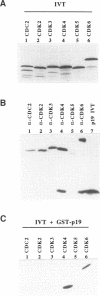
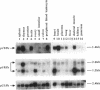
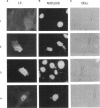
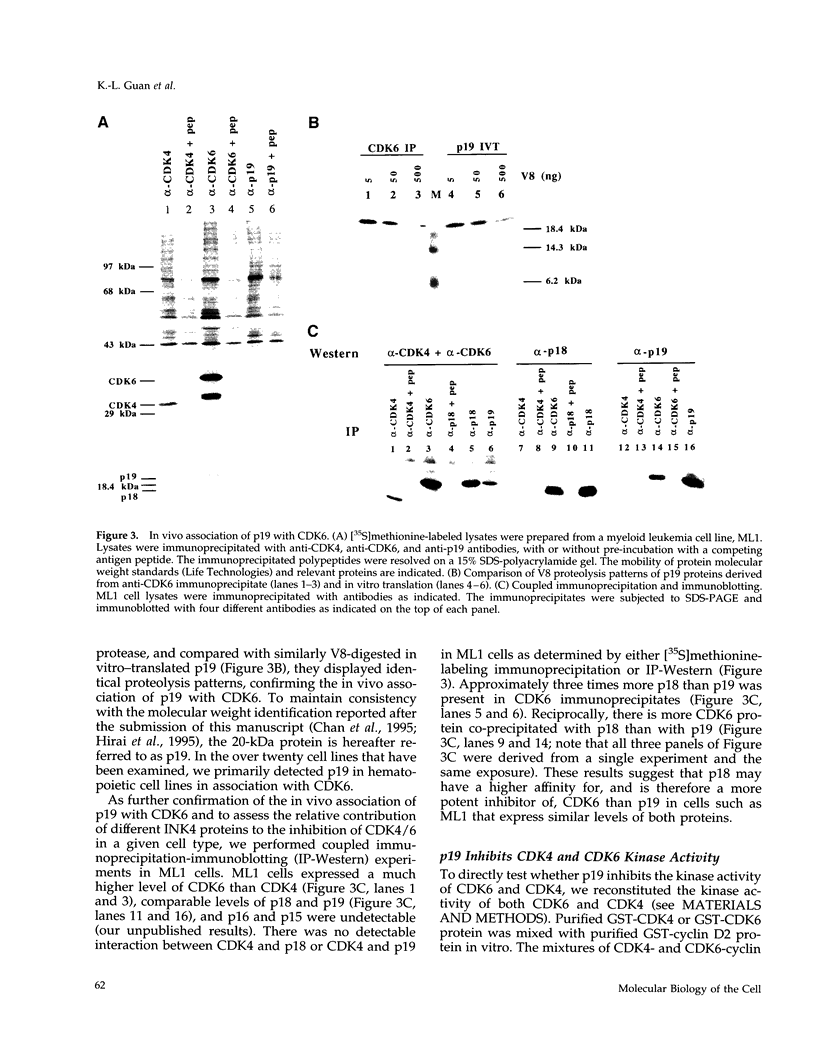
Abstract
Cyclin-dependent kinases 4 and 6 are complexed with many small cellular proteins in vivo. We have isolated cDNA sequences, INK4d, encoding a 19-kDa protein that is associated with CDK6 in several hematopoietic cell lines. p19 shares equal similarity and a common ancestor with other identified inhibitors of the p16/INK4 family. p19 interacts with and inhibits the activity of both CDK4 and CDK6 and exhibits no detectable interaction with the other known CDKs. p19 protein is present in both cell nuclei and cytoplasm. The p19 gene has been mapped to chromosome 19p13.2, and the level of its mRNA expression varies widely between different tissues. In contrast to p21 and p27 whose interaction with CDK subunits is dependent on or stimulated by the cyclin subunit, the interaction of p19 and p18 with CDK6 is hindered by the cyclin protein. Binary cyclin D1-p18/p19 or cyclin D1-CDK6 complexes are highly stable and cannot be dissociated by excess amounts of cyclin D1 or p19/p18 proteins, suggesting that p16 inhibitors and D cyclins may interact with CDKs 4 and 6 in a competing or potentially mutually exclusive manner.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aprelikova O., Xiong Y., Liu E. T. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J Biol Chem. 1995 Aug 4;270(31):18195–18197. doi: 10.1074/jbc.270.31.18195. [DOI] [PubMed] [Google Scholar]
- Baldin V., Lukas J., Marcote M. J., Pagano M., Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993 May;7(5):812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Chan F. K., Zhang J., Cheng L., Shapiro D. N., Winoto A. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol Cell Biol. 1995 May;15(5):2682–2688. doi: 10.1128/mcb.15.5.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Jackson P. K., Kirschner M. W., Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995 Mar 23;374(6520):386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- Flores-Rozas H., Kelman Z., Dean F. B., Pan Z. Q., Harper J. W., Elledge S. J., O'Donnell M., Hurwitz J. Cdk-interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase delta holoenzyme. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8655–8659. doi: 10.1073/pnas.91.18.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Turck C. W., Morgan D. O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993 Dec 16;366(6456):707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Jenkins C. W., Li Y., Nichols M. A., Wu X., O'Keefe C. L., Matera A. G., Xiong Y. Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 1994 Dec 15;8(24):2939–2952. doi: 10.1101/gad.8.24.2939. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Elledge S. J., Keyomarsi K., Dynlacht B., Tsai L. H., Zhang P., Dobrowolski S., Bai C., Connell-Crowley L., Swindell E. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995 Apr;6(4):387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Roussel M. F., Kato J. Y., Ashmun R. A., Sherr C. J. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol. 1995 May;15(5):2672–2681. doi: 10.1128/mcb.15.5.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Huret J. L., Brizard A., Slater R., Charrin C., Bertheas M. F., Guilhot F., Hählen K., Kroes W., van Leeuwen E., Schoot E. V. Cytogenetic heterogeneity in t(11;19) acute leukemia: clinical, hematological and cytogenetic analyses of 48 patients--updated published cases and 16 new observations. Leukemia. 1993 Feb;7(2):152–160. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Matsuoka M., Polyak K., Massagué J., Sherr C. J. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell. 1994 Nov 4;79(3):487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Kato J. Y., Matsuoka M., Strom D. K., Sherr C. J. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol Cell Biol. 1994 Apr;14(4):2713–2721. doi: 10.1128/mcb.14.4.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiechle-Schwarz M., Bauknecht T., Karck U., Kommoss F., du Bois A., Pfleiderer A. Recurrent cytogenetic aberrations and loss of constitutional heterozygosity in ovarian carcinomas. Gynecol Oncol. 1994 Nov;55(2):198–205. doi: 10.1006/gyno.1994.1277. [DOI] [PubMed] [Google Scholar]
- Koh J., Enders G. H., Dynlacht B. D., Harlow E. Tumour-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature. 1995 Jun 8;375(6531):506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Reynisdóttir I., Massagué J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995 Mar 15;9(6):639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- Li Y., Jenkins C. W., Nichols M. A., Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene. 1994 Aug;9(8):2261–2268. [PubMed] [Google Scholar]
- Li Y., Nichols M. A., Shay J. W., Xiong Y. Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res. 1994 Dec 1;54(23):6078–6082. [PubMed] [Google Scholar]
- Lukas J., Parry D., Aagaard L., Mann D. J., Bartkova J., Strauss M., Peters G., Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995 Jun 8;375(6531):503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- Luo Y., Hurwitz J., Massagué J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995 May 11;375(6527):159–161. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- Matera A. G., Ward D. C. Oligonucleotide probes for the analysis of specific repetitive DNA sequences by fluorescence in situ hybridization. Hum Mol Genet. 1992 Oct;1(7):535–539. doi: 10.1093/hmg/1.7.535. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Edwards M. C., Bai C., Parker S., Zhang P., Baldini A., Harper J. W., Elledge S. J. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995 Mar 15;9(6):650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Robetorye R. S., Adami G. R., Pereira-Smith O. M., Smith J. R. Identification of the active region of the DNA synthesis inhibitory gene p21Sdi1/CIP1/WAF1. EMBO J. 1995 Feb 1;14(3):555–563. doi: 10.1002/j.1460-2075.1995.tb07031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Demetrick D. J., Spillare E. A., Hagiwara K., Hussain S. P., Bennett W. P., Forrester K., Gerwin B., Serrano M., Beach D. H. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Serrano M., Gómez-Lahoz E., DePinho R. A., Beach D., Bar-Sagi D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science. 1995 Jan 13;267(5195):249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995 May 15;9(10):1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Warbrick E., Lane D. P., Glover D. M., Cox L. S. A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr Biol. 1995 Mar 1;5(3):275–282. doi: 10.1016/s0960-9822(95)00058-3. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992 Oct 30;71(3):505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation. Genes Dev. 1993 Aug;7(8):1572–1583. doi: 10.1101/gad.7.8.1572. [DOI] [PubMed] [Google Scholar]