Abstract
Objectives:
Patients with SSADH deficiency, a disorder of chronically elevated endogenous GABA and GHB, were studied for sleep symptoms and polysomnography. We hypothesized that patients would have excessive daytime somnolence and decreased REM sleep.
Design:
Polysomnography and MSLT were performed on patients enrolled for comprehensive clinical studies of SSADH deficiency.
Setting:
Sleep studies were obtained in the sleep laboratories at CNMC and NIH.
Patients:
Sleep recordings were obtained in 10 patients with confirmed SSADH deficiency.
Interventions:
Thirteen overnight polysomnograms were obtained in 10 patients (7 male, 3 female, ages 11-27 y). Eleven MSLT studies were completed in 8 patients.
Measurements and Results:
Polysomnograms showed prolongation of REM stage latency (mean 272 ± 89 min) and decreased percent stage REM (mean 8.9%, range 0.3% to 13.8%). Decreased mean sleep latency was present in 6 of 11 MSLTs.
Conclusions:
SSADH deficiency is associated with prolonged latency to stage REM and decreased percent stage REM. This disorder represents a model of chronic GABA and GHB accumulation associated with suppression of REM sleep.
Citation:
Pearl PL; Shamim S; Theodore WH; Gibson M; Forester K; Combs SE; Lewin D; Dustin I; Reeves P; Jakobs C; Sato S. Polysomnographic abnormalities in succinic semialdehyde dehydrogenase (SSADH) deficiency. SLEEP 2009;32(12):1645-1648.
Keywords: SSADH, GABA, REM
SUCCINIC SEMIALDEHYDE DEHYDROGENASE (SSADH) DEFICIENCY (OMIM # 271980) IS ONE OF THE FEW GENETIC DISORDERS AFFECTING GABA METABOLISM and homeostasis. The clinical phenotype is generally nonspecific, featuring seizures, developmental delay, intellectual disability, delayed or absent speech, hypotonia, behavioral problems, and ataxia, with a typically static clinical course.1 Neuroimaging has most consistently demonstrated abnormalities in the globus pallidus, subthalamic nucleus, and cerebellar dentate nucleus.2
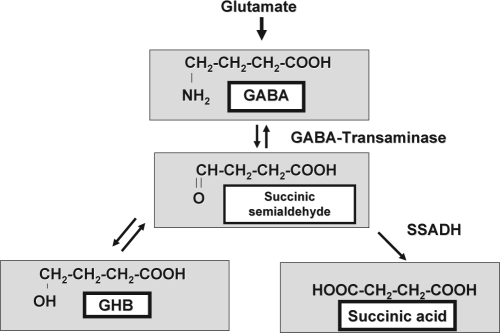
The biochemical hallmark is accumulation of γ-hydroxybutyric acid (GHB) (Figure 1). In addition, there is significant elevation in GABA in physiologic fluids as well as brain parenchyma, as measured by MR spectroscopy with special editing.3,4 GHB may act directly as a partial weak GABAB receptor agonist or as a direct neuromodulator on GHB receptors at pharmacologic concentrations, in addition to its interconversion to GABA.5 Exogenous GHB administration is associated with disinhibition, drowsiness, and ultimately coma at high doses.6 Chronic GHB administration in patients with narcolepsy-cataplexy increases delta sleep and suppresses REM intrusions with decreased awakenings and percentage of time awake during stage REM.7 We hypothesized that patients with SSADH deficiency have increased symptoms of sleep disorders and abnormal sleep architecture characterized by increased delta sleep and decreased stage REM sleep.
Figure 1.
Biochemical Pathway of GABA Metabolism.
METHODS
Patients with SSADH deficiency were recruited for comprehensive clinical, imaging, and neurophysiologic studies. Diagnosis of SSADH deficiency in all patients was based on a consistent phenotype, 4-hydroxybutyric aciduria, and confirmation utilizing enzymatic assay, mutation analysis, or both. Patients were not selected based on specific symptomatology. Ten patients were enrolled. Thirteen overnight PSG studies followed by daytime MSLTs were performed. Four patients were initially studied at CNMC (patients 2, 3, 7, and 10 in Table 2), and 9 patients were subsequently studied at NIH (3 patients were studied at both sites, separated by 3 years). All PSG recordings utilized EEG (4 channels at CNMC, 11 channels at NIH) plus 2 leg movement channels, EOG, EKG, oximetry, snore monitor, airflow, chest movements, abdominal movements, and chin EMG). MSLT was performed the day following overnight PSG; patients were offered five 20-minute naps 2 hours apart. Sleep latency was scored utilizing EEG, EOG, and EMG and compared to pediatric normative data as reported by Hoban and Chervin (2001).8 The studies were done using a Grass Telefactor machine in the NIH sleep laboratory and a SensorMedics SomnoStar Alpha system (version 4.2) at CNMC. Sleep was scored according to Rechtschaffen and Kales,9 and apneas and hypopneas were scored according to the 1998 American Thoracic Society criteria.10
Table 2.
Results of Polysomnography in Patients with SSADH Deficiency
| PID | Efficiency | Sleep Lat (min) | REM Lat (min) | Stage 1% | Stage 2% | Stage 3/4 % | REM % |
|---|---|---|---|---|---|---|---|
| 1 | 91.8 | 15.5 | 216.0 | 19.9 | 45.1 | 31.5 | 3.5 |
| 2* | 89 | 6.5 | 192 | 2.0 | 42.4 | 36.4 | 9.5 |
| 3* | 94 | 4.5 | prolonged | 3.7 | 41.1 | 30.6 | 19.7 |
| 3 | 87.4 | 34.5 | 235 | 9.7 | 47.5 | 31.0 | 11.7 |
| 4 | 92.1 | 8.5 | 242.5 | 18.7 | 49.9 | 24 | 9.4 |
| 5 | 78.9 | 42 | 363.0 | 13.4 | 41.6 | 44.7 | 0.3 |
| 6 | 81.8 | 9.5 | 358.5 | 19.9 | 39.2 | 30.4 | 10.5 |
| 7* | 84.0 | 12.5 | prolonged | 1.8 | 45 | 25.3 | 13.8 |
| 7 | 95 | 11 | 264.5 | 16 | 51.3 | 28.7 | 4 |
| 8 | 95 | 12.5 | 390.0 | 29.1 | 43.2 | 25.8 | 1.9 |
| 9 | 98 | 3.5 | 126.5 | 18.4 | 11.2 | 60.4 | 4.7 |
| 10* | 80.0 | 28 | 216.0 | 3.7 | 36.8 | 21.4 | 17.6 |
| 10 | 98.1 | 0 | 390.0 | 11.7 | 54.2 | 25 | 9.1 |
Bold: > 2 SD from mean.
Denotes Sleep study done at CNMC
RESULTS
Ten patients were enrolled into the study (7 male, 3 female, age range 11-27 years; Table 1). The results of polysomnography are shown in Table 2 along with published age- and gender-related normative data utilized by the sleep laboratories where these studies were performed.11 Thirteen overnight sleep studies were obtained. Of the 3 patients studied at both sites, there was a time period of 3 years between the studies. Eleven MSLTs were completed (Table 3). Two patients did not tolerate the daytime recordings (patients 5 and 6, Table 3). The mean sleep efficiency was 90.4% (range: 79% to 98.1%). The mean percent for each sleep stage was: stage 1, 2.9%; stage 2, 42.2%; stage, 31.9%; and stage REM, 8.21% (range 0.1% to 13.8%). Three patients had periodic leg movement indices greater than 5.0/h (Table 4). There was no evidence for significant sleep related respiratory disturbances; the apnea-hypopnea indices are reported in Table 4.
Table 1.
Demographic Data of Patients
| PID | Sex | Age | Tanner Stage | Medications |
|---|---|---|---|---|
| 1 | M | 10 | 1 | none |
| 2* | M | 10 | 2 | none |
| 3* | F | 11 | 2 | methylphenidate |
| 3 | F | 14 | 3 | none |
| 4 | M | 11 | 1 | none |
| 5 | M | 12 | 3 | trazodone, stopped 1 wk before study |
| 6 | F | 12 | 4 | levetiracetam |
| 7* | F | 13 | 3 | methylphenidate |
| 7 | F | 16 | 4 | none |
| 8 | M | 14 | 2 | methylphenidate, citalopram, polyethylene glycol |
| 9 | F | 19 | 5 | aripiprazole, benztropine, citalopram, metformin |
| 10* | M | 23 | 5 | carbamazepine, resperidone, fluoxetine |
| 10 | M | 27 | 5 | carbamazepine, fluoxetine, levothyroxine, risperidone |
Denotes Sleep study completed at CNMC
Table 3.
Multiple Sleep Latency Test Results
| PID | Sex | Age (years) | Mean sleep latency (min) | # of Naps with Sleep/ # of Nap Attempts | # of SOREM episodes/ # of naps |
|---|---|---|---|---|---|
| 1 | M | 10 | 8 | 3/4 | 0/4 |
| 2* | M | 10 | 15.8 | 1/4 | 0/4 |
| 3* | F | 11 | 3.4 | 5/5 | 2/5 |
| 3 | F | 14 | 9.4 | 5/5 | 0/5 |
| 4 | M | 11 | 6.9 | 5/5 | 2/5 |
| 5** | M | 12 | N/A | N/A | N/A |
| 6** | M | 12 | N/A | N/A | N/A |
| 7* | F | 13 | 15.6 | 2/4 | 0/4 |
| 7 | F | 16 | 19 | 1/5 | 0/5 |
| 8 | M | 14 | 16.9 | 4/4 | 0/4 |
| 9 | F | 19 | 4.25 | 4/4 | 0/4 |
| 10* | M | 23 | 10.9 | 4/5 | 0/5 |
| 10 | M | 27 | 5.7 | 4/4 | 0/4 |
Study completed at CNMC;
Daytime study cancelled at family's request;
Bold: > 2 SD from mean
Table 4.
Respiratory Data
| PID | PLMI | SpO2 (%) | Mean heart rate (bpm) | Apnea-hypopnea index |
|---|---|---|---|---|
| 1 | 3.2 | 98-100 | 76 | 0.4 |
| 2* | 3.4 | 92.5-100 | 84 | 0 |
| 3 | 0 | 97-99 | 61 | 0 |
| 3* | 10.9 | 91-99 | 93 | 0.3 |
| 4 | 0 | 96-100 | 80 | 0.5 |
| 5 | 0 | 99-100 | 57 | 0.6 |
| 6 | 0 | 98-99 | 75 | 0 |
| 7 | 0 | 98-100 | 61 | 0 |
| 7* | 0 | 95-100 | 83 | 0 |
| 8 | 14.1 | 96-98 | 70 | 0 |
| 9 | 0 | 98-100 | 84 | 0.2 |
| 10 | 6.1 | 94-97 | 66 | 0 |
| 10* | 1 | 89-99 | 70 | 1.4 |
Study done at CNMC.
The time latency to stage REM was prolonged, with a mean and standard deviation of 272 ± 89 minutes. This latency was prolonged by 2 standard deviations (SD) in 8 of 13 recordings (Table 2). This finding was consistent in 2 patients studied twice (patients 7 and 10). In the third patient studied twice (patient #3), the REM latency was prolonged on the first study and borderline prolonged (by 1 SD) on the repeat study. The time spent in stage REM was decreased by 2 SDs in 7 of 13 studies. This included one patient studied twice (patient 3), with a low percent REM on the second study and a borderline-low percent REM on the first study.
Eleven of 13 attempted diurnal MSLTs were completed. The numbers of naps achieved per attempt are shown in Table 3. Five patients had decreased mean sleep latency times compared to Tanner stage based control data (Table 3). Two of these 5 patients had been studied twice. One (patient #10) had decreased mean sleep latency by 2 SD on both studies, and the other (patient #3) had a decreased sleep latency by 2 SD on one study and by 1.5 SD on the repeat. Two MSLTs had 2 sleep onset rapid eye movement (SOREM) episodes.
DISCUSSION
SSADH deficiency is an autosomal recessive inherited disorder of GABA degradation that has a well characterized human phenotype and murine model.12 The models are consistent in biochemical alterations involving cerebral and CSF levels of GABA, GHB, and other amino acids and neurotransmitter metabolites.13,14 Animal models have demonstrated that hyperGABAergic states, e.g., via inhibition of GABA transaminase using L-cycloserine, are associated with reduction of REM sleep and prolongation of the transition phase between sleep stages NREM and REM.15 The biochemical hallmark of SSADH deficiency is accumulation of GHB. This compound has known sedative effects in humans, associated with potential for abuse as well as therapeutic value for consolidation of sleep and reduction in cataplexy in patients with narcolepsy. GHB has furthermore been associated with reduced stage REM and augmented delta sleep.
Sleep disturbances, therefore, may be anticipated in SSADH deficiency. Recent reports have emphasized sleep disorders in young adults with SSADH deficiency including prolonged sleep attacks with hallucinations in a pair of brothers.14,16 A single case report of polysomnography in a patient with SSADH deficiency revealed a slight increase (0.1%) in delta sleep the first night and an increase in slowing and delta sleep the second night following a generalized tonic-clonic seizure.17
We have previously reported an approximately 40% incidence of clinical sleep disturbances based on self-report by families in a database comprised of questionnaire-derived data.18 The symptoms are typically difficulty with maintaining sleep or excessive daytime sleepiness. We now report sleep study results of 13 PSG recordings in 10 patients with SSADH deficiency. Polysomnographic results did not substantiate consistent problems with sleep efficiency, overnight sleep fragmentation, or respiratory disturbances. Sleep architecture, however, showed a trend toward prolonged latency to achieve stage REM and a decreased amount of stage REM. Approximately half of the patients had decreased mean sleep latency on MSLT, consistent with excessive daytime somnolence. The results were concordant between repeat studies in the same patients, so that abnormalities were consistently abnormal by 2 SD, or were abnormal by 2 SD on one study and a trend in this direction without quite achieving this level of significance on the other study. While some patients were on antidepressant medications having the effect of potential REM suppression, 5 of the 8 patients with decreased REM percentage were on no medications at the time of the study (Table 1).
Thus, SSADH deficiency appears to be associated with suppression of REM sleep. This is consistent with experimental findings of decreased stage REM related to elevations of both GHB and GABA, although the mechanism for REM sleep inhibition in SSADH deficiency is unknown. Studies utilizing agents that augment stage REM, e.g., donepezil, may be considered in the murine model or clinical disorder.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Delman Fund for Pediatric Neurology Research, NS 40270/HD58553 (KMG, PLP), NINDS/NIH Intramural Program.
Institutions where work was performed: Children's National Medical Center, Washington, DC and NINDS, NIH, Bethesda, MD
REFERENCES
- 1.Pearl PL, Novotny EJ, Acosta MT, Jakobs C, Gibson KM. Succinic semialdehyde dehydrogenase deficiency in children and adults. Ann Neurol. 2003;54(suppl 6):S73–S80. doi: 10.1002/ana.10629. [DOI] [PubMed] [Google Scholar]
- 2.Pearl PL, Gibson KM, Quezado Z, et al. Decreased GABA-A binding in succinic semialdehyde dehydrogenase (SSADH) deficiency. Neurol. 2009 doi: 10.1212/WNL.0b013e3181b163a5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ethofer T, Seeger U, Klose U, et al. Proton MR spectroscopy in succinate semialdehyde dehydrogenase deficiency. Neurol. 2004;62:1016–18. doi: 10.1212/01.wnl.0000115385.45515.df. [DOI] [PubMed] [Google Scholar]
- 4.Novotny EJ, Fulbright RK, Pearl PL, Gibson KM, Rothman DL. Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol. 2003;54(suppl 6):S25–S31. doi: 10.1002/ana.10697. [DOI] [PubMed] [Google Scholar]
- 5.Snead OC. Evidence for a G protein-coupled gamma-hydroxybutyric acid receptor. J Neurochem. 2000;75:1986–96. doi: 10.1046/j.1471-4159.2000.0751986.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong CG, Gibson KM, Snead OC. From the street to the brain: neurobiology of the recreational drug gamma-hydroxybutyric acid. Trends in Pharmacol Sci. 2004;25:29–34. doi: 10.1016/j.tips.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Lammers GJ, Arends J, Declerck AC, Ferrari MD, Schouwink G, Troost J. Gammahydroxybutyrate and narcolepsy: a double-blind placebo-controlled study. Sleep. 1993;16:216–20. doi: 10.1093/sleep/16.3.216. [DOI] [PubMed] [Google Scholar]
- 8.Hoban TF, Chervin RD. Assessment of sleepiness in children. Sem Ped Neurol. 2001;8:216–28. doi: 10.1053/spen.2001.29043. [DOI] [PubMed] [Google Scholar]
- 9.Rechtschaffen A, Kales A, editors. A Manual of standardization terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: BIS/BIR, UCLA; 1968. [Google Scholar]
- 10.American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:855–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 11.Acebo C, Millman RP, Rosenberg C, Cavallo A, Carskadon MA. Sleep, breathing, and cephalometrics in older children and young adults. Part I – normative values. Chest. 1996;109:226–72. doi: 10.1378/chest.109.3.664. [DOI] [PubMed] [Google Scholar]
- 12.Gupta M, Hogema BM, Grompe M, et al. Murine succinate semialdehyde dehydrogenase deficiency. Ann Neurol. 2003;43(suppl 6):S81–90. doi: 10.1002/ana.10625. [DOI] [PubMed] [Google Scholar]
- 13.Gibson KM, Schor DS, Gupta M, et al. Focal neurometabolic alterations in mice deficient for succinate semialdehyde dehydrogenase. J Neurochem. 2002;81:71–9. doi: 10.1046/j.1471-4159.2002.00784.x. [DOI] [PubMed] [Google Scholar]
- 14.Gibson KM, Gupta M, Pearl PL, et al. Significant behavioral disturbances in SSADH deficiency. Biol Psychiatry. 2003;54:763–8. doi: 10.1016/s0006-3223(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 15.Scherschlicht R. Role for GABA in the control of the sleep-wakefulness cycle. In: Wauquier A, Gaillard J, Monti JM, et al., editors. Sleep: Neurotransmitters and neuromodulators. New York: Raven Press; 1985. pp. 237–49. [Google Scholar]
- 16.Philippe A, Deron J, Genevieve D, et al. Neurodevelopmental pattern of succinic semialdehyde deficiency (gamma-hydroxybutyric aciduria) Dev Med Child Neurol. 2004;46:564–8. doi: 10.1017/s0012162204000933. [DOI] [PubMed] [Google Scholar]
- 17.Arnulf I, Konafal E, Gibson KM, et al. Effect of genetically caused excess of brain GHB and GABA on sleep. Sleep. 2005;28:418–24. doi: 10.1093/sleep/28.4.418. [DOI] [PubMed] [Google Scholar]
- 18.Pearl PL, Taylor JL, Trcinski S, Sokohl A. The pediatric neurotransmitter disorders. J Child Neurol. 2007;22:606–16. doi: 10.1177/0883073807302619. [DOI] [PubMed] [Google Scholar]