Abstract
Axons and presynaptic nerve terminals of both invertebrate and mammalian SCG neurons contain a heterogeneous population of nuclear-encoded mitochondrial mRNAs and a local cytosolic protein synthetic system. Nearly one quarter of the total protein synthesized in these structural/functional domains of the neuron is destined for mitochondria. Acute inhibition of axonal protein synthesis markedly reduces the functional activity of mitochondria. The blockade of axonal protein into mitochondria had similar effects on the organelle’s functional activity. In addition to mitochondrial mRNAs, SCG axons contain approximately 200 different microRNAs (miRs), short, noncoding RNA molecules involved in the posttranscriptional regulation of gene expression. One of these miRs (miR-338) targets cytochrome c oxidase IV (COXIV) mRNA. This nuclear-encoded mRNA codes for a protein that plays a key role in the assembly of the mitochondrial enzyme complex IV and oxidative phosphorylation. Overexpression of miR-338 in the axon markedly decreases COXIV expression, mitochondrial functional activity, and the uptake of neurotransmitter into the axon. Conversely, the inhibition of endogeneous miR-338 levels in the axon significantly increased mitochondrial activity and norepinephrine uptake into the axon. The silencing of COXIV expression in the axon using short, inhibitory RNAs (siRNAs) yielded similar results, a finding that indicated that the effects of miR-338 on mitochondrial activity and axon function were mediated, at least in part, through local COXIV mRNA translation. Taken together, recent findings establish that proteins requisite for mitochondrial activity are synthesized locally in the axon and nerve terminal, and call attention to the intimacy of the relationship that has evolved between the distant cellular domains of the neuron and its energy generating systems.
Keywords: nuclear-encoded mitochondrial mRNAs, microRNA, cytochrome c oxidase IV, ATP synthesis, oxidative phosphorylation, sympathetic neurons
1 Introduction
One of the central tenets in neuroscience has been that the protein constituents of the distal structural/functional domains of the neuron (e.g., the axon and presynaptic nerve terminal) are synthesized in the nerve cell body and are subsequently transported to their ultimate sites of function. Although the majority of neuronal mRNAs are indeed translated in the neuronal cell soma, increasing attention is being focused on that subset of the transcriptome that is selectively transported to the distal domains of the neuron. The local translation of these mRNAs plays a key role in the development of the neuron and the function of the axon to include: navigation of the axon (Campbell and Holt 2001; Campbell et al. 2001; Ming et al. 2002; Zhang and Poo 2002, Wu et al. 2005; Leung et al. 2006), synthesis of membrane receptors employed as axon guidance molecules (Bruttis et al. 2002), axon regeneration (Zhang et al. 2001; Hanz et al. 2003; Verma et al. 2005), axon transport (Le et al. 2007), synapse formation (Schacher and Wu 2002), axon viability (Hu et al. 2003), neuronal survival (Cox et al. 2008), and activity-dependent synaptic plasticity (Martin et al. 1997; Casadio et al. 1999; Beaumont et al. 2001; Liu et al. 2003; Si et al. 2003).
Initial estimates of the number of mRNAs that are present in axon have derived from invertebrate model systems and approximate 200-400 different mRNAs (Perrone-Capano et al. 1987; Moccia et al. 2003). These messengers code for a diverse population of proteins to include cytoskeletal proteins, translation factors, ribosomal proteins, molecular motors, chaperone proteins and metabolic enzymes (for review, see Giuditta et al. 2008 and this volume). Results of early quantitative RT-PCR analyses established that the relative abundance of the mRNAs present in the axon differed markedly from that in the cell soma, a finding that suggested that these gene transcripts were being differentially transported into the axonal compartment (Chun et al. 1996). One surprising feature of the axonal mRNA population was the presence of a significant number of nuclear- encoded mitochondrial mRNAs (Gioio et al. 2001; Hillefors et al. 2007). Based in part upon this observation, it was hypothesized that proteins requisite for the maintenance of mitochondrial function are synthesized locally in the axon and presynaptic nerve terminal. In this communication, we review the evidence that both invertebrate and mammalian axons contain a heterogeneous population of nuclear-encoded mitochondrial mRNAs and that the local translation of these mRNAs plays a critical role in the regulation of the functional activity of the local mitochondrial population.
2 Nuclear-Encoded Mitochondrial mRNAs are Present in Invertebrate Axons and Nerve Endings
The initial evidence that mRNAs coding for nuclear-encoded mitochondrial proteins were present in the distal structural domains of the neuron derived from the squid giant axon and the large presynaptic nerve terminals of squid retinal photoreceptor neurons (Gioio et al. 2001, 2004). These structures were being employed as components of model invertebrate motor and sensory neurons, respectively.
In these early studies, differential mRNA display was used to compare the mRNAs present in the axon and neuronal cell soma. This comparison yielded approximately 150 sequences which manifested a higher relative abundance in the axon. These mRNA fragments were subsequently screened for evolutionary sequence conservation by dot-blot hybridization using cDNA prepared from mouse brain mRNA. Fifty of the squid sequences cross-hybridized to mouse brain cDNA and were subsequently subjected to DNA sequence analyses. Surprisingly, we were able to establish the identity of four nuclear-encoded mRNAs coding for mitochondrial proteins (COX 17, propionyl-OcA carboxylase, dihydrolipoamide dehydrogenase and CoQ7). In addition, mRNAs encoding the molecular chaperones Hsp70 and Hsp90, proteins facilitating the import of pre-proteins into the mitochondria, were also identified. The association of these mRNAs with polysomes prepared from presynaptic nerve terminals of photoreceptor neurons was subsequently established by RT-PCR and provided evidence to suggest that the mRNAs were being actively translated (Fig. 1).
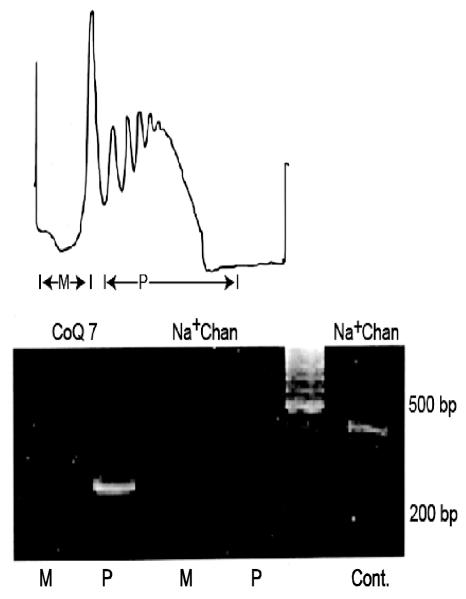
Fig. 1.
Nuclear-encoded m RNAs are actively translated in squid brain synaptosomes. (A) Polysomes were prepared from squid optic lobe synaptosomes and were displayed on linear sucrose density gradients. Gradients were divided into monosome (M) and polysome (P) fractions. UV absorbance of RNA was monitored continuously at 254 nm (B) RT-PCR analysis of monosome and polysome fractions using gene-specific primer sets for the nuclear-encoded mitochondrial protein CoQ7 and Na+ channel used as an internal control. PCR products were fractionated on agarose gels and visualized by ethidium bromide staining. The absence of amplicons generated from the Na+ channel primers indicates that the polysome fraction is devoid of RNA contamination from the neuronal cell soma. Cont, Na+ channel amplicons obtained from total RNA prepared from the optic lobe; bp, base-pairs; MW, molecular weight. Reproduced from Gioio et al. (2001).
To further evaluate the hypothesis that nuclear-encoded mitochondrial proteins were being locally synthesized, synaptosomes prepared from squid brain were incubated with [35S] methionine and the amount of newly synthesized protein associated with purified mitochondria was determined. Pretreatment of the synaptosomes with the antibiotic chloramphenicol was used to inhibit endogenous mitochondrial translational activity. Surprisingly, 20-25% of the total translational activity of squid brain synaptosomes was associated with the mitochondrial fraction. Hence, it appears that one of the major functions of the local protein synthetic system is to maintain the energy generating system present in the axon and nerve ending.
The results of a subsequent independent analysis of protein synthesized in squid brain synaptosomes confirmed the local synthesis of nuclear-encoded mitochondrial proteins (Jimenez et al. 2002). In this study, [35S] methionine-labeled synaptosomal proteins were fractionated by two-dimensional gel electrophoresis, and peptides generated by in-gel tryptic digestion were identified by mass spectrometry. Essentially, this proteomics study established the de novo synthesis of about 80 different proteins, several of which were nuclear-encoded mitochondrial proteins, as well as the molecular chaperone, Hsp70. Interestingly, in a high-throughput proteomic analysis of proteins synthesized in synaptoneurosomes derived from cultured rat cortical neurons, Liao et al (2007) reported that brain derived neurotrophic factor (BDNF) up-regulated the synthesis of approximately 200 different proteins. Eleven percent of these proteins were associated with mitochondria.
3 Axons of Sympathetic Neurons Contain a Heterogeneous Population of Nuclear-Encoded Mitochondrial mRNAs
To assess the general applicability of the early findings derived from invertebrate model systems, RT-PCR analyses and in situ hybridization histochemistry was employed to identify nuclear-encoded mitochondrial mRNAs in mammalian axons. In this study, primary sympathetic neurons were prepared from rat superior cervical ganglia (SGG) and were cultured in Campenot multi-compartment chambers (Campenot and Martin 2001). The use of this cell culture system, allows one to plate dissociated SCG neurons in the center compartment and grow axons into the two side compartments, providing pure axonal populations in both side compartments. One advantage of this culture system is that it permits the manipulation of axons in one of the side compartments, while axons in the opposite (contralateral) side compartments of the same culture dish serve as experimental controls (Hillefors et al. 2007).
Consistent with the findings derived from the squid giant axon and presynaptic nerve endings, SCG axons contain numerous nuclear-encoded mitochondrial mRNAs to include ATP synthase, cytochrome c oxidase IV and Va, DNA Polymerase γ, as well as the molecular chaperones Hsp70 and Hsp90 (for example, see Fig. 2A,B). The axonal localization of these mRNAs was confirmed by in situ hybridization (Fig. 2C).
Fig. 2.
SCG axons contain a heterogeneous population of mRNA. (A) RT-PCR analyses performed on total RNA isolated from SCG axons and somas. Note the shift in abundance of β-tubulin mRNA relative to H+-ATP synthase and DNA Polymerase γ mRNAs between axon and soma. (B) PCR products for β- tubulin and COXIV, a nuclear-encoded mitochondrial protein, were size-fractionated by agarose gel electrophoresis and visualized by ethidium bromide staining. MW, molecular weight. (C) COX IV subunit mRNA is present in SCG axons, as demonstrated with in situ hybridization histochemistry. Representative phase contrast photomicrographs of SCG axons after fixation and hybridization with antisense (COX IV antisense) and sense (Control; COX IV sense) riboprobes. Bar = 10 μm. From Hillefors et al. (2007).
In contrast to these findings, we were unable to detect the presence of several nuclear-encoded mitochondrial mRNAs in the SCG axons which were readily detected in the cell some located in the central compartment of the Campenot chambers (e.g., Mitochondrial topoisomerase 1, Cytochrome P450, Citrate lyase B subunit and Timm 10, a mitochondrial import inner membrane translocase subunit). This finding indicates that only a subset of the nuclear-encoded mitochondrial mRNAs that are expressed in the neuronal cell soma are translocated to the axon (Aschrafi et al. 2008).
The application of quantitative RT-PCR methodology allowed us to compare the relative abundance of different mRNAs present in the distal SCG axons present in the side compartments to that of the mRNAs present in the proximal axons and neuronal cell somas located in the central compartment. In somas and proximal axons, β-tubulin mRNA was more abundant than the mRNAs encoding ATP synthase and DNA polymerase γ, whereas the opposite was the case in the distal axon (Fig. 2A). The alterations in the relative abundance of these mRNAs in the different domains of the neurons suggest that there is a differential transport of nuclear-encoded mitochondrial mRNAs into SCG axons, an observation which is consistent with early findings reported in the squid giant axon (Chun et al. 1996). In this regard, mammalian axons may prove quite similar to those of cephalopod mollusks.
4 Axonal Protein Synthesis and Mitochondrial Activity
4.1 SCG Axons Contain a Local Protein Synthetic System
To test the hypothesis that SCG axons contain a local protein synthetic system, distal axons were exposed to low concentrations of the antibiotics cycloheximide, chloramphenicol or emetine prior to the addition of [35S] methionine. After a 4 hour labeling period, the amount of newly synthesized protein was assessed in the distal axons. The use of cycloheximide, emetine and chloramphenicol provides one the ability to differentiate between the axoplasmic and endogeneous mitochondrial translational activity, respectively. Cycloheximide and emetine markedly decreased the incorporation of [35S] methionine into protein (approximately 70-80%). In contrast, chloramphenicol had only modest effects on axonal protein synthesis, a finding that suggests that mitochondria, per se, contribute approximately 20-25% of the total protein synthetic active in the axon. Once again, the data obtained in a model mammalian neuron system were consistent with results obtained from the early metabolic labeling studies conducted in squid brain synaptosomes (Gioio et al. 2001; Jimenez et al. 2002).
4.2 Mitochondrial Membrane Potential and ATP Synthetic Capacity is Dependent on Local Protein Synthesis and Import of Axonal Protein
Mitochondrial viability and function are dependent on proteins synthesized from the cellular genome and these nuclear-encoded proteins are imported into the organelle from the cytosol (for review, see Bauer and Hoffman 2006). The import of mitochondrial pre-proteins utilizes the molecular chaperones Hsp70 and Hsp90, and a translocase complex situated on the outer membrane (TOM). The TOM70 receptor is located on the cytosolic side of the TOM complex, and disruption of the interaction of Hsp90 with the receptor inhibits the import of proteins into the mitochondria (Young et al. 2003). These events and inter-molecular interactions are schematized in Fig. 3. To evaluate the potential significance of axonal protein synthesis on mitochondrial function, we employed both protein synthesis inhibitors and a competitive inhibitor of the TOM70 receptor. The results of these experiments are summarized briefly below:
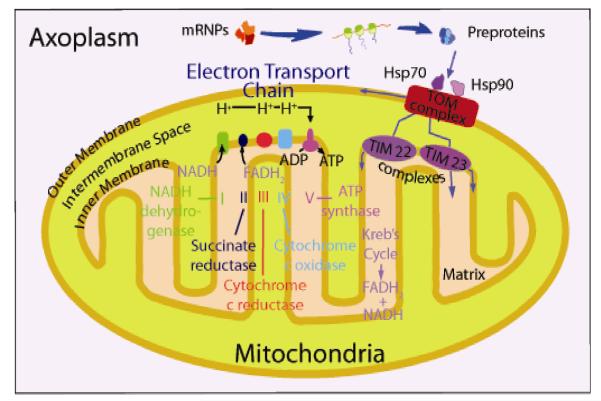
Fig. 3.
Model of a mitochondrion showing the outer and inner membrane, intramembrane space, and matrix. The Kreb’s cycle takes place in the matrix and the electron transport chain, generating ATP, is located in the inner membrane. Molecular chaperones such as Hsp70 and Hsp90 help facilitate the import of pre-proteins synthesized in the axoplasm into the organelle via the translocase of outermembrane (TOM) complex, which is located on the mitochondrion outer membrane.
4.2.1 Protein Synthesis Inhibition
The findings that SCG axons contain both nuclear-encoded mitochondrial mRNAs and a protein synthetic system, raised the possibility that proteins requisite for mitochondrial function are locally synthesized. To explore this postulate, axonal protein synthesis was inhibited by either cycloheximide or emetine and mitochondrial membrane potential was monitored using the mitochondrial-specific fluorescent dyes, JC-1 and TMRE. The fluorescense intensity of these molecular probes is proportional to the membrane potential of the organelle. Acute inhibition of local protein synthesis by either cycloheximide or emetine (3 hours) decreased mitochondrial membrane potential (Fig. 4A, B). Brief exposure to the protein synthesis inhibitors also inhibited the SCG axons ability to restore ATP levels after a depolarization stress induced by KCl treatment (Fig. 4C). These results suggest that the maintenance of axonal mitochondrial membrane potential and the organelle’s ability to generate ATP is dependent, at least in part, on locally synthesized proteins.
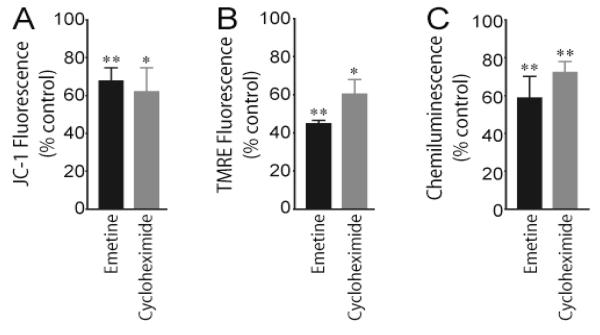
Fig. 4.
Inhibition of local protein synthesis decreases axonal mitochondrial membrane potential and generation of ATP. Axons in one side compartment of the Campenot cell culture chamber were exposed to the protein synthesis inhibitors emetine and cycloheximide for 3 hours and mitochondrial membrane potential assessed by subsequent treatment with the mitochondrial-specific fluorescent dyes JC-1 (A) or TMRE (B). Distal axons present in the contralateral side compartment served as vehicle-treated controls. Values for mitochondrial membrane potentials, as determined with each dye, are given as Mean ± S.E.M. (*P < 0.05, **P < 0.01) (C) Protein synthesis inhibitors impede the recovery of depolarization-induced decreases in axonal ATP levels. Depolarization was induced by exposing untreated SCG axons and axons exposed to emetine or cycloheximide for 3 hours followed by a 5 min exposure to 50 mM KCl. Axonal ATP levels were measured using ATPlite 1step kit and a microplate reader. Data shown are provided as the Mean ± S.E.M. (**P < 0.01). Reproduced with modification from Hillefors et al. (2007).
4.2.2 Blockade of Mitochondrial Protein Import
One means used to disrupt the import of axoplasmic proteins into mitochondria is to expose the TOM70 receptor to the carboxy terminal portion of Hsp90 (C90; Young et al. 1998). Although the C90 fragment can bind to the receptor, it is nonfunctional in that it cannot bind cargo proteins. When introduced into the axon, C90 molecules act as competitive inhibitors preventing full-length functional Hsp90 molecules from binding to the TOM70 receptor. To facilitate the detection of mitochondria with C90 molecules bound to their TOM70 receptors, C90 was labeled with fluorescent Alexa Fluor 488, and the inhibitor lipofected directly into the distal axons in one side compartment of the Campenot chambers. Axons in the contralateral compartment were treated with the reagent vehicle and served as controls. Alterations in mitochondrial membrane potential were subsequently employed to evaluate the effects of decreased protein import into the organelle. After 4 hours of exposure to C90, the membrane potential of Alexa-labeled mitochondria was reduced to 35% control values, indicating that mitochondria present in the distal axon require the import of local protein to maintain their functional integrity.
5 MicroRNAs in the Axon
MicroRNAs (miRs) are small (21-23 nucleotides), single-stranded, noncoding RNAs involved in the posttranscriptional regulation of gene expression. They are highly conserved and play a key role in the regulation of numerous biological processes such as cell proliferation and differentiation (for a neuronal perspective, see Kosik 2006). Results of a recent microarray analysis indicate that in the axons of primary sympathetic neurons there are approximately 200 different miRs. The axonal localization of twenty of these miRs was subsequently verified by quantitative RT-PCR methodology. Here, we focus on a brain-specific microRNA, miR-338, which modulates the expression of cytochrome c oxidase IV (COXIV), a nuclear- encoded protein which plays an important role in the assembly of the mitochondrial enzyme complex IV and oxidative phosphorylation (Fig. 3).
5.1 MicroRNAs Target Nuclear-encoded Mitochondrial mRNAs
As mentioned above, COXIV mRNA is one of several nuclear-encoded mRNAs that is present in axons of sympathetic neurons (Fig. 2). Sequence analysis of the relatively short 3′ untranslated region (3′UTR) of this mRNA revealed the presence of a putative target site for miR-338 (MTS). A RNA secondary structural analysis of the region indicated that the miR-338 MTS was positioned on a hairpin-loop structure, in an exposed position, that might facilitate miR accessibility. The transfection of axons of cultured SCG neurons with chimeric reporter gene constructs containing the COXIV 3′UTR with or without the putative MTS, established that miR-338 could specifically target COXIV mRNA (Aschrafi et al. 2008).
To explore the possibility that mature miRs might function in the axon, the presence of Dicer and the RNA-induced silencing complex (RISC) component eIF2c was visualized in the distal axons by immunocytochemistry. Upon fluorescence microscopy, Dicer and eIF2c antibodies revealed the presence of granule-like structures along the entire length of the axon. These findings support the hypothesis that microRNAs play a role in the regulation of mRNA levels in the axon and are consistent with the report of Hengst et al. (2006) who demonstrated that axons of dorsal root ganglion neurons were capable of autonomously silencing a gene without the contribution of the cell body.
In addition, SCG axons were transfected with precursor miR-338 (pre-miR-338) and levels of mature miR-338 determined by RT-PCR using specific primers for the mature form of the molecule. Transfection of axons with pre- miR-338 resulted in a 42-fold increase in mature miR-338 levels within 4 hours. After 24 hours, a 42,000-fold increase in mature miR-338 levels were observed in transfected axons compared to endogenous levels in sham-transfected control axons. Clearly, distal axons of SCG neurons contain Dicer and RISC and have the capability of processing miR precursors to the mature form of the molecule.
5.2 Regulation of Axonal COXIV Expression by miR-338
To evaluate whether miR-338 can modulate COXIV expression in the distal axon, COXIV mRNA and protein levels were monitored after transfecting SCG axons with pre-miR-338. COXIV mRNA and protein decreased by 80% and 60% control values 24 hours after transfection, respectively (Fig. 5A,B). Conversely, the inhibition of endogenous miR-338 by transfection of the axons with a competitor molecule (anti-miR-338) resulted in a 3.5-fold increase in axonal COXIV mRNA levels 24 hours after transfection (Fig. 5C). Two-fold increases in axonal COXIV protein levels were observed 24 hours after transfection with anti- miR-338 (Fig. 5D).
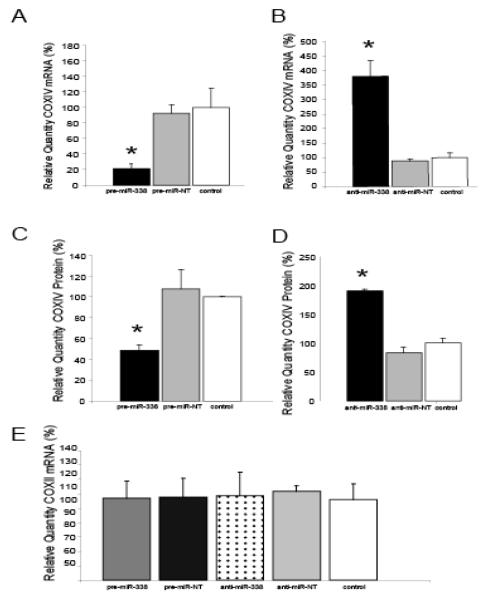
Fig. 5.
Introduction of miRNA-338 into distal SCG axons reduces COXIV expression . Quantification of COXIV mRNA levels in the distal axons of SCG neurons transfected with pre-miR-338 (A), or anti-miR-338 (B), as determined by quantitative RT-PCR 24 hours after oligonucleotide transfection. COXIV mRNA levels are expressed relative to β-actin mRNA. Values given are the Mean ± S.E.M. (*P<0.05). The introduction into the distal axons of pre-miR-338 (C) or anti-miR-338 (D) also altered axon COXIV protein levels 24 hours after transfection as determined by immunoblot analyses. Values shown are Mean ±S.E.M. (*P<0.05). Transfection of SCG axons with either precursor or anti-miR-338 oligonucleotides did not affect the relative abundance of COXII mRNA in the axon as shown by quantitative RT-PCR (E). Reproduced from Aschrafi et al. (2008) with modification.
The specificity of the COXIV response to anti-miR-338 was evaluated by assessing COXII mRNA levels by quantitative RT-PCR after transfection of the axons with anti-miR-338. This mRNA codes for one of the three subunits of the enzyme complex IV encoded by the mitochondrial genome. In contrast to the alterations in COXIV expression induced by anti- miR-338, no differences in COXII mRNA levels were observed 24 hours after transfection with anti- miR-338 (Fig. 5E).
5.3 Mir-338 Mediates Axonal Mitochondrial Activity
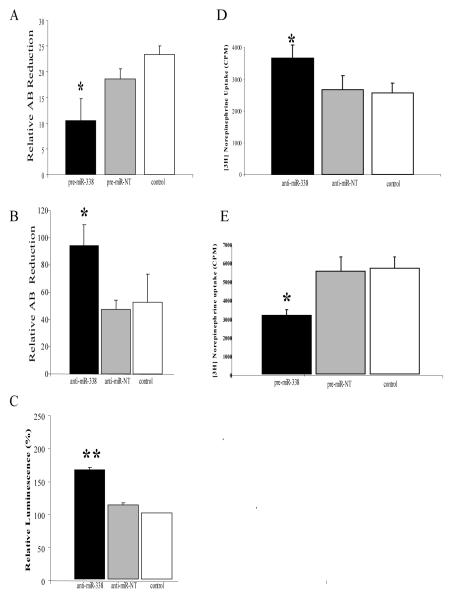
Alteration in the levels of miR-338 in the axon has a profound effect on the activity of the axonal mitochondrial population and the basal metabolic rate of the axon. For example, the overexpression of miR-338 in the axon significantly reduces mitochondrial oxygen consumption, as demonstrated by the reduction of Alamar Blue (AB), a redox dye used to assess the metabolic activity of mitochondria (Fig. 6A). The inhibition of miR-338 activity in the axon by the introduction of a competitor RNA (anti-miR-338) directly into the axon by lipofection increased oxygen consumption by 50-60% within 24 hours (Fig. 6B). Consistent with this finding, axonal ATP levels were elevated by 50% after the inhibition of endogenous axonal miR-338 (Fig 6C).
Fig. 6.
MiR-338 mediated alteration in COXIV levels modulates metabolic activity of mitochondria and neurotransmitter uptake into the distal axons of sympathetic neurons. SCG axons were transfected with either precursor miR-338 (A), anti-miR-338 (B), or non-targeting short oligonucleotides and metabolic activity of the axons assessed using the redox dye Alomar Blue (AB). Data represent Mean ±S.E.M. (* P < 0.05). ATP levels were measured in anti-miR-338 transfected axons using the luciferase cell viability assay (C). Values are given in arbitrary luminescence units and represent the Mean ±S.E.M. (** P <0.0001). Distal axons were also transfected with either anti-miR-338 (D), pre-miR-338 (E), or non-targeting short oligonucleotides (NT) and were subsequently exposed to radiolabeled norepinephrine (NE). NE uptake into distal treated axons was measured by liquid scintillation spectrometry. Data represent Mean ±S.E.M. (* P < 0.002). Reproduced with modification from Aschrafi et al. (2008).
To delineate the impact of the modulation of axonal miR-338 levels on ATP synthesis and respiration on the function of the axon and presynaptic nerve terminal, the distal axons present in the side compartments of the Campenot chambers were exposed to the neurotransmitter, [3H] norepinephrine (NE), and neurotransmitter uptake was assessed after introducing precursor miR-338 or anti-miR-338 directly into the distal axons. Under the experimental cell culture conditions employed in these experiments, desiprimine, a powerful NE uptake inhibitor, reduced the amount of [3H] NE in the distal axons by 85-95%. The introduction of anti-miR-338 or pre-miR-338 into the distal axons resulted in a 50% increase or decrease in the uptake of NE, respectively (Fig. 6D,E). These findings clearly established that modulation of axonal miR-338 levels has marked effects on axonal ATP synthesis, metabolic rate, and axonal function, as judged by catecholamine neurotransmitter uptake.
5.4 Knockdown of Local COXIV Expression Alters Mitochondrial Activity
Although the modulation of miR-338 levels in the axon has significant effects on axonal metabolic rate and function, this microRNA may have multiple target gene transcripts and it is unclear whether the effects described above derived from the miR-338-mediated regulation of COXIV expression per se. To further explore this phenomenon, short inhibitory RNAs (siRNA), designed to specifically silence COXIV expression, were introduced directly into the axons of primary sympathetic neurons cultured in Campenot chambers by lipofection. The introduction of these siRNAs into the axon resulted in marked reductions in the levels of COXIV mRNA and protein (Fig. 7A,B). Identical to the findings obtained with the overexpression of miR-338, reduction in axonal COXIV expression by RNA interference resulted in significant decrements in basal oxygen consumption (Fig.7C) and axonal ATP levels (Fig. 7D). In contrast, the introduction into distal axons of a nontargeted siRNA (NT) had no effect on these experimental outcomes. Moreover, the knockdown of local COXIV expression reduced axonal function by 25-30%, as judged by the reduction of [3H] NE uptake into the distal axons (Fig.7E). These findings provide persuasive evidence that the effects of MiR-338 on mitochondrial activity and axonal function are mediated, at least in part, by translational inhibition of COXIV mRNA.
Fig. 7. Knock-down of local COXIV levels decreases axonal respiration and ATP levels, and diminishes NE uptake into distal axons.
Two independent siRNA oligonucleotides targeted against COXIV mRNA were introduced into distal axons of SCG neurons by lipofection. COXIV mRNA (A) and protein levels (B) were quantitated 24 hours later by quantitative RT-PCR and immunoblot analyses, respectively. Knock-down of axonal COXIV expression reduced basal oxygen consumption (C), ATP levels (D), and [3H]NE uptake (E). Values are expressed as Mean ±S.E.M and statistical significance evaluated by One-Way ANOVA (* P<0.03). From Aschrafi et al. (2008).
6 Conclusion
The mitochondrial genome encodes only a few proteins (approximately 13) thus requiring the mitochondria to import several hundred nuclear-encoded proteins to maintain its structure, function and replicative activity. For example, 10 of the 13 subunits of enzyme complex IV, the proteins involved in the electron transport chain and ATP synthesis, derive from the nuclear genome (Heales et al. 2006). Numerous studies have demonstrated that mitochondrial functional activity requires a continuous import of nuclear-encoded proteins.
In neurons, it is well established that mitochondria are translocated via fast axonal transport from the cell soma to the axon and presynaptic nerve terminal (Hollenbeck and Saxton, 2005) and that mitochondrial trafficking can be regulated by neuronal activity ( for example, see Chang et al. 2006). However, little is known about the half-life or fate of these organelles once they reach their ultimate sites of function. If axons and nerve terminals lacked a cytosolic protein synthetic system then the resupply of protein for the local mitochondria must by default differ significantly from that in the cell soma. Alternatively, the organelles present in the distal domains of the neuron could be relatively, short-lived and continuously resupplied. The weight of the recent evidence would indicate that the mitochondria present in the axon and nerve ending are being sustained by local protein synthesis. Most significantly, it has recently been reported that mitochondria can replicate in the axon and nerve ending (Amiri and Hollenbeck 2008). The finding that the nuclear-encoded mitochondrial DNA polymerase γ mRNA is present in the axons of sympathetic neurons is consistent with this report (Hillefors et al. 2007), and raises the possibility that the local synthesis of nuclear-encoded mitochondrial proteins also plays an important role in the biogenesis of this organelle.
The local synthesis of both nuclear-encoded mitochondrial proteins and the molecular chaperones necessary for their import into the organelle suggests that a substantial portion of the protein synthesized in the axon and nerve terminal are directed toward fulfilling the biological demands of its energy generating and calcium buffering system. As mentioned above (Section 2), 20-25% of the total protein synthesized in these regions of the neuron is destined for the mitochondria. One surprising feature of the relationship between the axon and its mitochondrial population is the apparent rapid turnover rate of the locally synthesized protein. For example, only brief time periods of protein synthesis inhibition (3-4 hours) or the blockade of local protein import into the organelle was sufficient to induce mitochondrial membrane depolarization and diminish the organelle’s ability to generate ATP (Hillefors et al. 2007). These observations indicate that a significant portion of mitochondrial protein is being rapidly turned over in the axon, and raises the possibility that one of the major functions of the local protein synthetic system is to replace these highly labile constituents of this organelle. Interestingly, Li et al. (2006) demonstrated that COXIV plays a rate-limiting role in the assembly of enzyme complex IV, and that dysfunctional COXIV resulted in a compromised mitochondrial membrane potential, as well as decreased ATP levels and respiration. Such deficits could ultimately have deleterious effects on axon function and the ability of the neuron to respond to prolonged periods of activation or stress.
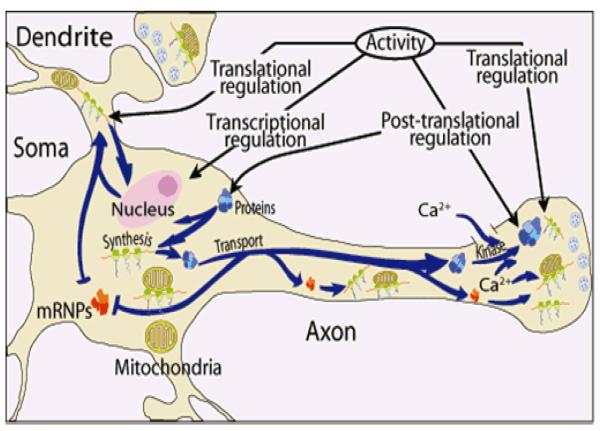
In summary, the findings reviewed here support the hypothesis that protein synthesis in the distal regions of the neuron is vital for mitochondrial activity and suggests a new model for the maintenance of mitochondrial function in the axon and nerve terminal (Fig. 8). In this schema, nuclear-encoded mitochondrial mRNAs are transported to the distal structural/functional domains of the neuron at a rapid rate in the form of relatively stable ribonucleoprotein particles (mRNPs). These complexes can serve as templates for the synthesis of mitochondrial pre-proteins, and their translation can be regulated by multiple mechanisms, to include microRNAs, at their site of function in response to local neuronal activity. In this regard, it has been reported that ribosome clusters (i.e., polysomes) in the squid giant axon are often visualized in association with mitochondria (Bleher and Martin 2001), and that the close approximation of mRNA to the vicinity of mitochondria is essential for respiratory function (Margeot et al. 2005). Hence, in this model, it is envisaged that pre-proteins are synthesized juxtaposed to or on the surface of axonal mitochondria and that this relationship plays a key role in the biogenesis, maintenance and functional activity of the organelle.
Fig. 8.
Model of neuronal protein synthesis. Protein synthesis occurs in multiple compartments within neurons to include the dendrite, axon, and nerve terminal. Key features of the model include the rapid and selective transport of stable messenger ribonucleoprotein complexes (mRNPs) to the neuronal periphery and the local regulation of mRNA translation in response to neuronal activity. The model also shows that synthesis of proteins occurs in the vicinity or on the surface of mitochondria in the distal parts of the neuron. The activity in the neuron can be modulated by transcriptional regulation in the soma, as well as translational and post-translational regulation in dendrites, axons, and nerve terminals. The down regulation of gene expression in the distal structural/functional domains of the neuron can also be effected by microRNAs. From Hillefors et al. (2007).
Acknowledgements
Work in the author’s laboratory was supported by the Division of Intramural Research Programs of the National Institute of Mental Health. The expert technical assistance of Ms. Orlangie Natera-Naranjo is greatly appreciated.
References
- Amiri M, Hollenbeck PJ. Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev Neurobiol. 2008;68:1348–1361. doi: 10.1002/dneu.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, Kaplan BB. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J Neurosci. 2008;28:12581–12590. doi: 10.1523/JNEUROSCI.3338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MF, Hofman S. Import of mitochondrial proteins. In: Shapira AHV, editor. Mitochondrial function and dysfunction. Academic Press; San Diego, CA: 2006. pp. 57–90. [Google Scholar]
- Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS. Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron. 2001;32:489–501. doi: 10.1016/s0896-6273(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Bleher R, Martin R. Ribosomes in the squid giant axon. Neuroscience. 2001;107:527–534. doi: 10.1016/s0306-4522(01)00366-9. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Campenot RN, Martin G. Construction and use of compartmented cultures for studies of cell biology in neurons. In: Federoff S, Richardson A, editors. Protocols for neural cell culture. Humana Press, Inc.; Totowa, NJ: 2001. pp. 49–57. [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Chang DTW, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JT, Gioio AE, Crispino M, Giuditta A, Kaplan BB. Differential compartmentalization of mRNAs in squid giant axon. J Neurochem. 1996;67:1806–1812. doi: 10.1046/j.1471-4159.1996.67051806.x. [DOI] [PubMed] [Google Scholar]
- Gioio AE, Eyman M, Zhang H, Lavina ZS, Giuditta A, Kaplan BB. Local synthesis of nuclear-encoded mitochondrial proteins in the presynaptic nerve terminal. J Neurosci Res. 2001;64:447–453. doi: 10.1002/jnr.1096. [DOI] [PubMed] [Google Scholar]
- Gioio AE, Lavina ZS, Jurkovicova D, Zhang H, Eyman M, Giuditta A, Kaplan BB. Nerve terminals of squid photoreceptor neurons contain a heterogeneous population of mRNAs and translate a transfected reporter mRNA. Eur J Neurosci. 2004;20:865–872. doi: 10.1111/j.1460-9568.2004.03538.x. [DOI] [PubMed] [Google Scholar]
- Giuditta A, Chun JT, Eyman M, Cefaliello C, Bruno AP, Crispino M. Local gene expression in axons and nerve endings: the glia-neuron unit. Physiol Rev. 2008;88:515–555. doi: 10.1152/physrev.00051.2006. [DOI] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Heales SJR, Gegg ME, Clark JB. Oxidative phosphorylation: structure, function, and Intermediary metabolism. In: Shapira AHV, editor. Mitochondrial function and dysfunction. Academic Press; San Diego, CA: 2006. pp. 25–56. [DOI] [PubMed] [Google Scholar]
- Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. Functional and selective RNA interference in developing axons and growth cones. J Neurosci. 2006;26:5727–5732. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillefors M, Gioio AE, Mameza MG, Kaplan BB. Axon viability and mitochondrial function are dependent on local protein synthesis in sympathetic neurons. Cell Mol Neurobiol. 2007;27:701–716. doi: 10.1007/s10571-007-9148-y. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez CR, Eyman M, Lavina ZS, Gioio A, Li KW, van der Schors RC, Geraerts WP, Giuditta A, Kaplan BB, van MJ. Protein synthesis in synaptosomes: a proteomics analysis. J Neurochem. 2002;81:735–744. doi: 10.1046/j.1471-4159.2002.00873.x. [DOI] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Leung KM, van Horck FP, Lin AC, Allison R, Standart N, Holt CE. Asymmetrical β-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Park JS, Deng JH, Bai Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr. 2006;38:283–291. doi: 10.1007/s10863-006-9052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Pilotte J, Xu T, Wong CCL, Edelman GM, Vanderklish P, Yates JR., III BDNF induces widespread changes in synaptic protein content and up-regulates components of the translational machinery: An analysis using high-throughput proteomic. J Proteome Res. 2007;6:1059–1071. doi: 10.1021/pr060358f. [DOI] [PubMed] [Google Scholar]
- Liu K, Hu JY, Wang D, Schacher S. Protein synthesis at synapse versus cell body: enhanced but transient expression of long-term facilitation at isolated synapses. J Neurobiol. 2003;56:275–286. doi: 10.1002/neu.10242. [DOI] [PubMed] [Google Scholar]
- Margeot A, Garcia M, Wang W, Tetaud E, di Rago JP, Jacq C. Why are many mRNAs translated to the vicinity of mitochondria: a role in protein complex assembly? Gene. 2005;354:64–71. doi: 10.1016/j.gene.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- Moccia R, Chen D, Lyles V, Kapuya E, E Y, Kalachikov S, Spahn CM, Frank J, Kandel ER, Barad M, Martin KC. An unbiased cDNA library prepared from isolated Aplysia sensory neuron processes is enriched for cytoskeletal and translational mRNAs. J Neurosci. 2003;23:9409–9417. doi: 10.1523/JNEUROSCI.23-28-09409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone Capano C, Giuditta A, Castigli E, Kaplan BB. Occurrence and sequence complexity of polyadenylated RNA in squid axoplasm. J Neurochem. 1987;49:698–704. doi: 10.1111/j.1471-4159.1987.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Poon MM, Choi SH, Jamieson CA, Geschwind DH, Martin KC. Identification of process-localized mRNAs from cultured rodent hippocampal neurons. J Neurosci. 2006;26:13390–13399. doi: 10.1523/JNEUROSCI.3432-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S, Wu F. Synapse formation in the absence of cell bodies requires protein synthesis. J Neurosci. 2002;22:1831–1839. doi: 10.1523/JNEUROSCI.22-05-01831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell. 2003;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Obermann WM, Hartl FU. Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90. J Biol Chem. 1998;273:18007–18010. doi: 10.1074/jbc.273.29.18007. [DOI] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Poo MM. Localized synaptic potentiation by BDNF requires local protein synthesis in the developing axon. Neuron. 2002;36:675–688. doi: 10.1016/s0896-6273(02)01023-1. [DOI] [PubMed] [Google Scholar]