Abstract
Body dysmorphic disorder (BDD) is an impairing and relatively common disorder that has high comorbidity with certain Axis I disorders. However, the longitudinal associations between BDD and comorbid disorders have not previously been examined. Such information may shed light on the nature of BDD’s relationship to putative “near-neighbor” disorders, such as major depression, obsessive-compulsive disorder (OCD), and social phobia. This study examined time-varying associations between BDD and these comorbid disorders in 161 participants over 1 to 3 years of follow-up in the first prospective longitudinal study of the course of BDD. We found that BDD had significant longitudinal associations with major depression – that is, change in the status of BDD and major depression were closely linked in time, with improvement in major depression predicting BDD remission, and, conversely, improvement in BDD predicting depression remission. We also found that improvement in OCD predicted BDD remission, but that BDD improvement did not predict OCD remission. No significant longitudinal associations were found for BDD and social phobia (although the results for analyses of OCD and social phobia were less numerically stable). These findings suggest (but do not prove) that BDD may be etiologically linked to major depression and OCD – i.e., that BDD may be a member of both the putative OCD spectrum and the affective spectrum. However, BDD does not appear to simply be a symptom of these comorbid disorders, as BDD symptoms persisted in a sizable proportion of subjects who remitted from these comorbid disorders. Additional studies are needed to elucidate the nature of BDD’s relationship to commonly co-occurring disorders, as this issue has important theoretical and clinical implications.
Keywords: body dysmorphic disorder, dysmorphophobia, course, obsessive-compulsive disorder, depression, social phobia
Body dysmorphic disorder is a relatively common somatoform disorder, affecting an estimated 0.7%-1.1% of the United States population (Bienvenu et al., 2000; Otto et al., 2001). BDD is also a severe disorder, with studies finding high rates of suicidal ideation and suicide attempts, marked impairment in social and academic/occupational functioning, and very poor quality of life (Phillips, 2001). However, BDD has been systematically studied for little more than a decade, and little is known about the nature of its relationship to putative “near-neighbor” disorders, such as major depression, obsessive-compulsive disorder (OCD), and social phobia.
Cross-sectional studies have generally found high comorbidity between BDD and these disorders, although findings have varied somewhat across studies. Most studies have found that major depression is the most common comorbid disorder in patients with BDD, with lifetime prevalence ranging from 36% of 50 subjects (Veale et al., 1996) to 76% of 293 subjects (Gunstad & Phillips, 2003). In the largest study (n=293), major depression was more than twice as common as any other Axis I disorder (Gunstad & Phillips, 2003). Conversely, although findings have varied, some studies have found a high prevalence of BDD among patients with major depression, especially the atypical subtype. Two studies both found that 14% of patients with atypical major depression had comorbid BDD (Nierenberg et al., 2002; Phillips et al., 1996), and another study found a rate of 42% (Perugi et al., 1998). Based on BDD’s high comorbidity with depression, and its response to antidepressants (SRIs specifically), BDD has been hypothesized to be related to affective disorders (Phillips et al., 1995). However, BDD and depression have also been noted to have important differences, suggesting that BDD is not simply a symptom of depression (Phillips, 1999). For example, BDD is characterized by prominent obsessions and compulsive behaviors, and it appears to respond to SRIs but not to non-SRI antidepressants (Hollander et al., 1999; Hollander et al., 1994; Phillips, 2001). In addition, clinical observations suggest that depressive symptoms in BDD patients often appear to be “secondary” to the distress and demoralization that BDD often causes. However, BDD’s relationship to depression has not been investigated and remains unclear.
BDD is also often comorbid with OCD, and BDD is widely conceptualized as an OCD-spectrum disorder (Cohen et al., 1997; Phillips et al., 1995). Indeed, BDD and OCD have many similarities (Frare et al., 2004; Phillips et al., 1998), including prominent obsessions and compulsions, and similarities in treatment response. In addition, BDD occurs with increased frequency in first-degree relatives of OCD probands, suggesting that these disorders may be related (Bienvenu et al., 2000). However, differences have also been found (Buhlmann et al., 2002; Eisen et al., 2004; Frare et al., 2004; Phillips et al., 1998), including poorer insight and higher rates of suicidality and comorbid depression in BDD, suggesting that they are not identical.
Although the relationship between BDD and social phobia has received less attention, social phobia is also highly comorbid with BDD, with the largest BDD study (n=293) finding a lifetime prevalence of 37% (Gunstad & Phillips, 2003). BDD and social phobia have many shared clinical features, including social avoidance and anxiety, introversion, and a negative interpretive bias for social scenarios (Buhlmann et al., 2002; Phillips & McElroy, 2000; Veale et al., 1996). In Eastern cultures, BDD is conceptualized as a form of social phobia (Kleinknecht et al., 1997).
Despite BDD’s high comorbidity and shared clinical features with these disorders, their relationship has received little investigation. Examination of comorbidity is limited to cross-sectional studies, and the meaning of their co-occurrence is unclear. A number of models may potentially explain comorbidity (Lyons et al., 1997). One possible explanation is that comorbid disorders are independent in terms of etiology and that comorbidity may be due to chance or high base rates of each disorder, especially in treatment-seeking individuals. Alternatively, two comorbid disorders may be etiologically distinct but produce non-specific overlapping symptoms, such as obsessions. Yet another model, the “predisposing,” or vulnerability, model, posits that comorbid disorders have a distinct etiology but that one disorder increases the likelihood of developing the second disorder. In contrast, comorbidity may reflect shared causality. For example, the hypothesis that BDD is a member of the OCD spectrum or the affective spectrum implies that these disorders have shared etiologic factors—i.e., shared genetic or environmental risk factors. In another sense, shared etiology could reflect an interaction between the disorders, with one occurring “secondary” to the other (for example, BDD causing demoralization and depressed mood which meets criteria for major depression). It is possible – perhaps likely -- that more than one of these models explains comorbidity in BDD. For example, it is possible that BDD and social phobia have a shared etiology (in the sense of shared genetic or environmental risk factors) and that social phobia also increases vulnerability to the subsequent development of BDD, perhaps by increasing sensitivity to perceived rejection by others. Furthermore, different models may potentially explain BDD’s comorbidity with different disorders.
One approach to determining whether two comorbid disorders are etiologically related is to examine changes in their course over time. If the disorders are related, then change in one disorder should be correlated with change in the other, and the changes should occur closely in time (Shea et al., 2004). (This would not necessarily be true for the predisposing model, as the disorder that increases the likelihood of developing the second disorder might precede onset of the second disorder for any length of time, and improvement in these disorders would not necessarily be closely temporally linked.) Longitudinal studies, unlike cross-sectional studies, have the advantage of enabling investigation of changes in comorbid disorders over time. In the present study, which is to our knowledge the only longitudinal study of BDD’s course, we examined longitudinal associations between the course of BDD and the course of comorbid major depression, OCD, and social phobia over 1 to 3 years of prospective follow-up using proportional hazard regression analyses with time varying covariates. This method has the advantage of tracking clinical events (e.g., remission) over brief intervals (e.g., weeks or months rather than years) and prospectively examining whether a change (e.g., improvement) in one disorder’s status is associated with a change (e.g., improvement) in the course of a second disorder (Shea et al., 2004). If two disorders change (e.g., improve) together, that strongly suggests they are etiologically linked to each other or to a third factor leading to the correlated change. On the other hand, if one disorder improves dramatically while the other is unchanged, that suggests the links between them are weak at best. We were also interested in examining the extent to which BDD symptoms would persist when comorbid disorders improved. We hypothesized that BDD would persist in a meaningful proportion of subjects whose comorbid major depression, OCD, or social phobia remitted, indicating that BDD is not simply a symptom of these disorders. We focused specifically on these three comorbid Axis I disorders because: 1) with the possible exception of substance use disorders, they appear to be the most commonly comorbid Axis I disorders in individuals with BDD (Gunstad & Phillips, 2003; Phillips et al., 2005), 2) there is discussion and controversy in the literature regarding BDD’s relationship to these three disorders (e.g., Phillips, 2001; Phillips et al., 1995), and 3) the above a priori hypothesis about the persistence of BDD pertained specifically to these three disorders.
METHODS
Subjects
Two hundred subjects were enrolled in this ongoing single-site prospective longitudinal study of the course of BDD. Inclusion criteria were DSM-IV BDD or its delusional variant (past or current), age 12 or older, and ability to be interviewed in person. Subjects with delusional BDD were included in our study and analyses because available data indicate that BDD’s delusional and nondelusional variants appear to constitute the same disorder, and they may be double coded according to DSM-IV (Phillips et al., in press). To maximize the generalizability of the study findings, the only exclusion criterion was the presence of a mental disorder, such as mental retardation or dementia, that would interfere with the collection of valid interview data. Subjects were obtained from a variety of sources: mental health professionals (46.0%), advertisements (38.6%), our program website and brochures (10.2%), the subject’s friends or relatives (3.4%) and nonpsychiatrist physicians (1.7%). (For a detailed description of the 200 subjects’ demographic and clinical characteristics at the intake interview, see Phillips et al., 2005). The study was carried out in accordance with the latest version of the Declaration of Helsinki and was approved by the hospital Institutional Review Board. All subjects signed statements of informed consent (assent plus parental consent for adolescents).
The current report presents data for the 161 subjects who met full DSM-IV BDD criteria at intake (n=176) and also had at least 1 year of follow-up data (91.5% of the 176 subjects). To increase power for analyses, we also included all two-year and three-year interview data presently available for analysis (for 109 subjects and 47 subjects, respectively). (Two-year and three-year interview data are not available for all 161 subjects because subjects were enrolled over 2.4 years and therefore currently have varying follow-up durations.) Of the 161 subjects, 70.2% (n=113) were female, and the mean age at intake was 32.9 ± 12.4. 16.4% (n=26) were members of a minority race, and 7.6% (n=12) were of Hispanic ethnicity. 64.0% (n=103) were single, the mean education level was “some college,” and 36.6% (n=59) were employed full time. Average BDD severity at the intake interview was moderate-severe (mean score of 30.2 ± 6.7 on the 48-point Yale-Brown Obsessive Compulsive Scale Modified for BDD) (Phillips et al., 1997). The most common comorbid disorders among the 161 subjects at the intake interview were major depressive disorder (current prevalence of 37.3%, lifetime prevalence of 73.3%), social phobia (32.9% current, 37.9% lifetime), OCD (26.1% current, 33.6% lifetime), a substance use disorder (17.4% current, 48.4% lifetime), specific/simple phobia (16.1% current, 19.9% lifetime), and an eating disorder (including eating disorder NOS) (9.9% current; 34.2% lifetime).
Assessments
Interviews were done by experienced clinical interviewers, who were closely supervised by the first author. Interviewers received extensive and rigorous training, as in similar longitudinal studies (e.g., Goisman et al., 1994). This training includes discussing videotapes, conducting mock interviews with experienced interviewers, and being closely supervised during training sessions and initial interviews. In addition, all interviews were thoroughly edited both clinically and clerically by senior staff. Of relevance to this report, Axis I disorders were evaluated at intake with the Structured Clinical Interview for DSM-IV—Non-Patient Version (SCID-I/NP) (First et al, 2002). The edition used in this study contains screening questions about psychotic symptoms but does not diagnose individual psychotic disorders. Five of the 161 subjects in this report had a comorbid lifetime psychotic disorder, and in no case were these subjects’ BDD symptoms considered to be better accounted for by the psychotic disorder. SCID diagnostic ratings had good agreement, with 100% agreement on the presence/absence of BDD. Percent agreement was 75% for OCD, 77% for social phobia, 83% for an eating disorder, 92% for major depressive disorder, 92% for alcohol abuse/ dependence, and 100% for drug abuse/dependence.
Follow-up interviews were then conducted annually using the Longitudinal Interval Follow-Up Evaluation (LIFE), which collects detailed information on course of illness. The LIFE is a reliable and valid semi-structured interview and rating system for assessing the longitudinal course of mental disorders. It is based on an approach originally used in the NIMH Collaborative Depression Study (e.g., Coryell et al., 1996) and has been used in many other longitudinal studies to track course of illness based on DSM criteria (e.g., Keller et al., 1987; Warshaw et al., 1994). The LIFE obtains information on symptom severity and diagnostic status, and it evaluates course of illness with Psychiatric Status Ratings (PSRs). PSRs are disorder-specific, global ratings of disorder severity with cutpoints for full DSM-IV criteria, partial remission, and full remission (Warshaw et al., 1994). PSRs are assigned for each week of follow-up, providing summaries of course (e.g., symptom improvement or worsening and remission). The range of PSR scores, and cutpoints for remission, for each of the disorders focused on in this report vary slightly. The BDD-PSR ranges from 1-7; a BDD-PSR score of 1 or 2 = full remission, 3 or 4 = partial remission, and 5-7 = full DSM-IV criteria. PSRs for major depression, OCD, and social phobia range from 1-6. For major depression and social phobia, 1 or 2 = full remission, 3 or 4 = partial remission, and 5 or 6 = full DSM-IV criteria. For OCD, 1 or 2 = full remission, 3 = partial remission, and 4-6 = full DSM-IV criteria.
PSRs have been shown to have good interrater reliability, test-retest reliability, and convergent validity (Warshaw et al., 1994). To determine interrater reliability for the disorders focused on in this report, we computed Shrout-Fleiss reliabilities for the maximum and minimum PSR values in our study during the first 8 weeks and the last 8 weeks of a year for each subject. Eight-week spans were used because this time period corresponds to our definition of remission. Mean inter-rater reliabilities were .96 for BDD, 1.0 for major depressive disorder, .74 for OCD, and 1.0 for social phobia. We also examined test-retest reliabilities for the BDD-PSR in our study. The test-retest reliability for the BDD-PSR over 1 year yielded a correlation for maximum BDD-PSRs of 0.79 (p<.0001) and for minimum BDD-PSRs of 0.78 (p<.0001).
Data Analyses
SAS version 8.0 was used for analyses. Subjects were judged to be remitted if they experienced remission as defined by the above PSR scores for at least 8 consecutive weeks. Eight weeks is the remission duration used in many other studies that have used the LIFE and PSR ratings (e.g., Keller et al., 1982). Because the probability of fully remitting from BDD in this study was very low (only .09 over the first year of follow-up), we combined BDD full remissions and partial remissions to increase power for analyses. Full remission was examined for major depression, OCD, and social phobia.
To examine the longitudinal association between improvement in BDD and improvement in each comorbid disorder, we used proportional hazard regression analyses (Allison, 1984; Cox, 1972) with time-varying covariates. This approach enables examination, among subjects with both disorders in a given pair at baseline (e.g., both BDD and major depression), of whether changes in course are correlated – that is, whether improvement in both disorders occurs at around the same time. As described in Shea et al. (2004), use of time-varying predictors allows examination of the course of one disorder subsequent to a change (in this case, improvement) in the other disorder (i.e., the time-varying predictor). As noted above, changes occurring closely in time, while not ruling out other models that may explain comorbidity, are most consistent with the model of shared etiology. In this report, we examined the association of change in BDD with change in the comorbid Axis I disorder. We examined associations in both directions -- i.e., BDD improvement as a predictor of remission of the comorbid Axis I disorder, and improvement in the comorbid Axis I disorder as a predictor of BDD remission. In the prediction of BDD remission, the time-varying predictor was the comorbid Axis I disorder’s PSR rating for the week preceding the time point being analyzed. In the prediction of remission of the comorbid Axis I disorder, the time-varying predictor was the BDD-PSR rating for the week preceding the time point being analyzed. For each pair of disorders being examined, only cases positive at baseline for both disorders were included. Cases positive for a particular disorder at baseline which subsequently remitted in the first week of follow-up (i.e., the first week after intake) were excluded from analyses examining that disorder as the dependent variable, since the value for the predictor variable (preceding week) would not be available.
Hazard (risk) ratios provide an estimate of strength of association between the predictor and dependent variables. The corresponding two-tailed p values from the Cox regression analysis test whether the risk ratio is different from 1.0. A hazard ratio less than 1.0 indicates that improvement (i.e., a lower value) in the predictor variable is associated with a higher likelihood of remission of the disorder that is the dependent variable – i.e., improvement in the “predictor” disorder predicts remission from the “dependent” disorder. The value of the hazard ratio is an estimate of the size of the association: we regard hazard ratios of .50-.67 and 1.5-2.0 as roughly equivalent to a medium effect size, and hazard ratios of less than .50 and greater than 2.0 as roughly equivalent to a large effect size. An alpha level of p < .05 designated statistical significance for all analyses.
RESULTS
Table 1 shows results from the proportional hazard regression analyses for improvement in the comorbid Axis I disorder as a predictor of BDD remission. Table 2 shows the converse: improvement in BDD as a predictor of remission from comorbid major depression, OCD, and social phobia. The results from Tables 1 and 2 are displayed graphically in figures 1-3.
Table 1.
Improvement in Comorbid Axis I Disorders as Predictors of Remission from Body Dysmorphic Disorder a
| Body Dysmorphic Disorder | ||||
|---|---|---|---|---|
| Hazard Ratio | p | Nb | Number Remittingc | |
| Major Depression | .604 | .0006 | 77 | 23 |
| OCD | .506 | .005 | 53 | 12 |
| Social Phobia | .832 | .336 | 66 | 18 |
Remission from BDD includes both partial and full remission
N’s indicate the number of subjects with both BDD and the comorbid disorder
Indicates the number of subjects partially or fully remitted from BDD
Table 2.
Improvement in Body Dysmorphic Disorder as a Predictor of Remission from Comorbid Major Depression, OCD, and Social Phobia a
| Major Depression | OCD | Social Phobia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | p | Nb | Number Remittingc | HR | p | Nb | Number Remittingc | HR | p | N b | Number Remitting c | |
| BDD | .745 | .0028 | 77 | 39 | .878 | .542 | 53 | 20 | .815 | .274 | 66 | 19 |
Remission from major depression, OCD, and social phobia included only full remission
N’s indicate the number of subjects with both BDD and the comorbid disorder
Indicates the number of subjects fully remitting from depression, OCD, or social phobia
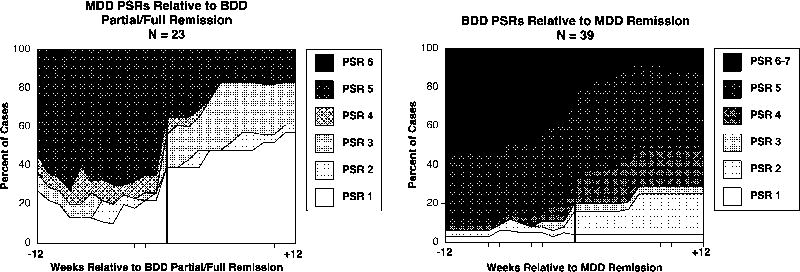
Figure 1.
Time-Varying Course of BDD Versus Major Depression
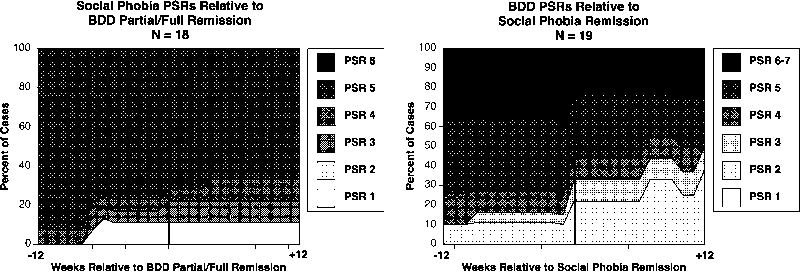
Figure 3.
Time-Varying Course of BDD Versus Social Phobia
As shown in Table 1, improvement in major depression significantly predicted remission from BDD, with a hazard ratio of .604 (p = .0006). Conversely, as shown in Table 2, improvement in BDD significantly predicted remission from major depression (hazard ratio = .745, p = .0028). These results are illustrated graphically in Figure 1. The left side of Figure 1 corresponds to Table 1 and shows changes in depression severity for the 12 weeks preceding BDD remission and for the 12 weeks after BDD remission (in the Figure, BDD remits at week 0). In the 12 weeks preceding BDD remission, depression severity is fairly stable; approximately 60%-70% of subjects meet full criteria for major depression (PSR 5 or 6), and approximately 20% are in full remission from major depression (PSR 1 or 2). Major depression starts improving around the time that BDD remits, and major depression continues to substantially improve after BDD remits. By 3 months after BDD remission, only about 20% of subjects still meet full criteria for major depression, and 61% of subjects are in full remission from depression. The right side of Figure 1 (which corresponds to the data in Table 2) shows the converse: changes in BDD severity before and after remission from major depression. Starting about 4-8 weeks before remission from major depression, BDD starts to gradually improve, and once depression remits, BDD continues to improve. Nonetheless, consistent with our hypothesis that BDD would persist in a substantial proportion of subjects for whom the comorbid disorder remits, 50% of cases still met full BDD criteria (PSR of 5-7) 3 months after remission of depression, and only 25% attained full remission from BDD following remission of depression.
Regarding treatment received, of the 28 subjects who had a remission from both BDD and major depression, 54% (n=15) were not receiving a serotonin-reuptake inhibitor (SRI) at the time of either remission, 32% (n=9) were taking an SRI at the time of both remissions, 7% (n=2) were taking an SRI when they remitted from major depression but not from BDD, and 7% (n=2) were taking an SRI when they remitted from BDD but not from major depression.
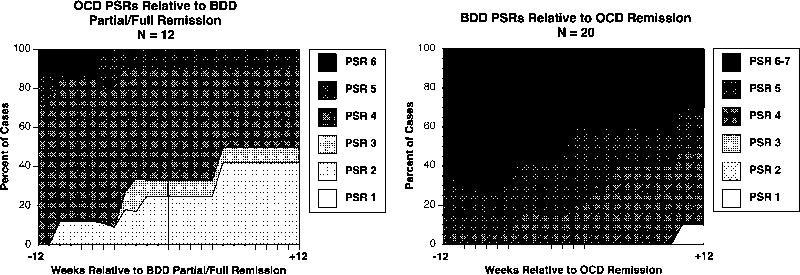
Time-varying analyses of BDD versus OCD and social phobia should be considered more preliminary, because fewer subjects had these disorders at baseline and subsequently remitted from them, and the results may therefore be less numerically stable. As shown in Table 1 and the left side of Figure 2, improvement in OCD significantly predicted BDD remission, with a hazard ratio of .506 (p = .005). However, as shown in Table 2, BDD improvement did not significantly predict OCD remission (hazard ratio = .878, p = .542). Supporting our hypothesis (see right side of Figure 2), 50% of subjects still met full BDD criteria (PSR 5-7) three months after OCD remission, and only about 10% of subjects fully remitted from BDD following OCD remission.
Figure 2.
Time-Varying Course of BDD Versus OCD
As shown in Table 1 and the left side of Figure 3, social phobia severity did not significantly predict BDD remission (hazard ratio = .832, p = .336). Nor did BDD severity significantly predict social phobia remission (hazard ratio = .815, p = .274; see Table 2 and the right side of Figure 3). Consistent with our hypothesis about the persistence of BDD, three months after remission of social phobia, 50% of cases still met full BDD criteria (right side of Figure 3).
DISCUSSION
We found several significant longitudinal associations between BDD and the comorbid disorders we examined. The association was strongest for depression. Time-varying associations showed that improvement in BDD and major depression were closely linked in time, with significant associations in both directions: improvement in major depression predicted BDD remission, and improvement in BDD predicted major depression remission. This finding suggests that the same etiologic processes contribute to both BDD and major depression in some subjects. The left side of Figure 1 further suggests that in our sample depression may be largely secondary to BDD for many subjects, as a substantial proportion remitted from major depression (PSR = 1 or 2) following BDD remission. Conversely, after remission of depression, BDD tended to improve but less markedly (right side of Figure 1).
Our findings additionally suggest that BDD is not simply a symptom of depression. If it were, BDD would be expected to remit around the time of depression remission and during subsequent months. As shown in the right side of Figure 1, during the 3 months after remission of major depression, BDD persisted for most subjects, with fewer than 30% of subjects attaining full remission from BDD (PSR of 1 or 2). This finding is consistent with clinical observations of differences between BDD and depression in terms of the disorders’ clinical features and treatment response, including BDD’s apparent response to SRIs but not to non-SRI antidepressants or electroconvulsive therapy (Hollander et al., 1999; Hollander et al., 1994; Phillips, 1999). In particular, a double-blind cross-over study in BDD found that the SRI clomipramine reduced depressive symptoms more than the non-SRI antidepressant desipramine; this finding suggests that depression in this sample may have been largely secondary to BDD, as depression improved significantly more with a medication that is efficacious for BDD (clomipramine) than with a medication that is usually efficacious for depression (desipramine) (Hollander et al., 1999). It is interesting that in the present study, a majority of subjects were not taking an SRI at the time of either remission, suggesting that the linkage we have observed between remission of BDD and major depression is not fully explained by receipt of medication. However, the relationship between treatment and remission of BDD versus that of comorbid disorders requires further investigation.
Although our results are less numerically stable for OCD, they were significant in only one direction, with improvement in OCD significantly increasing the likelihood of subsequent remission from BDD. This finding suggests that BDD and OCD symptoms may be linked – or that BDD may be secondary to OCD -- for some subjects. However, we found that full-criteria BDD (PSR of 5-7) persisted in approximately half of subjects after OCD remitted (right side of Figure 2), and that only about 10% fully remitted from BDD (PSR of 1 or 2) after OCD remitted, suggesting that BDD is not simply a symptom of OCD. If it were, BDD would be expected to remit in all subjects for whom OCD remitted. Taken together, these findings give some support to the hypothesis that BDD may be related to OCD and is an “OCD-spectrum disorder,” but that BDD and OCD are not identical. This finding is consistent with evidence suggesting that BDD and OCD have many similarities but also some differences (Frare et al., 2004; Phillips et al., 1998). For example, studies have found that BDD patients have poorer insight than those with OCD (Eisen et al., 2004; Simeon et al., 1995), that a higher proportion of BDD patients are unmarried, unemployed, and less educated (Frare et al., 2004), and that BDD patients have a higher prevalence of major depression, social phobia, and suicidal ideation and suicide attempts attributed primarily to their disorder (i.e., BDD or OCD) (Phillips et al., 1998). A small MRI study (n=16) found that BDD subjects had a leftward shift in caudate asymmetry and greater white matter volume than healthy controls, whereas some OCD studies have found the opposite (i.e., a rightward shift in caudate asymmetry and reduced white matter volume) (Rauch et al., 2003). BDD and OCD both appear to respond preferentially to SRIs (Hollander et al., 1999; Phillips, 2001), but preliminary data suggest that unlike OCD, BDD may not respond to SRI augmentation with antipsychotics (Phillips et al., 2005a, 2005b). However, additional research is needed to further examine the nature of BDD’s relationship to OCD.
We did not find significant longitudinal associations with BDD and social phobia. However, power for these analyses was more limited and the results may be less numerically stable than those for major depression. In addition, the absence of longitudinal associations between BDD and social phobia does not rule out the possibility of other causal associations (for example, the predisposing model). Clinical observations (Phillips, 2001) as well as Eastern conceptualizations (Kleinknecht, 1997) suggest that BDD and social phobia may be related disorders, and their relationship deserves further study.
Taken together, these findings suggest that BDD may be etiologically linked to major depression and OCD. Although they suggest that these disorders may have shared etiologic processes, they do not necessarily imply that they are identical disorders. Indeed, this seems unlikely, given that BDD persisted in a sizable proportion of subjects who remitted from the three co-occurring disorders. More than half of subjects who remitted from depression, OCD, or social phobia continued to meet full BDD criteria, and only a minority fully remitted from BDD. While our results are consistent with a model of shared etiologic mechanisms with major depression and OCD, they do not rule out the other models noted above which may potentially explain comorbidity. It is possible that BDD and major depressive disorder are etiologically distinct but produce non-specific overlapping symptoms, such as depressed mood and low self-esteem. The statistical approach used in this report is not as well suited to examining the “predisposing,” or vulnerability, model, as our primary analyses examined the relationship between already co-occurring disorders over only weeks to months. Longer-term prospective studies that examine new onsets of disorders are needed to determine whether the presence of one disorder increases the risk for developing another disorder (for example, whether social phobia predisposes to the subsequent development of BDD—even years later—perhaps by increasing sensitivity to scrutiny by other people). In addition, if one disorder predisposes to the development of a second disorder, the two disorders would not necessarily be expected to improve together, which is a focus of the analytic approach used in this report. Most important, studies of these disorders’ underlying etiopathology are needed to clarify their etiologic relationship to one another and determine which model is most valid. This relationship may be complex. For example, it is possible that multiple mechanisms (models) contribute to the co-occurrence of BDD and certain Axis I disorders. In addition, initial etiologic mechanisms may differ from pathophysiologic factors that are currently maintaining these disorders. It is also possible that there are subtypes of BDD – for example, subtypes that are etiologically linked to major depression or OCD and others that are not.
These findings have several theoretical and clinical implications. BDD’s classification in DSM is controversial and is likely to be addressed during the DSM-V development process (Phillips & Hollander, 1996). Should BDD be classified in a section of “OCD-spectrum” disorders, if such a section is added to DSM? Or should DSM include a category of “affective spectrum disorders” which might include BDD? Or should BDD continue to be classified as a somatoform disorder, despite little evidence that it is closely related to the other somatoform disorders (Phillips et al., 2003)? While more research is needed to answer classification questions such as these – in particular, elucidation of disorders’ underlying etiology and pathophysiology – our results offer some support for the hypothesis that BDD is a member of both the “OCD spectrum” and the “affective spectrum” (Phillips, et al., 1995). A clinical implication of our results is that if BDD improves, major depression tends to improve as well, and vice versa, and that improvement in OCD appears to be associated with subsequent improvement in BDD. However, while these associations are statistically significant, they do not necessarily apply to all individuals with BDD; as shown in the figures, some individuals remain ill after improvement in the comorbid disorder. From a clinical perspective, it seems important to differentiate BDD from major depression and OCD, as our findings suggest that BDD is not identical to these other disorders.
This study has a number of limitations. Because disorders in this study have been unexpectedly chronic, relatively few remissions occurred, which may result in some numerical instability for results for OCD and social phobia. In addition, with the existing sample it is possible only to detect larger effects. Additional studies with a larger sample or longer follow-up periods are needed to detect smaller effects, obtain more stable results, and examine less commonly comorbid disorders. The finding on the course of OCD relative to BDD remission is in particular need of replication because of the small sample size. Another study limitation is that because BDD was unusually chronic, we examined associations for partial or full BDD remission rather than full remission alone. Also, it is unclear how representative our sample is of individuals with BDD. Although we attempted to obtain a broad and diverse sample, it is a sample of convenience and may have unknown biases. Additional studies are needed in both clinical and epidemiologic samples. Also, although symptoms of BDD and comorbid disorders were rated for each week of the follow-up period, interviews covered the previous 12 months. Efforts were made to precisely determine the timing of symptom change, but the interviewers necessarily relied on subject’s memories of symptom changes over the previous year. Our test-retest reliability study of retrospective BDD-PSR ratings showed good agreement over a year (see above), but more frequent assessments might provide more accurate data. Finally, while changes in comorbid disorders occurring closely in time suggest that the disorders have a shared etiology (in the sense of underlying risk factors or an interaction between the disorders), they do not prove this. Family, twin, and adoption studies, and – most important -- studies of etiology and pathophysiology (e.g., underlying neurobiology) are needed to elucidate the nature of disorders’ relationship to one another (Hyman, 2003; Phillips et al., 2003; Lyons et al., 1997).
This study also has a number of strengths. The sample is larger and more diverse than most previous BDD samples, and it is to our knowledge the first prospective study of BDD’s course and the first to examine longitudinal associations between BDD and comorbid disorders. Course of illness was assessed using a standard and widely used method, which has the advantage of tracking symptom severity at weekly intervals. Additional research is needed to address this study’s limitations. As this ongoing study continues to accrue more data, the number of remissions of BDD and comorbid disorders will increase, which will increase power for analyses of the disorders in this report and other comorbid disorders. Longer durations of follow-up will also increase power to examine time-varying associations between BDD and the recurrence and new onsets of comorbid Axis I disorders. Longer follow-up will also enable further examination of possible course-based subtypes of BDD. For example, are the course subtypes we have observed -- for example, cases for whom BDD and major depression both seem to improve together versus those that seem to change independently -- consistent over time? For example, if BDD and major depression improve together, do they also relapse together? Do these subtypes respond similarly to treatment? And do subjects who remit from BDD when their comorbid OCD or major depression remit differ (e.g., in terms of demographic and clinical characteristics) from subjects who do not remit from BDD? Longitudinal studies that examine time-varying associations are also needed in other samples. Such research will shed important light on BDD’s relationship to commonly comorbid disorders, which has important implications for BDD’s classification and for patient care.
Acknowledgments
This study was supported by R01-MH60241 from the National Institute of Mental Health to Dr. Phillips. We thank William Menard and Christina Fay for their participation in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison P. Event history analysis: regression for longitudinal event data. Beverly Hills, CA: Sage Publications; 1984. [Google Scholar]
- Bienvenu OJ, Samuels JF, Riddle MA, Hoehn-Saric R, Liang KY, Cullen BA, Grados MA, Nestadt G. The relationship of obsessive-compulsive disorder to possible spectrum disorders: results from a family study. Biological Psychiatry. 2000;48:287–293. doi: 10.1016/s0006-3223(00)00831-3. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Wilhelm S, McNally RJ, Tuschen-Caffier B, Baer L, Jenike MA. Interpretive biases for ambiguous information in body dysmorphic disorder. CNS Spectrums. 2002;7:435–443. doi: 10.1017/s1092852900017946. [DOI] [PubMed] [Google Scholar]
- Cohen L, Hollander E. Obsessive-compulsive spectrum disorders. In: Stein DJ, editor. Obsessive-Compulsive Disorders. New York: Marcel Dekker Inc; 1997. [Google Scholar]
- Coryell W, Leon A, Winokur G, Endicott J, Keller M, Akiskal H, Solomon D. Importance of psychotic features to long-term course in major depressive disorder. American Journal of Psychiatry. 1996;153:483–489. doi: 10.1176/ajp.153.4.483. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life tables. Journal of the Royal Statistical Society Series B. 1972;34:187–220. [Google Scholar]
- Eisen JL, Phillips KA, Coles ME, Rasmussen SA. Insight in obsessive compulsive disorder and body dysmorphic disorder. Comprehensive Psychiatry. 2004;45:10–15. doi: 10.1016/j.comppsych.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version (SCID-I) [Google Scholar]
- Frare F, Perugi G, Ruffolo G, Toni C. Obsessive-compulsive disorder and body dysmorphic disorder: a comparison of clinical features. European Psychiatry. 2004;19:292–298. doi: 10.1016/j.eurpsy.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Goisman RM, Warshaw MG, Peterson LG, Rogers MP, Cuneo P, Tomlin-Albanese JM, Kazin A, Gollan J, Epstein-Kaye T, Reich JH, Keller MB. Panic, agoraphobia, and panic with agoraphobia: data from a multi-center anxiety disorder study. Journal of Nervous and Mental Disease. 1994;182:72–79. doi: 10.1097/00005053-199402000-00002. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Phillips KA. Axis I comorbidity in body dysmorphic disorder. Comprehensive Psychiatry. 2003;44:270–276. doi: 10.1016/S0010-440X(03)00088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Allen A, Kwon J, Aronowitz B, Schmeidler J, Wong C, Simeon D. Clomipramine vs desipramine crossover trial in body dysmorphic disorder. Archives of General Psychiatry. 1999;56:1033–1039. doi: 10.1001/archpsyc.56.11.1033. [DOI] [PubMed] [Google Scholar]
- Hollander E, Cohen L, Simeon D, Rosen J, DeCaria C, Stein DJ. Fluvoxamine treatment of body dysmorphic disorder (letter) Journal of Clinical Psychopharmacology. 1994;14:75–77. [PubMed] [Google Scholar]
- Hyman S. Foreward. In: Phillips KA, First MB, Pincus H, editors. Advancing DSM: Dilemmas in Psychiatric Diagnosis. Washington DC: American Psychiatric Publishing Inc; 2003. [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Keller MB, Shapiro RW, Lavori PW, Wolfe N. Recovery in major depressive disorder: analysis with the life table and regression models. Archives of General Psychiatry. 1982;39:905–910. doi: 10.1001/archpsyc.1982.04290080025004. [DOI] [PubMed] [Google Scholar]
- Kleinknecht RA, Dinnel DL, Kleinknecht EE, Hiruma N, Harada N. Cultural factors in social anxiety: A comparison of social phobia symptoms and Taijin Kyofusho. Journal of Anxiety Disorders. 1997;11:157–177. doi: 10.1016/s0887-6185(97)00004-2. [DOI] [PubMed] [Google Scholar]
- Lyons MH, Tyrer P, Gunderson J, Tohen M. Heuristic models of comorbidity of axis I and axis II disorders. Journal of Personality Disorders. 1997;11:260–269. doi: 10.1521/pedi.1997.11.3.260. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Phillips KA, Petersen TJ, Kelly KE, Alpert JE, Worthington JJ, Tedlow JR, Rosenbaum JF, Fava M. Body dysmorphic disorder in outpatients with major depression. Journal of Affective Disorders. 2002;69:141–148. doi: 10.1016/s0165-0327(01)00304-4. [DOI] [PubMed] [Google Scholar]
- Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. American Journal of Psychiatry. 2001;158:2061–2063. doi: 10.1176/appi.ajp.158.12.2061. [DOI] [PubMed] [Google Scholar]
- Perugi G, Akiskal HS, Lattanzi L, Cecconi D, Mastrocinque C, Patronelli A, Vignoli S, Bemi E. The high prevalence of “soft” bipolar (II) features in atypical depression. Comprehensive Psychiatry. 1998;39:63–71. doi: 10.1016/s0010-440x(98)90080-3. [DOI] [PubMed] [Google Scholar]
- Phillips KA. Body dysmorphic disorder and depression: theoretical considerations and treatment strategies. Psychiatric Quarterly. 1999;70:313–331. doi: 10.1023/a:1022090200057. [DOI] [PubMed] [Google Scholar]
- Phillips KA. Somatoform and factitious disorders. In: Oldham JM, Riba MB, editors. Review of Psychiatry Series. Washington DC: American Psychiatric Publishing; 2001. [Google Scholar]
- Phillips KA. Placebo-controlled study of pimozide augmentation of fluoxetine in body dysmorphic disorder. American Journal of Psychiatry. 2005a;162:377–379. doi: 10.1176/appi.ajp.162.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA. Olanzapine augmentation of fluoxetine in body dysmorphic disorder (letter) American Journal of Psychiatry. 2005b;162:1022–1023. doi: 10.1176/appi.ajp.162.5.1022-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Gunderson CG, Mallya G, McElroy SL, Carter W. A comparison study of body dysmorphic disorder and obsessive compulsive disorder. Journal of Clinical Psychiatry. 1998;59:568–575. doi: 10.4088/jcp.v59n1102. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E. Body dysmorphic disorder. In: Widiger TA, Frances AJ, Pincus HA, Ross R, First MB, Davis WW, editors. DSM-IV Sourcebook. Vol. 2. Washington, DC: American Psychiatric Association; 1996. [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin. 1997;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, McElroy SL. Personality disorders and traits in patients with body dysmorphic disorder. Comprehensive Psychiatry. 2000;41:229–236. doi: 10.1053/comp.2000.7429. [DOI] [PubMed] [Google Scholar]
- Phillips KA, McElroy SL, Hudson JI, Pope HG., Jr Body dysmorphic disorder: an obsessive compulsive spectrum disorder, a form of affective spectrum disorder, or both? Journal of Clinical Psychiatry. 1995;56:41–52. [PubMed] [Google Scholar]
- Phillips KA, Menard W, Fay C, Weisburg R. Demographic characteristics, phenomenology, comorbidity, and family history in 200 individuals with BDD. Psychosomatics. 2005;46:317–332. doi: 10.1176/appi.psy.46.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Menard W, Pagano M, Fay C, Stout RL. Delusional versus nondelusional body dysmorphic disorder: clinical features and course of illness. Journal of Psychiatric Research. doi: 10.1016/j.jpsychires.2005.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Nierenberg AA, Brendel G, Fava M. Prevalence and clinical features of body dysmorphic disorder in atypical major depression. Journal of Nervous and Mental Disease. 1996;184:125–129. doi: 10.1097/00005053-199602000-00012. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Price LH, Greenberg BD, Rasmussen SA. Should DSM’s diagnostic groupings be changed? In: Phillips KA, First MB, Pincus H, editors. Advancing DSM: Dilemmas in Psychiatric Diagnosis. Washington DC: American Psychiatric Publishing Inc; 2003. [Google Scholar]
- Rauch SL, Phillips KA, Segal E, Makris N, Shin LM, Whalen PJ, Jenike MA, Caviness VS, Jr, Kennedy DN. A preliminary morphometric magnetic resonance imaging study of regional brain volumes in body dysmorphic disorder. Psychiatry Research: Neuroimaging. 2003;20:13–19. doi: 10.1016/s0925-4927(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Shea MT, Stout RL, Yen S, Pagano ME, Skodol AE, Morey LC, Gunderson JG, McGlashan TH, Grilo CM, Sanislow CA, Bender DS, Zanarini MC. Associations in the course of personality disorders and Axis I disorders over time. Journal of Abnormal Psychology. 2004;113:499–508. doi: 10.1037/0021-843X.113.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeon D, Hollander E, Stein DJ, Cohen L, Aronowitz B. Body dysmorphic disorder in the DSM-IV field trial for obsessive-compulsive disorder. American Journal of Psychiatry. 1995;152:1207–1209. doi: 10.1176/ajp.152.8.1207. [DOI] [PubMed] [Google Scholar]
- Veale D, Boocock A, Gournay K, Dryden W, Shah F, Willson R, Walburn J. Body dysmorphic disorder: A survey of fifty cases. British Journal of Psychiatry. 1996;169:196–201. doi: 10.1192/bjp.169.2.196. [DOI] [PubMed] [Google Scholar]
- Warshaw MG, Keller MB, Stout RL. Reliability and validity of the Longitudinal Interval Follow-Up Evaluation for assessing outcome of anxiety disorders. Journal of Psychiatric Research. 1994;28:531–545. doi: 10.1016/0022-3956(94)90043-4. [DOI] [PubMed] [Google Scholar]