4002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jan JE, Freeman RD. Melatonin therapy for circadian rhythm sleep disorders in children with multiple disabilities: what have we learned in the last decade? Dev Med Child Neurol. 2004;46:776–82. doi: 10.1017/s0012162204001331. [DOI] [PubMed] [Google Scholar]
- 35.Rechtschaffen A. Current perspectives on the function of sleep. Perspect Biol Med. 1998;41:359–90. doi: 10.1353/pbm.1998.0051. [DOI] [PubMed] [Google Scholar]
- 36.Garstang J, Wallis M. Randomized controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child Care Health Dev. 2006;32:585–9. doi: 10.1111/j.1365-2214.2006.00616.x. [DOI] [PubMed] [Google Scholar]
- 37.Andersen IM, Kaczmarska J, McGrew SG, Malow BA. Melatonin for insomnia in children with autism spectrum disorders. J Child Neurol. 2008;23:482–5. doi: 10.1177/0883073807309783. [DOI] [PubMed] [Google Scholar]
- 38.Wasdell MB, Jan JE, Bomben MM, et al. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J Pineal Res. 2008;44:57–64. doi: 10.1111/j.1600-079X.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 39.Wimpory D, Nicholas B, Nash S. Social timing, clock genes and autism: a new hypothesis. J Intellect Disabil Res. 2002;46:352–8. doi: 10.1046/j.1365-2788.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- 40.Nicholas B, Rudrasingham V, Nash S, Kirov G, Owen MJ, Wimpory DC. Association of Per1 and Npas2 with autistic disorder: support for the clock genes/social timing hypothesis. Mol Psychiatry. 2007;12:581–92. doi: 10.1038/sj.mp.4001953. [DOI] [PubMed] [Google Scholar]
- 41.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 42.Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–45. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutt C, Hutt SJ, Lee D, Ounsted C. Arousal and childhood autism. Nature. 1964;204:908–9. doi: 10.1038/204908a0. [DOI] [PubMed] [Google Scholar]
- 44.Minderaa RB, Anderson GM, Volkmar FR, Akkerhuis GW, Cohen DJ. Noradrenergic and adrenergic functioning in autism. Biol Psychiatry. 1994;36:237–41. doi: 10.1016/0006-3223(94)90605-x. [DOI] [PubMed] [Google Scholar]
- 45.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 46.Richdale AL, Prior MR. The sleep/wake rhythm in children with autism. Eur Child Adolesc Psychiatry. 1995;4:175–86. doi: 10.1007/BF01980456. [DOI] [PubMed] [Google Scholar]