Abstract
Introduction
The tissue hemoglobin index (THI) is a hemoglobin signal strength metric provided on the InSpectra™ StO2 Tissue Oxygenation Monitor, Model 650. There is growing interest regarding the physiologic meaning of THI and whether a clinically useful correlation between THI and blood hemoglobin concentration exists. A series of in vitro and in vivo experiments was performed to evaluate whether THI has potential utility beyond its primary purpose of helping InSpectra™ device users optimally position a StO2 sensor over muscle tissue.
Methods
The THI and tissue hemoglobin oxygen saturation (StO2) were measured using the InSpectra™ StO2 Tissue Oxygenation Monitor, Model 650, with a 15 mm optical sensor. A THI normal reference range was established in the thenar eminence (hand) for 434 nonhospitalized human volunteers. In 30 subjects, the thenar THI was also evaluated during 5-minute arterial and venous blood flow occlusions, and with blood volume exsanguination in the hand induced with an Esmarch bandage. In addition, correlation of the THI to blood total hemoglobin concentration (Hbt) was studied in five pigs whose Hbt was isovolumetrically diluted from 13 to 4 g/dl systemically and 0.5 g/dl locally in the hind limb. The sensitivity and specificity of the THI to measure tissue hemoglobin concentration (THC) were characterized in vitro using isolated blood tissue phantoms.
Results
In human thenar tissue, the average THI was 14.1 ± 1.6 (mean ± standard deviation). The THI extrapolated to 100% blood volume exsanguination was 3.7 ± 2.0 units presumably from myoglobin. On average, the THI increased 1.5 ± 1.0 units with venous occlusion and decreased 4.0 ± 2.0 units with arterial occlusion. In porcine hind limbs, the THI weakly correlated with Hbt (r2 = 0.26) while ΔTHI during venous occlusion had a stronger correlation (r2 = 0.62). In vitro tests indicated that THI strongly correlated (r2 > 0.99) to phantom THC and was insensitive to StO2 changes.
Conclusions
Steady-state THI values do not reliably indicate Hbt. The THI is a reproducible quantitative index for THC, and THI trends can discriminate between arterial or venous blood flow occlusions. The THI magnitude permits the estimation of myoglobin's contribution to StO2.
Introduction
The InSpectra™ StO2 Tissue Oxygenation Monitor, Model 650 (Hutchinson Technology Inc., Hutchinson, MN, USA) provides continuous non-invasive assessment of tissue hemoglobin oxygen saturation (StO2) in the clinical setting. Depressed StO2 has been shown to correlate with the severity of systemic hypoperfusion and mortality in traumatic shock patients [1,2] and septic shock patients [3,4]. In addition to StO2, the InSpectra™ monitor displays the tissue hemoglobin index (THI), a measurement of hemoglobin signal strength useful for determining whether the StO2 sensor is optimally positioned over muscle.
There is growing interest as to whether the THI is clinically useful beyond guiding the placement of a StO2 sensor. More recently the THI has been studied during the clinical assessment of tissue oxygen perfusion status to convert an StO2 downward slope during arterial occlusion to an index of local oxygen consumption [5] and to assess microvascular reactivity when blood flow is re-established after arterial occlusion [6]. Since invasive blood draws are not always feasible in patients, researchers have sought to establish a link between non-invasive continuous tissue hemoglobin measurements (THCs) and blood total hemoglobin concentration (Hbt) [7-10].
The THI measured over the thenar eminence is potentially comparable with THC in muscle, typically <1 g/dl. Since the near-infrared spectroscopy (NIRS) method for measuring the THI assumes a constant but unknown optical path length, the measured tissue volume is unknown. Absolute units are therefore not assignable to the THI and it is not known whether THI values can be used to compare intermittent nontrended tissue hemoglobin values across a patient population. Also, since the THI signal includes an unknown contribution from myoglobin, there is uncertainty whether THI is sensitive to muscle THC and possibly Hbt.
To clarify the physiologic meaning of the THI and to provide InSpectra™ researchers with insight into whether the THI has value beyond StO2 sensor placement, a series of in vitro and in vivo experiments was performed. Since no gold standard for muscle hemoglobin concentration exists, we sought to demonstrate that the THI algorithm is specific and sensitive to Hbt in isolated blood-tissue phantoms. To evaluate THI variation and to establish a normal reference range for human thenar eminence muscle, the THI was measured in 434 healthy subjects. To estimate the potential contribution of myoglobin and to provide an estimate of the lowest obtainable THI measurement, 30 healthy subjects underwent hand/forearm blood volume exsanguination with an Esmarch bandage tourniquet procedure. Additionally, the same 30 subjects underwent head-of-bed elevation and pneumatic cuff-induced arterial/venous occlusion to evaluate variable tissue blood volume conditions that could be encountered in a clinical setting and could confound any correlation between the THI and Hbt. Isovolumetric hemodilution was performed in five pigs to evaluate the potential correlation of the THI to Hbt.
Materials and methods
Tissue hemoglobin index measurement equipment
The InSpectra™ StO2 Tissue Oxygenation Monitor emits and detects wavelengths of light at 680 nm, 720 nm, 760 nm, and 800 nm that transcutaneously illuminate and backscatter from human thenar eminence muscle at a maximum depth of 15 mm. Light returned to the monitor is converted into two second-derivative attenuation measurements centered at 720 nm and 760 nm. The optical hardware and calculations necessary for the second-derivative attenuation and StO2 measurements have been previously described [11].
Tissue hemoglobin index algorithm and calibration
It has been previously shown that 760 nm second-derivative attenuation measurements are specific to deoxyhemoglobin optical absorbance [12] and that the blood hemoglobin oxygen saturation (SO2), Hbt, and traversed distance of light (optical path length) are three primary physiologic variables that affect the 760 nm second-derivative optical attenuation signal (2D760) [11]. Hbt can therefore be determined for a given magnitude of SO2 and optical path length using 2D760 spectral measurements.
For the present study, the optical path length was not measured. The optical path length for a given wavelength, being grossly dependent upon the optical sensor's distance between the illumination and detection optical fibers, is assumed constant. The THI therefore represents the amount of total hemoglobin within an unknown volume of tissue, and accordingly has arbitrary measurement units. The mean signal depth is estimated to be one-half of the optical sensor spacing, with the maximum signal depth equal to the probe spacing distance (7.5 mm and 15 mm, respectively) [13].
At each possible level of SO2 there is a corresponding linear slope coefficient that empirically describes how changes with Hbt at a given hemoglobin oxygen 2D760 saturation and optical path length. The THI measurement first requires measurement of the StO2, before selecting the linear slope coefficient value 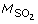 used to calculate the THI as follows:
used to calculate the THI as follows:
The probe scaling factor (PSF) can be used to obtain a common THI scale between different optical probe spacings or optical path lengths. Since all measurements in the present study were obtained with a 15 mm optical probe spacing, the PSF was set to 1.
A custom-made, isolated, dual-layer blood-tissue phantom apparatus [11] was used to acquire the linear slope coefficient values needed to calibrate the THI to Hbt in a tissue phantom. Whole bovine blood containing 10 units/ml heparin, and diluted to 10 g/dl Hbt with 0.9 wt% saline, was pumped through the blood-tissue phantom. The optical sensor was connected to the dual-layer flow cell apparatus. Paired values of StO2 and 2D760 were recorded and saved as the blood SO2 was slowly varied between 0 and 100%. For each paired recording of StO2 and 2D760, a linear slope coefficient value was calculated (Equation (1), PSF = 1, THI arbitrarily set to 10 at 10 g/dl Hbt). A nonlinear curve fit of linear slope coefficient versus StO2 was used to produce a calibration look-up table relating the linear slope coefficient to each StO2 level ranging from 0 to 99.9%, in 0.1% increments. The resultant look-up table was installed within the monitor's software to permanently calibrate THI to 2D760 for all possible levels of StO2.
Isolated blood-tissue phantom: tissue hemoglobin index sensitivity to total hemoglobin
To evaluate the robustness of the THI algorithm to StO2 changes at constant Hbt, the dual-layer blood loop apparatus was set up to create low THI, medium THI, and high THI conditions. Low THI (~5.8) was created with the flow cell having a 1.0 mm blood layer thickness and 6 g/dl inlet Hbt. To achieve medium THI (~11.4), the inlet Hbt was increased to 12 g/dl at 1.0 mm blood layer thickness. To obtain high THI (~18.0), the blood layer thickness was increased from 1.0 to 1.5 mm at 12 g/dl inlet Hbt. At each THI level, StO2 was adjusted by changing the membrane oxygenator inlet gas concentration from 21% to 0% oxygen, with 5% carbon dioxide and nitrogen for the remaining balance. StO2 and the THI were continuously recorded in order to obtain a linear regression model correlating the THI to StO2 for all three THI levels.
Since no standard exists for the in vivo THC, a homogeneous tissue phantom consisting of Intralipid (Fresenius Kabi Clayton L.P., Clayton, NC, USA) and blood was used to evaluate the correlation of the THI to THC. This correlation was studied from zero THC to the estimated upper limit of THC within a volume of muscle tissue, 0.2 mM [14] or 1.3 g/dl. Twenty milliliters of whole bovine blood, with 17.1 g/dl Hbt and 10 units/ml heparin, was added in 1 ml increments to a 500 ml mixture of 0.4 wt% Intralipid, 0.9% sodium chloride, 70% deionized water and 30% deuterium oxide. Deuterium oxide was used to optically adjust the tissue phantom to be optically equivalent to a muscle water concentration of 70 wt% [15]. The optical sensor was immersed within the Intralipid-blood mixture equilibrated to 6.2% oxygen, pH of 7.4 and a temperature of 37°C. The StO2 level of the mixture ranged from about 65 to 75%. To evaluate the THI sensitivity to different tissue scattering properties that could change the optical path length and the correlation of the THI to THC, the homogeneous tissue phantom procedure was replicated with 0.8 wt% and 0.2 wt% Intralipid.
The estimated optical scattering coefficients at 800 nm for the 0.2 wt%, 0.4 wt% and 0.8 wt% Intralipid solutions, prior to adding blood, are 2/cm, 4/cm, and 8/cm, respectively [16]. Red blood cells contribute to the overall scattering coefficient and are estimated to contribute another 2/cm to the 800 nm scattering coefficient at 2% hematocrit or about 0.67 g/dl [17]. An IL 682 Co-Oximeter (Instrumentation Laboratory, Lexington, MA, USA) was used to measure the Hbt of the whole blood added to the Intralipid solution.
Human study volunteers: normal tissue hemoglobin index range
This was a prospective, single-center, observational study in 434 nonhospitalized human volunteers who were employees of Hutchinson Technology Inc. All human studies were approved by the Western Institutional Review Board of Olympia, Washington. Males and females were enrolled who were 18 years and older, who had intact skin on the thenar eminence, and who gave written informed consent. There were no exclusion criteria.
Continuous thenar muscle StO2 and THI measurements were obtained from the right hand of resting subjects for 5 minutes. Heart rate and blood pressure were recorded before and after the StO2 and THI monitoring period. Collected demographic information included gender, age, ethnic group, smoking behavior, height, weight, and hand dominance.
Blood pressure and heart rate were measured in 271 study volunteers with a health station (Model 300; LifeClinic International, Rockville, MD, USA). During these measurements, study volunteers sat upright in the health station's chair with the measured arm resting on the station's arm rest and the elbow angled at 90 to 135°. In the remaining 163 individuals, blood pressure and heart rate were obtained with a different automatic blood pressure monitor (Model HEM-711ACN; Omron Healthcare Inc., Bannockburn, IL, USA). The subjects rested in a reclining chair with elbow angles of 135 to 180°. An interim analysis of the first 271 subjects revealed a thenar StO2 reference range lower than previously reported [18].
The previous study did not report the posture of their nonambulatory study subjects. After corresponding with an author of the previous study, we measured our remaining 163 study subjects in a reclined sitting posture to better replicate what was previously done [18].
Human study volunteers: induced upper-extremity ischemia and exsanguination
This was a prospective, single-center, observational study in 30 nonhospitalized human volunteers, all employed by Hutchinson Technology Inc. The sample population included an equal number of males and females aged 18 to 65 years who had intact skin on the thenar eminence, and who offered written consent. Exclusion criteria included history of limb injury or surgery, vascular disease, coagulopathy, or inability to ingest 325 mg acetylsalicylic acid before starting the study.
Continuous thenar StO2 and THI measurements were obtained from both thenar sites of volunteers at rest on a gurney. Head-of-bed elevation was adjusted from 60° to 30° to 0° with at least 5 minutes of rest between adjustments. An automated pneumatic tourniquet (A.T.S. 2000; Zimmer Inc., Warsaw, IN, USA) was placed around the upper arm and inflated to 200 mmHg for 5 minutes. Upon releasing the cuff pressure for 5 minutes and observing StO2 recovery, the pneumatic tourniquet was inflated to 50 mmHg to create venous blood flow occlusion for 5 minutes. After 5 minutes and StO2 recovery, the StO2 sensor was removed from the opposite hand to conduct the exsanguination procedure.
To accomplish exsanguination, the arm was supported in a vertical position for 1 minute. A 600 ml intravenous bag, filled with 375 ml water, was placed in the palm of the hand to evenly distribute the bandage pressure [19]. A 4 inch Esmarch bandage (Tetra Medical Supply Corp., Niles, IL, USA) was single wrapped with a one-half overlap from the finger tips to the upper forearm. The pneumatic cuff was then placed around the forearm, proximal to the elbow, and was inflated to 200 mmHg. After cuff inflation, the Esmarch andage was removed and the StO2 sensor was reapplied to the thenar site. The elapsed time from application of the Esmarch bandage to cuff deflation did not exceed 6 minutes. The left and right hands of both male and female groups were alternately assigned to either the blood vessel occlusion or exsanguination procedures.
Previous research using a scintigraphic technique with injected radiolabeled erythrocytes has shown that a similar exsanguination procedure applied to the upper limb of 10 healthy volunteers provides an average 69% reduction in tissue blood volume [20]. For our study, the estimated nadir THI for 100% blood volume reduction (THI100) was estimated from the nadir THI measured during exsanguination (THI69), having an assumed 69% volume reduction in blood. With THI prior to exsanguination (THI0) measured, the following equation was used to calculate THI100:
Heart rate and blood pressure were recorded before the and THI monitoring period. Collected demographic StO2 information included gender, age, ethnic group, height, weight, and hand dominance.
Porcine hind limb: blood hemoglobin dilution
The University of Minnesota Animal Use Committee, in accordance with established guidelines for the treatment of laboratory animals, approved this study of five male pigs weighing 18 to 28 kg each. Prior to anesthesia induction, a subcutaneous tissue depth ≤1.5 mm was verified with a skinfold caliper. Intramuscular ketamine 20 mg/kg and intravenous propofol 2 to 6 mg/kg were used to induce anesthesia. After intubation, anesthesia was maintained with 60% inhaled nitrous oxide and continued administration of propofol. One dose of intravenous heparin 100 units/kg was given, administered after surgery.
An InSpectra™ optical sensor was applied to the mid medial thigh of both hind limbs. A pulmonary artery catheter was placed via the internal jugular vein and an arterial line was placed into the carotid artery. During laparotomy, a splenectomy was performed and the distal aorta and vena cava were surgically accessed to facilitate cross-clamping to create acute hind limb ischemia. The femoral artery and vein of the right hind limb were accessed and fitted with annular ultrasonic flow transducers (Model TS420; Transonic Systems, Inc., Ithaca, NY, USA).
The total blood hemoglobin concentration was lowered by removing blood from the pulmonary artery catheter and replenishing the shed blood with Hextend® (Hospira, Inc., Lake Forest, IL, USA) to achieve targeted systemic hemoglobin levels of 13 g/dl, 10 g/dl, 7 g/dl, and 4 g/dl. A 20 mg bolus of furosemide was used to hemoconcentrate three of the five animals to elevate the baseline Hbt level to approximately 13 g/dl. To achieve 0.5 g/dl Hbt measured in the right femoral vein, the distal aorta was clamped and Hextend® was perfused below the cross-clamp site. The right femoral vein was incised to facilitate syringe sampling of the diluted blood effluent. After each targeted systemic Hbt level had been achieved, the StO2 THI, cardiac output, femoral artery and venous blood flows, blood pressures, temperature, pH, blood gases, oxygen saturation, lactate, hemoglobin, and base excess measurements were collected. StO2 and THI were subsequently measured during replicate aorta and vena cava 3-minute cross-clamp occlusions. StO2 and THI were recorded continuously.
Statistical methods
For the tissue phantom and porcine hind limb experiments, scatter plots and linear regression models were used to describe the relationships between the THI and the independent variables of Hbt and StO2. The squared Pearson correlation coefficient [21] was used to assess the degree of model fit. Squaring the correlation coefficient and multiplying it by 100 describes the percentage variability in observed THI attributable to changes in the independent variables.
For the human volunteer studies, mean and one standard deviation values were calculated for all measurement groups. The nonparametric Dunn's multiple-range test was used to evaluate differences between pairs of means for levels within a group. Correlation of StO2 and THI to numeric data, such as blood pressure, was performed with a Spearman rank two-tailed test. All mean tests were evaluated at 95% confidence. The coefficient of variation (standard deviation/mean) was used to evaluate THI variability in human volunteers.
Results
Isolated blood-tissue phantom: tissue hemoglobin index sensitivity to total hemoglobin
The linear regression models of Figure 1 describing the THI as a function of StO2 were used to predict THI values at zero and 100% StO2. This THI difference across the extreme StO2 range was then divided by the predicted THI at zero StO2 to obtain the percentage change in the THI reading for full-scale change in StO2. The resultant absolute crosstalk errors were 3.1%, 1.4%, and 10.2% for y-intercept THI values of 5.8, 11.4, and 18.0, respectively. Figure 1 also shows that the THI signal has more random noise at the highest tested THI level and has the greatest crosstalk error with StO2 >90%.
Figure 1.
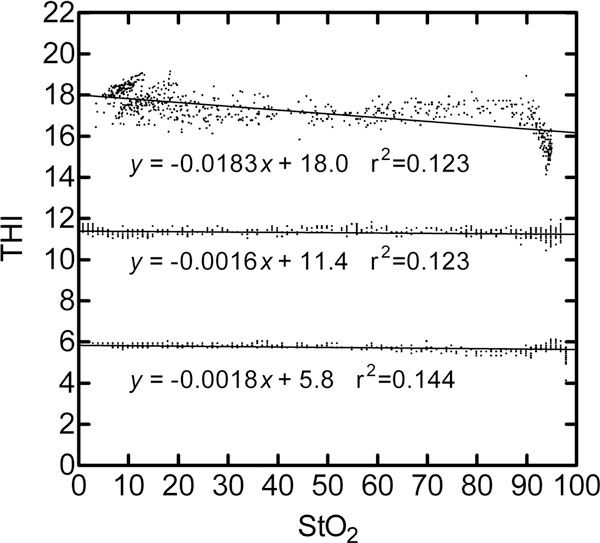
Tissue hemoglobin index at constant total hemoglobin absorption during variable hemoglobin oxygen saturation. The tissue hemoglobin index (THI) was measured at three constant total hemoglobin absorption conditions during variable hemoglobin oxygen saturation (StO2) conditions. The nominal THI measurements near 6 and 12 were obtained from 6 g/dl and 12 g/dl bovine blood flowing through the dual-layer flow cell apparatus [11] at a blood thickness of 1.0 mm. At 12 g/dl Hbt and 1.5 mm blood layer thickness, the nominal THI of 18 was obtained.
In Figure 2 the THI has a strong linear correlation (r2 > 0.99) to the hemoglobin concentration in a homogeneous suspension of Intralipid and red blood cells at three different scattering strength levels. A comparison of the linear equation slopes of Figure 2 reveals that a twofold increase in the optical scattering coefficient from 2 to 4/cm causes a 16% increase in THI. An increase in the scattering coefficient from 4 to 8/cm results in a 20% increase in THI. At 4/cm optical scattering, the sensitivity of THI to a change of ± 1/cm in optical scattering is estimated to be 5 to 8% of the THI reading.
Figure 2.
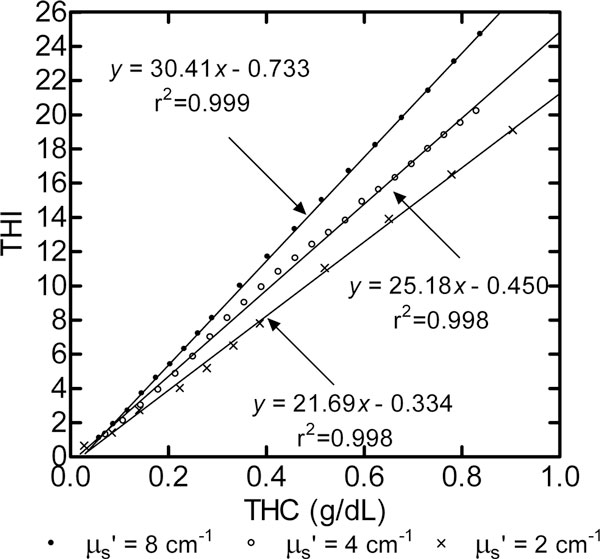
Tissue hemoglobin index at variable total hemoglobin concentration and optical scattering. Correlation of the tissue hemoglobin index (THI) to total tissue hemoglobin concentration (THC) within a homogenous mixture of bovine blood and Intralipid at three background optical scattering conditions, 0.2, 0.4 and 0.8 wt% Intralipid. Bovine blood having 17 g/dl total hemoglobin concentration was stepwise added in 1 ml increments to 500 ml Intralipid solution having 0.9 wt% NaCl, 30 vol% deuterium oxide, and deionized water. The mixture was pumped through a hollow flow cell apparatus [11] and was equilibrated to 6.2% oxygen, 5% carbon dioxide, 7.4 pH and 37°C. μs', optical scattering coefficient.
Human study volunteers: normal tissue hemoglobin index range
Tables 1 and 2 summarize the variable and attribute demographic study measurements. For 434 volunteers, the THI mean and one standard deviation limit was 14.1 ± 1.6. The THI weakly correlated with StO2 (Spearman r = 0.243, 95% confidence interval = 0.149 to 0.332). The THI did not correlate with age, height, weight, body mass index, systolic blood pressure, diastolic blood pressure, mean arterial pressure, or heart rate. The coefficient of variation for THI (11%) was less than that for blood pressure (14%) and that for heart rate (16%).
Table 1.
Variable demographic data for the 434 human volunteers enrolled in the normal THI range study
| Characteristic | Mean ± standard deviation | Coefficient of variation (%) |
|---|---|---|
| Age (years) | 42.3 ± 10.72 | 25 |
| Height (cm) | 172.0 ± 10.72 | 6 |
| Weight (kg) | 83.5 ± 18.95 | 23 |
| Body mass index (kg/m2) | 28 ± 6.1 | 22 |
| Systolic blood pressure (mmHg) | 128 ± 18.6 | 15 |
| Diastolic blood pressure (mmHg) | 80 ± 11.5 | 14 |
| Mean arterial pressure (mmHg) | 96 ± 13.0 | 14 |
| Heart rate (beats/minute) | 70 ± 11.2 | 16 |
| Thenar eminence StO2 (%) | 81.4 ± 5.25 | 6 |
| Thenar eminence THI | 14.1 ± 1.60 | 11 |
Coefficient of variation = (Mean/Standard deviation) × 100. Variation in the tissue hemoglobin index (THI) among subjects was less than blood pressure and heart rate. StO2, tissue hemoglobin oxygen saturation.
Table 2.
Attribute data for the 434 human volunteers enrolled in the tissue hemoglobin index range study
| Characteristic | Value | Tissue hemoglobin index | Tissue hemoglobin oxygen saturation (%) |
|---|---|---|---|
| Posture | * | * | |
| Sitting reclined | 271 | 13.9 ± 1.55 | 83.3 ± 4.01 |
| Sitting upright | 163 | 14.4 ± 1.62 | 78.3 ± 5.57 |
| Gender | * | ||
| Male | 235 | 14.1 ± 1.58 | 83.3 ± 4.29 |
| Female | 199 | 14.1 ± 1.63 | 79.3 ± 5.47 |
| Age (years) | 42.3 ± 10.7 | ||
| Ethnicity | |||
| White | 410 | 14.1 ± 1.62 | 81.4 ± 5.30 |
| Other | 11 | 13.5 ± 0.82 | 81.9 ± 3.14 |
| Hispanic | 6 | 14.3 ± 1.37 | 85.1 ± 6.19 |
| Indian | 6 | 12.9 ± 1.02 | 80.4 ± 2.86 |
| Black | 1 | 12.4 ± na | 78.0 ± na |
| Smoker | * | ||
| No | 392 | 14.7 ± 1.39 | 81.5 ± 5.25 |
| Yes | 42 | 14.0 ± 1.61 | 81.3 ± 5.35 |
| Hours last smoked | 4.4 ± 5.1 | ||
| Dominant hand measured | |||
| Yes | 395 | 14.1 ± 1.61 | 81.4 ± 5.35 |
| No | 39 | 13.9 ± 1.47 | 82.2 ± 4.04 |
Data presented as number of subjects or mean ± standard deviation. Posture and smoking habit influenced the tissue hemoglobin index.
*Characteristic parameter had a statistically significant effect, P < 0.05. na, not applicable.
The THI was lower in smokers versus nonsmokers (14.0 vs. 14.7, respectively; P < 0.01) and in reclined posture versus upright posture (13.9 vs. 14.4, respectively; P < 0.01).
Human study volunteers: induced upper-extremity ischemia and exsanguination
Table 3 summarizes the StO2 and THI results for all subjects and includes the patient demographic and hemodynamic measurements. A multiple-level comparison test for all rows within each experimental condition of Table 3 indicated statistically different mean StO2 and THI differences (P < 0.05), except for the baseline and baseline recovery measurements for the arterial occlusion, venous occlusion, and blood volume exsanguination conditions. Gender influenced StO2 baseline resting measurements (females had average StO2 about 3 units lower than males) but had no statistically significant influence on THI. The measured hand, left hand versus right hand, had no significant influence on the mean measurements (P > 0.05; results not shown).
Table 3.
THI and StO2 measurements in 30 healthy human volunteers before, during, and after acute ischemia
| Experimental condition | Tissue hemoglobin index | Tissue hemoglobin oxygen saturation |
|---|---|---|
| Head-of-bed elevation (no ischemia) | *0°, 60° | *0°, 30°, 60° |
| 0° | 14.6 ± 1.68 | 81.5 ± 5.11 |
| 30° | 15.0 ± 1.60 | 78.7 ± 5.17 |
| 60° | 15.2 ± 1.66 | 75.3 ± 5.86 |
| Arterial cuff occlusion | *All except baselines | *All except baselines |
| Baseline | 14.6 ± 1.68 | 82.0 ± 5.33 |
| End | 10.6 ± 1.81 | 28.2 ± 10.9 |
| Recovery peak | 18.7 ± 2.71 | 95.3 ± 1.86 |
| Recovery baseline | 14.8 ± 1.61 | 82.1 ± 4.57 |
| Venous cuff occlusion | *All except baselines | *All except baselines |
| Baseline | 14.8 ± 1.61 | 82.1 ± 4.45 |
| End | 16.2 ± 1.53 | 68.4 ± 6.84 |
| Recovery baseline | 14.5 ± 1.74 | 79.6 ± 4.90 |
| Esmarch bandage exsanguination | *All | *All |
| Nadir | 7.0 ± 1.56 | 31.7 ± 19.3 |
| Residual (calculated, Equation (2)) | 3.7 ± 2.0 | Not applicable |
| Recovery peak | 16.2 ± 2.49 | 92.1 ± 3.63 |
| Recovery baseline | 13.7 ± 1.90 | 78.1 ± 6.58 |
| Demographic characteristics | ||
| Age (years) | 41.8 ± 9.09 | |
| Height (inches) | 68.6 ± 3.47 | |
| Weight (pounds) | 171 ± 30.6 | |
| Body mass index (kg/m2) | 25.5 ± 3.68 | |
| Systolic blood pressure (mmHg) | 126 ± 7.9 | |
| Diastolic blood pressure (mmHg) | 78 ± 8.0 | |
| Mean arterial pressure (mmHg) | 94 ± 7.4 | |
| Heart rate (beats/minute) | 67 ± 11.6 |
Data presented as mean ± one standard deviation. Baseline measurements were obtained before inducing ischemia. All ischemia and ischemia recovery measurements were performed at 0° bed elevation. The tissue hemoglobin index (THI) mean was manipulated from 7.0 to 18.7 under conditions of constant blood hemoglobin concentration. *Rows having statistically different means, P < 0.05. StO2, tissue hemoglobin oxygen saturation.
The THI during arterial and venous occlusion exhibited different trends compared with the pre-occlusion THI. At the end of arterial occlusion the THI decreased 4.0 ± 2.0 units, while at the end of venous occlusion the THI increased 1.5 ± 1.0 units. Using Equation (2) with the individual THI values (not shown) measured at the 0° head-of-bed elevation condition prior to ishcemia (THI0) and the nadir condition during Esmarch bandage exsanguination (THI69), the estimated THI for 100% blood volume exsanguination (residual THI) would be 3.7 ± 2.0 units.
Porcine hind limb: blood hemoglobin dilution
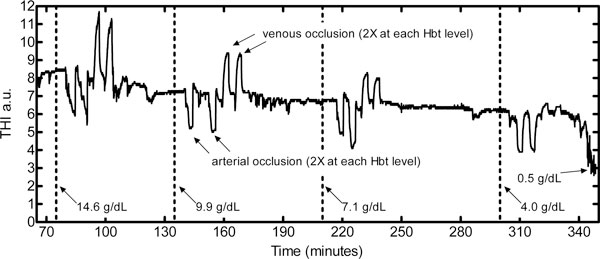
Figure 3 shows continuous hind limb THI measurements recorded during one experiment.
Figure 3.
Porcine hind limb tissue hemoglobin index measurements during isovolumetrically diluted arterial blood hemoglobin concentration. The last hemoglobin concentration condition involved purfusing Hextend® directly into the distal abdominal aorta with a cross-clamp upstream of the pressurized infusion point. Effluent from the femoral vein was then sampled to measure the resultant localized hind limb hemoglobin concentration (0.5 g/dl). a.u., arbitrary units; Hbt, total hemoglobin concentration.
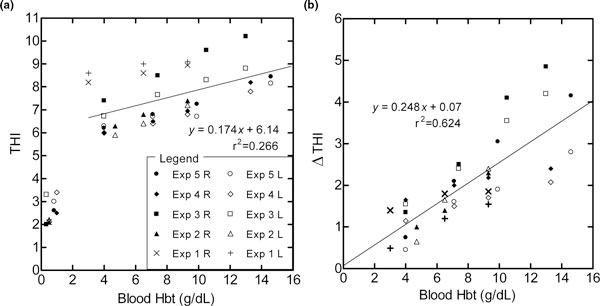
THI readings in five experiments weakly correlated to Hbt (r2 = 0.266), with Hbt ranging from 14 to 4 g/dl (Figure 4a). For individual experiments, the THI to Hbt correlation was best in Experiment 4 (THI = 0.317 Hbt + 6.16, r2 = 0.996) and was worst in Experiment 1 (THI = 0.08 Hbt + 8.39, r2 = 0.929). After locally perfusing the hind limbs with a nonhemoglobin perfusate (Hextend®) and obtaining an effluent femoral vein blood hemoglobin concentration near 0.5 g/dl, there was a remaining (residual) THI of 2.8 ± 0.6 units.
Figure 4.
Correlation of tissue hemoglobin index to arterial blood hemoglobin concentration during isovolumetric hemodilution. As shown in the legend the (a) baseline THI values, and (b) change in THI during 3-minute venous occlusion (ΔTHI) are from five animal experiments (Exp 1 to Exp 5) in which both right (R) and left (L) hind limbs were simultaneously measured. The right limb had the femoral artery and vein surgically accessed to locate annular ring flow transducers, and generally had higher (a) THI (mean difference 0.32, P = 0.05) and (b) ΔTHI (mean difference 0.44, P < 0.05).
With selective venous occlusion, the THI levels increased as shown in Figure 3. The magnitude of the THI increase with 3 minutes of venous occlusion (ΔTHI) had a stronger association with the blood hemoglobin concentration (ΔTHI = 0.248 Hbt + 0.07, r2 = 0.624; Figure 4b) than the steady-state baseline THI value (THI = 0.174 Hbt + 6.14, r2 = 0.266; Figure 4a). Table 4 summarizes all hemodynamic measurements recorded for each steady-state hemoglobin condition. No hemodynamic information other than the StO2, THI, and blood Hbt was measured during the 0.5 g/dl Hbt condition.
Table 4.
Hemodynamic measurements for five pigs having isovolumetric hemodilution
| Total hemoglobin concentration | ||||
|---|---|---|---|---|
| Characteristic | 13 g/dl | 10 g/dl | 7 g/dl | 4 g/dl |
| Number of measurements | 3 | 5 | 5 | 5 |
| Arterial total hemoglobin (g/dl)a | 13.6 (13 to 14.6) | 9.7 (9.3 to 10.5) | 6.9 (6.5 to 7.4) | 3.9 (3 to 4.7) |
| Cardiac output (ml/min/kg) | 110 ± 47 | 131 ± 37 | 160 ± 77 | 168 ± 98 |
| Femoral artery flow (ml/min/kg) | 4.8 ± 1.6 | 6.0 ± 2.2 | 5.9 ± 1.9 | 6.1 ± 2.0 |
| Heart rate (beats/minute) | 97 ± 9*4 | 113 ± 16 | 127 ± 29 | 155 ± 39*13 |
| Systolic blood pressure (mmHg) | 97 ± 2 | 99 ± 10 | 99 ± 10 | 91 ± 22 |
| Diastolic blood pressure (mmHg) | 69 ± 4 | 65 ± 9 | 69 ± 13 | 54 ± 16 |
| Mixed SmvO2 (%) | 83.0 ± 4.4*4 | 73.8 ± 5.5 | 69.0 ± 11.4 | 61.2 ± 19.3*13 |
| Hind limb StO2 (%) | 89.0 ± 2.8*10 and 7 | 80.0 ± 6.3*13 | 82.7 ± 5.6*13 | 84.1 ± 6.2 |
| Hind limb THI (arbitrary) | 8.3 ± 1.1*4 | 7.8 ± 1.2 | 7.3 ± 1.0 | 6.8 ± 1.0*13 |
Data presented as mean ± standard deviation. a Mean and range shown since the variable was controlled to have no overlap in values. A nonparametric Dunn's multiple comparison test was used to study differences in mean values. *P < 0.05 for the hemoglobin column(s) indicated.
The tissue hemoglobin index was statistically different only when total hemoglobin concentration changed from 13 to 4 g/dl. SmvO2, mixed venous hemoglobin oxygen saturation; StO2, tissue hemoglobin oxygen saturation.
Discussion
Isolated blood-tissue phantom: tissue hemoglobin index sensitivity to total hemoglobin
The experiments using the tissue phantom model provide evidence that the THI metric is specific to (Figure 1) and sensitive to (Figure 2) the total amount of hemoglobin for a given optical path length or volume that the detected light signal interrogates. The dual-layer phantom results of Figure 1 show that, at a fixed blood layer thickness, the THI signal doubled - changing from 5.8 to 11.4 - when Hbt was doubled from 6 to 12 g/dl. At constant Hbt (12 g/dl), an increase in the THI from 11.4 to 18.0 was similarly proportional to the increase in blood layer thickness (from 1.0 to 1.5 mm). These results suggest that both vascular Hbt and vascular density or thickness (diameter) would influence THI readings in vivo.
The results of Figure 2 show that the optical scattering properties of tissue can also influence a THI reading. As the optical scattering coefficient increases, the mean distance between scattering events (1/Optical scattering coefficient) increases and therefore the increased traversed distance of detected light (optical path length) results in more total hemoglobin absorption events and thus a greater THI value. The four-factor range in background optical scattering was chosen to estimate the sensitivity of THI to a change in tissue optical scattering. The resultant <10% THI reading change per 1/cm scattering coefficient change provides a basis for studying how THI might change in vivo using tissue optical scattering properties reported in the published literature.
The reported absolute value for tissue optical scattering measured in vivo on human limbs varies widely, from about 4 to 10/cm, and appears specific to a measured tissue bed, to the reporting research institution, or to the measurement equipment used [22-24]. Information regarding optical scattering changes for forearm tissue within a fixed NIRS measurement device suggests that optical scattering changes, observed in intersubject variability studies [22,23,25] or ischemia studies [26], does not exceed a factor of two. Although the authors have found no report of optical scattering variation in the thenar eminence tissue bed, the variability in this bed may be less than that in forearm since subcutaneous tissue thickness either from fat or edema is reported to be less variable at the thenar site [27]. The in vivo THI error from an extreme change in optical scattering (twofold change) therefore appears to be less than 20% of the THI reading.
Human study volunteers: normal tissue hemoglobin index range
The present study is the first investigation and report of the THI range in a nonhospitalized large group of human volunteers. The THI mean (14.1) and standard deviation (1.6) values are similar to the reference range for blood hemoglobin concentration (13.5 to 15.1 g/dl) [28]. This closeness of normal THI to normal Hbt reflects how the THI was calibrated against Hbt in a phantom tissue model mimicking the optical attenuation of tissue with normal levels of hemoglobin when measured with a 15 mm optical probe spacing. Different optical probe spacings and tissue locations can yield significantly different results. For instance, the author's stomach, which has about 1 inch of adipose thickness, measured a THI of 5 units compared with near 14 units on the thenar when using a 15 mm optical probe spacing distance. Adipose tissue has less dense vasculature than muscle and is estimated to have one-third of the THC of muscle (0.05 mM vs. 0.15 mM, respectively) [29]. A 25 mm optical spacing on the thenar can produce a normal THI value near 22 units [6] since the 25 mm probe, compared with a 15 mm probe, results in a significantly larger optical path length. The PSF of Equation (1) would need to be utilized to allow different optical probe spacings to have a common THI scale.
Our previous research identified that the mean THI from 10 healthy volunteer subjects and 10 sepsis patients closely resembled the mean Hbt measurements in both groups [5]. The correlation of THI to Hbt for individually paired measurements within the sepsis patient cohort, however, had only weak linear correlation to Hbt (r2 = 0.14) [30], similar to the results of the porcine hind limb hemodilution study.
Variation in optical scattering properties of the thenar eminence tissue between the 434 measurement subjects is unknown. The observed coefficient of variation for the THI, equal to 11%, is less than the calculated coefficients of variation for other hemodynamic variables of this study (15% for systolic blood pressure and 16% for heart rate) (Table 1). The relatively low coefficient of variation for normal THI indicates that optical scattering variation does not significantly confound THI measurements. We have seen THI values near 4 units in hospitalized sepsis patients [30], which indicates that the THI in patients can be well outside the normal reference range in nonhospitalized study subjects (14.1 ± 1.6 units). More investigation is needed to determine whether an abnormally low THI reading is diagnostically useful or relevant to a patient's health status or treatment.
Human study volunteers: induced upper-extremity ischemia and exsanguination
A total of 30 human subjects underwent acute arm ischemia conditions evoked by arterial occlusion, venous occlusion, and blood volume exsanguination. Head-of-bed elevation, used clinically to mitigate ventilator-associated pneumonia and elevated intracranial pressure [31,32], was evaluated for its effect on THI and StO2 variability. The main findings of the present study are that the THI trend during cuff-induced ischemia differentiated arterial and venous blood flow occlusions, and that the residual THI signal when extrapolated to 100% blood volume exsanguination was 3.7 ± 2.0 THI units. Since the blood hemoglobin concentration would be fairly constant during the study measurements, the results indicate that regional ischemia and posture could confound any correlation between the THI and Hbt.
Venous occlusion with a pneumatic cuff initially stops venous blood flow until the venous pressure increases above the occlusion pressure. A reduced venous flow resumes once the venous pressure rises above the cuff pressure [33]. This could explain why StO2 during venous occlusion had limited change (an approximately 14 StO2 unit decrease) compared with arterial occlusion (an approximately 54 StO2 unit decrease). While StO2 decreased during both venous and arterial occlusion, the THI increased 1.5 ± 1.0 units with venous occlusion and decreased 4.0 ± 2.0 units with arterial occlusion. These results suggest that the THI trend during ischemia might help to identify whether a flow resistance or blockage is emanating from the venous or arterial vascular compartment, similar to other studies measuring NIRS-derived relative THC changes in muscle free flaps [34]. The porcine hind limb THI readings always increased during distal vena cava cross-clamp occlusion (Figures 3 and 4b), while aorta cross-clamping caused the THI to always drop as indicated in Figure 3. While a rise in the THI during venous occlusion is expected because of venous pooling and blood volume congestion, decreases in the THI during arterial occlusion may have been caused by blood volume reduction. Other NIRS researchers have noted a decrease in total hemoglobin NIRS signals during arterial occlusion [35-37]. In compliant blood vessels, a decrease in arterial vascular pressure would reduce the vascular volume and hence cause the THC and THI to decrease.
The posture of the limb and the influence of gravity might also affect blood drainage and movement out of the measured vascular space. The head-of-bed elevation results of Table 3 show that StO2 decreased on average 6 units with 60° of elevation while the average THI slightly increased 0.6 units. The sitting upright and reclined results in Table 2 also show that the average StO2 was 5 units lower while the average THI was 0.5 units higher with an upright posture. These results confirm that limb posture can have a measurable influence on StO2 and THI measurements. With the arm positioned below heart level, the hand's venous pressure and venous blood volume would increase. The lower oxygen saturated venous blood could then become a larger fraction of the total blood volume that StO2 is measuring, and thus lower the StO2.
Other researchers have used scintillation X-ray measures to demonstrate that Esmarch bandage exsanguination of the lower forearm causes a 69% reduction in tissue blood volume [38]. We assume that the similar exsanguination technique of our study produces a similar 69% reduction in tissue blood volume. The nadir THI during the exsanguination procedure was 7.0 ± 1.6 units. Extrapolation to 100% blood volume reduction from a baseline THI value of 14.6 (Equation (2)) results in an estimated residual THI value of 3.7 ± 2.0 units. This residual value may indicate that the maximum potential contribution of myoglobin to the THI signals is approximately ≤25% of the baseline THI values in healthy volunteers. The 3.7 ± 2.0 nadir THI value in the present study compared with the 2.8 ± 0.6 residual THI value determined in the porcine hind limb study can possibly be explained by the higher concentration of myoglobin reported in human muscle compared with porcine muscle (4.7 mg/g and <1 mg/g wet weight, respectively) [39,40]. The nonzero venous Hbt level (0.5 g/dl) would elevate the residual THI in the porcine study and cause an overestimation of the myoglobin contribution. Deviations from the 69% blood volume exsanguination assumption for the human limb exsanguination experiments would change the estimated myoglobin contribution to human thenar THI measurements.
Since the average THI value in 434 human thenar tissue sites (14.1 ± 1.6) was greater than the average THI value in five porcine hind limbs (8.3 ± 1.1), the potential contribution of myoglobin to the human thenar THI is lower. However, it is evident that the contribution of myoglobin to a THI signal potentially changes for any given level of THI. For instance, in human thenar tissue with a normal THI value of 14.1, the myoglobin contribution may be limited to 25%; but at a THI level of 5, the myoglobin contribution might approach 75%. A THI measurement can therefore help to identify the tissue compartment, cellular versus vascular, where StO2 is pre-dominantly measured. It is not clear whether myoglobin and change concomitantly or independently in resting StO2 muscle [41]. Our previous work involving measurement of StO2 in oxygen consumption-inhibited porcine organs having myoglobin (heart and hind limb) and not having myoglobin (kidney) suggested that myoglobin did not significantly influence StO2 specificity to hemoglobin oxygen saturation measured from arterial and venous blood samples [11]. Regardless of myoglobin's contribution to NIRS signals, a low StO2 resulting from either myoglobin or hemoglobin desaturation would indicate a lower oxygen reserve available to the tissue.
Porcine hind limb: blood hemoglobin dilution
The isovolumetric hemodilution experiments indicate that the THI is not a direct measure of Hbt, although THI trends might indicate a changing Hbt. Whereas tissue phantom experiments indicated a strong linear correlation (r2 > 0.99; Figure 2) of the THI to total hemoglobin changes, the in vivo correlation of the THI to Hbt was nonlinear from 14 to near 0 g/dl and was only weakly linear (r2 = 0.26; Figure 4a) within the physiologic relevant range, from 4 to 16 g/dl.
NIRS optical signals originate primarily from the arteriolar, capillary, and venule microvascular compartments [42]. The relative amount of blood in each of these compartments determines where an attenuated or absorbed optical signal is being measured. In resting muscle tissue, one-third to one-half of the capillaries are open and perfused with blood [43]. Vascular tone reduction (vasodilation) and a resultant capillary recruitment from higher capillary pressure could explain why THC, and hence the THI, might be preserved even though dramatic changes in blood hemoglobin concentration occur. The general increase in cardiac output and femoral artery blood flow (Table 4) during Hbt dilution would result from microvascular arteriole vasodilation and additional blood flow to newly opened capillaries. In human volunteer subjects in whom the blood Hbt was reduced to 5.0 g/dl, researchers have observed decreased systemic vascular resistance and increased cardiac index [44]. A reduction in blood viscosity from hemodilution would decrease flow resistance and would also contribute to the observed increase in the systemic and local blood flows [45].
Myoglobin could be a significant factor contributing to the lack of correlation between the THI and Hbt as well as between StO2 and mixed venous hemoglobin oxygen saturation (Table 4) since hemoglobin and myoglobin have similar absorption characteristics in the near-infrared wavelength region (650 to 900 nm) [46]. The estimated contribution of myoglobin to NIRS-derived hemoglobin signals is unclear. Reports have ranged from suggesting that nearly all of the NIRS-derived signal is from myoglobin [47] to suggesting 90% of the signal is coming from hemoglobin [42,48]. Other studies have suggested that the myoglobin contribution is linked to the blood hemoglobin concentration since the constant concentration of tissue myoglobin becomes a larger fraction of the NIRS signal as the hemoglobin signal is diluted [49]. The results of our porcine study suggest that the THI may be useful in interpreting the potential contribution of myoglobin to NIRS-derived signals such as StO2. The residual THI of 2.8 ± 0.6 units, observed when the femoral vein hemoglobin concentration was 0.5 g/dl, suggests that the average myoglobin signal was no more than 40% of the average THI value (equal to 6.8 ± 1.0) when blood Hbt was near 4 g/dl, and was no more than 34% of the average THI value (equal to 8.3 ± 1.0) with blood Hbt near 13 g/dl. Other researchers have found in isolated porcine hearts that the myoglobin contribution may range from 63 to 46%, with one-half (5.1 ± 0.4 g/dl Hbt) and whole blood perfusate mixtures, respectively [49].
Venous cuff occlusion techniques have been used to isolate NIRS signals to the nonpulsating venous blood compartment [50,51]. The increased venous pressure causes venous pooling and a subsequent increase in THI. In Figure 4 the right limbs have slightly higher THI, and ΔTHI could be from venous obstruction from the flow transducer applied to the right hind limb femoral vein. The magnitude of the THI increase following a 3-minute period of 50 mmHg venous occlusion pressure produced a differential THI signal (ΔTHI) that had a significantly better correlation to Hbt compared with steady-state THI measurements. The y intercept of 0.07 in Figure 4b indicates that any offset from myoglobin absorption is potentially removed and a stronger linear relationship to Hbt is obtained (r2 = 0.62 vs. r2 = 0.26; Figure 4). Further studies may be warranted to examine whether the THI combined with venous occlusion techniques can be optimized to produce a reliable continuous non-invasive measurement indicative of Hbt status.
After surgical preparation and before beginning the porcine hemodilution experiments, it was observed that the starting hind limb THI was approximately one-half of the average THI value (14.1 ± 1.6 units) measured on the thenar eminence of human subjects. Postoperative porcine Hbt levels were low, about 10 g/dl, possibly stemming from the age of the pigs and from the fluids given during the surgical phase of the experiment. A 20 mg bolus of furosemide diuretic was used to hemoconcentrate three of the five animals to boost the starting hemoglobin level. At 13.6 ± 0.9 g/dl Hbt, the average hind limb THI value of 8.3 ± 1.1 units was still much lower than that observed in humans. Differences in tissue blood volume, resulting from differences in vascular density or myoglobin concentration between the human thenar and porcine hind limb, could account for the significantly different observed baseline THI values. The results of the human blood exsanguination experiments (see the exsanguination discussion section), however, indicate that myoglobin differences between human and porcine muscle may be less relevant to this THI difference. Differences in the optical scattering properties of porcine hind limb and human thenar eminence could also be a contributing factor for the lower observed THI baseline in the porcine hind limb. The subcutaneous tissue thickness of the hind limbs, more similar to that in the thenar eminence (about ≤1.5 mm), would make optical scattering differences less likely to have caused the THI differences.
Conclusion
Steady-state THI values do not reliably indicate the blood hemoglobin concentration. Regional ischemia and posture can significantly affect THI readings under conditions where the blood hemoglobin concentration is constant. The contribution of myoglobin to the THI and StO2 is dependent upon the THI magnitude. At a THI of 4, nearly all of the THI and StO2 signal measured on thenar eminence is from myoglobin. At normal THI (>10), the THI and StO2 signals are mostly from THC.
Abbreviations
2D760: 760 nm second-derivative optical attenuation signal; ΔTHI: tissue hemoglobin index increase with 3 minutes of venous occlusion; Hbt: total hemoglobin concentration; NIRS: near-infrared spectroscopy; PSF: probe scaling factor; StO2: tissue hemoglobin oxygen saturation; THC: tissue hemoglobin concentration; THI: tissue hemoglobin index.
Competing interests
DM is an employee of the company that manufactures the InSpectra™ device evaluated in the present study. DM owns stock in the company and is listed as an inventor on patents related to the content of this manuscript.
Acknowledgements
Funding for the present study was provided by Hutchinson Technology Inc., Hutchinson, Minnesota, USA.
This article is part of Critical Care Volume 13 Supplement 5: Tissue oxygenation (StO2) in healthy volunteers and critically-ill patients. The full contents of the supplement are available online at http://ccforum.com/supplements/13/S5. Publication of the supplement has been supported with funding from Hutchinson Technology Inc.
References
- McKinley BA, Marvin RG, Cocanour CS, Moore FA. Tissue hemoglobin O2 saturation during resuscitation of traumatic shock monitored using near infrared spectrometry. J Trauma. 2000;48:637–642. doi: 10.1097/00005373-200004000-00009. [DOI] [PubMed] [Google Scholar]
- Cohn SM, Nathens AB, Moore FA, Rhee P, Puyana JC, Moore EE, Beilman GJ. Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. J Trauma. 2007;62:44–54. doi: 10.1097/TA.0b013e31802eb817. discussion 54-55. [DOI] [PubMed] [Google Scholar]
- Pareznik R, Knezevic R, Voga G, Podbregar M. Changes in muscle tissue oxygenation during stagnant ischemia in septic patients. Intensive Care Med. 2006;32:87–92. doi: 10.1007/s00134-005-2841-8. [DOI] [PubMed] [Google Scholar]
- Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. 2007;33:1549–1556. doi: 10.1007/s00134-007-0739-3. [DOI] [PubMed] [Google Scholar]
- Skarda DE, Mulier KE, Myers DE, Taylor JH, Beilman GJ. Dynamic near-infrared spectroscopy measurements in patients with severe sepsis. Shock. 2007;27:348–353. doi: 10.1097/01.shk.0000239779.25775.e4. [DOI] [PubMed] [Google Scholar]
- Doerschug KC, Delsing AS, Schmidt GA, Haynes WG. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol. 2007;293:H1065–H1071. doi: 10.1152/ajpheart.01237.2006. [DOI] [PubMed] [Google Scholar]
- Dani C, Pezzati M, Martelli E, Prussi C, Bertini G, Rubaltelli FF. Effect of blood transfusions on cerebral haemodynamics in preterm infants. Acta Paediatr. 2002;91:938–941. doi: 10.1080/080352502760272623. [DOI] [PubMed] [Google Scholar]
- Torella F, Cowley R, Thorniley MS, McCollum CN. Monitoring blood loss with near infrared spectroscopy. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:199–203. doi: 10.1016/s1095-6433(01)00548-7. [DOI] [PubMed] [Google Scholar]
- Rendell M, Anderson E, Schlueter W, Mailliard J, Honigs D, Rosenthal R. Determination of hemoglobin levels in the finger using near infrared spectroscopy. Clin Lab Haematol. 2003;25:93–97. doi: 10.1046/j.1365-2257.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- Cerussi A, Van Woerkom R, Waffarn F, Tromberg B. Noninvasive monitoring of red blood cell transfusion in very low birth-weight infants using diffuse optical spectroscopy. J Biomed Opt. 2005;10:051401. doi: 10.1117/1.2080102. [DOI] [PubMed] [Google Scholar]
- Myers DE, Anderson LD, Seifert RP, Ortner JP, Cooper CE, Beilman GJ, Mowlem JD. Noninvasive method for measuring local hemoglobin oxygen saturation in tissue using wide gap second derivative near-infrared spectroscopy. J Biomed Opt. 2005;10:034017. doi: 10.1117/1.1925250. [DOI] [PubMed] [Google Scholar]
- Cooper CE, Elwell CE, Meek JH, Matcher SJ, Wyatt JS, Cope M, Delpy DT. The noninvasive measurement of absolute cerebral deoxyhemoglobin concentration and mean optical path length in the neonatal brain by second derivative near infrared spectroscopy. Pediatr Res. 1996;39:32–38. doi: 10.1203/00006450-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Cui W, Kumar C, Chance B. Expermental study of migration depth for the photons measured at sample surface. Proc SPIE. 1991;1431:180–191. [Google Scholar]
- Binzoni T, Quaresima V, Barattelli G, Hiltbrand E, Gurke L, Terrier F, Cerretelli P, Ferrari M. Energy metabolism and interstitial fluid displacement in human gastrocnemius during short ischemic cycles. J Appl Physiol. 1998;85:1244–1251. doi: 10.1152/jappl.1998.85.4.1244. [DOI] [PubMed] [Google Scholar]
- Matcher SJ, Cope M, Delpy DT. Use of the water absorption spectrum to quantify tissue chromophore concentration changes in near-infrared spectroscopy. Phys Med Biol. 1994;39:177–196. doi: 10.1088/0031-9155/39/1/011. [DOI] [PubMed] [Google Scholar]
- van Staveren HJ, Moes CJM, van Marle J, Prahl SA, van Gemert MJC. Light scattering in Intralipid-10% in the wavelength range of 400-100 nm. Appl Opt. 1991;30:4507–4514. doi: 10.1364/AO.30.004507. [DOI] [PubMed] [Google Scholar]
- Lovell AT, Hebden JC, Goldstone JC, Cope M. Determination of the transport scattering coefficient of red blood cells. Proc SPIE. 1999;3597:175–182. [Google Scholar]
- Crookes BA, Cohn SM, Bloch S, Amortegui J, Manning R, Li P, Proctor MS, Hallal A, Blackbourne LH, Benjamin R, Soffer D, Habib F, Schulman CI, Duncan R, Proctor KG. Can near-infrared spectroscopy identify the severity of shock in trauma patients? J Trauma. 2005;58:806–813. doi: 10.1097/01.ta.0000158269.68409.1c. discussion 813-816. [DOI] [PubMed] [Google Scholar]
- Harris PC, Scott S, Evans RA. Hand exsanguination: prospective randomised blind study of an established versus a modified technique. J Hand Surg Br. 2002;27:361–362. doi: 10.1054/jhsb.2002.0772. [DOI] [PubMed] [Google Scholar]
- Blond L, Madsen JL. Exsanguination of the upper limb in healthy young volunteers. J Bone Joint Surg Br. 2002;84:489–491. doi: 10.1302/0301-620x.84b4.12758. [DOI] [PubMed] [Google Scholar]
- Weisberg S. Applied Linear Regression. 2. New York: John Wiley and Sons; 1985. Simple linear regression; pp. 1–32. [Google Scholar]
- Franceschini MA, Gratton E, Hueber D, Fantini S. Near-infrared absorption and scattering spectra of tissues in vivo. Proc SPIE. 1999;3597:526–531. [Google Scholar]
- Matcher SJ, Kirkpatrick P, Nahid K, Cope M, Delpy DT. Absolute quantification methods in tissue near infrared spectroscopy. Proc SPIE. 1995;2389:486–495. [Google Scholar]
- Cheong S, Prahl SA, Welch AJ. A review of the optical properties of biological tissues. IEEE J Quantum Electron. 1990;256:2166–2185. [Google Scholar]
- Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol. 1995;40:295–304. doi: 10.1088/0031-9155/40/2/007. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Wei Q, Carraresi L, De Blasi RA, Zaccanti G. Time resolved spectroscopy of the human forearm. J Photochem Photobiol B. pp. 141–158. [DOI] [PubMed]
- Poeze M. Tissue-oxygenation assessment using near-infrared spectroscopy during severe sepsis: confounding effects of tissue edema on StO2 values. Intensive Care Med. 2006;32:788–789. doi: 10.1007/s00134-006-0121-x. [DOI] [PubMed] [Google Scholar]
- Bauer JD. Clinical Chemistry: Theory, Analysis, and Correlation. 2. St Louis, MO: CV Mosby Company; 1989. Hemoglobin; pp. 515–523. [Google Scholar]
- Duck FA. Physical Properties of Tissues: A Comprehensive Reference Book. San Diego, CA: Academic Press; 1990. pp. 320–321. [Google Scholar]
- Mulier KE, Skarda DE, Taylor JH, Myers DE, McGraw MK, Gallea BL, Beilman GJ. Near-infrared spectroscopy in patients with severe sepsis: correlations with invasive hemodynamic measurements. Surg Infect. 2008;9:515–519. doi: 10.1089/sur.2007.091. [DOI] [PubMed] [Google Scholar]
- Keeley L. Reducing the risk of ventilator-acquired pneumonia through head of bed elevation. Nurs Crit Care. 2007;12:287–294. doi: 10.1111/j.1478-5153.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- Blissitt PA, Mitchell PH, Newell DW, Woods SL, Belza B. Cere-brovascular dynamics with head-of-bed elevation in patients with mild or moderate vasospasm after aneurysmal sub-arachnoid hemorrhage. Am J Crit Care. 2006;15:206–216. [PubMed] [Google Scholar]
- Levick JR. An Introduction to Cardiovascular Physiology. 4. London: Arnold Publishers; 2003. Hemodynamics: flow, pressure and resistance; pp. 104–130. [Google Scholar]
- Repez A, Oroszy D, Arnez ZM. Continuous postoperative monitoring of cutaneous free flaps using near infrared spectroscopy. J Plast Reconstr Aesthet Surg. 2008;61:71–77. doi: 10.1016/j.bjps.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Irwin MS, Thorniley MS, Dore CJ, Green CJ. Near infra-red spectroscopy: a non-invasive monitor of perfusion and oxygenation within the microcirculation of limbs and flaps. Br J Plast Surg. 1995;48:14–22. doi: 10.1016/0007-1226(95)90024-1. [DOI] [PubMed] [Google Scholar]
- Hampson NB, Piantadosi CA. Near infrared monitoring of human skeletal muscle oxygenation during forearm ischemia. J Appl Physiol. 1988;64:2449–2457. doi: 10.1152/jappl.1988.64.6.2449. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Kobayashi R, Araki T, Sakamoto T, Fuchigami K, Kaku N, Kakegawa T. Clinical evaluation of severe ischemic limbs by tissue reflection spectrophotometry. Kurume Med J. 1994;41:187–191. doi: 10.2739/kurumemedj.41.187. [DOI] [PubMed] [Google Scholar]
- Blond L, Madsen JL. Exsanguination of the upper limb in healthy young volunteers. J Bone Joint Surg Br. 2002;84:489–491. doi: 10.1302/0301-620x.84b4.12758. [DOI] [PubMed] [Google Scholar]
- Newcom DW, Stalder KJ, Baas TJ, Goodwin RN, Parrish FC, Wiegand BR. Breed differences and genetic parameters of myoglobin concentration in porcine longissimus muscle. J Anim Sci. 2004;82:2264–2268. doi: 10.2527/2004.8282264x. [DOI] [PubMed] [Google Scholar]
- Moller P, Sylven C. Myoglobin in human skeletal muscle. Scand J Clin Lab Invest. 1981;41:479–482. doi: 10.3109/00365518109090486. [DOI] [PubMed] [Google Scholar]
- Ward KR, Ivatury RR, Barbee RW, Terner J, Pittman R, Filho IP, Spiess B. Near infrared spectroscopy for evaluation of the trauma patient: a technology review. Resuscitation. 2006;68:27–44. doi: 10.1016/j.resuscitation.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol. 1994;77:2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- Levick JR. An Introduction to Cardiovascular Physiology. 4. London: Arnold Publishers; 2003. Specialization in individual circulations; pp. 251–277. [Google Scholar]
- Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8:373–381. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick JR. An Introduction to Cardiovascular Physiology. 4. London: Arnold Publishers; 2003. Hemodynamics: flow, pressure and resistance; pp. 120–123. [Google Scholar]
- Schenkman KA, Marble DR, Burns DH, Feigl EO. Optical spectroscopic method for in vivo measurement of cardiac myoglobin oxygen saturation. Appl Spectrosc. 1999;53:332–338. [Google Scholar]
- Tran TK, Sailasuta N, Kreutzer U, Hurd R, Chung Y, Mole P, Kuno S, Jue T. Comparative analysis of NMR and NIRS measurements of intracellular PO2 in human skeletal muscle. Am J Physiol. 1999;276(6 Pt 2):R1682–R1690. doi: 10.1152/ajpregu.1999.276.6.R1682. [DOI] [PubMed] [Google Scholar]
- Boushel R, Piantadosi CA. Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiol Scand. 2000;168:615–622. doi: 10.1046/j.1365-201x.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- Nighswander-Rempel SP, Kupriyanov VV, Shaw RA. Relative contributions of hemoglobin and myoglobin to near-infrared spectroscopic images of cardiac tissue. Appl Spectrosc. 2005;59:190–193. doi: 10.1366/0003702053085106. [DOI] [PubMed] [Google Scholar]
- Yoxall CW, Weindling AM. Measurement of venous oxyhaemoglobin saturation in the adult human forearm by near infrared spectroscopy with venous occlusion. Med Biol Eng Comput. 1997;35:331–336. doi: 10.1007/BF02534086. [DOI] [PubMed] [Google Scholar]
- De Blasi RA, Ferrari M, Natali A, Conti G, Mega A, Gasparetto A. Noninvasive measurement of forearm blood flow and oxygen consumption by near-infrared spectroscopy. J Appl Physiol. 1994;76:1388–1393. doi: 10.1152/jappl.1994.76.3.1388. [DOI] [PubMed] [Google Scholar]