Abstract
Converging lines of evidence suggest that oligomers of amyloid-β play a role in the cognitive impairment characteristic of Alzheimer’s disease, but only three studies have provided experimental evidence of such impairment. To provide additional information about the effects of these oligomers on memory, the present study examined the memory of groups of rats exposed to ICV injections of the culture media (CM) of Chinese Hamster Ovary cells that were (7PA2) and were not (CHO-) transfected with a human mutation of amyloid precursor protein that appears to cause early-onset Alzheimer’s disease. The 7PA2 CM, which contained concentrations of soluble amyloid-β oligomers physiologically relevant to those found in human brain, significantly disrupted working memory in rats tested in a radial-arm maze. In contrast, CHO- CM, which did not contain such oligomers, had no effect on memory. The disruptive effects of 7PA2-derived amyloid-β oligomers, evident two hours after exposure, disappeared within a day. These findings are compared to results from 7PA2 CM tested under a complex procedure thought to measure aspects of executive function. The results confirm the disruptive effects of low-n amyloid-β oligomers and extend them to a well established rat model of memory.
Keywords: oligomers of amyloid-beta, memory, Alzheimer’s disease
Alzheimer’s disease (AD) is the most common human dementia, affecting approximately 4.5 million people in the USA in 2000[6]. The disease is characterized physiologically by its two proteinaceous brain inclusions [32]. These inclusions, called neuritic plaques and neurofibrillary tangles (NFT), characterize the post mortem neuropathology of the disease [32]. NFTs are intracellular filaments primarily composed of hyperphosphorylated microtubule-associated tau protein. Neuritic plaques are mainly composed of large extracellular aggregates of the amyloid-β protein (Aβ), which comprise a sequence of 39 to 42 amino acids derived from the transmembrane region of the amyloid precursor protein (APP) [10]. Behaviorally, AD is characterized by progressive deterioration in certain cognitive functions as well as changes in personality and behavior. Impairment of recent (i.e., working, or short-term) memory is one of the earliest detectable symptoms and a hallmark of the disease [25, 26, 37].
Most AD research in recent years has focused on amyloid plaques and the synthesis, cleavage, clearance, and deposition of Aβ, its main component. Indeed, while the percentage of total inherited AD cases is small, all the genetic mutations associated with inherited AD involve genes linked to APP and Aβ synthesis [32]. Further, recent evidence suggests that amyloid deposition likely precedes NFT formation in the development of the disease and data from transgenic mouse models of AD have shown that memory loss can be reversed in the presence of NFTs [24]. As for the large extracellular amyloid plaques, total plaque burden does not correlate well with the progression of cognitive decline that characterizes the disease [1].
Several lines of recent evidence, ably summarized elsewhere [32, 33], suggest that early AD memory loss may be better explained by the presence of small soluble forms of Aβ than by the presence of NFTs or large aggregated amyloid plaques. Under this hypothesis, single Aβ proteins (monomers) form into units of two (dimers), three (trimers), four (tetramers), or more molecules. Oda and colleagues [18] advanced the concept of soluble small soluble Aβ and Lambert and others demonstrated they could be formed from synthetic Aβ [11, 12]. Walsh and colleagues showed that Chinese hamster ovary cells (CHO) when over-expressing a human mutant form of APP secrete Aβ oligomers in the culture medium [35]. Conditioned media (CM) from these cells, called 7PA2 CM, disrupted rat hippocampal long term potentiation (LTP), a process often used to model short-term memory formation [35]. Degrading monomers, but not oligomers, otherwise present in 7PA2 CM did not alter its effect on LTP [34]. Similarly, research using size exclusion chromatography (SEC) to fractionate 7PA2 and CHO- demonstrated that the disruption of LTP was mediated by low-n oligomers, not by monomers or larger aggregates [36]. In addition, SEC fractions containing only monomers have proven non-toxic in cell culture and have been not affected behavior in previous studies (3, 30, 36). These findings indicate that oligomers of Aβ, not other elements contained in 7PA2 CM, are responsible for its effects on memory.
While a number of studies have correlated levels of oligomeric Aβ in brain with poor memory or cognitive performance [e.g., 14, 15, 16], very few experiments have assessed the cognitive effects of Aβ oligomers. In 2005, it was demonstrated that 7PA2 CM, containing Aβ dimers and trimers but not monomers, disrupted the memory of complex learned behavior when injected into the lateral ventricle of behaving rats [3]. This study employed the alternating lever cyclic ratio (ALCR) schedule of food reinforcement, which has proven to be very sensitive to a variety of psychoactive drugs and insoluble aggregated forms of Aβ [19, 23]. Townsend and colleagues [30] subsequently showed that a scyllo-inositol compound significantly reduced Aβ oligomer-induced errors under the ALCR assay. The task requires rats to switch from one lever to the other each time a specified response requirement is met. In addition to the reference memory features of the lever alteration requirement, it has been shown that rats track the variable but regular changes in the response requirement [19]. This feature of the ALCR is suggestive of behavior measuring general aspects of executive function.
In 2006, Lesné and colleagues identified a large (56 kDa) Aβ assembly, called Aβ*56 (abeta star 56), that was negatively correlated with cognitive performance in the APP over-expressing Tg2576 mouse model of AD [13]. When Aβ*56 was injected into the lateral ventricle of rats performing in a Morris Water Maze, reference memory was disrupted [13]. Synthetic oligomeric Aβ1–40-induced disruption of Morris water maze performance has also been demonstrated in hyperglycemic mice [8].
The studies just described provide the only direct experimental evidence of the detrimental effects of oligomeric Aβ on cognitive performance. In view of the remarkable sensitivity of the ACLR procedure [39], however, it is not clear whether Aβ oligomers that disrupt performance under that procedure also disrupt cognitive performance in other more commonly used models of animal memory. To make this determination, the present study compared effects of natural oligomers of amyloid-β protein in separate groups of rats tested in different laboratories under an ALCR schedule and in a radial arm maze, which has been used previously with rats to demonstrate memory impairment induced by exogenous Aβ(1–40) [4, 5, 29] and of Aβ(25–35 [7, 27, 28]. In previous studies of natural oligomers of amyloid-β, only reference memory performance or performance under tasks influenced by executive function has been assessed under Aβ oligomer challenge. The current study extends the assessment of cognitive properties of such oligomers by examining their effects in rats tested in a radial arm maze, which is a relatively simple task (hence involving minimal executive function) that is an established test of spatial working memory [9, 20, 38]. The effects of the oligomers were also examined in an ALCR procedure similar to that used by Cleary et al. [3]. To increase the potential generality of findings, the ALCR and radial-arm maze procedures were conducted with different rat strains tested in two different laboratories (Queens University and Western Michigan University, respectively) and the Aβ oligomers were obtained from a third laboratory (University of Minnesota). In addition, different experimental designs (within-subject for ALCR, between-subjects for the radial-arm maze) were used in the two studies.
MATERIALS AND METHODS
Subjects
Radial-Arm Maze
Twenty male Sprague-Dawley rats, purchased from Charles River (Portage, MI) and approximately one year old at the beginning of the study (mean weight =448 g) were used. They had been handled extensively before the experiment and prior to surgery were housed in pairs with unlimited access to water in plastic cages (24 cm long × 31.5 cm wide × 21 cm high) located in a colony room maintained on a 12-h light/12-h dark cycle and kept at a relatively consistent temperature (20–22° C). Following surgery, the rats were housed individually in similar cages with elevated tops that provided sufficient space to accommodate the animal’s guide cannula and head cap. Training and testing occurred during the light portion of the cycle. Throughout the experiment the rats were restricted to 1 h of access to Purina Rat Chow (Ralston-Purina, St. Louis) each day; access occurred 20 hours before the first experimental session of the following day. The radial arm maze portion of this study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals promulgated by the National Research Council [17] and was approved by the Institutional Animal Care and Use Committee at Western Michigan University.
ALCR
Fourteen male Sprague-Dawley rats (Harlan, UK) weighing 250 g at the beginning of the experiment were used. They were housed individually and trained and tested under an ALCR schedule of food reinforcement using the method previously described by Cleary et al [3] and by Richardson et al. [23]. Housing, surgery, injections and injectates were as described in detail above for rats tested in the radial arm maze. The ALCR portion of this study was conducted under Home Office license (UK).
Apparatus
Radial-Arm Maze
An 8-arm maze constructed of clear plastic 18 cm high was used. Each arm was 60 cm long and 12 cm wide. The arms radiated outward symmetrically from a circular start box 36 cm in diameter. When an arm was baited, a piece of Cocoa Pellets cereal (Post Cereals, Battle Creek, MI) was placed in a plastic receptacle affixed to the wall of the arm opposite the start box. When desired, an individual arm could be blocked with plastic doors located at the intersection of that arm and the start box. To provide visual cues for the rats, a sheet of white poster board printed with a black and white design (e.g., straight vertical line, three squiggly horizontal line)s was located immediately behind the end of each arm. Hinged clear plastic tops covered each arm and a clear plastic top was put over the start box as soon as a rat was placed therein. The floor of the maze was constructed of perforated metal and a line was inscribed on each arm 53 cm from the start box; crossing this line defined entry into an arm (see Dependent Variables below).
ALCR
Ten two-lever Campden Instruments rat test chambers (Camden Instruments, Loughborough, UK), enclosed in sound attenuating chambers, were employed under the ALCR assay. The reinforcer was 0.01 ml 10% sucrose, which was delivered into a tray situated midway between the two levers, 1.0 cm above the test chamber floor. A Siemens 486 computer, programmed in MED-PC (Med Associates, Fairfield, VT), controlled the experiment and collected data.
Behavioral Procedures
Radial-Arm Maze
All pre-training sessions began with the rat being placed in the start box, facing in a randomly determined direction. For the first five pre-training sessions all arms were open and eight Cocoa Pebbles were placed at random locations around the maze. On the sixth session, a single bit of cereal was placed at the entrance to each arm. On the seventh, eight, ninth, and tenth pre-training sessions, respectively, a single piece of cereal was placed approximately 25, 50, 75, and 100% of the way to the end each arm. Fourteen sessions were then arranged in which all arms were open and baited. All pre-training sessions continued until the rat had consumed all of the food, after which it was immediately removed from the maze and returned to the home cages. Pre-training, training (see below), and testing (see below) were arranged daily seven days a week at about the same time each day, in the light portion of the light/dark cycle.
Training and testing procedures were similar to those used by Chappell et al. [2] to examine the effects of cholinergic lesions of the basal forebrain on the spatial working memory of rats. Training began immediately after the 24 pre-training days. During training, two sessions were arranged each day. During the first session, four arms were blocked and the remaining four were baited. The arms that were blocked were selected at random each day, with the exception that no more than two consecutive arms could be blocked. Each rat was placed in the start box, facing in a randomly selected direction, when the session began and was allowed access to the maze until it had consumed all four pieces of cereal. At that time, it was removed from the maze and placed in its home cage, and the maze was washed with warm soapy water. Two hours later, a second session was arranged. In this session, used to index memory, each rat was returned to the start box, again facing in a randomly determined direction. All arms of the maze were open and the four arms that initially were blocked were baited, whereas the arms that initially were open (and baited) were unbaited. The rat was allowed access to the maze until it had consumed all four pieces of cereal, at which time it was removed and returned to its home cage. The maze was then washed. Training continued for 14 days, after which surgery was arranged.
During test sessions, an observer quantified the rats’ behavior by recording the sequence in which arms were entered and whether or not entries, defined as any part of the rat crossing a line located inside each arm 53 cm from the start box, constituted errors. Two kinds of errors were recorded. Proactive errors involved entering an arm that had been baited during the first daily session and was not currently baited. Retroactive errors involved re-entering arms from which food had been obtained in the current session.
ALCR
The Alternating Lever Cyclic Ratio (ALCR) test has been described in detail previously [3, 23]. Briefly, under this task rats must learn a complex sequence of lever-pressing requirements in a two-lever experimental chamber. Subjects must alternate between two levers by switching to the other lever after pressing the first lever enough times to get a food reward. The exact number of presses required for each food reward changes, first increasing from 2 responses per food pellet up to 56 presses per food pellet, then decreasing back to 2 responses per pellet. Intermediate values are based on the quadratic function, x2 − x. One cycle is an entire ascending and descending sequence of lever press requirements (e.g., 2, 6, 12, 20, 30, 42, 56, 56, 42, 30, 20, 12, 6, and 2 presses per food reward). Six such complete cycles are presented during each daily session. Errors are of two types. Switching errors were of two types and occurred when the subject a) did not alternate to the other lever after reward or b) switched from the correct lever to the incorrect lever before completing the response requirement and getting rewarded. Perseveration errors occurred when the subject ‘persevered’ on the incorrect lever after making an initial response (switching error) on the incorrect lever. If, for example, the left lever was correct and a rat responded six consecutive times on that lever before switching to the right (correct) lever, those six responses would comprise one switching error (the first response) and five perseveration errors (the remaining responses).
Surgical Procedure
The rats were given atropine (1.0 mg/kg, IP) and subsequently anesthetized with sodium pentobarbital (50 mg/kg, IP). Once a rat was fully anesthetized, as indicated by the absence of hind-paw pinch reflex, a unilateral guide cannula (C313G, 22 gauge, Plastics One, Roanoke, VA) was surgically implanted in the dorsal lateral ventricle at stereotaxic coordinates of AP = −0.4, ML = + 1.3, DV= − 4.2 [21] and anchored to the skull with skull screws and cranioplastic cement.
Injection Material and Procedure
Oligomers of amyloid-β peptide
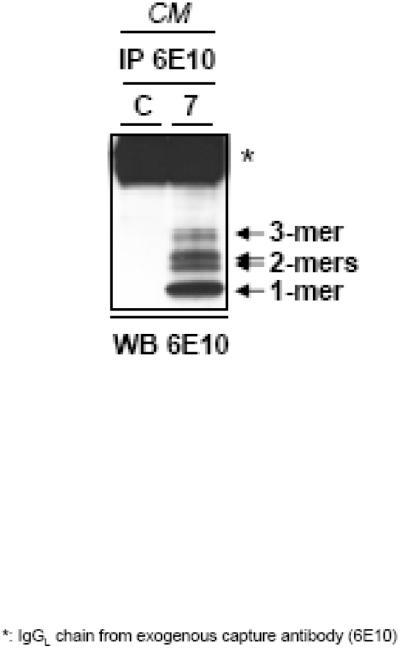
Concentrations of soluble Aβ oligomers that are physiologically relevant to those found in human brain (<10−9 M) are contained in the culture media (CM) of Chinese Hamster Ovary cells (CHO) transfected with a human mutation of APP that causes early-onset AD [22]. These cultured cells, called 7PA2 cells, excrete Aβ oligomers, primarily dimers and trimers, into the CM as the cells mature. The lane labeled 7 of Figure 1 shows a western blot of the Aβ monomers, dimers and trimers that are present in 7PA2 CM. Lane C from Figure 1 shows Western Blot results of the CM from normal untransfected CHO cells (CHO-).
Figure 1.
Western blot of CM from 7PA2 ( 7 ) and CHO- ( C ) cells using antibody 6E10 against the amyloid-β peptide. CHO- CM shows no soluble oligomeric Aβ, while CM from 7PA2 cells show monomers, dimers and trimers of Aβ.
Radial-Arm Maze Injection Procedure
No sessions were arranged for three days immediately following cannula implantation surgery. On days four through six post-surgery, two sessions were conducted each day under the 8-arm peocedure, as described above. Immediately after the first session ended on the seventh day following surgery, all rats received an intracerebroventricular (ICV) injection of either 7PA2 CM or CHO- CM (n=10 / group, with random assignment). Two hours after the injection, they received a second daily (test) session, as described previously. Arranging a two-hour delay between 7PA2 CM injections and test sessions had previously been shown to increase errors under the ALCR procedure [3]. Micro-infusion pumps (CMA/100, CMA, Stockholm, Sweden) were used to infuse 20 μl of 7PA2 CM or CHO- CM through 28 gauge injectors (C313I, Plastics One) into the lateral ventricle slowly, at a maximum rate of 5 μl/minute.
The initial test session under 7PA2 CM and CHO- CM was followed by two weeks of twice per day training and testing sessions. During this time both the 7PA2 CM group and the CHO- CM group received six days without any injections, followed by a day with an ICV injection prior to the second daily session. This same exact routine was repeated over the next week. Animals that received 7PA2 CM initially always received 7PA2 CM in subsequent injections and animals that initially received CHO- CM always received CHO- CM subsequently. Two rats in the 7PA2 group did not receive the third injection because their cannula skull caps loosened.
ALCR Injection Procedure
Behavioral testing begun 2 hours after ICV injections. All ICV injections were 20μl and were slowly administered over a 5-minute period in freely moving animals.
Confirmation of Cannula Placement
Following behavioral testing, all rats were sacrificed and their brains were extracted and stored in 10% formalin solution until sectioned. In sectioning, brains were sliced at a thickness of 60 microns and mounted on slides. Cannula placement in the ventricles of all rats was confirmed by visual inspection of slides under a microscope.
RESULTS
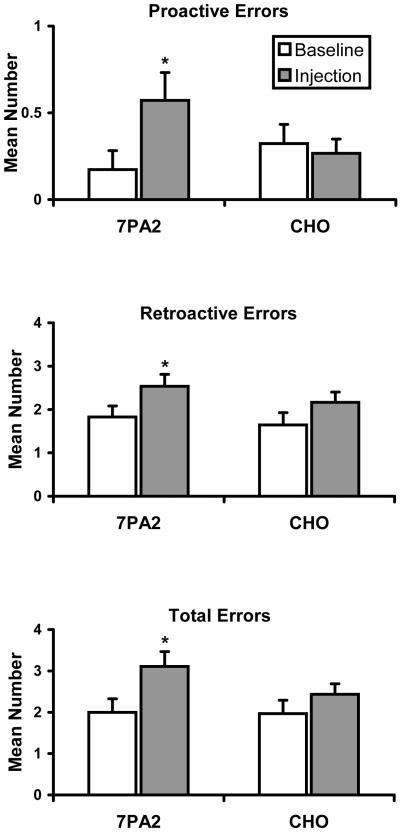
Figure 1 illustrates the low molecular weight soluble species of Aβ present in 7PA2 CM, which typically includes monomers, dimers, and trimers. CHO- CM contains no soluble low molecular weight Aβ. Figure 2 shows the mean (± SEM) number of proactive errors, retroactive errors, and total errors made during the three test sessions in which 7PA2 CM or CHO- CM was administered two hours earlier (injection) and during the three control sessions immediately preceding those test sessions (baseline). Performance of the two groups of rats during control sessions was comparable. The mean control value for total errors was 2.0 for the 7PA2 CM groups and 1.97 for the CHO- CM group. Each group made relatively few proactive errors (M = 0.17 and 0.32 for the 7PA2 and CHO- groups, respectively) and substantially more retroactive errors (M = 1.80 and 1.65 for the 7PA2 and CHO groups, respectively). Mean values for number of proactive (0.27), retroactive (2.10), and total (2.37) errors that occurred two hours after ICV injections of CHO- CM did not differ appreciably from control values. For rats that received 7PA2 CM, the mean numbers of proactive, retroactive, and total errors made during sessions preceded by injections (0.57, 2.55, and 3.125, respectively) were substantially above control levels.
Fig. 2.
Mean number of proactive, retroactive, and total errors made by groups of 10 rats tested in a radial-arm maze during sessions 2 h after ICV injections of 7PA2 CM and CHO- CM and during the immediately preceding control sessions. Asterisks indicate significant (p < 0.05) differences between injection and control means.
Gain scores calculated from the data in Figure 2 were analyzed statistically by repeated measures analysis of variance followed by planned comparisons by the Bonferroni method; the alpha level was set at 0.05 for all tests. Results indicated that the mean number of proactive, retroactive, and total errors differed significantly across the four conditions of interest (7PA2 CM baseline, 7PA2 CM injection, CHO- CM baseline, CHO- CM injection) (F[2, 94] = 3.38, p = 0.038; F[2, 94] = 4.31, p = 0.016; F[2, 94] = 6.02, p = 0.003, respectively). Planned comparisons revealed that 7PA2 CM and CHO- CM baseline accuracy did not differ for proactive, retroactive, or total errors (all ps > 0.05). For rats that received 7PA2 CM, the mean number of proactive, retroactive, and total errors during injection sessions was significantly greater than the number made during baseline sessions (p = 0.036, p = 0.0169, and p = 0.0026, respectively). In contrast, for rats that received CHO- CM, the mean number of proactive, retroactive, and total errors made during injection and baseline sessions did not significantly differ (all ps > .05). The error-increasing effects of 7PA2 CM in the radial-arm maze did not increase in magnitude across administrations, and were in fact greatest following the first administration.
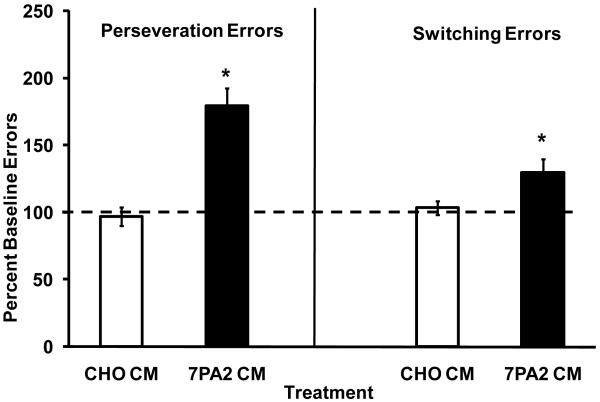
Under ALCR, error rates following injections were compared to baseline error rates consisting of at least 3 non-treatment days temporally contiguous to the injection. Student’s T test of statistical inference was used for analysis of effects. Figure 3 illustrates the mean (± SEM) number of incorrect lever Perseveration Errors and incorrect lever Switching Errors as a percentage of the mean baseline error rate (3 days prior to testing) for 7PA2 CM or CHO CM under the ALCR cognitive assay. Both Switching and Perseveration errors were significantly increased relative to baseline, while CHO- CM had no effect. The error-increasing effects of 7PA2 CM under the ALCR did not vary systematically in magnitude across three exposures.
Figure 3.
Effect of 7PA2 CM and CHO CM on Perseveration Errors and Switching Errors under the ALCR assay. Data are expressed as a percentage of the mean baseline error rate prior to compound testing (± S.E.M.). *Indicates significant differences (p < 0.05).
DISCUSSION
Cleary et al. [3] have reported previously that naturally produced Aβ oligomers from 7PA2 cells, but not Aβ monomers or non-Aβ CM, produced cognitive impairment in rats tested under an ALCR procedure. In the current study, CM from Aβ oligomer-containing 7PA2 cells significantly increased Switching and Perseveration errors under ALCR. These findings are consistent with those of Cleary et al. Aβ oligomer-induced errors under ALCR have been associated with reference memory [4] and such errors in the Morris Water Maze [13] also are thought to be related to reference memory.
Under the radial-arm maze procedure in the present study, oligomers of Aβ significantly increased proactive and retroactive errors in rats. Both measures are assumed to assess spatial working memory [2]. Errors did not increase when CM containing no Aβ oligomers was administered. Therefore, it appears that the Aβ oligomers per se, not ICV injections or non-specific components of the CM, were responsible for the effect. In sum, the present data provide further evidence of the deleterious cognitive effects of oligomers of Aβ and extent those effects to a well established animal model of spatial working memory, the radial arm maze [2, 9, 20, 38]. This extension is significant because a deficit in working memory has been considered to be one of the earliest detectable symptoms of AD and is a clinical hallmark of the disease [25, 26, 37].
In the current study, Aβ oligomers increased ALCR Perseveration errors to 179% of the control level and Switching errors to 130% of control. Aβ oligomers increased Proactive and Retroactive errors to 190% and 142% of control, respectively. Thus, although the manner in which memory is indexed in the radial-arm maze and ALCR procedures are very different, the degree of cognitive disruption observed under the two behavioral procedures was comparable in magnitude. The ALCR and radial-arm maze procedures vary substantially in many regards, but both appear to involve spatial memory, in that successful performance requires subjects to move from place to place and to base their movements on the consequences of prior actions. It is tempting to suggest that oligomers of Aβ generally impair rats’ spatial memory, but an unpublished pilot study that we conducted failed to find a disruptive effect of oligomers of Aβ (ICV) in rats exposed to a delayed-nonmatching-to-position procedure, which also involves spatial memory [31]. Injection of the prototypic amnestic drug scopolamine significantly increased errors in that study. Thus, while the current study expanded the class of behaviors affected by Aβ oligomers, the range of conditions under which memory is disrupted and the specific nature of the disruption is still largely unexplored. It is, however, clear that the disruptive effects of oligomeric Aβ are not limited to measures of reference memory and common animal behavior tests may be sensitive to these effects under some conditions.
The present data are consistent with those reported by Cleary et al. [3] in that the effects of Aβ oligomers were transient, that is, there was no evidence of significant cognitive impairment on the day following the injection under either the radial-arm maze or the ALCR lever pressing task. Moreover, there was no evidence that oligomeric Aβ was neurotoxic in the present study, because there was no increase in errors as a result of repeated exposure. In fact, the oligomers appeared to act similarly to pharmacological agents that are cleared over time. This aspect of the oligomers’ action is important because it offers hope that cognitive deficits due to Aβ oligomers in people with AD may be reversible. Reversible effects were also reported in an animal model by Holscher et al. [7], who found that three daily ICV injections of soluble Aβ(25–35) produced short- and long-term memory impairment in rats tested in a radial-arm maze on post-injection days 12–20, but not on post-injection days 3–11 or 20–28. The difference in the time-course of effects with soluble synthetic Aβ(25–35) and with oligomers of naturally derived Aβ(1–42) from 7PA2 cells is noteworthy and merits investigation with respect to mechanism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Braak H, Braak E. Argyrophilic grain disease: Frequency of occurrence in different age categories and neuropathological diagnostic criteria. J Neural Trans. 1998;105:801–819. doi: 10.1007/s007020050096. [DOI] [PubMed] [Google Scholar]
- [2].Chappell J, McMahan R, Chiba A, Gallagher M. A re-examination of the role of basal forebrain cholingergic neurons in spatial working memory. Neuropharmacology. 1998;37:481–487. doi: 10.1016/s0028-3908(98)00032-x. [DOI] [PubMed] [Google Scholar]
- [3].Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- [4].Hashimoto M, Hossain S, Shimada T, Sugioka K, Yamasaki H, Fujii Y, Ishibashi Y, Oka J-I, Shido O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J Neurochem. 2002;81:1084–1091. doi: 10.1046/j.1471-4159.2002.00905.x. [DOI] [PubMed] [Google Scholar]
- [5].Hashimoto M, Tanabe Y, Fikoo Y, Kikuta T, Shibata H, Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognitive learning ability in amyloid β-infused rats. Nutritional Neurosci. 2005;135:549–555. doi: 10.1093/jn/135.3.549. [DOI] [PubMed] [Google Scholar]
- [6].Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. State-specific projections through 2025 of Alzheimer disease prevalence. Neurology. 2003;62:1645. doi: 10.1212/01.wnl.0000123018.01306.10. [DOI] [PubMed] [Google Scholar]
- [7].Holscher D, Gengler S, Galut VA, Harriott P, Mallot HA. Soluble beta-amyloid[25–35] reversibly impairs hippocampal synaptic plasticity and spatial learning. Euro J Neuropharm. 2007;561:85–90. doi: 10.1016/j.ejphar.2007.01.040. [DOI] [PubMed] [Google Scholar]
- [8].Huang HJ, Liang KC, Chen CP, Chen CM, Hsieh-Li HM. Intrahippocampal administration of A beta(1–40) impairs spatial learning and memory in hyperglycemic mice. Neurobiol Learn Mem. 2007;87:483–494. doi: 10.1016/j.nlm.2006.11.006. [DOI] [PubMed] [Google Scholar]
- [9].Jaffard R, Bontempi B, Menzaghi F. Theoretical and practical considerations for the evaluation of learning and memory in mice. In: Buffacusco J, editor. Methods of Behavior Analysis in Neuroscience. CRC Press LLC; Boca Raton, FL: 2000. pp. 295–323. 295–323. [Google Scholar]
- [10].Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Crzesckik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein reembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- [11].Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: The solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- [12].Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- [14].Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smit MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999:4860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [16].Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. J Amer Med Assoc. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- [17].National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- [18].Oda T, Wals P, Osterburg H, Johnson S, Pasinetti G, Morgan T, Rozovsky I, Stine WB, Holzman T, Kraft G, Finch C. Clusterin (apoJ) alters the aggregation of amyloid beta peptide (A beta 1–42) and forms slowly sedimenting A beta complexes that cause oxidative stress. Exp Neuol. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- [19].O’Hare E, Levine AS, Semotuk MT, Tierney KJ, Shephard RA, Grace MK, Cleary J. Utilization of a novel model of food reinforced behavior involving neuropeptide Y, insulin, 2-deoxy-d-glucose and naloxone. Behav Pharmacol. 1996;7:742–753. [PubMed] [Google Scholar]
- [20].Olton DS. The radial arm maze as a tool in behavioral pharmacology. Physio Behav. 1985;40:793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- [21].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. p. 256. [Google Scholar]
- [22].Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydel RE, Teplow DB, Selkoe DJ. Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995;270:9564–70. doi: 10.1074/jbc.270.16.9564. [DOI] [PubMed] [Google Scholar]
- [23].Richardson RL, Kim E-M, Shepard RA, Gardiner T, Cleary J, O’Hara E. Behavioural and histopathological analysis of ibuprofen treatment on the effect of aggregated Aβ -sub((1–42)) injections in the rat. Brain Res. 2002;954:1–10. doi: 10.1016/s0006-8993(02)03006-8. [DOI] [PubMed] [Google Scholar]
- [24].Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2002;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Selkoe DJ. Amyloid protein and Alzheimer’s disease. Sci Amer. 1991;265:68–78. doi: 10.1038/scientificamerican1191-68. [DOI] [PubMed] [Google Scholar]
- [26].Small BJ, Gagnon E, Robinson B, Neugroschi JA. Early identification of cognitive deficits: Preclinical Alzheimer’s disease and mild cognitive impairment. Geriatrics. 2007;62:19–23. [PubMed] [Google Scholar]
- [27].Stepanichev MY, Moiseeva YV, Lazareva Na, Onufriev VM, Gulyaeva NV. Single intracerebroventricular administration of amyloid-beta(25–35) peptide induced impairment in short-term rather than long-term memory in rats. Brain Res Bu. 2003;l 61:197–205. doi: 10.1016/s0361-9230(03)00118-7. [DOI] [PubMed] [Google Scholar]
- [28].Stepanichev MY, Zdobnova M, Zarubenko II, Lazareva NA, Gulyaeva NV. Studies of the effects of central administration of β-amyloid peptide (25–35): Pathomorphological changes in the hippocampus and impairment of spatial memory. Neuroscience Behav Physio. 2006;36:101–106. doi: 10.1007/s11055-005-0167-1. [DOI] [PubMed] [Google Scholar]
- [29].Sweeney WA, Luedtke J, McDonald MP, Overmier JB. Intrahippocampal injections of exogenous β-amyloid induce postdelay errors in an eight-arm radial maze. Neurobio Learning Mem. 1997;68:97–101. doi: 10.1006/nlme.1997.3770. [DOI] [PubMed] [Google Scholar]
- [30].Townsend M, Cleary J, Mehta T, Hofmeister J, Lesné S, O’Hare E, Walsh DM, Selkoe DJ. Orally available compound prevents deficits in memory caused by the Alzheimer amyloid-beta oligomers. Ann Neurol. 2006;60:668–676. doi: 10.1002/ana.21051. [DOI] [PubMed] [Google Scholar]
- [31].Vardigan JD, Parraghi JW, Panos JJ, Porritt M, Kueh D, Cleary J, Baker LE, Poling A. 265.20 Abstract Viewer and Itinerary Planner. Society for Neuroscience; Atlanta, GA: 2006. Effects of 7-PA2 beta-amyloid oligomer microinjections in a delayed non-matching to procedure in rats. Online. [Google Scholar]
- [32].Walsh DM, Selkoe DJ. Oligomers on the brain: The emerging role of soluble protein aggregates in neurodegeneration. Pro Pep Letters. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- [33].Walsh DM, Selkoe DJ. Aβ oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- [34].Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, Agnaf OE, Hartley DM, Selkoe DJ. Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerizaiton of natural Abeta and thereby rescue long-term potentiation. J. Neurosci. 2005;25:2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: Their production, toxicity and therapeutic inhibition. Biochem Soc Tran. 2002;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- [36].Walsh DM, Klyubin I, Fadeeva J, William K, Cullen W, Anwyl R, Wolfe M, Roman M, Selkoe DJ. Naturally secreted oligomers of the Alzheimer amyloid beta-protein potently inhibit hhippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- [37].Walsh DM, Klyubin I, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ. The role of cell-derived oligimers of Aβ in Alzheimer’s disease and avenues for therapeutic intervention. Biochem Soc Trans. 2005;33:1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- [38].Wenk GL. Assessment of spatial memory using the radial arm maze and the Morris water maze. In: Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, Wray S, editors. Short Protocols in Behavioral Neuroscience: Systems and Behavioral Methods. John Wiley & Sons; Hoboken, NJ: 2007. pp. 370–375. 2007. [Google Scholar]
- [39].Weldon DT, O’Hare E, Kuskowswki MA, Cleary J, Mach JR., Jr. Alternating lever cyclic-ratio schedule analysis of the effects of atropine sulface. Pharmacol Biochem Behav. 1996;54:753–757. doi: 10.1016/0091-3057(96)00014-7. [DOI] [PubMed] [Google Scholar]