Abstract
Study Objectives.
To describe the semiological features of NREM arousal parasomnias in detail and identify features that can be used to reliably distinguish parasomnias from nocturnal frontal lobe epilepsy (NFLE).
Design.
Systematic semiologial evaluation of parasomnias and NFLE seizures recorded on video-EEG monitoring.
Patients.
120 events (57 parasomnias, 63 NFLE seizures) from 44 subjects (14 males).
Interventions.
The presence or absence of 68 elemental clinical features was determined in parasomnias and NFLE seizures. Qualitative analysis of behavior patterns and ictal EEG was undertaken. Statistical analysis was undertaken using established techniques.
Results.
Elemental clinical features strongly favoring parasomnias included: interactive behavior, failure to wake after event, and indistinct offset (all P < 0.001). Cluster analysis confirmed differences in both the frequency and combination of elemental features in parasomnias and NFLE. A diagnostic decision tree generated from these data correctly classified 94% of events. While sleep stage at onset was discriminatory (82% of seizures occurred during stage 1 or 2 sleep, with 100% of parasomnias occurring from stage 3 or 4 sleep), ictal EEG features were less useful. Video analysis of parasomnias identified three principal behavioral patterns: arousal behavior (92% of events); non-agitated motor behavior (72%); distressed emotional behavior (51%).
Conclusions
Our results broadly support the concept of confusion arousals, somnambulism and night terrors as prototypical behavior patterns of NREM parasomnias, but as a hierarchical continuum rather than distinct entities. Our observations provide an evidence base to assist in the clinical diagnosis of NREM parasomnias, and their distinction from NFLE seizures, on semiological grounds.
Citation:
Derry CP; Harvey AS; Walker MC; Duncan JS; Berkovic SF. NREM arousal parasomnias and their distinction from nocturnal frontal lobe epilepsy: a video EEG analysis. SLEEP 2009;32(12):1637-1644.
Keywords: Parasomnia, sleep terror, confusional arousal, somnambulism, nocturnal frontal lobe epilepsy, EEG
NREM AROUSAL PARASOMNIAS ARE PAROXYSMAL BEHAVIORS WITHOUT CONSCIOUS AWARENESS, USUALLY ARISING FROM STAGE 3 OR 4 NREM SLEEP. THEY are classically subdivided into three main forms: confusional arousals are associated with little motor or autonomic involvement; somnambulism is associated with motor activity but little autonomic involvement; and sleep terrors involve prominent autonomic involvement with variable motor activity.1,2 While parasomnias are generally benign, frequent or unusual episodes may sometimes be confused with epilepsy, particularly nocturnal frontal lobe epilepsy (NFLE).3–5
In cases of diagnostic uncertainty video-EEG monitoring may be required. Even this, however, may not result in a confident diagnosis, as interictal and ictal EEG findings are frequently unremarkable or nonspecific in both parasomnias and NFLE.5,6 In practice, diagnosis is often based primarily on the ictal semiology (that is, the ictal symptoms, signs, and behaviours) of recorded events. However, although frontal lobe seizure semiology has been extensively described.5,7–9 the semiology of parasomnias has not. Initial neurophysiological studies of the parasomnias contained general descriptions, but predated video-EEG monitoring technology and the recognition of NFLE10–12; more recent reports contain only limited semiological information.6,13 Thus, while the broad behavioral characteristics of parasomnias are accepted, surprisingly little detail of their ictal manifestations is known.
In this study we have compiled a video-EEG monitoring series with the aim of accurately describing the semiological features of the NREM arousal parasomnias. In addition we have directly compared these with NFLE, providing an evidence base for the accurate diagnosis of these disorders on semiological grounds. This knowledge is important for the increasingly common clinical situation of diagnosing attacks recorded by family members on home videos or mobile phone cameras.14
METHODS
Subjects
Subjects were recruited from three centers (National Hospital for Neurology and Neurosurgery, London; Austin Hospital, Melbourne and Royal Children's Hospital, Melbourne). Diagnoses were made on the basis of historical, imaging and video-EEG findings. All subjects had at least one habitual sleep-related event recorded.
Parasomnias are quasi-physiological phenomena with no “gold standard” biological marker. Investigations in NFLE, including ictal EEG, are also frequently nondiagnostic.5 In order to avoid circularity in this study (i.e., simply describing clinical features already used subjectively to define these disorders) we adopted stringent exclusion and inclusion criteria.
Subjects with parasomnias were included only if:
(i) history was consistent with a diagnosis of parasomnias according to a validated diagnostic scale15
(ii) there was consensus agreement regarding the diagnosis by all clinicians involved in the management (neurophysiologists and neurologists with experience in sleep disorders and epilepsy), following review of all diagnostic information.
Subjects were excluded if there was any evidence of epilepsy, specifically: a history of paroxysmal events suggestive of seizures (other than nocturnal episodes under investigation); epileptiform abnormalities on EEG; cerebral lesions on neuroimaging.
Subjects were included in a comparison group of “pure” NFLE (for parallel analysis) if they fulfilled all the following criteria:
(i) the history was compatible with a diagnosis of NFLE according to a validated clinical scale15
(ii) there was consensus agreement regarding the diagnosis by the clinicians involved in the patient's management (neurophysiologists and neurologists with experience in sleep disorders and epilepsy), following review of all diagnostic information.
(iii) ≥ 1 of the following biological correlates was present (with more than one being present in some subjects): an epileptogenic frontal lobe lesion on neuroimaging (e.g., tumor or dysplasia, 14% of subjects); an established diagnosis of autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) with a proven genetic mutation (14% of subjects); a robust ictal rhythm (evolving rhythmic fast, sharp, or spike-wave activity) on EEG during the episodes (38% of subjects); or an ictal SPECT scan demonstrating focal frontal hyperperfusion (43% of subjects).
(iv) ≥ 90% of the subject's seizures arose from sleep.
Patients with NFLE who had no definite biological correlate on investigations, or who had convulsive or diurnal seizures, were excluded to ensure that the comparison group was robust and accurately reflected those NFLE seizures which cause diagnostic confusion in practice.
Video EEG Monitoring
Simultaneous video-EEG data were acquired during nocturnal events in all subjects. EEG was recorded using at least 21 electrodes, placed according to the International 10-20 system, with single-channel ECG. Data from additional EEG channels, chin EMG, and EOG were recorded some patients.
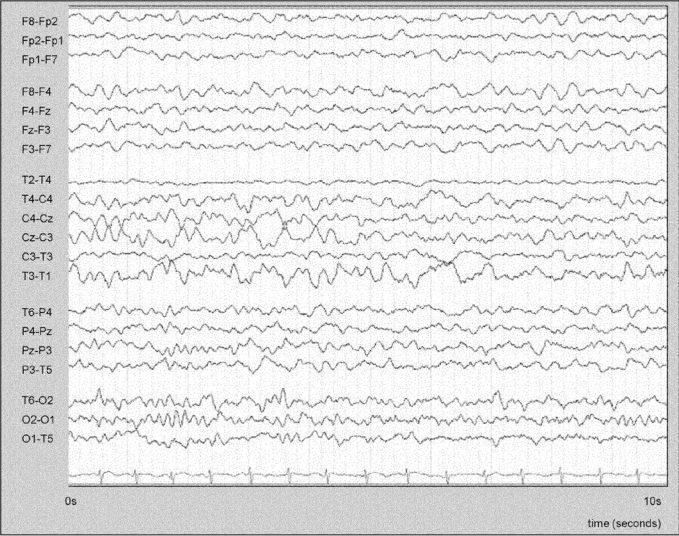
Ictal EEG findings were categorized as: prominent artifact; partial arousal to lighter sleep; dissociative pattern (posterior dominant rhythm plus simultaneous sleep patterns in anterior leads, Figure 3); rhythmic slow activity; focal or diffuse attenuation; robust ictal rhythm (recruiting rhythm or rhythmic spike or spike-wave ictal discharges). These categories were not mutually exclusive.
Figure 3.
EEG (transverse montage) during a prolonged parasomnia, showing a dissociation pattern; a clear α rhythm is seen in posterior channels consistent with the subject's posterior dominant rhythm, with anterior and midline theta activity and vertex sharp activity consistent with light NREM sleep. Timescale: 1 page = 10 seconds.
Video data for each event were reviewed in detail. The presence or absence of 68 elemental clinical features was recorded, using an approach similar to that used previously in seizure semiology studies.16,17 These features were initially generated following analysis of the literature and modified following a pilot study of 9 subjects. They were grouped into 6 categories: respiratory; autonomic; simple motor; complex motor; vocal; and others. A comprehensive list of features examined is included in Supplementary Table 1. Each event was analyzed by 2 observers (CD and ASH or MW). In individuals with multiple recorded events, only data from the first 3 were included in the study.
The total number of events and the maximum number of events per night were recorded for each subject. For each event, total duration and duration of arousal behaviors preceding the major motor behaviors were recorded. As parasomnias may be ameliorated by an unfamiliar sleeping environment,12,18 the occurrence or non-occurrence of events on the first night of monitoring was documented.
Statistical Analysis
For elemental clinical features, data for a binary outcome (parasomnias or NFLE) were analyzed using logistic regression. For each subject, the number of events was the denominator and the number of events showing the feature of interest was the numerator; this ensured statistical independence between the observations, effectively generating a single observation for each subject.
Logistic regression could not be applied for those features which never occurred in one group. In such cases, the association between group and feature of interest was analyzed by recording whether a subject demonstrated the feature of interest in any event, and using Fisher exact test to compare between NFLE and parasomnias. This approach also assessed the strength of association at the “subject” level, not the “event” level, preserving statistical independence between observations.
For continuous variables, median values were compared between the NFLE and parasomnia groups using the Mann-Whitney U test.
As recommended by several authorities,19,20 no statistical adjustments were made for multiple comparisons, but the dataset with all comparisons is presented in its entirety (Supplementary Table 1).
The natural grouping of NFLE seizures and parasomnias according to elemental clinical features was examined with cluster analysis on SPSS software using Ward's method with Euclidean squared distance measurements. This hierarchical cluster technique has previously been adopted in studies of seizure semiology.17 Internal validation was undertaken using k-means cluster analysis, an alternative, non-hierarchical technique.
A model for event classification based on elemental clinical features was generated using the exhaustive CHAID decision tree algorithm21 on SPSS Answer Tree 3.0 software.
RESULTS
Video EEG monitoring data from 120 nocturnal events (57 parasomnias, 63 NFLE seizures) in 44 patients (23 with parasomnias, 21 with NFLE) were studied. The general characteristics of the subjects studied, the events recorded and the ictal EEG features are summarized in Table 1. Video examples of nocturnal events are included as supplemental online material.
Table 1.
General Patient Characteristics and EEG Features in Parasomnia and NFLE Groups
| Subject/ event characteristic | NREM parasomnia group | NFLE comparison group | P value |
|---|---|---|---|
| Subjects: | |||
| Number of subjects | 23 | 21 | |
| Median age at onset (range) | 8.5 years (range 1.5–39 years) | 7.0 years (range 0.5–34 years) | n.s. |
| Median age at video-EEG monitoring (range) | 12 years (range 4–69 years) | 21 years (range 3–38 years) | n.s. |
| Male sex (%) | 14 (61%) | 13 (62%) | n.s. |
| Events: | |||
| Total number of recorded during monitoring | 57 | 63 | |
| Median number of recorded events per subject | 2 | 8 | P <0.001 |
| Percentage of individuals with events on first night of monitoring | 42% | 76% | P = 0.03 |
| Median number of events recorded per night (range) | 2 (1–5) | 7 (1–10) | P <0.001 |
| Median event duration (range) | 60 sec (range 11s–14 min) | 37 sec (range 9 s – 125 s) | P <0.001 |
| Ictal EEG findings: | |||
| Stage of sleep at onset: | |||
| NREM stage 1 or 2 | 0% | 87% | P <0.001 |
| NREM stage 3 or 4 | 100% | 13% | P <0.001 |
| REM | 0% | 0% | |
| Robust ictal rhythm (%age of subjects) | 0% | 38% | P <0.001 |
| Light sleep patterns during event (% of subjects) | 52% | 0% | P <0.001 |
| Dissociative pattern* during event (% of subjects) | 27% | 0% | P <0.001 |
| Rhythmic non-epileptiform slow activity (% of subjects) | 52% | 61% | n.s. |
| Focal or diffuse attenuation of EEG amplitude (% of subjects) | 39% | 43% | n.s. |
| Prominent muscle/ movement artefact (% of subjects) | 93% | 87% | n.s. |
n.s. = not statistically significant.
evidence of EEG state dissociation was defined as posterior dominant α rhythm (suggestive of resting wakefulness) in posterior channels, with simultaneous sleep patterns in anterior channels (Figure 3).
The results are presented here in 4 sections: firstly, a comparison of the elemental clinical features seen in the 2 groups, including statistical analyses; secondly, a comparison of the various temporal aspects of parasomnias (onset, progression, offset) with those of NFLE; thirdly, a description of the broad behavior patterns seen in parasomnias; and finally, an analysis of EEG findings.
1. Elemental Clinical Features
Sixty-nine elemental features were analyzed in 120 events. Features strongly favoring a diagnosis of parasomnias included crying or sobbing, waxing and waning quality, physical or verbal interaction with the environment, modification of the event by individuals present, coherent speech in sentences, and “normal” arousal behaviors such as scratching and face rubbing (all P < 0.001). In contrast, bicycling movements, thrashing, grunting, grimacing, and dystonic posturing clearly favored NFLE (all P < 0.001). The full dataset with comparative statistics is displayed in Supplementary Table 1.
To objectively assess the grouping of elemental features, Ward's cluster analysis was applied to the data set. This divided the 120 nocturnal events into two major groups (using visual inspection of the dendrogram output and graphical examination of cluster coefficient against cluster number). Internal validation using k-means analysis was subsequently performed for 2 clusters, giving 97% concordance with Ward's method. The clusters identified corresponded to a parasomnia group and an NFLE group. Overall, 91% of nocturnal events were correctly categorized using Ward's method.
The diagnostic classification tree, based on video features only, is shown in Figure 1. In our dataset this algorithm correctly classified 113 of 120 (94%) events.
Figure 1.
Results of the exhaustive CHAID Tree Analysis for the diagnosis of nocturnal events. This algorithm correctly identified 94% of the 120 nocturnal events in the study.
As the individual, clinical features do not adequately portray the semiology of parasomnias, detailed descriptions, and comparison with NFLE, follow.
2. Temporal Aspects of Parasomnias
(i) Onset
Parasomnias usually began with arousal behavior (79% of events), comprising eye opening, head elevation and staring or looking around. This lasted from 2 sec to several minutes, and in 65% of subjects was followed by more dramatic manifestations. Less commonly (21%) there was an explosive motor onset (typically sitting forward with a frightened expression, agitated searching behaviors, and distressed coherent speech).
Events were triggered by a clear external stimulus (such as a noise) or an internal stimulus (such as a cough or snore) in 39% of parasomnias. Tachycardia was almost universal at onset.
Comparison with NFLE.
Brief arousal behaviors, indistinguishable from those in parasomnias, also preceded the major behaviors in 49% of NFLE seizures (P = 0.10); these were of comparable duration in the two conditions (median 7 seconds). Abrupt onset with no preceding arousal was seen in 51% of NFLE seizures (video 1), indistinguishable from that seen in 21% of parasomnias. Seizures were also universally associated with tachycardia, but triggers (such as noise) were identified in only 8%.
(ii) Progression
Evidence of increasing interaction during parasomnias was common. At onset, there was typically minimal interactive behavior or speech, but these often developed as the event progressed. Over one-third of parasomnias were modified (exacerbated or terminated) by the actions of individuals present; 39% of events had a waxing and waning intensity.
Comparison with NFLE.
Environmental interaction was present in 11% of seizures, but was usually simple (e.g., repeatedly grabbing bedsides) and did not increase through the event (video 1). Coherent speech was unusual in NFLE, and when present was frenetic without a discernible interactive quality. Seizures did not wax and wane in intensity.
(iii) Offset
Parasomnias terminated with either full wakefulness (26%) or light NREM sleep (74%). A clear and distinct offset was uncommon (16% of events). Subjects who did not waken fully usually showed a “tapering off” of motor behavior and rapid return to slow wave sleep. In subjects who did wake, the precise point at which full “normal” consciousness was reached was difficult to determine.
Comparison with NFLE.
The offset pattern in NFLE was strikingly different to that of parasomnias, with 88% of seizures ending in full wakefulness and 76% showing distinct offset (video 1). All events, with the exception of some episodes of brief dystonic posturing only, caused the subject to fully waken.
3. Behavior Patterns and Combinations of Behavior Patterns in Parasomnias
A relatively narrow repertoire of behaviors was seen, and recorded events were often shorter and less elaborate than those described in the history; this contrasted with NFLE, in which the historical accounts and recorded events were very similar. We identified 3 fundamental patterns of behavior in parasomnias, with most events (79%) comprising a composite of more than one pattern.
The first pattern comprised arousal behaviors (video 2) and was seen at some stage in almost all events (92%), typically around onset or offset. Simple arousal behaviors included eye opening, head elevation, and staring; face rubbing, yawning, scratching, moaning and mumbling also sometimes occurred. In some subjects hypnic jerks precipitated events, and in others trembling or shivering formed occurred throughout the parasomnia (video 3).
The second pattern, non-agitated motor behavior, was present in 72% of events. This comprised predominantly sitting forward, manipulation of nearby objects (such as EEG equipment), and searching behaviors (e.g., looking over the side of the bed) (videos 4, 5, 6). Although standing and walking on the bed were occasionally observed, no frank somnambulism occurred as subjects were restrained by EEG equipment or other individuals. Facial expression was impassive or perplexed. Speech, typically coherent, was common. A clear interactive component to speech and behavior was frequently observed (44% of events).
The third pattern comprised distressed emotional behavior (51% of events), with predominantly fearful behavior, facial expression and speech content (e.g., “they're going to kill me,” video 8). Sitting or standing, screaming, and frantic searching, recoiling or evasive behaviors (video 7), were prominent. Attempts to console or restrain the subject were often resisted, sometimes provoking aggressive responses such as hitting or foot stamping. In some events, inconsolable sobbing and anguish (rather than fear) were prominent. All behaviors in this pattern reflected “negative” emotions (fear or anguish), with concordant facial expression, speech and motor activity.
The three behavior patterns occurred in various combinations and sequences with an apparent hierarchy (Figure 2). The most common combinations observed were broadly, but not entirely, congruent with traditional subclassifications. The first combination (19% of events; Figure 2, panel i), would be traditionally described as confusional arousal, with prolonged arousal behaviors only. The second combination (35% of recorded events; Figure 2 panel ii) often started and ended with arousal behaviors, but non-agitated motor behavior predominated. This could be classified somnambulism (although standing and walking were uncommon). The third combination (26% of events; Figure 2 panel iii) was dominated by distressed emotional behavior, sometimes with an explosive onset, and would be traditionally classified sleep terrors. The fourth and final combination (19% of events; Figure 2 panel iv) observed was not classifiable using the classical subtypes. These episodes contained all 3 behavior patterns, alternating in a waxing and waning fashion over a prolonged period (in some subjects up to 14 minutes).
Figure 2.
Schematic representation of common parasomnias, displayed as hierarchical combinations of the 3 fundamental behavior patterns on the y axis, and time (typically 1-10 min) on the x axis. Panel 1 represents a typical confusional arousal, comprising only normal arousal behaviours but of abnormal duration (19% of recorded events); panel 2 shows classical somnambulism with non-agitated motor behaviour, and normal arousal behaviours at onset, offset or both (35% of recorded events); panel 3 represents typical sleep terrors, with predominantly negative emotional behaviour often of sudden onset; calm motor and normal arousal behaviours are usually also seen during these events, either at onset or offset (26% of events); panel 4 is a mixed type, but comprising waxing and waning of the four behaviour types (19% of events). All events usually start in stage 3 or 4 NREM sleep, and end either in wakefulness or lighter NREM sleep. Sometimes episodes are brief (solid lines) and at other times prolonged (hatched lines).
4. EEG Findings
Both parasomnias and seizures arose exclusively from NREM sleep. In contrast to parasomnias, most seizures arose from light NREM sleep (Table 1). Definitive ictal rhythms were seen in 38% of NFLE subjects, although this figure may be artificially high due to our inclusion criteria (see Methods). In 52% of individuals with parasomnias, light sleep patterns (vertex waves, sleep spindles, and θ activity) were seen at some point during events; in a subgroup of these (27% of subjects) evidence of EEG state dissociation was seen at some point, with posterior dominant α rhythm suggestive of resting wakefulness in posterior electrodes but anterior θ activity, and sometimes vertex waves or spindles, consistent with light sleep (Figure 3). These findings were not seen in NFLE seizures (Table 1).
Other findings showed considerable overlap between NFLE and parasomnias; muscle and movement artifact, rhythmic non-epileptiform θ or δ activity (arousal patterns) over the anterior quadrants, and diffuse attenuation in EEG amplitude (a manifestation of seizure onset or state change) were all common findings in both conditions and did not have significant discriminatory value (Table 1).
DISCUSSION
The differential diagnosis of nocturnal seizures and parasomnias is often successfully achieved based on the clinical context, the timing and frequency of events, and results of EEG and polysomnography. In challenging cases, however, the diagnosis depends upon an analysis of ictal semiology of events recorded on video-EEG monitoring. Even this may not yield a clear diagnosis, largely because the features of parasomnias (as compared to that of nocturnal frontal lobe seizures), although well known in outline, have not been adequately described in detail. In this study we have addressed this issue by analyzing the semiology of the NREM arousal parasomnias in detail, and identifying important features which help distinguish them from the seizures of NFLE.
Parasomnias: Broad Concepts
NREM arousal parasomnias are traditionally grouped into distinct types (confusional arousals, sleep terrors and somnambulism), and the results of this study partially support this classification. The 3 basic behavior patterns were observed, but with individual events usually comprising a combination of these behavior patterns. The behavior patterns were observed in a hierarchical fashion (Figure 2), with arousal behaviors being the fundamental component. The next level of behavior is abnormal but non-agitated motor behavior, and the third and most abnormal tier is distressed emotional behavior (either fearful or anguished in nature). Although any pattern may predominate, it is rare for a single pattern to occur in isolation; components from the lower level are almost always present at some stage (92% of our series). In other words, while an event may comprise arousal behaviors alone, non-agitated motor behaviors are not seen without arousal features, and agitated activity is not seen without both arousal and non-agitated motor behavior. While clinical events are often broadly consistent with the three traditional subtypes, the labels of sleep terrors, somnambulism and confusional arousal are an oversimplification. Furthermore, they give the false impression of 3 nosologically and biologically distinct entities. Rather, NREM parasomnias comprise a composite of the 3 main behavioral patterns, on a hierarchical continuum, the proportion of which may vary between events and patients.
Semiology of NFLE and Parasomnias: Useful Features and Potential Pitfalls
In general, despite the variety of behaviors reported in parasomnias, the observed behavioral repertoire was relatively small. This is possibly because the restrained environment of video-EEG monitoring restricts elaboration of complex behaviors, including somnambulism. Discrepancies between historical account and recorded events were prominent in parasomnias, whereas in NFLE the history and video-EEG findings were generally concordant.
Cluster analysis, based on the presence or absence of elemental behaviors, divided events relatively cleanly into seizures and parasomnias. This suggests broad semiological differences clearly exist between these 2 biologically distinct conditions. The clinical decision tree generates a diagnostic model using features of greatest discriminatory value (Figure 1). This provides a framework for analyzing video recordings of nocturnal events, correctly identifying 94% of events in our series, and with the other potentially discriminatory features, may be useful in the assessment of video-EEG monitoring data or home video recordings. Although the onset of nocturnal events is often missed on home video recordings, our study indicated that onset behaviors were not discriminatory between parasomnias and NFLE seizures, in contrast to the evolution and the offset of the events.
Characteristics which may have diagnostic value are summarized in Table 2. These conclusions would, however, benefit from further prospective validation, as our deliberate methodological strategy of using “pure cultures” of NFLE and parasomnias, while minimizing the risk of misdiagnosis, may have excluded part of the spectrum of nocturnal events and artificially separated NFLE and parasomnias.
Table 2.
Important Quantitative and Qualitative Features which can be Used in the Positive Identification of Parasomnias
| Features strongly favoring parasomnias | Features moderately favoring parasomnias | Features which do not discriminate between parasomnias and NFLE |
|---|---|---|
| Yawning | Tremor/ trembling | Brevity |
| Scratching and prominent nose-rubbing | Myoclonic jerks | Sitting |
| Rolling over in bed | Coughing | Standing or walking* |
| Internal or external trigger (noise, cough, snore) | Semipurposeful behaviors, fumbling, manipulation of nearby objects | Preceding “normal” arousal |
| Waxing and waning pattern | Variability/ absence of stereotypy | Brief arousals (up to 10 seconds) without definite semiological features of epilepsy |
| Physical or verbal interaction | No events recorded on first night of monitoring | Fearful emotional behavior |
| Sobbing, sad emotional behavior | Few events recorded in total (less than 3) | |
| Indistinct offset | ||
| Failure to fully arouse after event with complex behavior | ||
| Prolonged duration ( > 2 minutes) | ||
| Discordance between severity and duration of reported event and recorded event |
Standing and walking do not, in usual circumstances, discriminate between parasomnias and NFLE. However, in individuals who rouse to full wakefulness after their events, and in whom events have an indistinct offset, standing or walking suggests a diagnosis of parasomnias over NFLE (see decision tree algorithm).
Three important potential diagnostic pitfalls in the assessment of nocturnal events were identified:
Firstly, the diagnosis of recurrent brief arousals from sleep may be extremely difficult. Paroxysmal arousals are a recognized presentation of NFLE, in which they are typically highly stereotyped and regarded as “fragments” of habitual seizures.4,5,22–25 However, other, non-epileptic conditions (such as restless legs syndrome) may also cause recurrent arousals. Although individuals with brief arousals alone were not included in this study, our observations of the initial arousal behaviors in both parasomnias and NFLE suggest that these behaviors are often indistinguishable in the 2 conditions (video 9). It has previously been suggested that it may be impossible, even for experts, to differentiate brief epileptic motor events in NFLE from physiological arousals,26–28 and our findings support this. It seems likely that in some situations, brief subclinical seizures may act simply as an arousing stimulus, producing arousal behaviors without specific ictal epileptic behaviors. Again, this is concordant with published stereo-EEG data indicting that non-epileptic arousal behaviors in NFLE may be associated with underlying epileptiform activity.27–29 A conceptual framework illustrating the proposed relationship of arousal behaviors, NFLE and parasomnias is shown in Figure 4. An alternative view, not necessarily mutually exclusive to this, is that arousals and even brief motor events in NFLE are not always epileptic seizures in themselves. Rather the epileptic process, at particular times in the cyclic alternating pattern observed on EEG, causes a disorder of arousal mechanisms with nonspecific cortical disinhibition; a similar disorder of arousal mechanisms may occur in parasomnias. Both conditions, therefore, result in the occurrence of innate motor patterns during sleep, generated by central pattern generators.30–32 From a practical perspective, these finding suggests that accurate diagnosis of isolated recurrent arousals may not be possible on semiological grounds alone.
Figure 4.
Schematic representation of the postulated relationship of arousal behavior to parasomnias and nocturnal seizures. During sleep, physiological stimuli (external or internal) or subclinical seizure discharges can induce indistinguishable arousal behaviors. In parasomnia subjects, these may evolve (heavy black arrows) to a clinical parasomnia or terminate with return to sleep or full waking. In individuals with nocturnal epilepsy (heavy grey arrows), clinically evident seizures with distinguishing characteristic behaviours and marked stereotypy may occur, or the event may terminate with full waking or return to sleep; it appears possible that subclinical seizures may also induce clinical parasomnias (dashed gray arrow).
Secondly, postictal behaviors, when present in NFLE, showed striking similarities in quality and nature to parasomnias. Staring, looking around, semipurposeful fumbling and partially interactive speech were all seen postictally in NFLE (video 10). It is therefore important to bear this in mind when reviewing videos in which the early features of the event may not have been captured.
Finally, although NFLE and NREM parasomnias are the most frequently confused nocturnal motor events, there are other conditions which may sometimes present in a similar way and should be considered within a differential diagnosis. These include REM behavior disorder, obstructive sleep apnea (which may also exacerbate parasomnias) and gastroesophageal reflux.33,34
Despite these caveats, the data presented indicate that parasomnias and NFLE usually have distinct semiological features, and provide an evidence base to assist in confident clinical diagnosis of these conditions.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank Ms Catherine Scott (EEG Technologist, National Hospital for Neurology and Neurosurgery, London), Ms Catherine Bailey (EEG Technologist, Royal Children's Hospital, Melbourne) and the EEG technologists at the Austin Hospital, Melbourne, for their assistance with this work, as well as A/Prof Ian Gordon of the University of Melbourne for statistical advice. This work was financially supported by the Jack Brockoff Foundation (Australia) and the National Society for Epilepsy (UK).
All coauthors have read and agree with the contents of this manuscript. The authors declare no financial interests. Informed consent was obtained from all individuals interviewed for the purposes of this study, and releases have been obtained for all video recordings presented. The submission is not under review at any other publication
Supplemental Table 1.
Frequencies of elemental semiological features recorded in parasomnias and NFLE. The table is ordered according to the strength of association of each feature to diagnosis. Raw percentages of events in each group showing the feature are given. The statistical significance of these values, according to binary logistic regression or Fisher's exact test, accounts for the number of events in each subject as described in the methods section. The logistic regression coefficient (B), Wald statistic, odds ratio and uncorrected p values are given for those elemental features analysed using logistic regression. For those assessed using Fisher's exact test, p values only are given. Light shading represents p values of 0.05 – 0.001; dark shading represents p values of < 0.001.
Supplementary Videos are available online at www.journalsleep.org
Seizure in NFLE, with vocalisation and apparent fear. Note the abrupt onset and offset, and the minimally interactive quality of speech and behaviour.
Brief segment of normal arousal behaviour in a parasomnia. The subject rouses, looks around and mumbles; other than the prolonged durationof the behaviour and electrographic sleep on EEG this is normal arousal behaviour.
Segments of a prolonged parasomnia. Onset is with confused mumling and swearing, with some non-agitated motor behaviour. This alternates with emotional distress through the event. At one point, tremor and some low amplitude myoclonic jerks are seen.
Non-agitated motor behaviour. The subject sits forward, and licks his hands apparently purposefully during sleep.
Parasomnia, triggered by a snore, with brief apparent fear and vocalisation, followed by apparently purposeful fumbling with nearby objects whilst asleep by electrographic criteria.
Early somnambulism. The subject stands but is restricted by EEG leads and does not leave the bed.
Apparently fearful behaviour at arousal, with the patient backing away from an unseen object, but without vocalisation. This rapidly settles to non-agitated behaviour.
Fearful and distressed speech, with clear interaction, during a prolonged parasomnia.
Nocturnal frontal lobe seizure,with clear dystonic posturing, but commencing with several seconds of arousal behaviour; this arousal is indistinguishable from the onset of many parasomnias.
Postictal behaviour mimicking parasomnias. The seizure itself is quite subtle, with eye opening and repeated left arm flexion and extension. The video clip shows the last few seconds of the seizure, which was associated with a robust ictal rhythm. This is followed be postictal behaviour with sitting forward, searching, fumbling with EEG electrodes and some oral automatisms. The beahviour looks very similar to many parasomnias.
Footnotes
Funding Sources: This study was financially supported by the Jack Brockhoff Foundation (Australia) and the National Society for Epilepsy (UK).
REFERENCES
- 1.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Mahowald MW. Arousal and sleep-wake transition parasomnias. In: Lee-Chiong TJ, Sateia MJ, Carskadon MA, editors. Sleep medicine. Philadelphia: Hanley and Belfus; 2002. pp. 207–13. [Google Scholar]
- 3.Montagna P. Nocturnal paroxysmal dystonia and nocturnal wandering. Neurology. 1992;42:61–7. [PubMed] [Google Scholar]
- 4.Provini F, Plazzi G, Montagna P, Lugaresi E. The wide clinical spectrum of nocturnal frontal lobe epilepsy. Sleep Med Rev. 2000;4:375–86. doi: 10.1053/smrv.2000.0109. [DOI] [PubMed] [Google Scholar]
- 5.Provini F, Plazzi G, Tinuper P, et al. Nocturnal frontal lobe epilepsy. A clinical and polygraphic overview of 100 consecutive cases. Brain. 1999;122(Pt 6):1017–31. doi: 10.1093/brain/122.6.1017. [DOI] [PubMed] [Google Scholar]
- 6.Zucconi M, Ferini-Strambi L. NREM parasomnias: arousal disorders and differentiation from nocturnal frontal lobe epilepsy. Clin Neurophysiol. 2000;111(Suppl 2):S129–35. doi: 10.1016/s1388-2457(00)00413-2. [DOI] [PubMed] [Google Scholar]
- 7.Williamson PD, Spencer DD, Spencer SS, et al. Complex partial seizures of frontal lobe origin. Ann Neurol. 1985;18:497–504. doi: 10.1002/ana.410180413. [DOI] [PubMed] [Google Scholar]
- 8.Waterman K, Purves SJ, Kosaka B, et al. An epileptic syndrome caused by mesial frontal lobe seizure foci. Neurology. 1987;37:577–82. doi: 10.1212/wnl.37.4.577. [DOI] [PubMed] [Google Scholar]
- 9.Salanova V, Morris HH, Van Ness P, et al. Frontal lobe seizures: electroclinical syndromes. Epilepsia. 1995;36:16–24. doi: 10.1111/j.1528-1157.1995.tb01659.x. [DOI] [PubMed] [Google Scholar]
- 10.Broughton RJ. Sleep disorders: disorders of arousal? Science. 1968;159:1070–8. doi: 10.1126/science.159.3819.1070. [DOI] [PubMed] [Google Scholar]
- 11.Gastaut H, Broughton, R A clinical and polygraphic study of episodic phenomena during sleep. Recent Adv Biol Psychiatry. 1965;7:197–221. [Google Scholar]
- 12.Jacobson A, Kales A, Lehmann D, Zweizig JR. Somnambulism: all-night electroencephalographic studies. Science. 1965;148:975–7. doi: 10.1126/science.148.3672.975. [DOI] [PubMed] [Google Scholar]
- 13.Kavey NB, Whyte J, Resor SR, Jr, Gidro-Frank S. Somnambulism in adults. Neurology. 1990;40:749–52. doi: 10.1212/wnl.40.5.749. [DOI] [PubMed] [Google Scholar]
- 14.Tinuper P, Provini F, Bisulli F, et al. Movement disorders in sleep: guidelines for differentiating epileptic from non-epileptic motor phenomena arising from sleep. Sleep Med Rev. 2007;11:255–67. doi: 10.1016/j.smrv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Derry CP, Davey M, Johns M, et al. Distinguishing sleep disorders from seizures: diagnosing bumps in the night. Arch Neurol. 2006;63:705–9. doi: 10.1001/archneur.63.5.705. [DOI] [PubMed] [Google Scholar]
- 16.Kotagal P, Arunkumar G, Hammel J, Mascha E. Complex partial seizures of frontal lobe onset statistical analysis of ictal semiology. Seizure. 2003;12:268–81. doi: 10.1016/s1059-1311(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 17.Manford M, Fish DR, Shorvon SD. An analysis of clinical seizure patterns and their localising value in frontal and temporal lobe epilepsies. Brain. 1996;119:17–40. doi: 10.1093/brain/119.1.17. [DOI] [PubMed] [Google Scholar]
- 18.Joncas S, Zadra A, Paquet J, Montplaisir J. The value of sleep deprivation as a diagnostic tool in adult sleepwalkers. Neurology. 2002;58:936–40. doi: 10.1212/wnl.58.6.936. [DOI] [PubMed] [Google Scholar]
- 19.Rothman KJ. Modern epidemiology. Boston/ Toronto: Little, Brown and Company; 1986. pp. 147–51. [Google Scholar]
- 20.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biggs D, deVille B, Suen E. A method of choosing multiway partitions for classification and decision trees. J Appl Stats. 1991;18:49–62. [Google Scholar]
- 22.Valenti MP, Froelich S, Rudolf G, et al. Isolated paroxysmal arousals as focal epilepsy. Epileptic Disord. 2006;8:45–52. [PubMed] [Google Scholar]
- 23.Zucconi M, Oldani A, Ferini-Strambi L, et al. Nocturnal paroxysmal arousals with motor behaviors during sleep: frontal lobe epilepsy or parasomnia? J Clin Neurophysiol. 1997;14:513–22. doi: 10.1097/00004691-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Montagna P, Sforza E, Tinuper P, et al. Paroxysmal arousals during sleep. Neurology. 1990;40:1063–6. doi: 10.1212/wnl.40.7.1063. [DOI] [PubMed] [Google Scholar]
- 25.Nobili L, Sartori I, Terzaghi M, et al. Intracerebral recordings of minor motor events, paroxysmal arousals and major seizures in nocturnal frontal lobe epilepsy. Neurol Sci . 2005;26(Suppl 3):s215–9. doi: 10.1007/s10072-005-0490-x. [DOI] [PubMed] [Google Scholar]
- 26.Vignatelli L, Bisulli F, Provini F, et al. Interobserver reliability of video recording in the diagnosis of nocturnal frontal lobe seizures. Epilepsia. 2007;48:1506–11. doi: 10.1111/j.1528-1167.2007.01121.x. [DOI] [PubMed] [Google Scholar]
- 27.Terzaghi M, Sartori I, Mai R, et al. Sleep-related minor motor events in nocturnal frontal lobe epilepsy. Epilepsia. 2007;48:335–41. doi: 10.1111/j.1528-1167.2006.00929.x. [DOI] [PubMed] [Google Scholar]
- 28.Nobili L, Sartori I, Terzaghi M, et al. Intracerebral recordings of minor motor events, paroxysmal arusals and major seizures in nocturnal frontal lobe epilepsy. Neurol Sci. 2005;26(Suppl3):s215–9. doi: 10.1007/s10072-005-0490-x. [DOI] [PubMed] [Google Scholar]
- 29.Nobili L, Sartori I, Terzaghi M, et al. Relationship of epileptic discharges to aroual instability and periodic leg movements in a case of nocturnal frontal lobe epilepsy: a stereo EEG study. Sleep. 2006;29:701–4. doi: 10.1093/sleep/29.5.701. [DOI] [PubMed] [Google Scholar]
- 30.Terzaghi M, Sartori I, Mai R, et al. Coupling of minor motor events and epileptiform discharges with arousal fluctuations in NFLE. Epilepsia. 2008;49:670–6. doi: 10.1111/j.1528-1167.2007.01419.x. [DOI] [PubMed] [Google Scholar]
- 31.Montagna P, Provini F, Bisulli F, Tinuper P. Nocturnal epileptic seizures versus the arousal parasomnias. Somnologie. 2008;12:25–37. [Google Scholar]
- 32.Tassinari C, Rubboli G, Gardella E, et al. Central pattern generators for a common semiology in fronto-limbic seizures and in parasomnias. A neuroethologic approach. Neurol Sci. 2005;26(supp 3):s225–s232. doi: 10.1007/s10072-005-0492-8. [DOI] [PubMed] [Google Scholar]
- 33.Guilleminault C, Kirisoglu C, Bao G, et al. Adult chronic sleepwalking and its treatment based on polysomnography. Brain. 2005;128:1062–9. doi: 10.1093/brain/awh481. [DOI] [PubMed] [Google Scholar]
- 34.Derry CP, Duncan JS, Berkovic SF. Paroxysmal motor disorders of sleep: the clinical spectrum and differentiation from epilepsy. Epilepsia. 2006;47:1775–91. doi: 10.1111/j.1528-1167.2006.00631.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Videos are available online at www.journalsleep.org
Seizure in NFLE, with vocalisation and apparent fear. Note the abrupt onset and offset, and the minimally interactive quality of speech and behaviour.
Brief segment of normal arousal behaviour in a parasomnia. The subject rouses, looks around and mumbles; other than the prolonged durationof the behaviour and electrographic sleep on EEG this is normal arousal behaviour.
Segments of a prolonged parasomnia. Onset is with confused mumling and swearing, with some non-agitated motor behaviour. This alternates with emotional distress through the event. At one point, tremor and some low amplitude myoclonic jerks are seen.
Non-agitated motor behaviour. The subject sits forward, and licks his hands apparently purposefully during sleep.
Parasomnia, triggered by a snore, with brief apparent fear and vocalisation, followed by apparently purposeful fumbling with nearby objects whilst asleep by electrographic criteria.
Early somnambulism. The subject stands but is restricted by EEG leads and does not leave the bed.
Apparently fearful behaviour at arousal, with the patient backing away from an unseen object, but without vocalisation. This rapidly settles to non-agitated behaviour.
Fearful and distressed speech, with clear interaction, during a prolonged parasomnia.
Nocturnal frontal lobe seizure,with clear dystonic posturing, but commencing with several seconds of arousal behaviour; this arousal is indistinguishable from the onset of many parasomnias.
Postictal behaviour mimicking parasomnias. The seizure itself is quite subtle, with eye opening and repeated left arm flexion and extension. The video clip shows the last few seconds of the seizure, which was associated with a robust ictal rhythm. This is followed be postictal behaviour with sitting forward, searching, fumbling with EEG electrodes and some oral automatisms. The beahviour looks very similar to many parasomnias.