Abstract
This mini review highlights issues associated with the use of dendrimers as drug delivery vehicles. The review introduces dendrimers and summarizes findings on their use in vivo and in vitro. Specifically, this review is limited to examples wherein the drug is non-covalently associated with the dendrimer. Examples wherein the drug is covalently attached to the dendrimer are not discussed.
Keywords: dendrimer, non-covalent, drug, delivery, review
INTRODUCTION
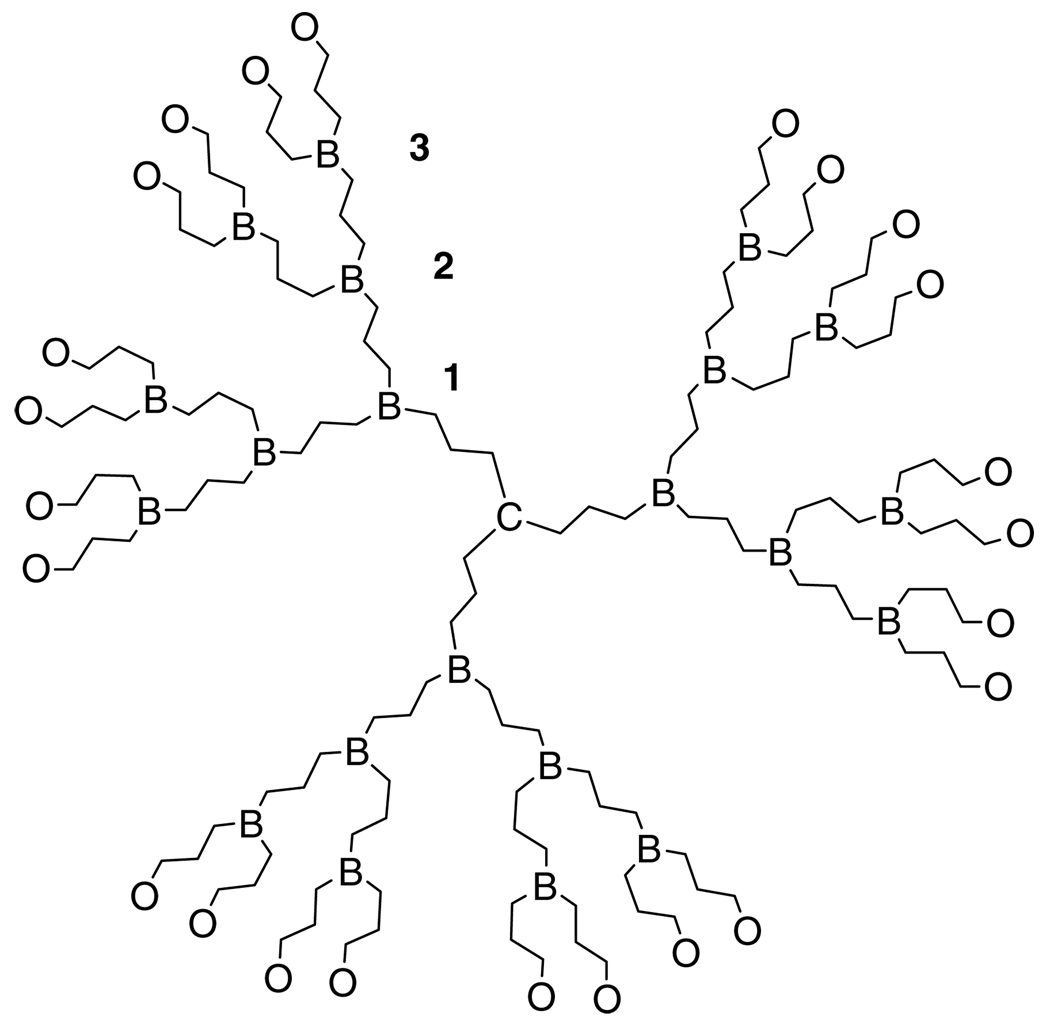
The word dendrimer is derived from the Greek words dendron (‘tree’ or ‘branch’) and meros (‘part’). Dendrimers are hyperbranched macromolecules comprising a multifunctional core (C), several branching points (B), and outer surface moieties (O) (Fig. 1). Dendrimer size is classified by ‘generation’, wherein each generation corresponds to a layer of branching units. Unlike traditional linear polymer synthesis that produces a mixture of materials ranging in molecular weight, dendrimers are the product of multistep organic synthesis. This difference means that dendrimers can in theory, but not always in practice, be single chemical entities. In the extreme, dendrimers are synthesized divergently from a multifunctional core outward,1 or convergently from the surface groups inward to form a dendron that is reacted with the multifunctional core.2 These routes have been reviewed extensively.3–6 The results are macromolecules that have a very low polydispersity, and a defined number of reactive surface groups. An opportunity to control dendrimer size, shape, and surface reactivity has brought these molecules to the forefront of biomedicine and drug delivery in particular.
Figure 1.
Generalized generation-3 dendrimer. C = core, B = branching points, O = outer surface groups.
At a generation dependent on the monomer used, the very regular hyperbranched structure of dendrimers leads to a densely packed, globular macromolecule that can have a unique, well-defined outer and inner three-dimensional architecture.7 At lower generations, however, the dendritic branches may backfold into the dendrimer at certain pH values and solvent polarities, dependent on the surface groups of the dendrimer.8,9 With large numbers of surface groups (a G-4 polyamidoamine (PAMAM) dendrimer has 64 surface amines), dendrimers have the capacity to take place in multivalent interactions. Multivalency has been shown to result in an increase in activity compared to a monomeric interaction, called the ‘cluster’ or ‘dendritic’ effect.6 The dendritic effect occurs when the simultaneous binding of n molecules to the same ligand results in a synergistic increase of affinity of the binding molecules, with a maximum binding affinity equal to that of the single binding affinity, Ka, raised to the power of n, expressed as (Ka)n.
Dendrimers have been synthesized for examination in numerous biomedical applications including gene delivery, chemotherapy, ocular adhesives, anti-infectives, and in vivo diagnostics.10 This mini review addresses only non-covalent interactions of drugs and dendrimers, with a brief discussion of the factors that anchor the merits of these investigations; namely, dendrimer toxicity and biodistribution. Covalent interactions of drugs and dendrimers have been reviewed elsewhere recently.11
DENDRIMER TOXICITY AND BIODISTRIBUTION
Of the many considerations for in vivo use of any molecule, cytotoxicity, hemocompatibility, immunogenicity, and organ accumulation are critical. Dendrimer cytotoxicity has been related to generation, concentration, and the chemistry of surface groups. The cytotoxicity and cell permeability of dendrimers has been found to increase with increasing generation and concentration.12 Duncan et al.13 found that cationic PAMAM dendrimers showed increased toxicity compared to anionic dendrimers. These results were supported by a study by Chen et al.,14 in which cationic melamine-based dendrimers were much more cytotoxic than poly(ethylene glycol)-derived (PEGylated) or anionic dendrimers. Recent studies have shown that generation 7 cationic PAMAM dendrimers interact with lipid bilayers comprising dimyristoylphosphatidylcholine, causing the formation of 15–40 nm wide holes in the bilayers.15 The formation of holes in cell membranes creates a disturbance in electrolyte flux, causing cell death.16 Although the cytotoxicity of a dendrimer is most strongly influenced by its surface charge, a computational study by Pricl and co-workers17 suggests that cytotoxicity may also be related to the radius of gyration, molecular shape, and dimensions of a dendrimer. In a series of polyamidoamine dendrimers, the non-cytotoxic dendrimers were characterized by a dense, globular shape and a smooth surface pattern. Cationic PAMAM dendrimers larger than generation 1 exhibited generation-dependent hemolysis above a concentration of 1mg mL−1; however, diaminobutane (DAB) and diaminoethane (DAE) dendrimers did not show generation-dependent hemolytic activity, and anionic PAMAM and DAB showed no hemolysis.13 Following the results for cytotoxicity, cationic melamine dendrimers caused more hemolysis than anionic or PEGylated dendrimers.14
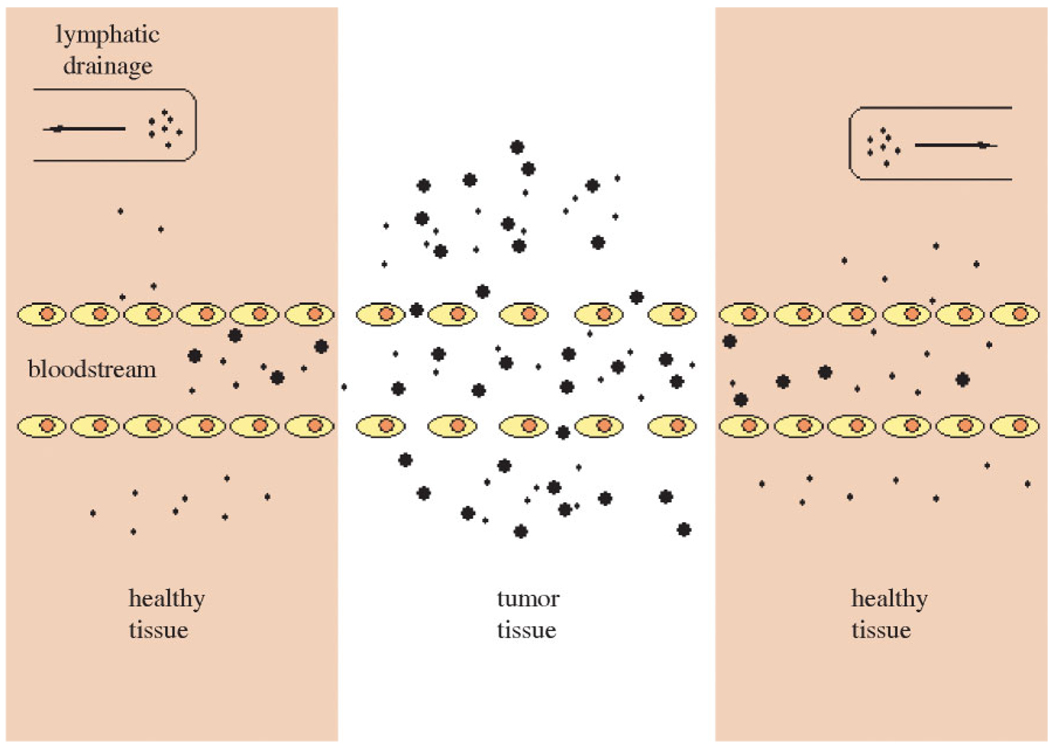
The biodistribution of parenterally administered dendrimers has been widely studied due in large part to the development of dendritic-based imaging agents.4,10,13 In general, smaller generation dendrimers show rapid renal elimination, while higher molecular weight dendrimers, as well as those with charged or hydrophobic surfaces, are cleared by the liver.18 Dendrimers bearing hydrophilic or PEGylated surfaces are not cleared rapidly. Selective accumulation of dendrimers in tumor tissue may be accomplished through the enhanced permeability and retention (EPR) effect.19 The discontinuous endothelium of tumor vasculature (their so-called ‘leaky’ vasculature), which allows large molecules circulating in the bloodstream to pass through, and the lack of effective lymphatic drainage in tumors (Fig. 2) together cause the passive accumulation of macromolecules in solid tumor tissue. This may increase the tumor concentration of antitumor drugs up to 70-fold when drug delivery systems are injected intravenously.20
Figure 2.
Schematic of the EPR effect.
Albumin is the most abundant protein in mammals, and is very important to the transport and deposition of many substances in the blood. Albumin is a highly charged biomolecule with a formal charge of −15.21 It has been proposed that the interaction of cationic and anionic dendrimers with serum albumin is the cause of much of the immunogenicity of these dendrimers. Dendrimers can also trigger the release of cytokines and chemokines, which can cause inflammation and cytotoxicity; however, their production can be utilized and directed to localize in tumor tissue22 or reduce scar tissue after glaucoma filtration surgery in an animal model.23
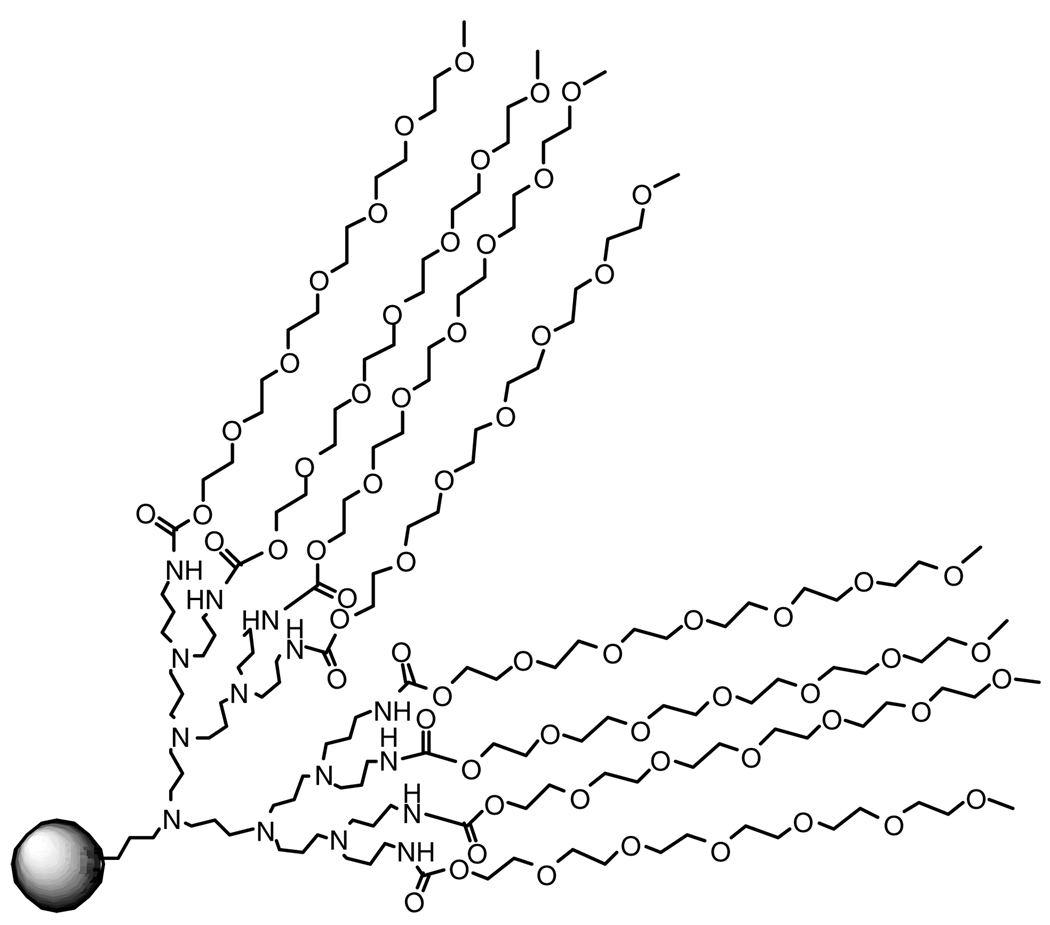
Many of the adverse affects displayed by dendrimers can be attenuated through the conjugation of poly(ethylene glycol) (PEG) to their surface. PEGylated materials have shown greatly reduced cyto- and hemotoxicity,14,24,25 as well as increased circulation time in the blood and decreased accumulation in the liver and kidneys.26 These effects are attributed to the reduction or shielding of the positive charge on the dendrimer surface by the PEG chains (Fig. 3).25
Figure 3.
Poly(ethylene glycol) shields the ionizable dendrimer groups.
COMPLEXATION OF DNA
The multitude of surface sites of dendrimers can present ionizable groups, such as primary amines, that are ideal for forming complexes with many drugs. The first human clinical trials of gene transfection were initiated in 1990, but so far success has been limited by a shortage of methods for delivering the genetic material. The development of efficient and nontoxic vectors that can transport exogenous DNA into cells is required to complement the original viral vectors.27 Dendrimers may prove to be suitable in this capacity.
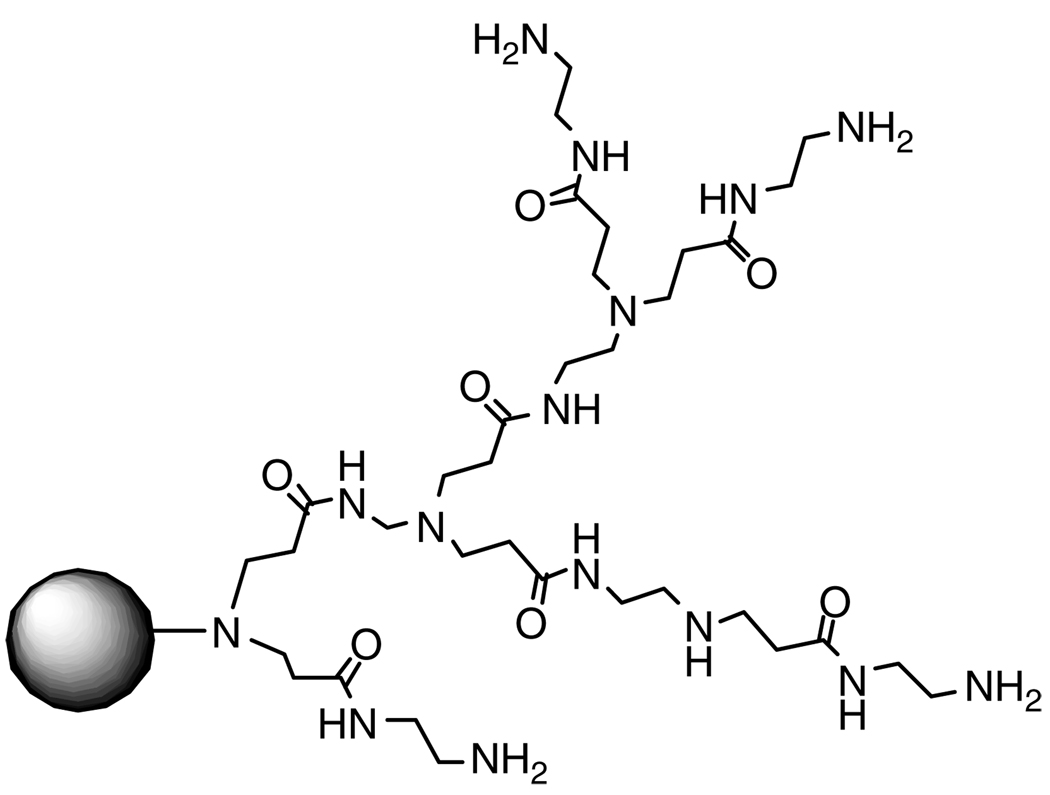
The most common dendrimers used today in gene delivery are polyethyleneimine and fractured PAMAM, the commercially available carrier Superfect®(Fig. 4).28,29
Figure 4.
Fractured PAMAM dendrimer Superfect®.
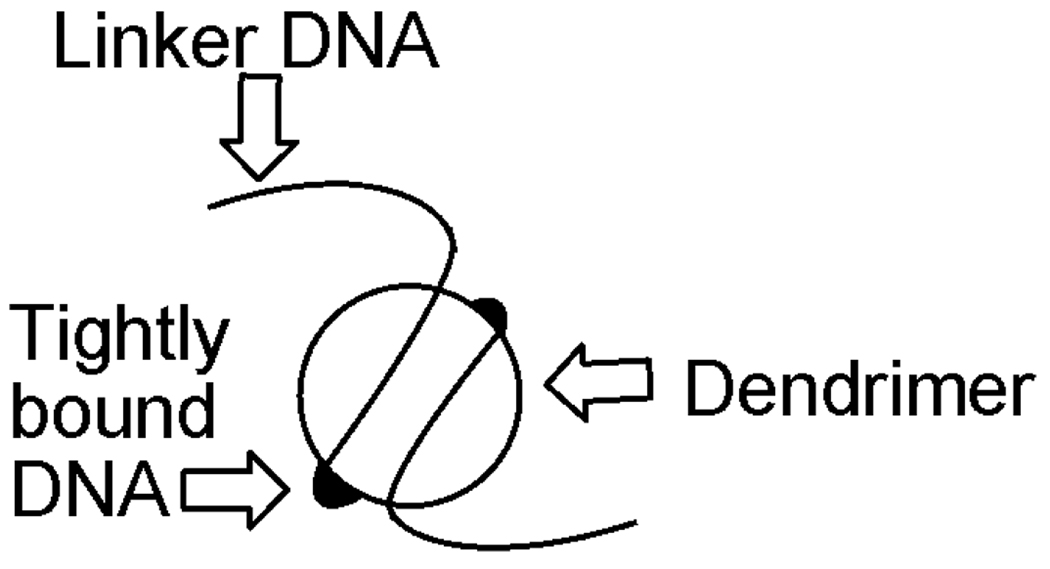
DNA binding is the first requirement for gene delivery. The formation of DNA–dendrimer complexes is typically embodied by an electrostatic interaction (polyplex) between a cationic dendrimer and the negatively charged phosphate backbone of DNA. A study by Chen et al.30 investigated the binding of DNA to polyamidoamine Starburst® dendrimers through ethidium bromide binding and fluorescence. The ethidium bromide did not readily displace the dendrimer, and only intercalated with unbound regions. A model emerged wherein ‘tightly bound DNA’ regions were calculated to increase from 3.2 base pairs for a G2 dendrimer to 106 base pairs for a G7 dendrimer (Fig. 5). Analogies between histones and dendrimers have been drawn.30 Several studies have shown that dendrimers that can form more compact complexes with DNA, such as highly flexible and partially degraded dendrimers, are better transfection agents as they can more easily enter the cell through endocytosis.31–34
Figure 5.
Schematic of dendrimer-bound DNA.
There have been numerous studies on the effects that structural changes to a particular dendrimer will have on DNA binding. Polypropylene dendrimers are ideal as DNA binding agents because all of their nitrogens are protonable. A study of polypropyleneimine (PPI) dendrimers found that DNA binding increased as dendrimer generation increased.34 However, cytotoxicity also increased as generation increased, making the higher generation dendrimers not useful for gene therapy. A separate study has found that PPI dendrimers with methylated quaternary surface amines showed improved DNA complexation and decreased cytotoxicity.35
A study by Kim et al.36 investigated the effects of increasing generations of a PAMAM–PEG–PAMAM triblock copolymer on DNA binding. Dendrimers were characterized by N/P ratios (primary, tertiary amines of dendrimer/phosphates of DNA), but it was found that it was not the total nitrogen density of the dendrimer, but the number of surface primary amines that is important for DNA binding. One hypothesis is that the internal tertiary amines of the dendrimers were too sterically hindered to take part in DNA condensation.36 A study with a series of amphiphilic dendrimers with a rigid diphenylethylene core and saturated C12 chains showed that as the number of C12 chains increased, the DNA binding ability of the dendrimers decreased.37 However, there have been studies that showed that above a limiting size, DNA condensation with poly(l-lysine) is independent of dendrimer size and shape,38 and that for many cationic dendrimers it is the electrostatic interactions, not size and shape of dendrimers that mediates DNA binding.33
To complement binding studies, other efforts have focused on enhancing cellular uptake. In a study by Toth and co-workers39 the conjugation of a lipophilic lipoamino acid tail to a lysine dendrimer showed improved membrane partitioning. The lipophilic tail acted as a lipid solubilizer, facilitating cellular entry of a cationic dendrimer–DNA complex and inhibited production of the human vascular endothelial growth factor better than the commercially available transfection agent cytofectin GSV. A generation-5 PAMAM dendrimer conjugated with 14 PEG chains showed a 20-fold increase in transfection compared to Superfect®, although a high conjugate:DNA ratio was necessary.40 The authors believe that the PEG chains provide steric stabilization to the dendrimer–DNA electrostatic interactions, and that terminal t-Boc groups aid in cellular internalization, improving the transfection ability of the PEG conjugate. A ternary complex of DNA, cationic peptides, and an anionic dendrimer containing phthalocyanine (Fig. 6) was also investigated to improve cellular uptake.41 The design was based on the belief that the ternary complex would be taken into the endosome, where the dendrimer would release the DNA and interact with the endosomal membrane. Excitation of the phthalocyanine with light would induce photochemical degradation to the endosomal membrane, allowing escape of the DNA to the nucleus.41
Figure 6.
Dendrimer containing phthalocyanin.
The surface of dendrimers may also be modified with a ligand to target gene delivery to specific cells. A DAB-8 dendrimer was conjugated to galactose residues that target asialoglycoprotein receptors of hepatocytes.42 The transfection ability of a series of dendrimer complexes with either one, two, or three galactose residues was compared for BL-6 cells, which do not present asialoglycoprotein receptors, and HepG2 cells, which present receptors. The number of galactose residues had no effect on the transfection ability in the BL-6 cells, but the Di-Gal and Tri-Gal conjugates showed a 20-fold increase in transfection ability in the HepG2 cells, compared to Mono-Gal. These results suggest that the transfection activity is mediated by interactions between the galactose residues and asialoglycoprotein receptors. To confirm these results, asialofetuin, a natural substrate that binds to asialoglycoprotein receptors, was added to the HepG2 cells before transfection. The decrease in transfection ability of Di-Gal and Tri-Gal was attributed to a competition for asialoglycoprotein receptors.42
Interactions of cationic dendrimers with serum albumin are believed to contribute significantly to the immunogenicity of these dendrimers. Even in gene therapy uses, polyplexes of cationic dendrimers and DNA usually still carry positive charge, and interactions with serum proteins have been shown to significantly reduce the transfection efficiency of carriers.43 PEGylation increases the transfection efficiency of cationic dendrimers.36,44
In contrast to the cationic polyplexes discussed above, Maksimenko et al.45 reported that the addition of anionic oligonucleotides to dendrimer–DNA polyplexes increased cell transfection. When the oligonucleotides were first mixed with the plasmids, subsequent addition of Superfect® resulted in the formation of much less condensed conjugates than those formed without oligonucleotides, or when the oligonucleotides were added after DNA–dendrimer complexation. These smaller conjugates provided a higher level of transfection, consistent with results from previous studies.31,32 Although no definitive explanation for the formation of smaller particles could be given, it is possible that a kinetic competition between the negatively charged plasmids and oligonucleotides for cationic dendrimer sites may be taking place.45 An anionic PAMAM dendrimer has also recently been developed for gene silencing in cancer cells.46 The anionic dendrimer showed no cytotoxic effects, and had greater membrane transport than cationic PAMAM dendrimers. If DNA binding can be accomplished with other anionic dendrimers, the possibility of a new class of noncytotoxic gene transfer vectors exists.
COMPLEXATION OF DRUGS WITH POLAR GROUPS ON THE DENDRIMER SURFACE
There are several examples of the complexation of small-molecule drugs to dendrimers. This technique was given precedence in studies by Twyman and co-workers47,48 in which small acidic molecules, such as benzoic acid, were encapsulated in TRIS-terminated water-soluble PAMAM dendrimers. The acidic molecules were bound within the interior of the dendrimer, ion paired with the tertiary nitrogens, as evidenced by the precipitation of solubilized guests at pH 2 at which the interior nitrogen would be protonated.
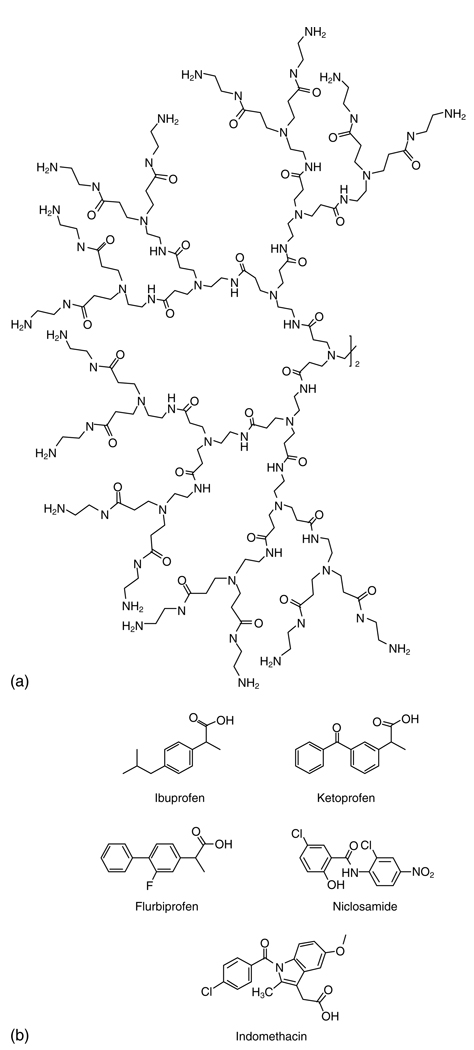
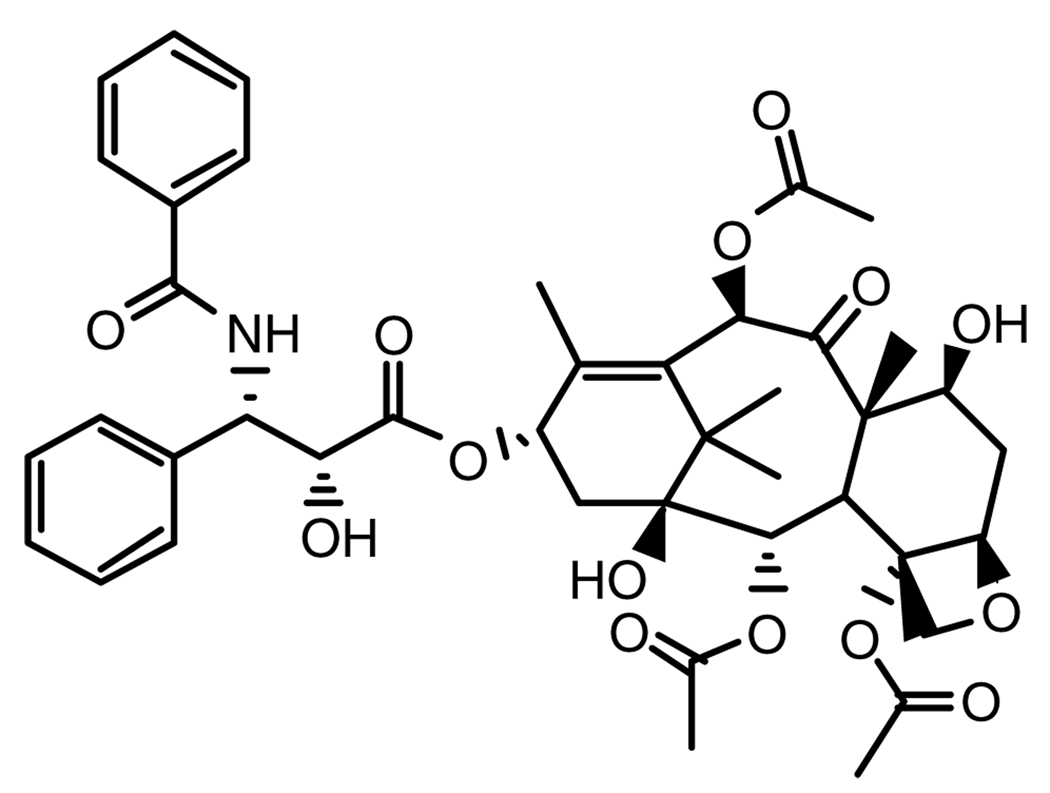
Kolhe et al.49 report that the carbonyl group of the nonsteroidal anti-inflammatory drug ibuprofen (Fig. 7) forms electrostatic interactions with the amine groups of PAMAM dendrimers, based on NMR and Fourier transform infrared (FTIR) spectroscopy. A G3 PAMAM dendrimer binds 32 molecules of ibuprofen (equal to the 32 surface amines) while the G4 dendrimer with 64 surface amines binds 78 molecules of ibuprofen (50% by weight), suggesting that there may also be some encapsulation in the interior of the larger dendrimer. The complex showed facilitated entry into A549 cells as well as more rapid suppression of COX-2 mRNA levels than free drug.50
Figure 7.
(a) Generation-3 PAMAM dendrimer and (b) several drugs that interact with its surface.
Ketoprofen (Fig. 7) has also been complexed to PAMAM through electrostatic interactions.51–53 The drug showed concentration- and generation-dependent solubilization with the dendrimer, increasing with dendrimer concentration.51 In pH studies ketoprofen was least soluble at pH 3 and most soluble at pH 6, supporting the conclusion that it is electrostatically complexed to the dendrimer, as the drug would not be fully ionized at low pH and would not interact as strongly with the dendrimer.52 In vitro release of ketoprofen from the complex into water was significantly slower than diffusion of free drug across the dialysis membrane, and the complex showed prolonged pharmocodynamic in vivo activity.53
This trend in pH and solubility of weakly acidic drugs with PAMAM has also been seen by others. The solubility of flurbiprofen (Fig. 7) with a G4-NH2 dendrimer was highest at pH 7, lower at pH 10, and lowest at pH 2.54 Niclosamide (Fig. 7) is most soluble with PAMAM in water, where the primary amines of the dendrimer are positively charged, increasing the solution pH to 8–9. Niclosamide has a pKa value of 7.3, so it is completely ionized at pH 8 and interacts fully with the dendrimer. At lower pH the drug is nonionized, and at higher pH the drug is fully ionized, but PAMAM is almost completely nonionized.55
Chauhan et al.56 have developed a series of PAMAM dendrimer conjugates for transdermal delivery of indomethacin (Fig. 7). All dendrimers showed an increase in the flux of indomethacin across the skin in vitro and in vivo. G4-NH2 and G4-OH dendrimers showed a 1.6- and 1.5-fold increase in the percentage inhibition of paw volume, compared to a suspension of pure indomethacin.
Cationic dendrimers can also bind heparin, an anticoagulant. Heparin is completely associated with the dendrimer at a weight ratio of 0.9:1, and the anticoagulant activity of heparin is neutralized at a weight ratio of 1:1.57 This suggests possible use as a heparin antidote. Heparin is also necessary for angiogenesis: arginine dendrimers have been used to bind heparin and stop angiogenesis.58
ENCAPSULATION OF DRUGS WITHIN A DENDRIMER
Dendrimers comprising a hydrophobic core and a polar surface are often referred to as ‘unimolecular micelles’ lacking a lower limit (critical micellar concentration) of formation.59–62 The hydrophilic and hydrophobic properties of a dendrimer, along with branching and rotational angles and the length of the repeat units will determine host–guest interactions.63 PEG is often conjugated to the surface of dendrimers to create a hydrophilic shell around the hydrophobic dendrimer, forming a unimolecular micelle capable of solubilizing hydrophobic drugs in the interior of the dendrimer or hydrophilic drugs in the PEG coat.64–70 The anticancer drug 5-fluorouracil67 and the blood schizonticide chloroquine phosphate68 have been encapsulated in PEGylated dendrimers, resulting in increased drug entrapment with an increase in molecular weight of PEG68 and versus non-PEGylated dendrimers.67 They also showed decreased cytotoxicity and hemolytic activity.
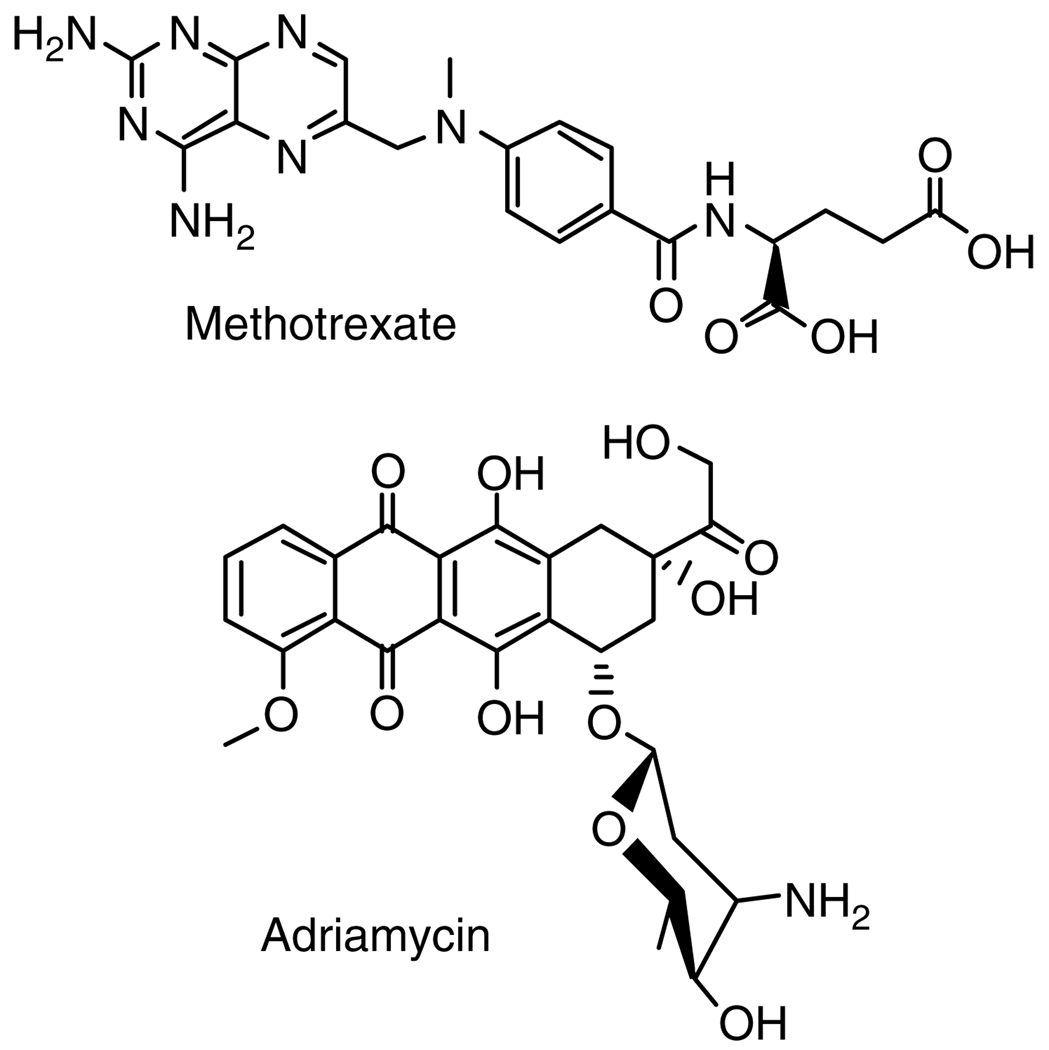
PAMAM dendrimers with PEG grafts of varying lengths were studied for the encapsulation of methotrexate (MTX) and adriamycin (Fig. 8).64 Encapsulation ability increased with increasing dendrimer generation and increasing length of PEG. A G4 dendrimer with PEG2000 encapsulated the highest amount of drug: 26 MTX molecules or 6.5 adriamycin molecules per dendrimer molecule. MTX was released slowly in low ionic strength aqueous solutions, but rapidly in isotonic solutions.64 Similar characteristics were found in the release of MTX from non-PEGylated PAMAM dendrimers conjugated with folate.71 PEGylated dendrimers have also been used to solubilize and release betamethasone corticosteroids,69 5-aminosalicylic acid, mefenamic acid, and diclofenac.70 Solubilizants other than PEG have also been attached to dendrimers. β-Cyclodextrins attached to a polymannosidic dendrimer solubilized docetaxel and formed glycoclusters with increased lectin affinity.72 PAMAM dendrimers with a poly(N,N-dimethylaminoethyl methacrylate) shell showed solubilization and controlled, pH-dependent release of chlorambucil.73
Figure 8.
Methotrexate and adriamycin.
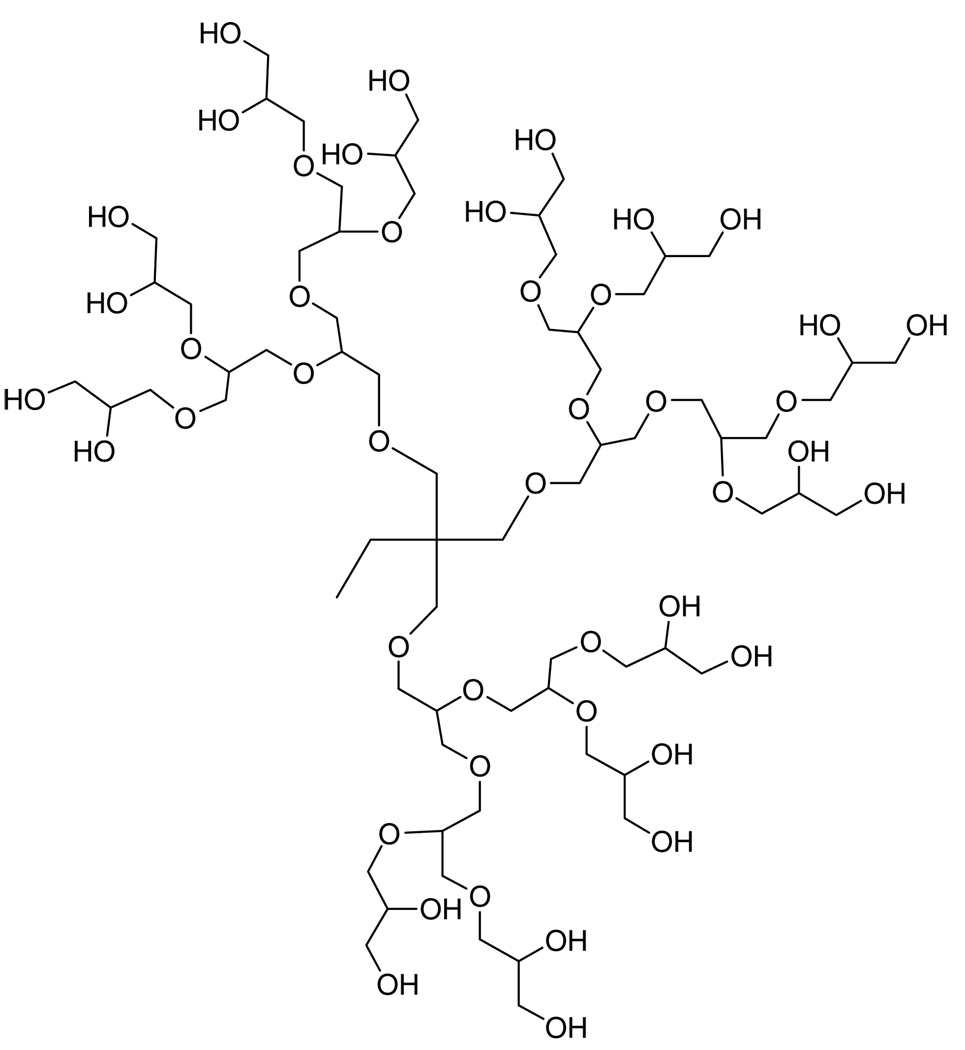
The ability of PEG to increase the solubility of insoluble drugs has been attributed to its structure-altering affects on water (hydrotropic solubilization).74 Paclitaxel (Fig. 9) is a commonly used antitumor agent that has very poor water solubility. Hydrotropic solubilization of paclitaxel in polyglycerol dendrimers (Fig. 10) resulted in a 10 000-fold increase in solubilization at an aqueous concentration of 80 wt% dendrimer.65 The enhancement was not due to a molecular weight effect, suggesting that the high density of ethylene glycol units in the dendrimer is causing the solubilization. 1H NMR spectra revealed that the aromatic rings, methyne groups, and acetyl groups of paclitaxel are surrounded by the dendrimer, and as the generation of dendrimer increased, the number of hydroxyl groups participating in hydrogen bonding with the paclitaxel increased.75 The conjugation of a cholesterol molecule to the dendrimers did not effect the solubilization of paclitaxel.76
Figure 9.
Anticancer drug paclitaxel.
Figure 10.
Generation-3 polyglycerol dendrimer.
The anticancer drug 10-hydroxycamptothecin (10-HCPT) has been encapsulated within a carboxylated poly(glycerol succinic acid) dendrimer77 and a tri-block macromolecule composed of two poly(glycerol succinic acid) dendrons linked by PEG3400.66 The triblock polymer increased the water solubility of 10-HCPT 20-fold. Silver salts78 and ciprofloxacin79 have been conjugated to PAMAM and polyproline-based dendrimers, respectively, to give new antibiotic conjugates that exhibit controlled release. The antimalarial drug primaquine phosphate has been encapsulated in PPI dendrimers of varying generation, and showed generation-dependent drug loading and controlled release.80
CONCLUSIONS
Molecular recognition in dendrimers has evolved significantly from the preliminary reports of the complementary host–guest strategy described by Newkome using barbituric acid.81 Instead of guest-specific dendrimers, more general methods suitable for interactions with a range of guests have appeared, including hydrophobicity and ion pairing, which have become the most widely adopted methods and have helped move the field forward. Two reasons for this shift seem motivating. The first reason is maturity. Both molecular recognition (with its pinnacle in the late 1980s and early 1990s) and dendrimer synthesis (turn of the century) are mature fields and many lessons have already been learned from either earlier or more fundamental studies. The second reason is synthetic burden. Most of the examples described rely on relatively trivial manipulations of commercially available materials. Instead of synthetically demanding variations in dendrimer host, studies favor generality over a range of guests. This approach also reflects a shift in emphasis from fundamental investigations into areas that are or will lead into translation research and ultimately the clinic. All bodes well for the future of dendrimers in medicine.
ACKNOWLEDGEMENTS
This work was supported by a grant to EES (NIH GM 64560). HC is an NSF Pre-doctoral Fellow.
REFERENCES
- 1.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. Polym J. 1985;17:117. [Google Scholar]
- 2.Hawker CJ, Fréchet JMJ. J Am Chem Soc. 1990;112:7638. [Google Scholar]
- 3.Newkome GR, Moorefield CN, Vögtle F. Dendritic Molecules. New York: VCH Publishers; 1996. pp. 1–261. [Google Scholar]
- 4.Stiriba S-E, Frey H, Haag R. Angew Chem Int Ed. 2002;41:1329. doi: 10.1002/1521-3773(20020415)41:8<1329::aid-anie1329>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Aulenta F, Hayes W, Rannard S. Eur Polym J. 2003;39:1741. [Google Scholar]
- 6.Boas U, Heegaard PMH. Chem Soc Rev. 2004;33:43. doi: 10.1039/b309043b. [DOI] [PubMed] [Google Scholar]
- 7.Bosman AW, Janssen HM, Meijer EW. Chem Rev. 1999;99:1665. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 8.Lee I, Athey BD, Wetzel AW, Meixner W, Baker JR. Macromolecules. 2002;35:4510. [Google Scholar]
- 9.Chen W, Tomalia DA, Thomas JL. Macromolecules. 2000;33:9169. [Google Scholar]
- 10.Duncan R, Izzo L. Adv Drug Deliv Rev. 2005;57:2215. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Svenson S, Tomalia DA. Adv Drug Deliv Rev. 2005;57:2106. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Jevprasesphant R, Penny J, Attwood D, Mckeown NB, D’Emanuele A. Pharm Res. 2003;20:1543. doi: 10.1023/a:1026166729873. [DOI] [PubMed] [Google Scholar]
- 13.Duncan R, Malik N, Lorenz K, Weener JW, Paulus W. J Cont Rel. 2000;65:133. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 14.Chen H-T, Neerman MF, Parrish AR, Simanek EE. J Am Chem Soc. 2004;126:10044. doi: 10.1021/ja048548j. [DOI] [PubMed] [Google Scholar]
- 15.Hong SP, Bielinska AU, Mecke A, Keszler B, Beals JL, Shi XY, et al. Bioconj Chem. 2004;15:774. doi: 10.1021/bc049962b. [DOI] [PubMed] [Google Scholar]
- 16.Hong S, Leroueil PR, Janus EK, Peters JL, Kober M-M, Islam MT, et al. Bioconj Chem. 2006;17:728. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 17.Metullio L, Ferrone M, Coslanich A, Fuchs S, Fermeglia M, Paneni MS, et al. Biomacromolecules. 2004;5:1371. doi: 10.1021/bm049858x. [DOI] [PubMed] [Google Scholar]
- 18.Padilla De Jesús OL, Ihre HR, Gagne L, Fréchet JMJ, Szoka FCJ. Bioconj Chem. 2002;13:453. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 19.Matsumura Y, Maeda H. Cancer Res. 1986;6:6387. [PubMed] [Google Scholar]
- 20.Duncan R. Nature. 2003;2:360. [Google Scholar]
- 21.Purohit G, Sakthivel T, Florence AT. Int J Pharm. 2003;254:37. doi: 10.1016/s0378-5173(02)00679-8. [DOI] [PubMed] [Google Scholar]
- 22.Vanucci L, Fiserova A, Sadalapure K, Lindhorst TK, Kuldova M, Rossman P, et al. Int J Oncol. 2003;23:285. [PubMed] [Google Scholar]
- 23.Shaunak S, Thomas S, Gianasi E, Godwin A, Jones E, Duncan R, et al. Nature Biotechnol. 2004;22:1. doi: 10.1038/nbt995. [DOI] [PubMed] [Google Scholar]
- 24.Bhadra D, Bhadra S, Jain S, Jain NK. Int J Pharm. 2003;257:111. doi: 10.1016/s0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 25.Jevprasesphant R, Penny J, Jalal R, Attwood D, Mckeown NB, D’Emanuele A. Int J Pharm. 2003;252:263. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi H, Kawamoto S, Saga T, Sato N, Hiraga A, Ishimori T, et al. Magn Res Med. 2001;46:781. doi: 10.1002/mrm.1257. [DOI] [PubMed] [Google Scholar]
- 27.Verma IM, Somia N. Nature. 1997;389:239. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 28.Eichman JD, Bielinska AU, Kukowska-Latallo JF, Baker JRJ. PSTT. 2000;3:232. doi: 10.1016/s1461-5347(00)00273-x. [DOI] [PubMed] [Google Scholar]
- 29.Tang MX, Redmann CT, Szoka FCJ. Bioconj Chem. 1996;7:703. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Turro NJ, Tomalia DA. Langmuir. 2000;16:15. [Google Scholar]
- 31.Bielinska AU, Chen C, Johnson J, Baker JRJ. Bioconj Chem. 1999;10:843. doi: 10.1021/bc990036k. [DOI] [PubMed] [Google Scholar]
- 32.Gebhart CL, Kabanov AV. J Cont Rel. 2001;73:401. doi: 10.1016/s0168-3659(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 33.Tang MX, Szoka FC. Gene Ther. 1997;4:823. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- 34.Zinselmeyer BH, Mackay SP, Schatzlein AG, Uchegbu IF. Pharm Res. 2002;19:960. doi: 10.1023/a:1016458104359. [DOI] [PubMed] [Google Scholar]
- 35.Schatzlein AG, Zinselmeyer BH, Elouzi A, Dufes C, Chim YTA, Roberts CJ, et al. J Cont Rel. 2005;101:247. doi: 10.1016/j.jconrel.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Kim T, Seo HJ, Choi JS, Jang H-S, Baek J, Kim K, et al. Biomacromolecules. 2004;5:2487. doi: 10.1021/bm049563j. [DOI] [PubMed] [Google Scholar]
- 37.Joester D, Losson M, Pugin R, Heinzelmann H, Walter E, Merkle HP, et al. Angew Chem Int Ed. 2003;42:1486. doi: 10.1002/anie.200250284. [DOI] [PubMed] [Google Scholar]
- 38.Männistö M, Vanderkerken S, Toncheva V, Elomaa M, Ruponen M, Schacht E, et al. J Cont Rel. 2002;83:169. doi: 10.1016/s0168-3659(02)00178-5. [DOI] [PubMed] [Google Scholar]
- 39.Wimmer N, Marano RJ, Kearns PS, Rakoczy EP, Toth I. Bioorg Med Chem Lett. 2002;12:2635. doi: 10.1016/s0960-894x(02)00511-5. [DOI] [PubMed] [Google Scholar]
- 40.Luo D, Haverstick K, Belcheva N, Han E, Saltzman WM. Macromolecules. 2002;35:3456. [Google Scholar]
- 41.Nishiyama N, Iriyama A, Jang W-D, Miyata K, Itaka K, Inoue Y, et al. Nature. 2005;4:934. doi: 10.1038/nmat1524. [DOI] [PubMed] [Google Scholar]
- 42.Ren T, Zhang G, Liu D. Bioorg Med Chem. 2001;9:2969. doi: 10.1016/s0968-0896(01)00203-6. [DOI] [PubMed] [Google Scholar]
- 43.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. Gene Ther. 1999;6:595. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 44.Loup C, Zanta M-A, Caminade A-M, Majoral J-P, Meunier B. Chem Eur J. 1999;5:3644. [Google Scholar]
- 45.Maksimenko AV, Mandrouguine V, Gottikh MB, Bertrand J-R, Majoral J-P, Malvy C. J Gene Med. 2003;5:61. doi: 10.1002/jgm.319. [DOI] [PubMed] [Google Scholar]
- 46.Hussain M, Shchepinov MS, Sohail M, Benter IF, Hollins AJ, Southern EM, et al. J Cont Rel. 2004;99:139. doi: 10.1016/j.jconrel.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Twyman LJ, Beezer AE, Esfand R, Hardy MJ, Mitchell JC. Tetrahedron Lett. 1999;40:1743. [Google Scholar]
- 48.Beezer AE, King ASH, Martin IK, Mitchel JC, Twyman LJ, Wain CF. Tetrahedron. 2003;59:3873. [Google Scholar]
- 49.Kolhe P, Misra E, Kannan RM, Kannan S, Lieh-Lai M. Int J Pharm. 2003;259:143. doi: 10.1016/s0378-5173(03)00225-4. [DOI] [PubMed] [Google Scholar]
- 50.Kannan S, Kolhe P, Raykova V, Glibatec M, Kannan RM, Lieh-Lai M, et al. J Biomater Sci Polymer Edn. 2004;15:311. doi: 10.1163/156856204322977201. [DOI] [PubMed] [Google Scholar]
- 51.Yiyun C, Tongwen X. Eur J Med Chem. 2005;40:1188. doi: 10.1016/j.ejmech.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 52.Yiyun C, Tongwen X, Rongqiang F. Eur J Med Chem. 2005;40:1390. doi: 10.1016/j.ejmech.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Na M, Yiyun C, Tongwen X, Yang D, Xiaomin W, Zhenwei L, et al. Eur J Med Chem. 2006;41:670. doi: 10.1016/j.ejmech.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Asthana A, Chauhan AS, Diwan PV, Jain NK. AAPS Pharm Sci Technol. 2005;27:536. doi: 10.1208/pt060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Devarakonda B, Hill RA, Liebenberg W, Brits M, de Villiers MM. Int J Pharm. 2005;304:193. doi: 10.1016/j.ijpharm.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 56.Chauhan AS, Sridevi S, Chalasani KB, Jain AK, Jain SK, Jain NK, et al. J Cont Rel. 2003;90:335. doi: 10.1016/s0168-3659(03)00200-1. [DOI] [PubMed] [Google Scholar]
- 57.Al-Jamal KT, Ramaswamy C, Florence AT. Adv Drug Deliv Rev. 2005;57:2238. doi: 10.1016/j.addr.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 58.Kasai S, Nagasawa H, Shimamura M, Uto Y, Hori H. Bioorg Med Chem Lett. 2002;12:951. doi: 10.1016/s0960-894x(02)00066-5. [DOI] [PubMed] [Google Scholar]
- 59.Newkome GR, Yao Z, Baker GR, Gupta VK. J Org Chem. 1985;50:2003. [Google Scholar]
- 60.Tomalia DA, Berry V, Hall M, Hedstrand DM. Macromolecules. 1987;20:1164. [Google Scholar]
- 61.Hawker CJ, Wooley KL, Fréchet JMJ. J Chem Soc Perkin Trans. 1993;1:1287. [Google Scholar]
- 62.Newkome GR, Moorefield CN, Baker GR, Saunders MJ, Grossman SH. Angew Chem Int Ed. 1991;30:1178. [Google Scholar]
- 63.Esfand R, Tomalia DA. Drug Discov Today. 2001;6:427. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 64.Kojima C, Kono K, Maruyama K, Takagishi T. Bioconj Chem. 2000;11:910. doi: 10.1021/bc0000583. [DOI] [PubMed] [Google Scholar]
- 65.Ooya T, Lee J, Park K. J Cont Rel. 2003;93:121. doi: 10.1016/j.jconrel.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Morgan MT, Carnahan MA, Finkelstein S, Prata CAH, Degoricija L, Lee SJ, et al. Chem Commun. 2005:4309. doi: 10.1039/b502411k. [DOI] [PubMed] [Google Scholar]
- 67.Bhadra D, Bhadra S, Jain S, Jain NK. Int J Pharm. 2003;257:111. doi: 10.1016/s0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 68.Bhadra D, Bhadra S, Jain NK. Pharm Res. 2006;23:623. doi: 10.1007/s11095-005-9396-9. [DOI] [PubMed] [Google Scholar]
- 69.Sideratou Z, Tsiourvas D, Paleos CM. J Colloid Interf Sci. 2001;242:272. [Google Scholar]
- 70.Namazi H, Adeli M. Biomaterials. 2005;26:1175. doi: 10.1016/j.biomaterials.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 71.Patri AK, Kukowska-Latallo JF, Baker JR. Adv Drug Deliv Rev. 2005;57:2203. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 72.Benito JM, Gómez-García M, Mellet CO, Baussanne I, Defaye J, Fernndez JMG. J Am Chem Soc. 2004;126:10355. doi: 10.1021/ja047864v. [DOI] [PubMed] [Google Scholar]
- 73.Hui H, Xiao-dong F, Zhong-lin C. Polymer. 2005;46:9514. [Google Scholar]
- 74.Sato T, Niwa H, Chiba A. J Chem Phys. 1998;108:4138. [Google Scholar]
- 75.Ooya T, Lee J, Park K. Bioconj Chem. 2004;15:1221. doi: 10.1021/bc049814l. [DOI] [PubMed] [Google Scholar]
- 76.Ooya T, Huh KM, Saitoh M, Tamiya E, Park K. Sci Technol Adv Mater. 2005;6:452. [Google Scholar]
- 77.Morgan MT, Carnahan MA, Immoos CE, Ribiero AA, Finkelstein S, Lee SJ, et al. J Am Chem Soc. 2003;125:15485. doi: 10.1021/ja0347383. [DOI] [PubMed] [Google Scholar]
- 78.Balogh L, Swanson DR, Tomalia DA, Hagnauer GL, McManus AT. Nano Lett. 2001;1:18. [Google Scholar]
- 79.Crespo L, Sanclimens G, Montaner B, Peréz-Tomás R, Royo M, Pons M, et al. J Am Chem Soc. 2002;124:8876. doi: 10.1021/ja020364m. [DOI] [PubMed] [Google Scholar]
- 80.Bhadra D, Yadav AK, Bhadra S, Jain NK. Int J Pharm. 2005;295:221. doi: 10.1016/j.ijpharm.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 81.Newkome GR, Woosley BD, He E, Moorefield CN, Guether R, Baker GR, et al. Chem Commun. 1996;24:2737. [Google Scholar]