Abstract
The cAMP response element modulator (CREM)α is a widely expressed transcriptional repressor that is important for the termination of the T cell immune response and contributes to the abnormal T cell function in patients with systemic lupus erythematosus. We present evidence that APCs of Crem−/− mice express increased amounts of the costimulatory molecule CD86 and induce enhanced Ag-dependent and Ag-independent T cell proliferation. Similarly, human APCs in which CREMα was selectively suppressed expressed more CD86 on the surface membrane. CREMα was found to bind to the CD86 promoter and suppressed its activity. Transfer of APCs from Crem−/− mice into naive mice facilitated a significantly stronger contact dermatitis response compared with mice into which APCs from Crem+/+ mice had been transferred. We conclude that CREMα is an important negative regulator of costimulation and APC-dependent T cell function both in vitro and in vivo.
We have recently identified the transcriptional repressor cAMP response element modulator α (CREMα)3 as a negative regulator of IL-2 production and T cell proliferation in normal T cells (1, 2). CREMα down-regulates IL-2 production by a chromatin-dependent mechanism mediated by recruitment of histone deacetylase (HDAC)1 (3). Additionally, CREMα is involved in the expression of abnormal T cell function in patients with systemic lupus erythematosus (SLE). Specifically, T cells from patients with SLE express increased amounts of CREMα, which binds to the Il2 promoter and suppresses its activity resulting in limited production of this cytokine (4, 5). In addition, CREMα binds to the c-fos promoter, which results in decreased AP-1 activity (6). Suppression of CREMα expression in SLE T cells results in increased production of IL-2 and correction of effector cell function (4). Thus CREMα is of importance in the T cell pathophysiology of SLE and it is a general negative mediator of T cell physiology.
CREM, like the transcriptional activator CREB, belongs to the superfamily of bZip proteins containing a basic domain/leucine zipper domain, which binds an 8-bp palindromic DNA sequence cAMP response element (CRE, TGACGTCA) (7, 8). These nuclear factors are activated following phosphorylation by several protein kinases in response to different signaling routes, including protein kinases A and C, MAPK, and stress-activated protein kinase, Ca2+/calmodulin-dependent kinases IV and II (7, 9, 10). In patients with SLE, increased expression and activity of CaMKIV accounts for the increased binding of CREMα to the Il2 promoter.
The effect of CREM on transcription is complex because CREM is a multiexonic gene that encodes both activator (τ) and repressor (α,β,γ) isoforms of transcriptional regulation (7). The two groups of CREM differ in the number of exons that are excluded by alternative splicing (7). The repressor isoforms are shorter and lack exons, generated by alternative splicing, alternative initiation codon and alternative polyadenylation, which contribute to the transactivating N terminus of the protein. Activated normal T cells and T cells from patients with SLE express primarily CREMα, which occupies promoters of Il2 and c-fos, resulting in decreased IL-2 production and aberrant T cell function, contributing to disease pathology.
Nothing is known about the role of CREMα in APCs so far. Recently, it was shown that the activating transcription factor ATF3, which belongs to the family of CREM/CREB transcription factors, is involved in TLR-mediated gene transcription in macrophages (11). Moreover, an activator isoform of CREM participates in macrophage maturation.
In this study, we report that CREMα binds to a specific site of the CD86 promoter in a chromatin-dependent manner and suppresses its activity. CREMα -deficient human monocytic U937 cells and murine dendritic cells (DCs) express more CD86 on their surface membrane and promote Ag-specific T cell proliferation both in vitro and in vivo animal model of contact dermatitis.
Materials and Methods
Generation of bone marrow-derived DCs (BMDCs)
Mouse bone marrow cells were flushed from femurs and tibias with Dulbecco medium. Erythrocytes were lysed with lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) for 5 min at room temperature, and the remaining cells were washed once with PBS. Cells were cultured in Dulbecco medium with 1 g/L glucose supplemented with 10% heat-inactivated FCS (Life Technologies) and 2 mM L-glutamine. DCs were generated by culturing bone marrow cells in the presence of IL-4 and GM-CSF for 6 days. FACS analysis showed that cells were 98% positive for CD11c and negative for BM-8. Cells were thereafter stimulated with LPS (100 ng/ml), R848 (0.5 μg/ml), or CpG oligonucleotides for different time points and analyzed by Western blot, RT-PCR, and flow cytometry.
Quantitative real-time PCR
Five million mouse DCs or U937 monocytes were used for extracting RNA (RNA Easy Mini kit; Qiagen). cDNA was synthesized from 5 μg of total RNA using RevertAid H Minus transcriptase (Fermentas). Primers were designed using the Primer Express software package (Applied Biosystems) and obtained from Qiagen. Real-time RT-PCR was performed using the Quanti-Tect SYBR Green PCR kit (Qiagen) and data were acquired with the ABI PRISM 7900 HT (Applied Biosystems). Each measurement was set up in duplicates, and three independent experiments were performed. After normalization to endogenous housekeeping control genes for mouse bone marrow cells (GAPDH and ribosomal protein L) and for human U937 cells (GAPDH), the relative expression was calculated. Selected genes and primers for U937 cells were as follows: CREM 5′-GAA ACA GTT GAA TCC CAG CAT GAT GGA AGT-3′ (reverse) 5′-TGC CCC GTG CTA GTC TGA TAT ATG-3′; GAPDH (forward) 5′-GCAAATTTC CATG GCACCGT-3′, (reverse) 5′-GCCCCACTTGATTTTGGAGG-3′; and ribosomal protein L (forward) 5′-AGGTATGCT GCCCCACAAAAC-3′, (reverse) 5′-TGTAGGCTTCAGACGCACGAC-3′.
Chromatin immunoprecipitation (ChIP) analysis
Five million U937 cells were used per investigated Ab. The cells were treated with formalin (1%) for 10 min, washed, lysed and sonicated. The DNA-protein complexes were immunoprecipitated with an Ab (anti-CREM; Santa Cruz Biotechnology) or unspecific isotypic rabbit control Ab and extracted by protein A/G-Sepharose beads (Pierce). After several washing steps the protein was digested with proteinase K, the cross-link between DNA and protein was reversed at 65°C overnight, and the DNA was extracted with a special elution step in 30 μl (QiaAmp PCR kit; Qiagen). The DNA was amplified with primers flanking the putative CREM binding site of the CD86 promoter (forward) 5′-CACTCCCAATCCA CATCCACAATA-3′, (reverse) 5′-ATGACGTTAAGATGAGCCTGAATA-3′ by semiquantitative PCR method. DNA from approximately one million cells was used per PCR study.
Transfection of U937 cells
A total of ~1 × 106 U937 cells were used for transfection with polyethyleneimine. Plasmids encoding CREMα antisense and CREMα expression plasmid was a gift from Dr. P. Sassone-Corsi (University of California, Irvine, CA) and CREB expression plasmids or corresponding empty vector plasmids were used for transfection. The plasmid encoding the CD86 luciferase was a gift from Dr. N. Suciu-Foca (Columbia University, New York, NY). A total of 4 μg of each plasmid was used per transfection (12). The 10 μl of 0.1% polyethyleneimine plus 190 μl of OptiMem were prepared for each transfection (13). In a second tube DNA was prepared and filled up to a 100 μl with OptiMem. Subsequently polyethyleneimine solution was added to DNA medium solution and incubated for 20 min at room temperature. Cells were incubated with the solution overnight, washed, and replaced with fresh medium. After 24 h, cells were harvested for different purposes like luciferase assay, FACS analysis, ChIP assay, or RT-PCR. For luciferase assays cells were harvested and luciferase activity was measured with Promega firefly luciferase kit according to the manufacturer’s instructions. As an internal control for transfection efficiency, Renilla luciferase constructs were used and cotransfected. Activity was measured with Promega Renilla luciferase kit according to the manufacturer’s instructions.
Reporter ChIP
Reporter ChIP technique enables the study of the in vivo binding of transcription factors to a specific binding site on a promoter of a reporter construct (14, 15). Mutagenesis of the −21 site of the CD86 promoter was performed with a site-directed mutagenesis kit (Stratagene) and primers including the mutagenesis site (5′-GGACTCTAATCCAGGGTTC TGG TATTTTGCCAGCC ACCAT-3′). The mutated construct was transfected into 5 × 106 U937 cells and the nonmutated construct was transfected into another 5 × 106 cells for control purposes. At 24 h after transfection, ChIP analysis of the cells was performed with an anti-CREM Ab, anti-CREB Ab, or an isotypic control (Santa Cruz Biotechnology). Semiquantitative PCR was performed with a primer specific for the CD86 promoter and another one specific for the luciferase plasmid (forward) 5′-CACTC CCAATCCACATCCACAATA-3′, (reverse) 5′-TCACCTCGATATGTG CATC-3′ to ensure that only reporter specific DNA and not genomic DNA is amplified.
Flow cytometric analysis (FACS analysis)
Cells were stained with PE-labeled anti-CD86, anti-CD80, anti-CD40, CD11c, BM-8, (BD Biosciences) Ab or an isotype-specific control (Santa Cruz Biotechnology). A minimum of 10,000 events were collected for evaluation on a FACSCalibur (BD Biosciences) and data were analyzed using CellQuest Pro software.
Animals
Crem−/− animals were originally cloned and provided by Prof. G. Schütz (Deutsches Krebsforschungszentrum, Heidelberg, Germany) (16). Animals are on a mixed background of SV129/Bl6 for over 30 generations. Male Crem−/− animals are infertile, so only heterozygous animals can be used for breeding. Age- and gender-matched littermates/siblings were used for the experiments according to approved protocols of the animal welfare committee of the University of Münster (Münster, Germany).
For the experiments to evaluate the Ag-specific T cell proliferation in Fig. 1d, Crem−/− animals were crossed into the DO11.10 background and heterozygous DO11.10-positive Crem mice were the founder generation of the Crem−/− and Crem+/+ animals, which were used for generation of BMDCs. Again, age-matched siblings were used for experiments.
FIGURE 1.
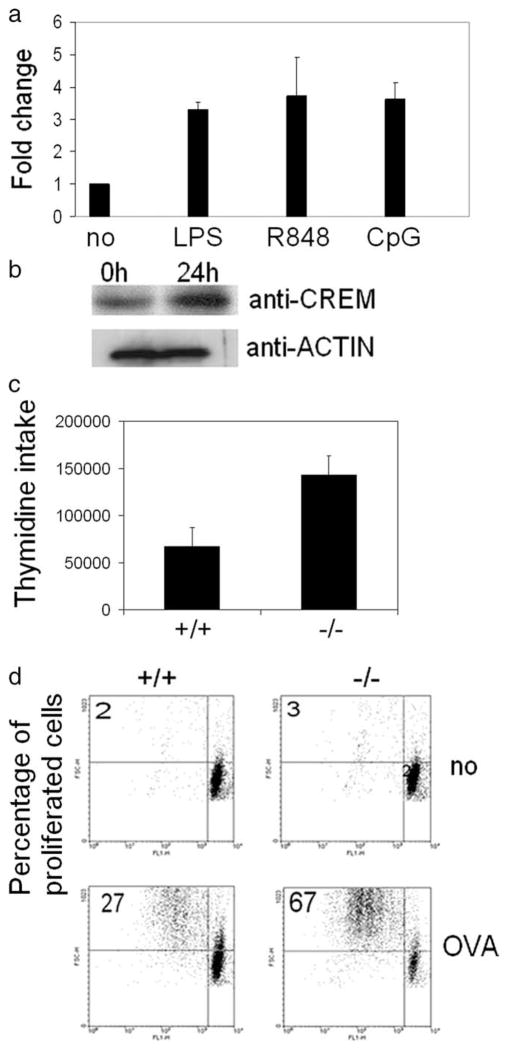
CREM-deficient DC provide enhanced Ag-specific T cell proliferation. a, BMDCs of C57BL/6 animals were treated with the TLR4 agonist LPS, the TLR7 agonist R848, and the TLR9 agonist CpG for 8 h. RNA was extracted and real-time PCR for CREM was performed and equalized to GAPDH and ribosomal protein L values (n = 3 experiments). b, BMDCs of C57BL/6 animals were stimulated with the TLR4 agonist LPS for 24 h and nuclear proteins were extracted. CREM protein expression was detected by Western blot (n = 2 experiments). c, BMDCs of Crem−/− or Crem+/+ animals were incubated together with T cells from allogenic BALB/c mice. Three days later, T cells were labeled with thymidine and its incorporation was measured 24 h later (n = 5 experiments, p = 0.0065, paired t test). d, BMDCs of DO11.10 Crem−/− or DO11.10 Crem+/+ animals were either left untreated or were pulsed with OVA peptide (1 μM) for 2 h and extensively washed with PBS. BMDCs were cocultured with CFSE-labeled (1 μM) T lymphocytes of OVA-transgenic DO11.10 CREM heterozygote (HZ) mice in ratios ranging from 1:10 to 1:100. FACS analysis of T cells was performed after 3–5 days. Number represents percentage of proliferated cells in each quadrant (n = 4 experiments).
Contact hypersensitivity reaction
To haptenize BMDCs in vitro, day 7 BMDCs were incubated for 3 h with 1 mM dinitrobenzene sulfonic acid (Sigma-Aldrich). In a second set of experiments BMDCs were incubated either with an anti-CD86 Ab (BD Biosciences) or with an isotypic IgG control for 1 h before transfer. Cells were extensively washed with PBS and 1 × 106 BMDCs in PBS/mouse were injected i.v. into the tail veins of naive mice of the same genetic background on day 0. Mice were challenged on day 6 by painting 10 μl of 0.2% DNFB (2,4-dinitro-1-fluorobenzene; Sigma-Aldrich) in an acetone to olive oil ratio of 4:1 on the ventral and dorsal surface of the right ear. The left ears were treated with vehicle alone and served as an internal control for these studies. Contact hypersensitivity reaction was determined by the degree of ear swelling measured with a micrometer of the hapten-exposed ear minus that of the vehicle-treated contralateral ear at 24 h after challenge. Ears were obtained from sacrificed mice and processed for histological examination.
Isolation of T cells and assessment of Ag-specific T cell proliferation
For experiments with OVA-transgenic animals, BMDCs were fed with OVA peptide (1 μM) for 2 h and extensively washed with PBS. BMDCs from Crem−/− or Crem+/+ mice were cocultured with CFSE-labeled (1 μM) T lymphocytes (pan T cell kit; Miltenyi Biotec) of OVA-transgenic Crem+/+ mice in ratios ranging from 1:10 to 1:100. FACS analysis of T cells was performed after 3–5 days.
Histology
For histological H&E stained tissue studies, mouse ears were fixed with 10% PBS-buffered formalin using a Tissue Tek Tissue Processor and Embedding Station (Miles). Sections were cut at 3- to 5-μm and mounted on superfrost slides (Fisher Scientific), deparaffinized, rehydrated, and stained with H&E (Richard Allen). Sections were mounted under Permount (Fisher Scientific) and examined by light microscopy to assess histological changes and immune cell infiltration.
Results
Enhanced CD86 expression on the surface of Crem−/− DCs
We first determined whether CREM was expressed in murine APCs. We generated DCs by culturing bone marrow cells in the presence of IL-4 and GM-CSF for 6 days and treated them with different TLR agonists for 24 h. Similarly to human T cells, in which CREM was induced following stimulation with a combination of anti-CD3 and anti-CD28 Abs, we found that stimulation of mouse DCs with TLR4, TLR7, and TLR9 agonists resulted in a 3-to 5-fold enhancement of CREM transcription (Fig. 1a). In accordance, Western blot analysis of DC nuclear lysates following stimulation with the TLR4 agonist LPS for 24 h revealed a strong enhancement of CREMα protein expression (Fig. 1b).
Next we asked whether the presence of CREM changes the function of APCs. We generated DCs from Crem−/− and Crem+/+ mice and cocultured them with allogenic T cells from C57BL/6 mice. T cells showed a significantly enhanced proliferation in the presence of Crem−/− DCs compared with Crem+/+ DCs (Fig. 1c). To determine whether CREM also influenced the function of APCs in an Ag-dependent manner we crossed Crem−/− and Crem+/+ mice into a DO11.10 OVA-TCR transgenic background and primed the DO11.10 Crem−/− and DO11.10 Crem+/+ DCs with OVA peptide. Subsequently after 3–5 days of coculture, proliferation of OVA-TCR-expressing T cells was measured. We did not see any difference in the proliferation of T cells cocultured with unprimed DCs. In contrast, we observed significantly enhanced Ag-dependent proliferation of T cells, which have been cocultured with DCs derived from DO11.10 Crem−/− mice compared with DO11.10 Crem+/+ mice (Fig. 1d).
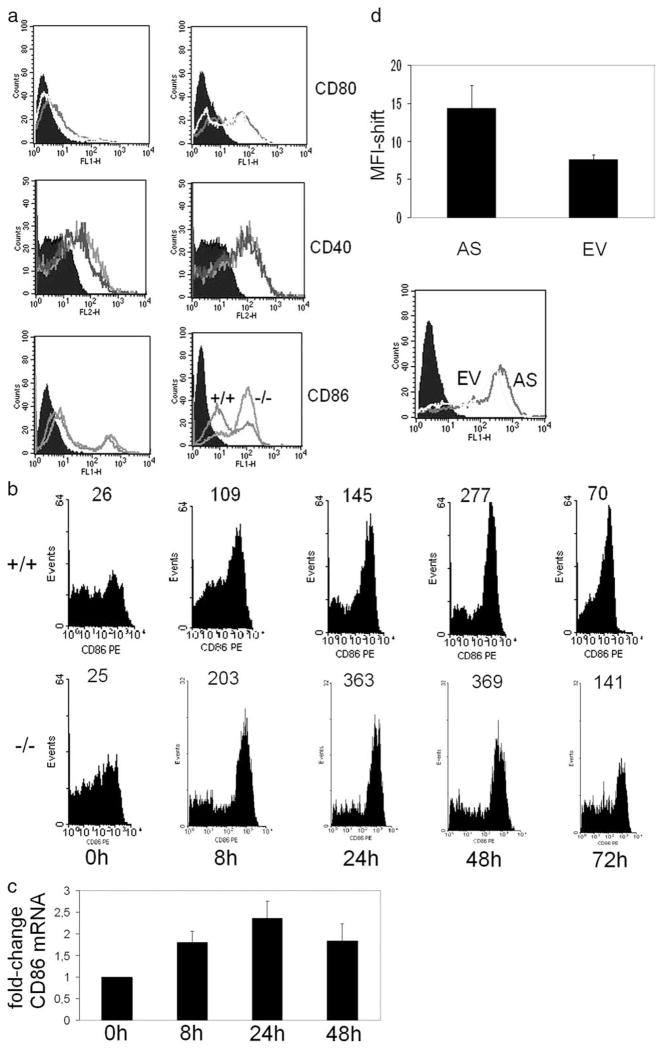
Subsequently, we asked whether increased expression of co-stimulatory molecules was responsible for the increased T cell proliferation induced by DCs of Crem−/− mice. We found enhanced CD86 expression on the surface of DCs of Crem−/− mice, which had been stimulated with LPS, whereas the expression of CD80 or CD40 (Fig. 2a) was not affected. We additionally studied the expression of CD86 over time and found an up-regulation during the first 48 h after LPS treatment, which resolved after 72 h in Crem+/+ and Crem−/− DCs (Fig. 2b). Nevertheless, the transcription of CD86 peaks after 24 h (Fig. 2c), which is the time point of highest protein expression of CREM (Fig. 1b).
FIGURE 2.
Enhanced expression of CD86 by CREM-deficient DCs. a, BMDCs from Crem−/− and Crem+/+ animals were either left unstimulated or were stimulated with LPS for 24 h and stained with Abs against CD40, CD80, and CD86. CD86 staining shows the isotypic control (filled histogram), the CD86 expression of the Crem+/+ mice, and the CD86 expression of the Crem−/− mice (n = 3 animals each) for four experiments completed. b, BMDCs from Crem−/− and Crem+/+ animals were either left unstimulated or were stimulated with LPS for depicted time points with Abs against CD86. Number at top of peaks represents mean fluorescent intensity (n = 3 animals each) for two experiments performed. c, BMDCs from Crem+/+ animals were either left unstimulated or were stimulated with LPS for depicted time points. mRNA was extracted and real-time PCR was performed with primers specific for CD86 (n = 3 animals each) for two experiments completed. Data show fold-change relative to gapdh expression. d, U937 cells were transfected with an antisense CREM construct (AS) or an empty-vector construct (EV) together with a GFP construct. CD86 expression on cell surface was measured by flow cytometric analysis 24 h after transfection within a gate set on GFP-positive cells. Mean fluorescence intensity was calculated with error bar representing SEM (n = 6 experiments, p = 0.006, paired t test). Empty vector (gray line histogram) and antisense CREM (black line histogram) are shown (bottom).
Next we studied the expression of CD86 in human APCs, in which CREMα was transiently knocked out by an antisense CREMα construct. To this end, we transfected the human U937 monocytic cell line with an antisense CREMα plasmid and a GFP-construct and gated on GFP-positive cells. Antisense CREMα enhanced the expression of CD86 on the surface of U937 (n = 6 experiments, mean fluorescent intensity 14 vs 7, p = 0.035) (Fig. 2b). This response indicates that CREMα is involved in the transcription and expression of CD86 on human monocytic cells.
CREMα binds to the −21 CRE site of the CD86 promoter and regulates its activity
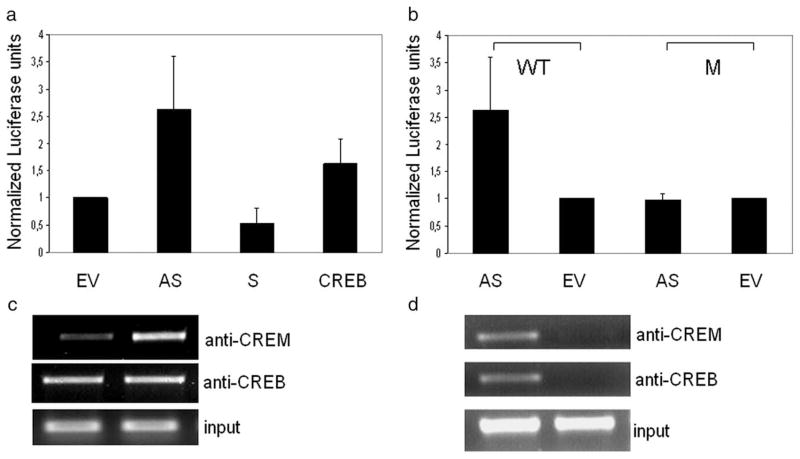
We transfected a luciferase reporter construct driven by the human CD86 promoter into U937 cells and knocked down the expression of CREM using an antisense CREMα construct. Cells were stimulated with LPS for additional 24 h. Transfection of antisense CREMα strongly enhanced CD86 promoter activity (Fig. 3, a and b). In addition, we cotransfected a CREMα as well as a CREB expression plasmid together with the CD86 luciferase reporter. Although the CREMα plasmid down-regulated CD86 luciferase expression, the CREB plasmid enhanced CD86 promoter-driven lu-ciferase expression (Fig. 3a).
FIGURE 3.
CREM binds to the −21 CRE site of the CD86 promoter. a, The wild-type CD86 promoter luciferase construct has been cotransfected into U937 cells together with an antisense CREMα (AS), a CREMα expression plasmid (S), a CREB expression plasmid or a control plasmid (EV) and cells were stimulated with LPS for 24 h. Luciferase activity was measured and relative luciferase activity was normalized to luciferase activity of the control plasmid. b, The wild-type (WT) or the mutated (M) CD86 luciferase construct was transfected into U937 cells together with either an antisense CREM (AS) plasmid or a control (EV) plasmid and stimulated with LPS. Luciferase activity was measured 24 h after stimulation with LPS (n = 4) and values were normalized to transfection with the control plasmid. c, CD86 promoter luciferase construct has been transfected into U937 cells together with an antisense CREMα (AS) construct or an empty vector (EV) and reporter ChIP has been performed with Abs against CREM and CREB. DNA was amplified with primers specific for the CD86 luciferase construct and run on a 1.5% agarose gel (n = 3). d, CD86 luciferase construct was mutated at the −21 CRE site (M) and transfected into U937 cells. As control, the wild-type luciferase construct (WT) was transfected and an reporter ChIP was performed with Abs against CREM and CREB. DNA was amplified with primers specific for the CD86 luciferase construct and run on a 1.5% agarose gel (n = 3).
We performed a reporter ChIP (14) using the CD86 promoter reporter construct and observed decreased binding of CREM after co-transfection with antisense CREMα. In contrast, the binding of the transcriptional activator CREB, which is also able to bind CRE sites and thereby induces promoter activity, was unaffected by the antisense CREMα plasmid (Fig. 3c). We identified a putative CRE (GTCAC) half-site at −21 bp of the CD86 promoter, which is very close to the transcription initiation site and destroyed it by site-directed mutagenesis. We used the −21 site-mutated CD86 reporter construct to perform reporter ChIP (Fig. 3d) and we found that neither CREM nor CREB bound to the CD86 promoter (Fig. 3d). As expected, the luciferase activity of this mutated reporter construct could not be up-regulated by a knockout of CREM (Fig. 3b) any further. These data demonstrate that the functional −21 CRE site of the CD86 promoter is critical for its regulation by CREM and CREB.
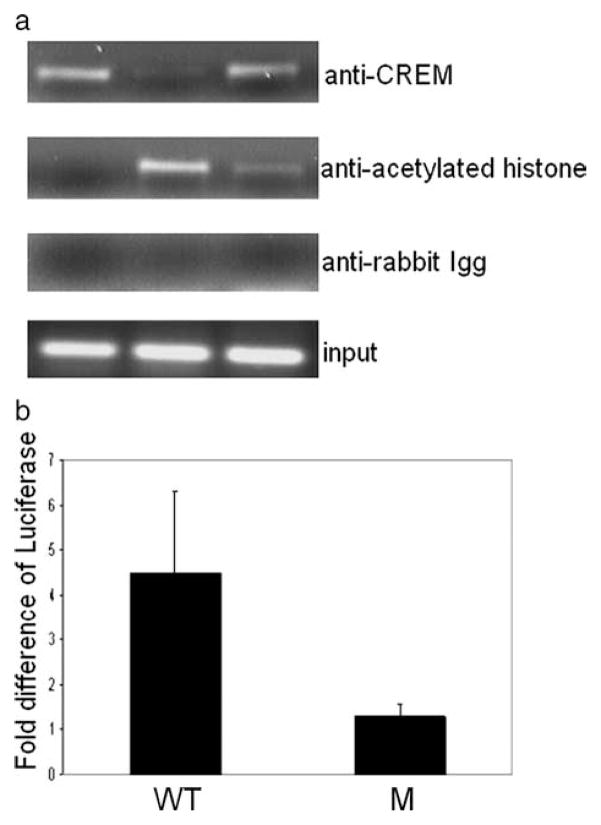
Previously, we reported that in T cells binding of CREM to the Il2 promoter recruits HDAC1. Accordingly, we asked whether CREM regulates CD86 expression also in a chromatin- dependent manner. Using ChIP assays we determined the binding of CREM and acetylated histone 4 (a marker of transcriptional activation). As shown in Fig. 4a, CREM bound to the CD86 promoter constitutively but its binding decreased after LPS stimulation and increased again 24 h later. In contrast, acetylated histone 4 binding appeared 6 h after stimulation, when CREM binding was minimal, and decreased 24 h later. Subsequently, we transfected U937 cells with a mutated or a nonmutated CD86 promoter-driven reporter construct and treated them with the HDAC inhibitor SAHA. As shown in Fig. 4b the nonmutated, wild-type CD86 promoter-driven construct showed stronger activity after HDAC inhibitor treatment compared with the −21 site-mutated CD86 promoter-driven construct.
FIGURE 4.
CREM regulates gene expression by a chromatin-dependent mechanism. a, U937 cells were stimulated with LPS for 0, 6, and 24 h and cells were harvested for ChIP analysis with Abs against CREM, acetylated histone 4, and an isotypic control, subsequently semiquantitative PCR with primers specific for the CD86 promoter was performed and the products were run on a 1.5% agarose gel (n = 3 experiments). b, −21 CRE mutated (M) or nonmutated (WT) CD86 promoter luciferase constructs were tranfected into U937 cells and subsequently treated with the HDAC inhibitor SAHA. Luciferase activity after treatment with SAHA was measured 24 h later and values were normalized to nontreated transfected cells. Number shows fold increase of luciferase after treatment with SAHA (n = 3 experiments).
Crem−/− DCs induce enhanced contact dermatitis
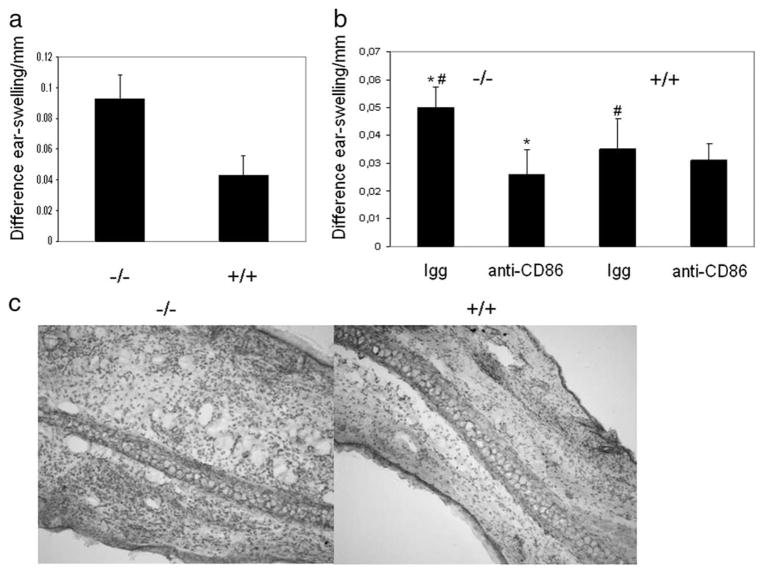
Having demonstrated the importance of CREM in the regulation of the expression of CD86 and APC function in in vitro assays, we sought to determine whether CREM is important for APC function in Ag-specific T cell-dependent disease. Contact dermatitis represents T cell-dependent skin damage in which Ag presentation is critical. To analyze APC function we adoptively transferred dinitrobenzene sulfonic acid haptenized DCs from either Crem−/− or Crem+/+ mice into naive heterozygous recipients. This approach induces a sensitization, which is solely dependent on the Ag presentation by DCs. The animals were challenged 1 wk later with the analog DNFB. At 24 h later, ear thickness was measured and the animals were sacrificed for histological evaluation. Indeed, in this model, Crem−/− DCs induced a significantly stronger inflammatory response as measured by ear thickness and by histological evaluation (Fig. 5, a and c). We additionally asked whether a blockade of CD86 would result in a comparable inflammatory response in both the Crem−/− and Crem+/+ mice and incubated the haptenized DCs either with an anti-CD86 Ab or an isotypic control before injection into naive animals. The blockade of CD86 resulted in a reduction of disease severity in both animals and equalized the inflammatory response as measured by ear thickness (Fig. 5b).
FIGURE 5.
CREM-deficient DC promote contact dermatitis pathology. a, To haptenize BMDCs in vitro, day 7 BMDCs of Crem−/− and Crem+/+ mice were incubated for 3 h with 1 mM dinitrobenzene sulfonic acid. Cells were extensively washed with PBS and 1 × 106 BMDCs in PBS/mouse were injected i.v. into the tail veins of heterozygous CREM mice on day 0 and mice were challenged on day 6 by painting 10 μl of 0.2% DNFB with an acetone to olive oil ratio of 4:1 on the ventral and dorsal surface of the right ear. The left ear was treated with vehicle alone and served as an internal control for these studies. Contact hypersensitive reaction was determined by the degree of ear swelling measured with a micrometer of the hapten-exposed ear minus that of the vehicle-treated contralateral ear at 24 h after challenge. The y-axis shows difference of ear swelling before and 24 h after challenge with DNFB (n = 5 animals each) in three experiments. Error bar represents SD (p = 0.005, paired t test). b, BMDCs were treated as in a and additionally incubated with an anti-CD86 Ab or an IgG control for 1 h. BMDCs were extensively washed and injected into the tail veins of naive mice. Contact allergy was elicited and measured on day 6 (n = 5 animals each) in two experiments. Error bar represents SD. #, p = 0.028 for isotypic blockade between Crem−/− and Crem+/+ mice.*, p = 0.007 between Crem−/− isotypic control and CD86 blockade. No significant (n.s.) difference was found between Crem+/+ isotypic control and CD86 blockade (by paired t test). c, Animals were sacrificed after 24 h, mouse ears were fixed and stained with H&E. Enhanced cellular infiltration in the ear is shown after transfer of haptenized Crem−/− DCs.
Discussion
We have presented novel evidence that CREMα is involved in the regulation of APC function by controlling the expression of CD86 which is a well established costimulatory molecule regulating the magnitude of the responding T cells. Using molecular approaches, we demonstrate that CREMα binds to a CRE site located close to the transcription initiation site (−21) of the CD86 promoter and limits its activity. APCs that lack CREMα exhibit an increased ability to promote the proliferation of both Ag-specific and non-specific T cells in vitro. More importantly, when transferred into animals after hapten-sensitization Cremα-deficient APCs promote an enhanced contact dermatitis upon proper rechallenge. It has been suggested before that cAMP regulates nonspecified mechanisms that might influence CD86 expression (17, 18). Apart from cAMP-mediated mechanisms, which have been defined in this study, NF-KB has been previously identified as a regulator of CD86 promoter activity (12).
Previously, we had shown that CREMα represents a potent suppressor of IL-2 production by means of repressing the transcriptional activity of the Il2 promoter. Stimulation of human peripheral blood T cells with anti-CD3 and anti-CD28 Ab resulted in an early increase of CREMα expression, which bound to the −180 site of the Il2 promoter effectively terminating its transcription (2). In one experiment (Fig. 1), we show a similar induction of CREMα in DCs following stimulation with TLR ligands. Because the induced CREMα binds to the CD86 promoter and suppresses the expression of CD86 on the surface membrane of DCs, it effectively limits their ability to provide costimulation to T cells. Thus, CREMα appears to be an inducible negative regulator of both T cell and DC function and its timely up-regulation after stimulation is surmised to end the initiated immune response.
Dysregulated CREMα expression is of importance in the pathogenesis of SLE. Specifically, SLE T cells express increased amounts of CREMα that binds to the Il2 promoter and suppresses its activity in a direct manner (4, 19). In addition, CREMα was found to bind to the c-fos promoter and limit the production of c-fos protein. Consequently, the formation and activity of the c-fos/c-jun (AP-1) heterodimer is diminished (6), which is a known enhancer of the Il2 promoter activity (20, 21). These studies as well as our experiments have provided hints for the development of tools to control the immune response. Suppression of CREMα expression with an antisense CREM construct resulted in correction of IL-2 production by SLE T cells. Similarly, (Fig. 2b) transfer of antisense CREMα resulted in increased promoter activity and expression of CD86. CD86 expression has been claimed to be important in the pathogenesis of several human diseases including SLE (22, 23, 24), atopic dermatitis (25, 26), and diabetes (27, 28). In SLE, the expression of CD86 on APCs is controversial and seems to depend on the cell type. Whereas monocyte-derived DCs have been reported to display increased expression of CD86 (24), B cells have been reported to have either increased or decreased expression of CD86 (29). In atopic dermatitis the expression of CD86 on B cells was significantly enhanced and correlated with a Th2 switch and IgE production (26). In the NOD mouse model of diabetes, CD86 knockout mice were protected from diabetes. Specifically, CD86 deficiency caused a profound diminishment in the generation of spontaneously activated CD4 T cells and islet-specific CD4 T cell expansion (27, 28). In a model of contact dermatitis, transgenic overexpression of CD86 in keratinocytes resulted in significantly enhanced disease activity (30). This is analogous to our contact dermatitis disease model in which transfer of hapten-sensitized Crem−/− DCs, which express increased levels of CD86, resulted in enhanced skin thickness after rechallenge with hapten (Fig. 5).
In this study, we have transferred haptenized Crem−/− DCs into naive mice and thus we have been able to specifically dissect the role of CREM in the regulation of APC function. Indeed, DCs deficient in CREM were able to stimulate T cells much stronger than CREM-sufficient DCs. This enhanced costimulation could be blocked in vivo by an anti-CD86 Ab. This in vivo demonstration complements our in vitro data, which demonstrated that CREM bound to the promoter of CD86 and suppressed its expression. In general, newly synthesized CREMα binds to promoters, which contain a CRE site. If the production of CREM is inhibited (e.g., by antisense CREMα) or in Crem−/− mice this resulted in an enhanced and prolonged transcription and expression of genes. Target genes among others include CREM itself (our unpublished observation), whereby it provides negative feedback to control its production.
Comparable to the mechanism in T cells, in which CREMα is a negative regulator of IL-2 transcription, the effect on the CD86 promoter seems to be mediated by chromatin-dependent mechanisms. CREMα and the transcriptional activator CREB are both involved in chromatin remodelling (31). Closed chromatin structure is a barrier for transcription and opening of chromatin can be achieved by acetylation of histones (32). We showed that binding of CREM was correlated inversely with acetylation of histone 4 on the CD86 promoter. CREB and CREM can bind CREB-binding protein and p300, which have histone acetylating transferase activities, with their kinase inducible domain (33, 10, 34). Unlike CREB, CREMα lacks both Q domains (31). The Q2 domain is necessary to activate histone acetylating transferase activities of p300 and CREB-binding protein and therefore, CREMα is unable to promote histone acetylating transferase activities of p300 and CREB-binding protein (31). Additionally, our findings suggest mechanisms similar to those identified in T cells where CREM regulates transcription by recruitment of HDAC1 (3). This claim is corroborated by an enhanced promoter activity after treatment with the HDAC inhibitor SAHA of cells transfected with the CD86 reporter construct. This enhancement was clearly dependent on the existence of a functional CRE site.
In conclusion, we have shown that CREM binds to the CD86 promoter and suppresses its expression. CREM-deficient DCs promote T cell proliferation in vitro and present allergen in vivo much stronger than CREM-sufficient DC. We present evidence that the CREM-mediated suppression of the CD86 promoter activity depends on histone acetylation. Thus CREM is an important negative regulator of APC function and its manipulation may be useful in the control of pathology that results from excessive Ag presentation to T cells.
Footnotes
This work is supported in part by a Public Health Service Grant R01 AI 49954, a Deutsche Forschungsgemeinschaft Grant TE 339/4-3, and an Interdisziplinäres Zentrum für Klinische Forschung Münster Grant FG5.
Abbreviations used in this paper: CREM, cAMP response element modulator; SLE, systemic lupus erythematosus; DC, dendritic cell; ChIP, chromatin immunoprecipitation; BMDC, bone marrow-derived DC; HDAC, histone deacetylase; CRE, cAMP response element.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Powell JD, Lerner CG, Ewoldt GR, Schwartz RH. The −180 site of the IL-2 promoter is the target of CREB/CREM binding in T cell anergy. J Immunol. 1999;163:6631–6639. [PubMed] [Google Scholar]
- 2.Tenbrock K, Juang YT, Tolnay M, Tsokos GC. The cyclic adenosine 5′-monophosphate response element modulator suppresses IL-2 production in stimulated T cells by a chromatin-dependent mechanism. J Immunol. 2003;170:2971–2976. doi: 10.4049/jimmunol.170.6.2971. [DOI] [PubMed] [Google Scholar]
- 3.Tenbrock K, Juang YT, Leukert N, Roth J, Tsokos GC. The transcriptional repressor cAMP response element modulator alpha interacts with histone deacetylase 1 to repress promoter activity. J Immunol. 2006;177:6159–6164. doi: 10.4049/jimmunol.177.9.6159. [DOI] [PubMed] [Google Scholar]
- 4.Tenbrock K, Juang YT, Gourley MF, Nambiar MP, Tsokos GC. Antisense cyclic adenosine 5′-monophosphate response element modulator up-regulates IL-2 in T cells from patients with systemic lupus erythematosus. J Immunol. 2002;169:4147–4152. doi: 10.4049/jimmunol.169.8.4147. [DOI] [PubMed] [Google Scholar]
- 5.Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, Kyttaris VC, Tsokos GC. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyttaris VC, Juang YT, Tenbrock K, Weinstein A, Tsokos GC. Cyclic adenosine 5′-monophosphate response element modulator is responsible for the decreased expression of c-fos and activator protein-1 binding in T cells from patients with systemic lupus erythematosus. J Immunol. 2004;173:3557–3563. doi: 10.4049/jimmunol.173.5.3557. [DOI] [PubMed] [Google Scholar]
- 7.Sassone-Corsi P. Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol. 1995;11:355–377. doi: 10.1146/annurev.cb.11.110195.002035. [DOI] [PubMed] [Google Scholar]
- 8.De Cesare D, Sassone-Corsi P. Transcriptional regulation by cyclic AMP-responsive factors. Prog Nucleic Acid Res Mol Biol. 2000;64:343–369. doi: 10.1016/s0079-6603(00)64009-6. [DOI] [PubMed] [Google Scholar]
- 9.Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 10.Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- 11.Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, Kennedy K, Hai T, Bolouri H, Aderem A. Systems biology approaches identify ATF3 as a negative regulator of Toll-like receptor 4. Nature. 2006;441:173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Liu Z, Jiang S, Cortesini R, Lederman S, Suciu-Foca N. T suppressor lymphocytes inhibit NF-κB-mediated transcription of CD86 gene in APC. J Immunol. 1999;163:6386–6392. [PubMed] [Google Scholar]
- 13.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenbrock K, V, Kyttaris C, Ahlmann M, Ehrchen JM, Tolnay M, Melkonyan H, Mawrin C, Roth J, Sorg C, Juang YT, Tsokos GC. The cyclic AMP response element modulator regulates transcription of the TCR æ-chain. J Immunol. 2005;175:5975–5980. doi: 10.4049/jimmunol.175.9.5975. [DOI] [PubMed] [Google Scholar]
- 15.Lavrrar JL, Farnham PJ. The use of transient chromatin immunoprecipitation assays to test models for E2F1-specific transcriptional activation. J Biol Chem. 2004;279:46343–46349. doi: 10.1074/jbc.M402692200. [DOI] [PubMed] [Google Scholar]
- 16.Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schutz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380:162–165. doi: 10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- 17.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-κB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- 18.Suzuki M, Shinohara F, Sato K, Taniguchi T, Takada H, Rikiishi H. Interleukin-1βconverting enzyme subfamily inhibitors prevent induction of CD86 molecules by butyrate through a CREB-dependent mechanism in HL60 cells. Immunology. 2003;108:375–383. doi: 10.1046/j.1365-2567.2003.01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J Immunol. 2001;166:4216–4222. doi: 10.4049/jimmunol.166.6.4216. [DOI] [PubMed] [Google Scholar]
- 20.Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992;356:801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- 21.Jain J, V, Valge-Archer E, Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J Immunol. 1992;148:1240–1250. [PubMed] [Google Scholar]
- 22.Tsokos GC, Kovacs B, Liossis SN. Lymphocytes, cytokines, inflammation, and immune trafficking. Curr Opin Rheumatol. 1997;9:380–386. doi: 10.1097/00002281-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Liossis SN, Sfikakis PP, Tsokos GC. Immune cell signaling aberrations in human lupus. Immunol Res. 1998;18:27–39. doi: 10.1007/BF02786511. [DOI] [PubMed] [Google Scholar]
- 24.Decker P, Kotter I, Klein R, Berner B, Rammensee HG. Monocyte-derived dendritic cells over-express CD86 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2006;45:1087–1095. doi: 10.1093/rheumatology/kel061. [DOI] [PubMed] [Google Scholar]
- 25.Ohki O, Yokozeki H, Katayama I, Umeda T, Azuma M, Okumura K, Nishioka K. Functional CD86 (B7-2/B70) is predominantly expressed on Langerhans cells in atopic dermatitis. Br J Dermatol. 1997;136:838–845. [PubMed] [Google Scholar]
- 26.Jirapongsananuruk O, Hofer MF, Trumble AE, Norris DA, Leung DY. Enhanced expression of B7.2 (CD86) in patients with atopic dermatitis: a potential role in the modulation of IgE synthesis. J Immunol. 1998;160:4622–4627. [PubMed] [Google Scholar]
- 27.Yadav D, Judkowski V, Flodstrom-Tullberg M, Sterling L, Redmond WL, Sherman L, Sarvetnick N. B7-2 (CD86) controls the priming of autoreactive CD4 T cell response against pancreatic islets. J Immunol. 2004;173:3631. doi: 10.4049/jimmunol.173.6.3631. [DOI] [PubMed] [Google Scholar]
- 28.Yadav D, Sarvetnick N. B7-2 regulates survival, phenotype, and function of APCs. J Immunol. 2007;178:6236–6241. doi: 10.4049/jimmunol.178.10.6236. [DOI] [PubMed] [Google Scholar]
- 29.Chang NH, McKenzie T, Bonventi G, Landolt-Marticorena C, Fortin PR, Gladman D, Urowitz M, Wither JE. Expanded population of activated antigen-engaged cells within the naive B cell compartment of patients with systemic lupus erythematosus. J Immunol. 2008;180:1276–1284. doi: 10.4049/jimmunol.180.2.1276. [DOI] [PubMed] [Google Scholar]
- 30.Burns R, Luzina I, Nasir A, Haidaris CG, Barth RK, Gaspari AA. Keratinocyte-derived, CD80-mediated costimulation is associated with hapten-specific IgE production during contact hypersensitivity to TH1 haptens. J Allergy Clin Immunol. 2005;115:383–390. doi: 10.1016/j.jaci.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Asahara H, Santoso B, Guzman E, Du K, Cole PA, Davidson I, Montminy M. Chromatin-dependent cooperativity between constitutive and inducible activation domains in CREB. Mol Cell Biol. 2001;21:7892–7900. doi: 10.1128/MCB.21.23.7892-7900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 33.Chakravarti D, V, LaMorte J, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM. Role of CBP/P300 in nuclear receptor signalling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 34.von Mikecz A, Zhang S, Montminy M, Tan EM, Hemmerich P. CREB-binding protein (CBP)/p300 and RNA polymerase II colocalize in transcriptionally active domains in the nucleus. J Cell Biol. 2000;150:265–273. doi: 10.1083/jcb.150.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]