Abstract
Black currant is an important material for food industry, but little research has been reported on the isolation of phenolic acids because of their low content. In present study, high-speed countercurrent chromatography (HSCCC) has been successfully used for the preparative isolation of the minor phenolic compounds from the ethyl acetate extracts of black currant fruit. The HSCCC separation was performed with a two-phase solvent system composed of n-hexane/EtOAc/MeOH/H2O (5:15:4:7 v/v) at a flow rate of 1.5 mL/min. From 500 mg crude sample 0.8 mg of protocatechuic acid, 1.0 mg of caffeic acid, 0.5 mg of 4-hydroxybenzoic acid and 2.5 mg of myricetin were purified by one-step HSCCC operation,. Their chemical structures were confirmed by MS and NMR.
Keywords: Black currant, High-speed countercurrent chromatography (HSCCC), Protocatechuic acid, Caffeic acid, 4-Hydroxybenzoic acid, Myricetin
INTRODUCTION
Black currant (Ribes nigrum L.) is an important plant material for food industry for preparing juice, liquors, jams and other food products because of its distinctive color and high antioxidant content.[1] The fruits of black currant (Ribes nigrum L.) have an array of phenolic compounds, including anthocyanins, phenolic acids and flavonoids. Anthocyanins are the major group of phenolics (250 mg/100 g of fresh fruit) which contribute about 80% to the total compounds,[2] and have attracted much attention during the past decades. The study on phenolic acids and flavonoids of black currant is scarce due to their low content: caffeic acid (~10.6mg/kg, dry weight), protocatechuic acid (~7.1mg/kg, dry weight), 4-hydroxybenzoic acid (~3.2mg/kg, dry weight).[3] These phenolic acids were reported to have biological activities such as antioxidant and antimutagenic activities.[4, 5] Myricetin was reported to have health-promoting effects such as anticarcinogenic, antiviral activities, cytoprotective capacity, and therapeutic benefit of cardiovascular diseases.[6–9] Phenolic acids and myricetin probably play an important role in the biological activity of black currant. Therefore, it is necessary to isolate and identify these minor constituents for further investigation of their molecular mechanism about biological activities or pharmacological functions.
Up to now, it is still a challenge to separate minor compounds by the conventional chromatographic methods such as column chromatography, and the overall yield of these methods is usually poor because the target compounds tend to be irreversibly adsorbed onto the solid support during separation., High-speed countercurrent chromatography (HSCCC) which uses no solid support has a great advantage over the conventional liquid-solid methods since it gives near 100% sample recovery. This unique technique has been widely applied for the separation and purification of various natural compounds.[10–13] The present study describes successful isolation of minor phenolic acids and myricetin from black currant fruit using HSCCC with an optimized two-phase solvent system composed of n-hexane/ethyl acetate/methanol/water (5:15:4:7 v/v) at a flow rate of 1.5 mL/min. The chemical structures of these compounds were elucidated by ESI-MS, 1H NMR and 13C NMR. As far as we know, this is the first report about the isolation of minor phenolic compounds from black currant using HSCCC.
EXPERIMENTAL
Apparatus
The preparative HSCCC instrument employed in this study was a model TBE-300A high-speed countercurrent chromatograph (Tauto Biotech, Shanghai, China) with a set of three polytetrafluoroethylene (PTFE) preparative coils (internal diameter of tubing: 2.6 mm; total column capacity: 290 mL). The revolution radius or the distance between the holder axis and the central axis of the centrifuge (R) was 5 cm, and the β-value varied from 0.5 at the internal terminal to 0.8 at the external terminal (β = r/R, where r is the distance from the coil to the holder shaft). An optimum speed of 850 rpm was used in this study. The solvent was pumped into the column with a model TBP-50A constant-flow pump (Tauto Biotech, Shanghai, China). Continuous monitoring of the effluent was achieved with a Model 8823A-UV monitor at 254 nm, and a manual sample injection valve with a 20-ml loop for the preparative HSCCC was used to introduce the sample into the column. Model N2000 workstation (Zhejiang University, Hangzhou, China) was used to draw the chromatogram.
The high-performance liquid chromatography equipment (DIONEX, USA) used was a DIONEX system including a P680 pump, an ASI-100 Automated sample injector, a TCC-100 thermostatted column compartment, and a UVD170U detector. The analysis was carried out with an Inertsil ODS-SP column (5 µm, 4.6 × 250 mm GL Sciences Inc, Japan). Evaluation and quantification were made on a Chromeleon WorkStation.
Reagents
All organic solvents used for HSCCC were of analytical grade and purchased from Tianjin chemical Factory (Tianjin, China). Methanol and acetonitrile used for HPLC were of HPLC-grade and purchased from Fisher Scientific Company (Park Lawn, NJ, USA). Sephadex LH-20 gel was purchased from Pharmacia Fine Chemicals Inc. (Nanjing, China).
The dry ripe black currant fruit was purchased from Emin County, Xinjiang, China in August, 2008.
Preparation of Crude Sample
Dry black currant berries (1 kg) were extracted with 5 L of methanol containing 0.1% trifluoroacetic acid for 24 h. The extraction process was repeated three times. After concentration under reduced pressure, the extract was diluted with water to a total volume of 1L and partitioned with petroleum ether (boiling point: 60–90°C) (3 × 1 L) first, and then the water solution was extracted with ethyl acetate (3 × 1L) to give ethyl acetate fraction. This fraction was concentrated by a rotary evaporator at 40°C yielding 9.8 g crude sample which was used for further HSCCC separation and purification.
Measurement of Partition Coefficient (K)
The two-phase solvent system was selected according to the partition coefficient (K) of the target components. Different volume ratios of n-hexane-ethyl acetate-methanol-water were prepared and equilibrated in a separation funnel at room temperature. The K values were determined by HPLC analysis as follows: a suitable amount of samples (1mg) was added to 4.0 mL of the mixture of equal volume of each phase of the two-phase solvent system followed by thorough mixing. After equilibration was established, the upper phase and the lower phase were each analyzed by HPLC. The peak area corresponding to the target compounds obtained from the upper phase was recorded as AU and that from the lower phase was recorded as AL. The K value was calculated according to the following equation: K=AU/AL.
HSCCC Separation
The preparative HSCCC was similarly performed with a model TBE-300A HSCCC instrument as follows: the multiplayer coiled column was first entirely filled with the upper phase as stationary phase. The lower phase was then pumped into the head end of the column at a given flow rate, while the apparatus was run at a revolution speed of 850 rpm. When hydrodynamic equilibrium had been reached, the sample solution (500 mg of the crude sample in 4 mL of a mixture of upper and lower phases) was injected through the sample port. The effluent from the outlet of the column was monitored with a UV detector at 254 nm. Peak fractions were manually collected according to the chromatogram.
HPLC Analysis and Identification of Crude Sample and HSCCC Peak Fractions
The crude sample and the peak fraction from HSCCC were analyzed by HPLC. The analyses were performed with an Inertsil ODS-SP column (4.6 mm × 250 mm, 5 µm) at column temperature of 35°C. The mobile phase was a linear gradient of acetonitrile (A), methanol (B) and 0.2% formic acid (C) as follows: A-B-C (7.5:7.5:85, v/v ) to A-B-C (17.5:17.5:65, v/v ) in 40 min, then to A-B-C (38:37:25, v/v ) in 20 min. The flow-rate was 1.0 mL/min and the effluent was monitored at 254 nm by a UV detector.
Identification of the HSCCC peak fractions was carried out by MS, 1H- and 13C-NMR.
RESULTS AND DISCUSSION
HPLC Analysis of Crude Sample
The crude extract of black currant was analyzed by HPLC first. The result indicated that it contained several flavonoids, including protocatechuic acid (retention time: 7.58 min), caffeic acid (retention time: 14.04 min), 4-hydroxybenzoic acid (retention time: 11.59 min) and myricetin (retention time: 37.09 min) with some other unknown compounds, as shown in Figure 1.
Figure 1.
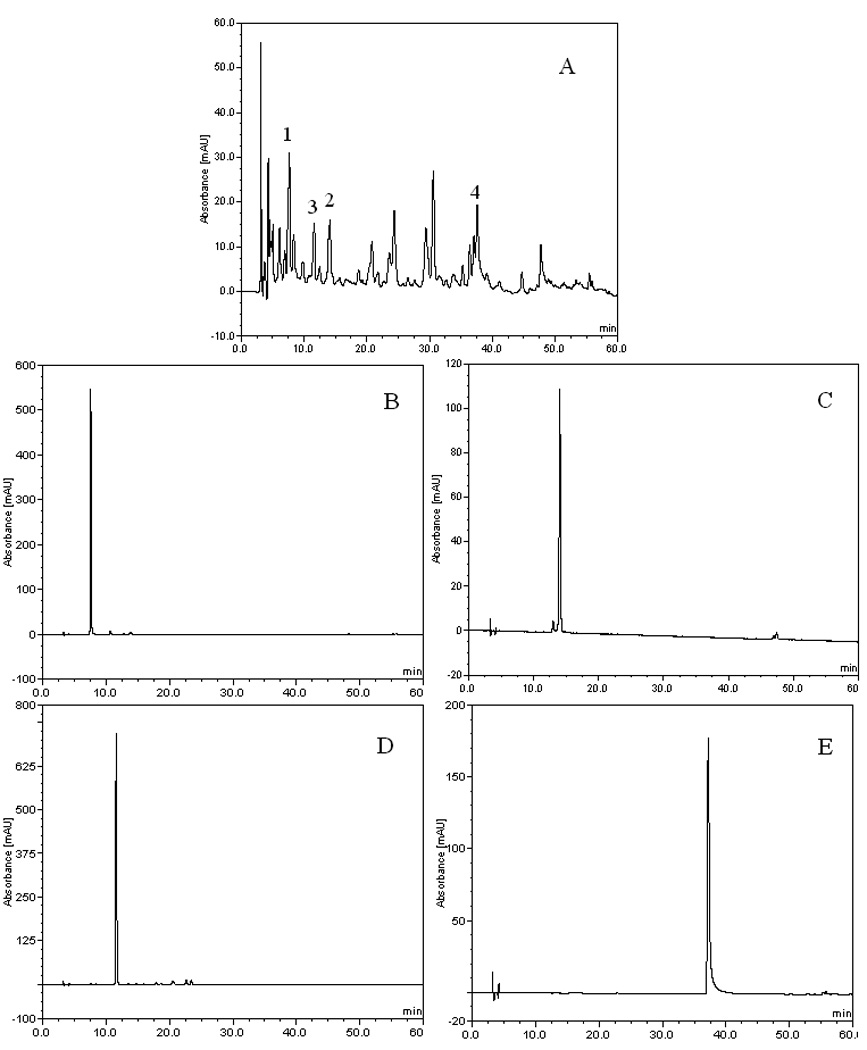
HPLC chromatogram of the acetic ether fraction and HSCCC fractions from black currant. Column: inertsil ODS-SP column (4.6 mm × 250 mm, 5 µm); the mobile phase A: acetonitrile, B: methanol, C: 0.2% formic acid, the gradient was as follows: A-B-C (7.5:7.5:85, v/v ) to A-B-C (17.5:17.5:65, v/v ) in 40 min, then to A-B-C (38:37:25, v/v ) in 20 min. detection: 254 nm; flow rate: 1.0 mL/min. (A) acetic ether fraction from black currant, (B) protocatechuic acid (1), (C) caffeic acid (2), (D) 4-hydroxybenzoic acid (3), (E) myricetin (4).
Selection of Two-Phase Solvent System and Other Conditions of HSCCC
In HSCCC, successful separation depends upon the selection of a suitable two-phase solvent system, which requires the following considerations: retention of the stationary phase should be satisfactory which is attained with the short settling time of the solvent system (<25 sec) and the partition coefficient of the target compound is between 0.4 – 2.5. [14, 15] A smaller K value elutes the solute closer to the solvent front with lower resolution while a larger K value tends to give better resolution but broader, more dilute peaks with a longer elution time.[16] Because the crude sample was obtained from the complex natural product, the larger K value (<3.5) can be accepted. Although it will tend to give the longer elution time and broader peak, better resolution is necessary to separate complex crude samples.
Several reviews and chapters in the cited references listed various two-phase solvent systems successfully used for CCC. [11, 17] According to the properties of phenolic acids, we chose the two-phase solvent systems of n-hexane-ethyl acetate-methanol-water based on these references. In this study, different volume ratios of n-hexane-ethyl acetate-methanol-water were prepared and tested. At last, the two-phase solvent system of n-hexane-ethyl acetate-methanol-water (5:15:4:7, v/v) was found to be suitable for the separation of phenolic compounds of black currant. In this system, the HSCCC run yielded four separate peaks which were combined into fraction I, II, III, IV, corresponding to compound 1, 2, 3, 4, respectively as shown in Figure 2, and their K values were 0.71, 1.06, 1.75 and 3.08, respectively.
Figure 2.
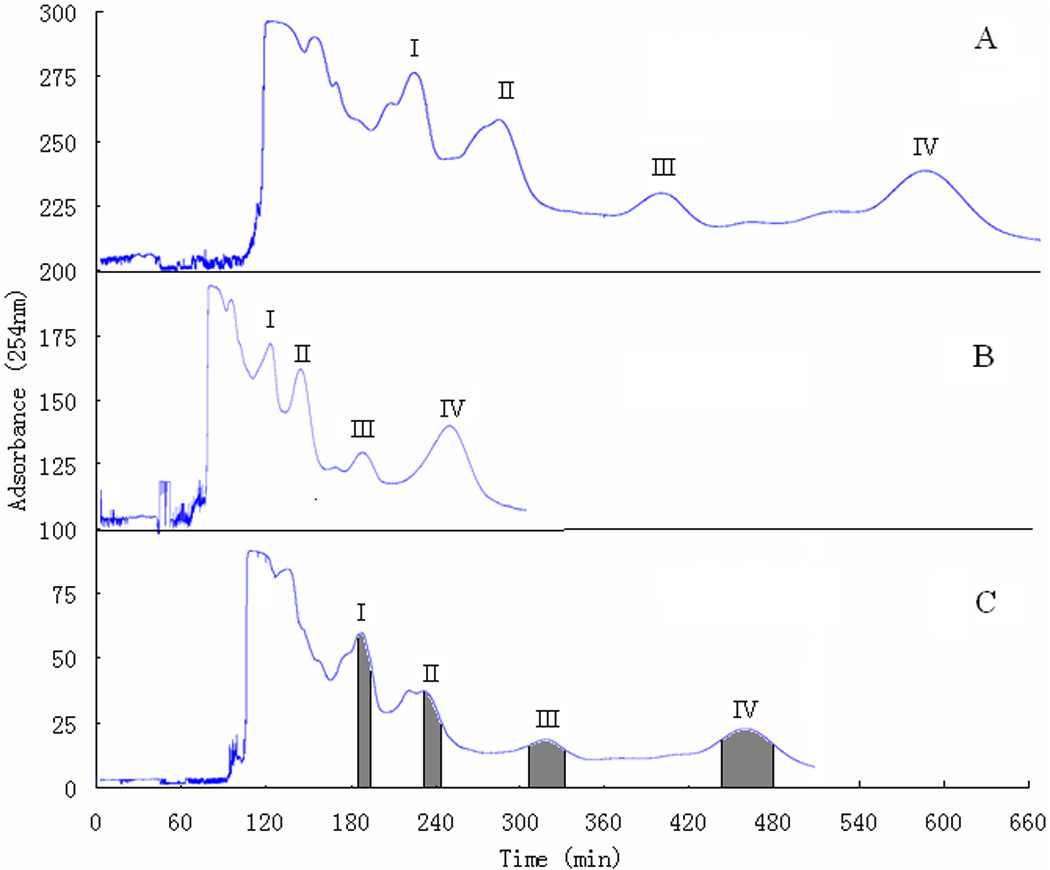
Comparative analysis of HSCCC chromatograms of 500 mg of the crude sample of black currant, at different flow rates: (A) 1.0 mL/min, (B) 2.0 mL/min, (C) 1.5 mL/min. Two-phase solvent system: n-hexane/EtOAc/MeOH/H2O (5:15:4:7 v/v); stationary phase: upper organic phase; rotation speed: 850 rpm; detection wavelength: 254 nm; peaks I, II, III, IV corresponding to the following compounds: protocatechuic acid (1), caffeic acid (2), 4-hydroxybenzoic acid (3), myricetin (4), respectively.
In order to obtain good separation peak resolution within acceptable separation time, other factors such as sample size and flow rate were also investigated. When the amount of sample was 300 – 500 mg, good separation was obtained. The compound 1 – 4 could be successfully separated from the crude sample. However, when the amount of sample exceeded 500 mg, the resolution of peak I and II decreased. Then the flow rate of mobile phase was optimized to shorten the separation time. As shown in Figure 2A, at the flow rate of 1.0 mL/min peaks I – IV were eluted within 680 minutes with the retention of stationary phase at 65.5%. When the flow rate was increased to 2.0 mL/min, the whole retention time was shortened to 310 minutes (Figure 2B) while the retention of stationary phase reduced to 57.9% and the resolution of peaks I and II also decreased where compound 1 and impurity peaks were overlapped thoroughly. So was peak of compound 2. However, when the flow rate was set at 1.5 mL/min both the retention time and the resolution were acceptable (Figure 2C). The overall results of the above studies showed that when the amount of sample was 500 mg, good separation could be obtained at a flow rate of 1.5 mL/min.
HSCCC Separation
The HSCCC run yielded four fractions: I (185 – 195 min), II (231 – 247 min), III (308 – 332 min), IV (440 – 480 min), corresponding to compound 1, 2, 3, 4, respectively, as shown in Figure 2C where 0.8 mg of compound 1, 1.0 mg of compound 2, 0.5 mg of compound 3, 2.5 mg of compound 4 were yielded from 500 mg of crude sample in one-step separation. The purity of compound 1, 2, 3, and 4 was 89, 94, 95, and 96%, respectively, which was determined by HPLC, as shown in Figure 1. Since the amounts of 1 and 3 were relatively low, the HSCCC separation was repeated three times, and 3.2 mg of 1 and 2.0 mg of 3 were obtained from 2.0 g of crude sample. Compound 1 was further purified to 96% (Figure 1B) by Sephadex LH-20 column chromatography (1 × 50 cm), eluted by methanol at a flow rate of 5.0 mL/h. Compounds 1, 2, 3, and 4 were determined to be protocatechuic acid, caffeic acid, 4-hydroxybenzonic acid and myricetin, respectively, by ESI–MS and NMR analysis.
Structural Identification
The identification of compounds 1, 2, 3 and 4 was carried out by MS, 1H- and 13C- NMR as follows:
Protocatechuic acid (3, 4-dihydroxybenzoic acid) (1): ESI-MS, m/z: 153 [M-H]−. 1H-NMR (600 MHz, CD3OD) δ ppm: 7.44 (1H, d, J=1.2 Hz, H-2), 7.41 (1H, dd, J=7.8 Hz, 1.2 Hz, H-6), 6.79 (1H, d, J=7.8 Hz, H-5). 13C-NMR (150 MHz, CD3OD) δ ppm: 168.85 (C=O), 150.10 (C-4), 144.63 (C-3), 122.47 (C-6), 121.69 (C-1), 116.29 (C-2), 114.33 (C-5). The 1H NMR and 13C NMR spectral data were in agreement with literature.[18, 19]
Caffeic acid (2): ESI-MS, m/z: 179 [M-H]−. 1H NMR (600MHz, CD3OD) δ ppm: 7.41 (1H, d, J =15.6 Hz, H-7), 6.93 (1H, d, J = 1.8 Hz, H-2), 6.83 (1H, dd, J = 8.4, 1.8Hz, H-6), 6.67 (1H, d, J = 8.4 Hz, H-5), 6.12 (1H, d, J = 15.6 Hz, H-8). 13C NMR (150 MHz, CD3OD) δ ppm: 170.04 (C=O), 147.91 (C-3), 145.36 (C-4), 145.15 (C-7), 126.48 (C-1), 121.32 (C-6), 115.03 (C-5), 114.72 (C-8), 113.58 (C-2). The 1H NMR and 13C NMR spectral data were in agreement with literature.[20, 21]
4-Hydroxybenzoic acid (3): ESI-MS, m/z: 137 [M-H]−, 1H NMR (600 MHz, CD3OD) δ ppm: 7.87 (2H, d, J = 8.4 Hz, H-2, 6), 6.81 (2H, d, J = 8.4 Hz, H-3, 5). 13C NMR (150 MHz, CD3OD) δ ppm: 168.75 (C=O), 161.90 (C-4), 131.57 (C-2, 6), 121.41 (C-1), 114.57 (C-3, 5). The 1H NMR and 13C NMR spectral data were in agreement with literature.[19]
Myricetin (4): ESI-MS, m/z: 317 [M-H]−, 1H NMR(600 MHz, DMSO-d6) δ ppm :7.24 (2H, s, H-2′, 6′), 6.37 (1H, d, J = 1.8 Hz, H-8), 6.18 (1H, d, J = 2.4 Hz, H-6). 13C NMR (150MHz, DMSO-d6) δ ppm: 175.71 (C-4), 164.08 (C-7), 160.69 (C-5), 156.07 (C-9), 146.75 (C-2), 145.73 (C-3′, 5′), 135.88 (C-3), 135.84 (C-4′), 120.74 (C-1′), 107.11 (C-2′, 6′), 102.86 (C-10), 98.20 (C-6), 93.21 (C-8). The 1H NMR and 13C NMR spectral data were in agreement with literature.[24]
CONCLUSIONS
High-speed counter-current chromatography (HSCCC) is successfully applied for separation and purification of four minor phenolic acids including protocatechuic acid, caffeic acid, 4-hydroxybenzoic acid and myricetin from black currant using a two-phase solvent system composed of n-hexane/EtOAc/MeOH/H2O (5:15:4:7, v/v). The results demonstrated that HSCCC is a powerful technique for the isolation of minor compounds from crude plant extracts.
Figure 3.
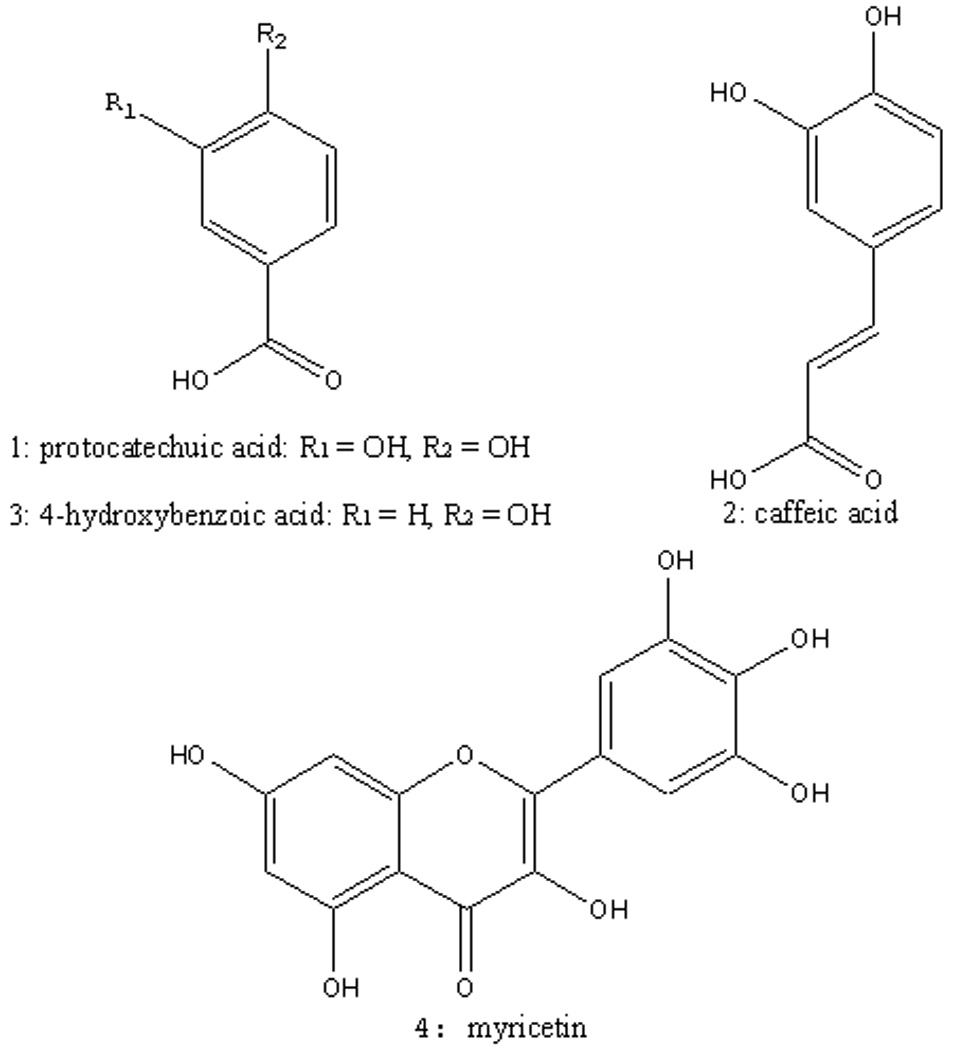
The chemical structures of compounds 1–4 from black currant isolated by HSCCC.
ACKNOWLEDGEMENTS
This work was financially supported by a grant (code: KSCX2-YWR-132) from Key Project of Knowledge Innovation Program of Chinese Academy of Sciences and another grant from the International Partnership Program for Creative Teams “Study on Bioactive Ingredients and Application of Arid Region Edible Plant” of Chinese Academy of Sciences and the State Administration of Foreign Experts Affairs.
REFERENCES
- 1.Heinonen IM, Lehtonen PJ, Hopia AI. Antioxidant activity of berry and fruit wines and liquors. J. Agric. Food Chem. 1998;46:25–31. doi: 10.1021/jf970489o. [DOI] [PubMed] [Google Scholar]
- 2.Koeppen BH, Herrmann K. Flavonoid glycosides and hydroxycinnamic acid esters of blackcurrants (Ribes nigrum). Phenolics of fruits 9. Zeitschrift für Lebensmitteluntersuchung und -Forschung A. 1977;164:263–268. doi: 10.1007/BF01147302. [DOI] [PubMed] [Google Scholar]
- 3.Ryszard Z, Marian N, Jaroslaw N. Phenolic acid profiles in some small berries. J. Agric. Food Chem. 2005;53(6):2118–2124. doi: 10.1021/jf040411p. [DOI] [PubMed] [Google Scholar]
- 4.Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002;50:2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 5.Yamada J, Tomita Y. Antimutagenic activity of caffeic acid and related compounds. Bioscience, Biotechnology, and Biochemistry. 1996;60:328–329. doi: 10.1271/bbb.60.328. [DOI] [PubMed] [Google Scholar]
- 6.Ko CH, Shen SC, Lee TJ, Chen YC. Myricetin inhibits matrix metalloproteinase 2 protein expression and enzyme activity in colorectal carcinoma cells. Molecular Cancer Therapeutics. 2005;4(2):281–290. [PubMed] [Google Scholar]
- 7.Ono K, Nakane H, Fukushima M, Chermann JC, Barre Sinoussi F. Differential inhibitory effects of various flavonoids on the activities of reversetranscriptase and cellular DNA and RNA polymerases. European Journal of Biochemistry. 1990;190(3):469–476. doi: 10.1111/j.1432-1033.1990.tb15597.x. [DOI] [PubMed] [Google Scholar]
- 8.Dajas F, Rivera F, Blasina F, Arredondo F, Echeverry C, Lafon L, Morquio A, Heizen H. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotoxicity Research. 2003;5(6):425–432. doi: 10.1007/BF03033172. [DOI] [PubMed] [Google Scholar]
- 9.Ong KC, Khoo HE. Biological effects of myricetin. General Pharmacology. 1997;29(2):121–126. doi: 10.1016/s0306-3623(96)00421-1. [DOI] [PubMed] [Google Scholar]
- 10.Gu DY, Yang Y, Zhong J, Aisa HA, Zhang TY. High-Speed Counter-Current Chromatography Combined with Column Chromatography for Isolation of Methyllycaconitine from Delphinium pseudocyanthum. Chromatographia. 2007;66:949–951. [Google Scholar]
- 11.Ito Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter current chromatography. J. Chromatogr. A. 2005;1065(2):145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Gu DY, Wu HK, Aisa HA, Zhang TY, Ito Y. Application of preparative high-speed countercurrent chromatography for separation of Elatine from Delphinium shawurense. J. Liq. Chromatog. & Rel. Technol. 2008;31(19):3012–3019. doi: 10.1080/10826070802424956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Wu T, Yang WX, Aisa HA, Zhang TY, Ito Y. Preparative isolation and purification of four flavonoids from Flos Gossypii by high-speed countercurrent chromatography. J. Liq. Chromatog. & Rel.Technol. 2008;31(10):1523–1531. [Google Scholar]
- 14.Oka H, Suzuki M, Harada K, Iwaya M, Fujii K, Goto T, Ito Y, Matsumoto H, Ito Y. Purification of Food Color Red No. 106 (acid red) using pH-zone-refining counter-current chromatography. J. Chromatogr. A. 2002;946(1–2):157–162. doi: 10.1016/s0021-9673(01)01548-5. [DOI] [PubMed] [Google Scholar]
- 15.Brent FJ, Pauli G. G.U.E.S.S. A generally useful estimate of solvent systems for CCC. J. Liq. Chromatog. & Rel. Technol. 2005;28:2777–2806. [Google Scholar]
- 16.Oka H, Harada K, Ito Y, Ito Y. Separation of antibiotics by countercurrent chromatography. J. Chromatogr. A. 1998;812(1–2):35–52. doi: 10.1016/s0021-9673(97)01277-6. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Conway WD. High-speed Countercurrent Chromatography. New York, USA: Press: Wiley-Interscience; 1996. pp. 36–39. 1996. [Google Scholar]
- 18.Derek G, Wray V, Winterhalter P, Jerz G. Preparative Isolation and Purification of Flavonoids and Protocatechuic Acid from Sea Buckthorn Juice Concentrate (Hippophaë rhamnoides L. ssp. rhamnoides) by High-Speed Counter-Current Chromatography. Chromatographia. 2007;65(1–2):1–7. [Google Scholar]
- 19.Katherine N. Carbon-13 nuclear magnetic resonance of biologically important aromatic acids. I. Chemical shifts of benzoic acid and derivatives. Journal of the American Chemistry Society. 1972;94(24):8564–8568. doi: 10.1021/ja00779a045. [DOI] [PubMed] [Google Scholar]
- 20.Vassiliki E, Anastasios T, Gerothanassis Ioannis P, Maria T, Dimitrios B. Identification and quantification of caffeic and rosmarinic acid in complex plant extracts by the use of variable-temperature two-dimensional nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2001;49:2–8. doi: 10.1021/jf990928e. [DOI] [PubMed] [Google Scholar]
- 21.Tomoyuki N, Yukihito A, Seiichi M, Yasuyuki U, Keiji S, Hirokazu K. Purification of Caffeic Acid as an Antioxidant from Submerged Culture Mycelia of Phellinus linteus (Berk. et Curt.) Teng (Aphyllophoromycetideae) International Journal of Medicinal Mushrooms. 2003;5:163–167. [Google Scholar]
- 22.Shen CC, Chang YS, Ho LK. Nuclear magnetic resonance studies of 5,7-dihydroxyflavonoids. Phytochemistry. 1993;34(3):843–845. [Google Scholar]


