Abstract
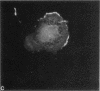
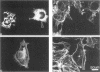
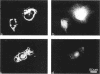
We have analyzed the interactions between two types of sarcomeric proteins: myosin heavy chain (MyHC) and members of an abundant thick filament-associated protein family (myosin-binding protein; MyBP). Previous work has demonstrated that when MyHC is transiently transfected into mammalian nonmuscle COS cells, the expressed protein forms spindle-shaped structures consisting of bundles of myosin thick filaments. Co-expression of MyHC and MyBP-C or -H modulates the MyHC structures, resulting in dramatically longer cables consisting of myosin and MyBP encircling the nucleus. Immunoelectron microscopy indicates that these cable structures are more uniform in diameter than the spindle structures consisting solely of MyHC, and that the myosin filaments are compacted in the presence of MyBP. Deletion analysis of MyBP-H indicates that cable formation is dependent on the carboxy terminal 24 amino acids. Neither the MyHC spindles nor the MyHC/MyBP cables associate with the endogenous actin cytoskeleton of the COS cell. While there is no apparent co-localization between these structures and the microtubule network, colchicine treatment of the cells promotes the formation of longer assemblages, suggesting that cytoskeletal architecture may physically impede or regulate polymer formation/extension. The data presented here contribute to a greater understanding of the interactions between the MyBP family and MyHC, and provide additional evidence for functional homology between MyBP-C and MyBP-H.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett P., Craig R., Starr R., Offer G. The ultrastructural location of C-protein, X-protein and H-protein in rabbit muscle. J Muscle Res Cell Motil. 1986 Dec;7(6):550–567. doi: 10.1007/BF01753571. [DOI] [PubMed] [Google Scholar]
- Bähler M., Eppenberger H. M., Wallimann T. Novel thick filament protein of chicken pectoralis muscle: the 86 kd protein. I. Purification and characterization. J Mol Biol. 1985 Nov 20;186(2):381–391. doi: 10.1016/0022-2836(85)90112-3. [DOI] [PubMed] [Google Scholar]
- Bähler M., Eppenberger H. M., Wallimann T. Novel thick filament protein of chicken pectoralis muscle: the 86 kd protein. II. Distribution and localization. J Mol Biol. 1985 Nov 20;186(2):393–401. doi: 10.1016/0022-2836(85)90113-5. [DOI] [PubMed] [Google Scholar]
- Callaway J. E., Bechtel P. J. C-protein from rabbit soleus (red) muscle. Biochem J. 1981 May 1;195(2):463–469. doi: 10.1042/bj1950463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. Structure of A-segments from frog and rabbit skeletal muscle. J Mol Biol. 1977 Jan 5;109(1):69–81. doi: 10.1016/s0022-2836(77)80046-6. [DOI] [PubMed] [Google Scholar]
- Davis J. S. Interaction of C-protein with pH 8.0 synthetic thick filaments prepared from the myosin of vertebrate skeletal muscle. J Muscle Res Cell Motil. 1988 Apr;9(2):174–183. doi: 10.1007/BF01773739. [DOI] [PubMed] [Google Scholar]
- Dennis J. E., Shimizu T., Reinach F. C., Fischman D. A. Localization of C-protein isoforms in chicken skeletal muscle: ultrastructural detection using monoclonal antibodies. J Cell Biol. 1984 Apr;98(4):1514–1522. doi: 10.1083/jcb.98.4.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S., Fischman D. A. Isolation and characterization of a cDNA clone encoding avian skeletal muscle C-protein: an intracellular member of the immunoglobulin superfamily. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2157–2161. doi: 10.1073/pnas.87.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghali R., Leinwand L. A. Molecular genetic characterization of a developmentally regulated human perinatal myosin heavy chain. J Cell Biol. 1989 May;108(5):1791–1797. doi: 10.1083/jcb.108.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann P. A., Greaser M. L., Moss R. L. C-protein limits shortening velocity of rabbit skeletal muscle fibres at low levels of Ca2+ activation. J Physiol. 1991 Aug;439:701–715. doi: 10.1113/jphysiol.1991.sp018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden H. M., Ito M., Hartshorne D. J., Rayment I. X-ray structure determination of telokin, the C-terminal domain of myosin light chain kinase, at 2.8 A resolution. J Mol Biol. 1992 Oct 5;227(3):840–851. doi: 10.1016/0022-2836(92)90226-a. [DOI] [PubMed] [Google Scholar]
- Jeacocke S. A., England P. J. Phosphorylation of a myofibrillar protein of Mr 150 000 in perfused rat heart, and the tentative indentification of this as C-protein. FEBS Lett. 1980 Dec 15;122(1):129–132. doi: 10.1016/0014-5793(80)80418-2. [DOI] [PubMed] [Google Scholar]
- Karsch-Mizrachi I., Feghali R., Shows T. B., Leinwand L. A. Generation of a full-length human perinatal myosin heavy-chain-encoding cDNA. Gene. 1990 May 14;89(2):289–294. doi: 10.1016/0378-1119(90)90020-r. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretz J. F. Effects of C-protein on synthetic myosin filament structure. Biophys J. 1979 Sep;27(3):433–446. doi: 10.1016/S0006-3495(79)85227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy-Mintz P., Kielian M. Mutagenesis of the putative fusion domain of the Semliki Forest virus spike protein. J Virol. 1991 Aug;65(8):4292–4300. doi: 10.1128/jvi.65.8.4292-4300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Teal S. B., Stockdale F. E. Evolutionarily conserved sequences of striated muscle myosin heavy chain isoforms. Epitope mapping by cDNA expression. J Biol Chem. 1989 Aug 5;264(22):13122–13130. [PubMed] [Google Scholar]
- Moncman C. L., Rindt H., Robbins J., Winkelmann D. A. Segregated assembly of muscle myosin expressed in nonmuscle cells. Mol Biol Cell. 1993 Oct;4(10):1051–1067. doi: 10.1091/mbc.4.10.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos C., Feng I. N. Effect of C-protein on actomyosin ATPase. Biochim Biophys Acta. 1980 Oct 1;632(2):141–149. doi: 10.1016/0304-4165(80)90071-9. [DOI] [PubMed] [Google Scholar]
- Moos C. Fluorescence microscope study of the binding of added C protein to skeletal muscle myofibrils. J Cell Biol. 1981 Jul;90(1):25–31. doi: 10.1083/jcb.90.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos C., Mason C. M., Besterman J. M., Feng I. N., Dubin J. H. The binding of skeletal muscle C-protein to F-actin, and its relation to the interaction of actin with myosin subfragment-1. J Mol Biol. 1978 Oct 5;124(4):571–586. doi: 10.1016/0022-2836(78)90172-9. [DOI] [PubMed] [Google Scholar]
- Moos C., Offer G., Starr R., Bennett P. Interaction of C-protein with myosin, myosin rod and light meromyosin. J Mol Biol. 1975 Sep 5;97(1):1–9. doi: 10.1016/s0022-2836(75)80017-9. [DOI] [PubMed] [Google Scholar]
- Obinata T., Kitani S., Masaki T., Fischman D. A. Coexistence of fast-type and slow-type C-proteins in neonatal chicken breast muscle. Dev Biol. 1984 Sep;105(1):253–256. doi: 10.1016/0012-1606(84)90283-5. [DOI] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Okagaki T., Weber F. E., Fischman D. A., Vaughan K. T., Mikawa T., Reinach F. C. The major myosin-binding domain of skeletal muscle MyBP-C (C protein) resides in the COOH-terminal, immunoglobulin C2 motif. J Cell Biol. 1993 Nov;123(3):619–626. doi: 10.1083/jcb.123.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson N. J., Pearson R. B., Needleman D. S., Hurwitz M. Y., Kemp B. E., Means A. R. Regulatory and structural motifs of chicken gizzard myosin light chain kinase. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2284–2288. doi: 10.1073/pnas.87.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinach F. C., Masaki T., Fischman D. A. Characterization of the C-protein from posterior latissimus dorsi muscle of the adult chicken: heterogeneity within a single sarcomere. J Cell Biol. 1983 Jan;96(1):297–300. doi: 10.1083/jcb.96.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinach F. C., Masaki T., Shafiq S., Obinata T., Fischman D. A. Isoforms of C-protein in adult chicken skeletal muscle: detection with monoclonal antibodies. J Cell Biol. 1982 Oct;95(1):78–84. doi: 10.1083/jcb.95.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee D., Sanger J. M., Sanger J. W. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton. 1994;28(1):1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein L., Webster S. G., Travis M., Blau H. M. Developmental progression of myosin gene expression in cultured muscle cells. Cell. 1986 Sep 26;46(7):1075–1081. doi: 10.1016/0092-8674(86)90707-5. [DOI] [PubMed] [Google Scholar]
- Starr R., Almond R., Offer G. Location of C-protein, H-protein and X-protein in rabbit skeletal muscle fibre types. J Muscle Res Cell Motil. 1985 Apr;6(2):227–256. doi: 10.1007/BF00713063. [DOI] [PubMed] [Google Scholar]
- Starr R., Offer G. H-protein and X-protein. Two new components of the thick filaments of vertebrate skeletal muscle. J Mol Biol. 1983 Nov 5;170(3):675–698. doi: 10.1016/s0022-2836(83)80127-2. [DOI] [PubMed] [Google Scholar]
- Starr R., Offer G. Polypeptide chains of intermediate molecular weight in myosin preparations. FEBS Lett. 1971 Jun 2;15(1):40–44. doi: 10.1016/0014-5793(71)80075-3. [DOI] [PubMed] [Google Scholar]
- Starr R., Offer G. The interaction of C-protein with heavy meromyosin and subfragment-2. Biochem J. 1978 Jun 1;171(3):813–816. doi: 10.1042/bj1710813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano-Ohmuro H., Goldfine S. M., Kojima T., Obinata T., Fischman D. A. Size and charge heterogeneity of C-protein isoforms in avian skeletal muscle. Expression of six different isoforms in chicken muscle. J Muscle Res Cell Motil. 1989 Oct;10(5):369–378. doi: 10.1007/BF01758433. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan K. T., Weber F. E., Einheber S., Fischman D. A. Molecular cloning of chicken myosin-binding protein (MyBP) H (86-kDa protein) reveals extensive homology with MyBP-C (C-protein) with conserved immunoglobulin C2 and fibronectin type III motifs. J Biol Chem. 1993 Feb 15;268(5):3670–3676. [PubMed] [Google Scholar]
- Vaughan K. T., Weber F. E., Fischman D. A. cDNA cloning and sequence comparisons of human and chicken muscle C-protein and 86kD protein. Symp Soc Exp Biol. 1992;46:167–177. [PubMed] [Google Scholar]
- Vaughan K. T., Weber F. E., Ried T., Ward D. C., Reinach F. C., Fischman D. A. Human myosin-binding protein H (MyBP-H): complete primary sequence, genomic organization, and chromosomal localization. Genomics. 1993 Apr;16(1):34–40. doi: 10.1006/geno.1993.1136. [DOI] [PubMed] [Google Scholar]
- Vikstrom K. L., Rovner A. S., Saez C. G., Bravo-Zehnder M., Straceski A. J., Leinwand L. A. Sarcomeric myosin heavy chain expressed in nonmuscle cells forms thick filaments in the presence of substoichiometric amounts of light chains. Cell Motil Cytoskeleton. 1993;26(3):192–204. doi: 10.1002/cm.970260303. [DOI] [PubMed] [Google Scholar]
- Weber F. E., Vaughan K. T., Reinach F. C., Fischman D. A. Complete sequence of human fast-type and slow-type muscle myosin-binding-protein C (MyBP-C). Differential expression, conserved domain structure and chromosome assignment. Eur J Biochem. 1993 Sep 1;216(2):661–669. doi: 10.1111/j.1432-1033.1993.tb18186.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. Characterization of H-protein, a component of skeletal muscle myofibrils. J Biol Chem. 1984 Jun 10;259(11):7163–7168. [PubMed] [Google Scholar]
- Yamamoto K. Effect of H-protein on the formation of myosin filaments and light meromyosin paracrystals. J Biochem. 1988 Feb;103(2):274–280. doi: 10.1093/oxfordjournals.jbchem.a122260. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. The binding of skeletal muscle C-protein to regulated actin. FEBS Lett. 1986 Nov 10;208(1):123–127. doi: 10.1016/0014-5793(86)81545-9. [DOI] [PubMed] [Google Scholar]