Abstract
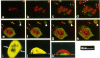
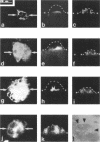
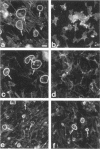
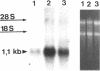
Protein sorting in eukaryotic cells is mainly done by specific targeting of polypeptides. The present evidence from oocytes, neurons, and some other polarized cells suggests that protein sorting can be further facilitated by concentrating mRNAs to their corresponding subcellular areas. However, very little is known about the mechanism(s) involved in mRNA targeting, or how widespread and dynamic such mRNA sorting might be. In this study, we have used an in vitro cell culture system, where large multinucleated osteoclasts undergo continuous structural and functional changes from polarized (resorbing) to a nonpolarized (resting) stage. We demonstrate here, using a nonradioactive in situ hybridization technique and confocal microscopy, that mRNAs for several vacuolar H(+)-ATPase subunits change their localization and polarity in osteoclasts according to the resorption cycle, whereas mRNA for cytoplasmic carbonic anhydrase II is found diffusely located throughout the osteoclast during the whole resorption cycle. Antisense RNA against the 16-kDa or 60-kDa V-ATPase subunit inhibits polarization of the osteoclasts, as determined by cytoskeleton staining. Antisense RNA against carbonic anhydrase II, however, has no such effect.
Full text
PDF


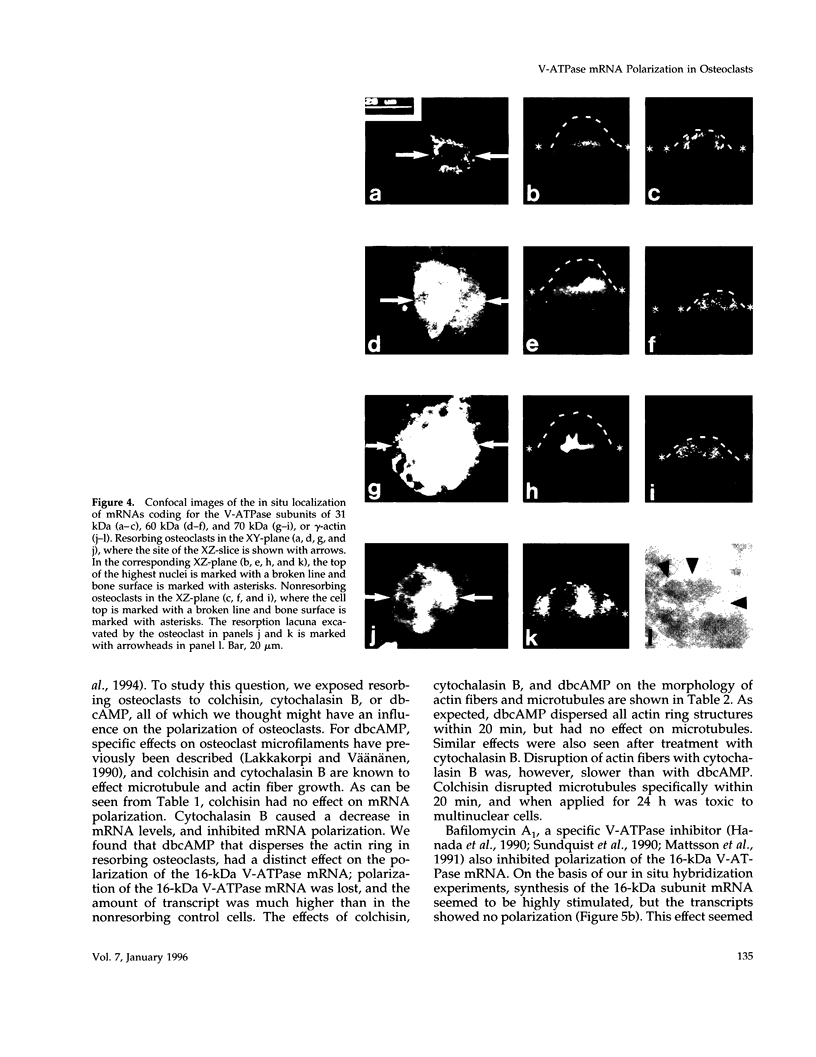
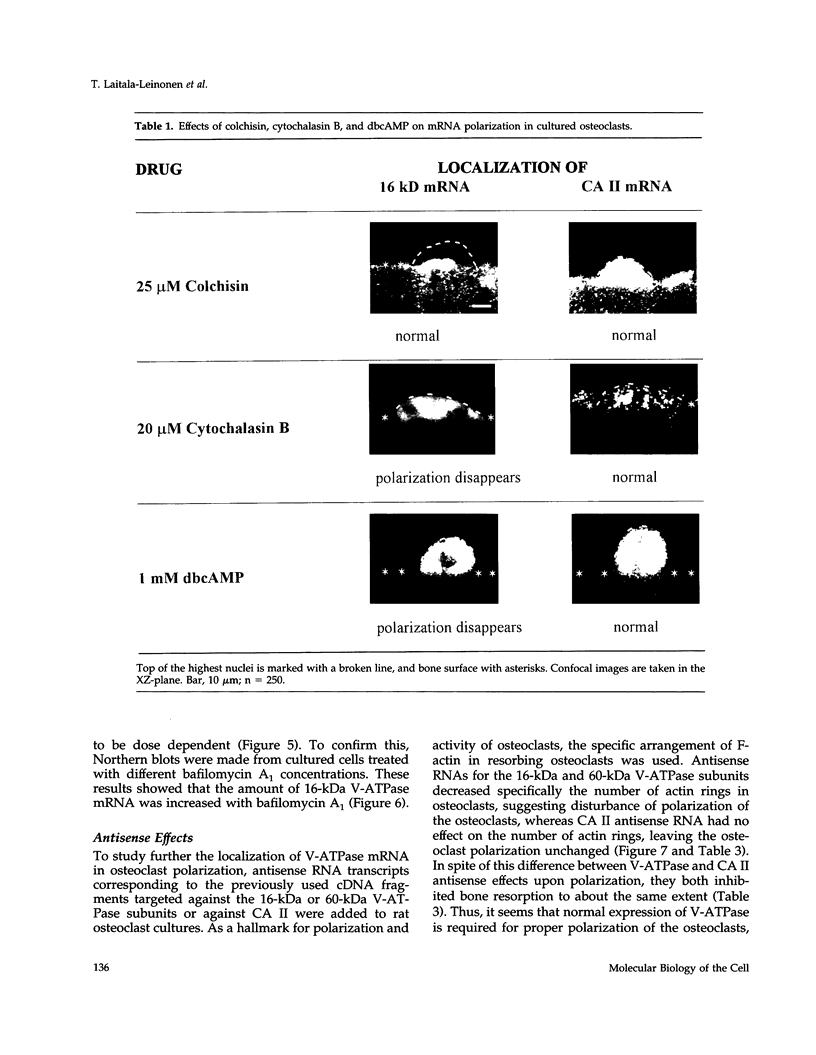
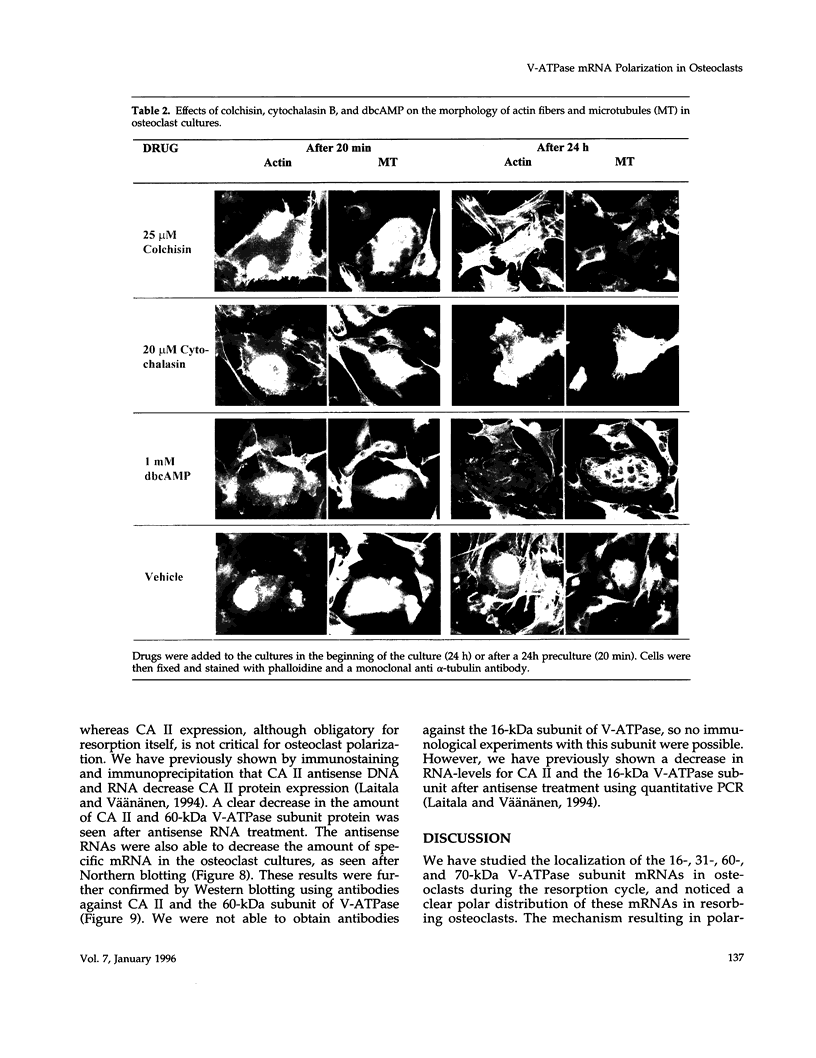
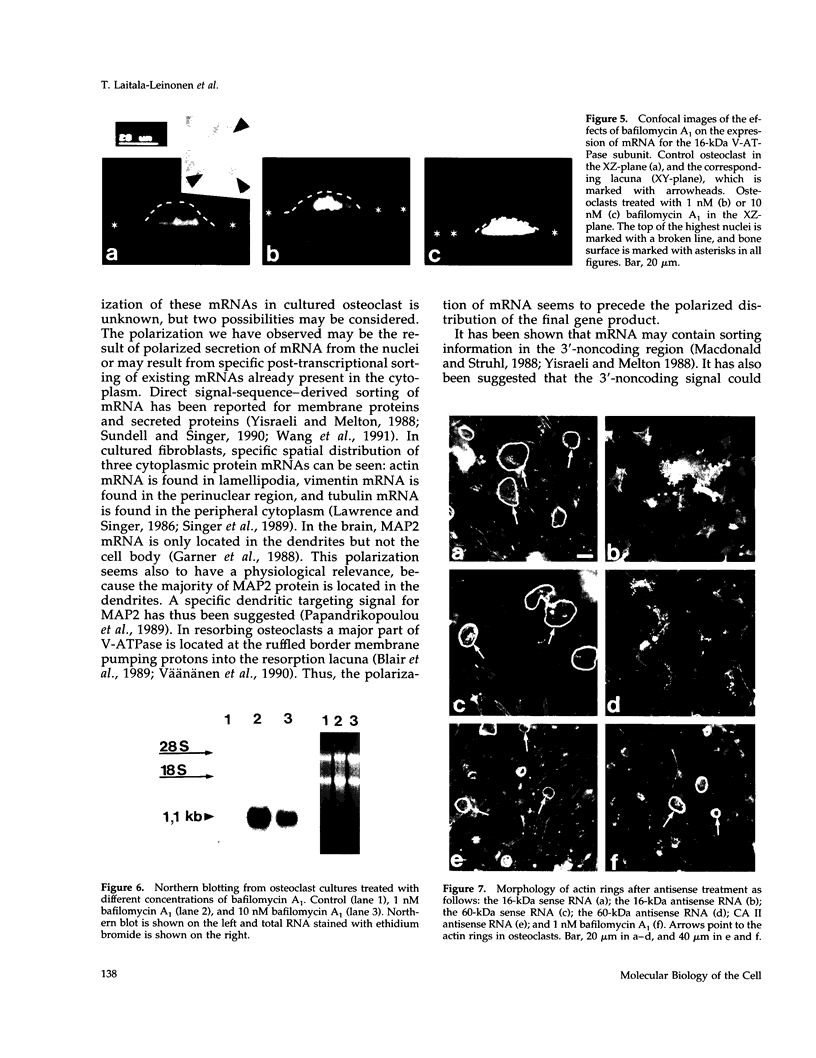
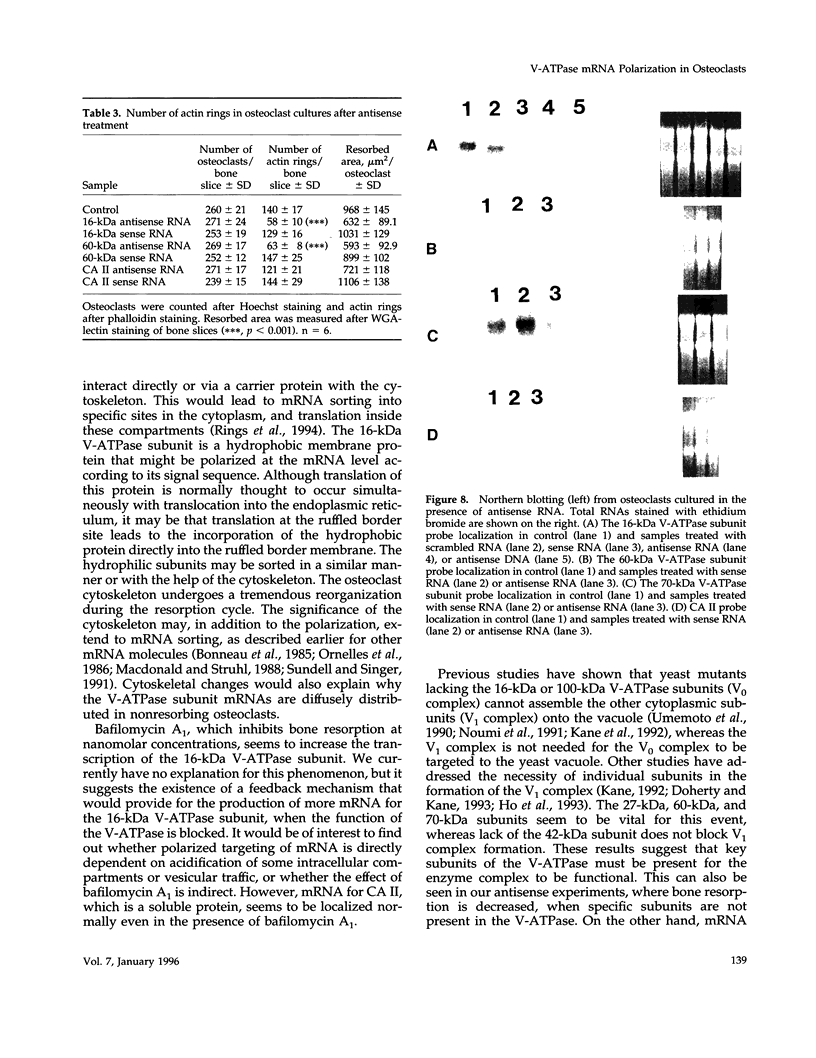
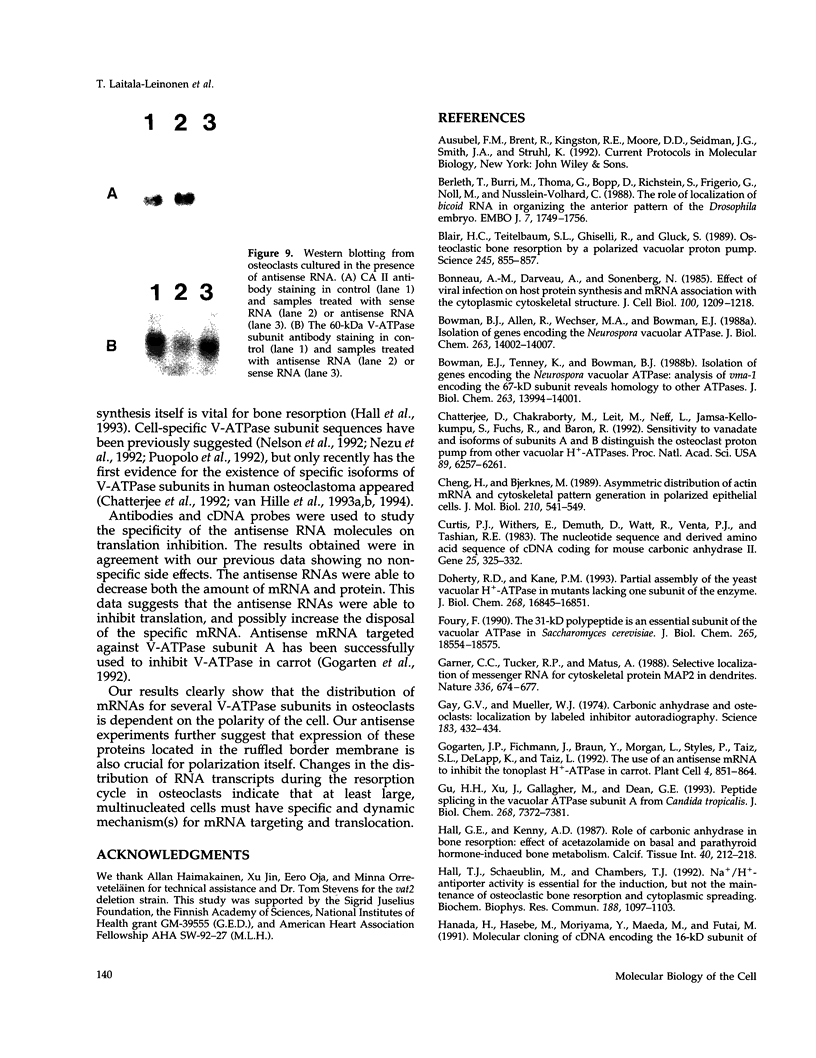


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berleth T., Burri M., Thoma G., Bopp D., Richstein S., Frigerio G., Noll M., Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 1988 Jun;7(6):1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. C., Teitelbaum S. L., Ghiselli R., Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989 Aug 25;245(4920):855–857. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- Bonneau A. M., Darveau A., Sonenberg N. Effect of viral infection on host protein synthesis and mRNA association with the cytoplasmic cytoskeletal structure. J Cell Biol. 1985 Apr;100(4):1209–1218. doi: 10.1083/jcb.100.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Allen R., Wechser M. A., Bowman E. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-2 encoding the 57-kDa polypeptide and comparison to vma-1. J Biol Chem. 1988 Oct 5;263(28):14002–14007. [PubMed] [Google Scholar]
- Bowman E. J., Tenney K., Bowman B. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-1 encoding the 67-kDa subunit reveals homology to other ATPases. J Biol Chem. 1988 Oct 5;263(28):13994–14001. [PubMed] [Google Scholar]
- Chatterjee D., Chakraborty M., Leit M., Neff L., Jamsa-Kellokumpu S., Fuchs R., Baron R. Sensitivity to vanadate and isoforms of subunits A and B distinguish the osteoclast proton pump from other vacuolar H+ ATPases. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6257–6261. doi: 10.1073/pnas.89.14.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Bjerknes M. Asymmetric distribution of actin mRNA and cytoskeletal pattern generation in polarized epithelial cells. J Mol Biol. 1989 Dec 5;210(3):541–549. doi: 10.1016/0022-2836(89)90130-7. [DOI] [PubMed] [Google Scholar]
- Curtis P. J., Withers E., Demuth D., Watt R., Venta P. J., Tashian R. E. The nucleotide sequence and derived amino acid sequence of cDNA coding for mouse carbonic anhydrase II. Gene. 1983 Nov;25(2-3):325–332. doi: 10.1016/0378-1119(83)90237-8. [DOI] [PubMed] [Google Scholar]
- Doherty R. D., Kane P. M. Partial assembly of the yeast vacuolar H(+)-ATPase in mutants lacking one subunit of the enzyme. J Biol Chem. 1993 Aug 5;268(22):16845–16851. [PubMed] [Google Scholar]
- Foury F. The 31-kDa polypeptide is an essential subunit of the vacuolar ATPase in Saccharomyces cerevisiae. J Biol Chem. 1990 Oct 25;265(30):18554–18560. [PubMed] [Google Scholar]
- Garner C. C., Tucker R. P., Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988 Dec 15;336(6200):674–677. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- Gay C. V., Mueller W. J. Carbonic anhydrase and osteoclasts: localization by labeled inhibitor autoradiography. Science. 1974 Feb 1;183(4123):432–434. doi: 10.1126/science.183.4123.432. [DOI] [PubMed] [Google Scholar]
- Gogarten J. P., Fichmann J., Braun Y., Morgan L., Styles P., Taiz S. L., DeLapp K., Taiz L. The use of antisense mRNA to inhibit the tonoplast H+ ATPase in carrot. Plant Cell. 1992 Jul;4(7):851–864. doi: 10.1105/tpc.4.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H. H., Xu J., Gallagher M., Dean G. E. Peptide splicing in the vacuolar ATPase subunit A from Candida tropicalis. J Biol Chem. 1993 Apr 5;268(10):7372–7381. [PubMed] [Google Scholar]
- Hall G. E., Kenny A. D. Role of carbonic anhydrase in bone resorption: effect of acetazolamide on basal and parathyroid hormone-induced bone metabolism. Calcif Tissue Int. 1987 Apr;40(4):212–218. doi: 10.1007/BF02556624. [DOI] [PubMed] [Google Scholar]
- Hall T. J., Schaeublin M., Chambers T. J. Na+/H(+)-antiporter activity is essential for the induction, but not the maintenance of osteoclastic bone resorption and cytoplasmic spreading. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1097–1103. doi: 10.1016/0006-291x(92)91344-p. [DOI] [PubMed] [Google Scholar]
- Hanada H., Moriyama Y., Maeda M., Futai M. Kinetic studies of chromaffin granule H+-ATPase and effects of bafilomycin A1. Biochem Biophys Res Commun. 1990 Jul 31;170(2):873–878. doi: 10.1016/0006-291x(90)92172-v. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E., Pryme I. F. Interaction between mRNA, ribosomes and the cytoskeleton. Biochem J. 1991 Jul 1;277(Pt 1):1–10. doi: 10.1042/bj2770001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata R., Ohsumk Y., Nakano A., Kawasaki H., Suzuki K., Anraku Y. Molecular structure of a gene, VMA1, encoding the catalytic subunit of H(+)-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1990 Apr 25;265(12):6726–6733. [PubMed] [Google Scholar]
- Hirsch S., Strauss A., Masood K., Lee S., Sukhatme V., Gluck S. Isolation and sequence of a cDNA clone encoding the 31-kDa subunit of bovine kidney vacuolar H+-ATPase. Proc Natl Acad Sci U S A. 1988 May;85(9):3004–3008. doi: 10.1073/pnas.85.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. N., Hirata R., Umemoto N., Ohya Y., Takatsuki A., Stevens T. H., Anraku Y. VMA13 encodes a 54-kDa vacuolar H(+)-ATPase subunit required for activity but not assembly of the enzyme complex in Saccharomyces cerevisiae. J Biol Chem. 1993 Aug 25;268(24):18286–18292. [PubMed] [Google Scholar]
- Hoock T. C., Newcomb P. M., Herman I. M. Beta actin and its mRNA are localized at the plasma membrane and the regions of moving cytoplasm during the cellular response to injury. J Cell Biol. 1991 Feb;112(4):653–664. doi: 10.1083/jcb.112.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J. Cytoplasmic regulation of mRNA function: the importance of the 3' untranslated region. Cell. 1993 Jul 16;74(1):9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Standart N. Do the poly(A) tail and 3' untranslated region control mRNA translation? Cell. 1990 Jul 13;62(1):15–24. doi: 10.1016/0092-8674(90)90235-7. [DOI] [PubMed] [Google Scholar]
- Kane P. M., Kuehn M. C., Howald-Stevenson I., Stevens T. H. Assembly and targeting of peripheral and integral membrane subunits of the yeast vacuolar H(+)-ATPase. J Biol Chem. 1992 Jan 5;267(1):447–454. [PubMed] [Google Scholar]
- Laitala T., Vänänen H. K. Inhibition of bone resorption in vitro by antisense RNA and DNA molecules targeted against carbonic anhydrase II or two subunits of vacuolar H(+)-ATPase. J Clin Invest. 1994 Jun;93(6):2311–2318. doi: 10.1172/JCI117235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitala T., Vänänen K. Proton channel part of vacuolar H(+)-ATPase and carbonic anhydrase II expression is stimulated in resorbing osteoclasts. J Bone Miner Res. 1993 Jan;8(1):119–126. doi: 10.1002/jbmr.5650080115. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi P. T., Vänänen H. K. Calcitonin, prostaglandin E2, and dibutyryl cyclic adenosine 3',5'-monophosphate disperse the specific microfilament structure in resorbing osteoclasts. J Histochem Cytochem. 1990 Oct;38(10):1487–1493. doi: 10.1177/38.10.2169493. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi P. T., Vänänen H. K. Kinetics of the osteoclast cytoskeleton during the resorption cycle in vitro. J Bone Miner Res. 1991 Aug;6(8):817–826. doi: 10.1002/jbmr.5650060806. [DOI] [PubMed] [Google Scholar]
- Lakkakorpi P., Tuukkanen J., Hentunen T., Järvelin K., Vänänen K. Organization of osteoclast microfilaments during the attachment to bone surface in vitro. J Bone Miner Res. 1989 Dec;4(6):817–825. doi: 10.1002/jbmr.5650040605. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H., Villnave C. A., Stein J. L., Stein G. S. Intracellular distribution of histone mRNAs in human fibroblasts studied by in situ hybridization. Proc Natl Acad Sci U S A. 1988 Jan;85(2):463–467. doi: 10.1073/pnas.85.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P. M., Struhl G. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988 Dec 8;336(6199):595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Moriyama Y., Hulmes J. D., Pan Y. C., Nelson H., Nelson N. cDNA sequence encoding the 16-kDa proteolipid of chromaffin granules implies gene duplication in the evolution of H+-ATPases. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5521–5524. doi: 10.1073/pnas.85.15.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolson M. F., Ouellette B. F., Filion M., Poole R. J. cDNA sequence and homologies of the "57-kDa" nucleotide-binding subunit of the vacuolar ATPase from Arabidopsis. J Biol Chem. 1988 Dec 5;263(34):17987–17994. [PubMed] [Google Scholar]
- Mattsson J. P., Vänänen K., Wallmark B., Lorentzon P. Omeprazole and bafilomycin, two proton pump inhibitors: differentiation of their effects on gastric, kidney and bone H(+)-translocating ATPases. Biochim Biophys Acta. 1991 Jun 18;1065(2):261–268. doi: 10.1016/0005-2736(91)90238-4. [DOI] [PubMed] [Google Scholar]
- Nelson H., Mandiyan S., Nelson N. A bovine cDNA and a yeast gene (VMA8) encoding the subunit D of the vacuolar H(+)-ATPase. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):497–501. doi: 10.1073/pnas.92.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H., Mandiyan S., Nelson N. A conserved gene encoding the 57-kDa subunit of the yeast vacuolar H+-ATPase. J Biol Chem. 1989 Jan 25;264(3):1775–1778. [PubMed] [Google Scholar]
- Nelson N. Structure, molecular genetics, and evolution of vacuolar H+-ATPases. J Bioenerg Biomembr. 1989 Oct;21(5):553–571. doi: 10.1007/BF00808113. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Guo X. L., Masood K., Brown D., Kalkbrenner M., Gluck S. Selectively amplified expression of an isoform of the vacuolar H(+)-ATPase 56-kilodalton subunit in renal intercalated cells. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3541–3545. doi: 10.1073/pnas.89.8.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezu J., Motojima K., Tamura H., Ohkuma S. Molecular cloning of a rat liver cDNA encoding the 16 kDa subunit of vacuolar H(+)-ATPases: organellar and tissue distribution of 16 kDa proteolipids. J Biochem. 1992 Aug;112(2):212–219. doi: 10.1093/oxfordjournals.jbchem.a123879. [DOI] [PubMed] [Google Scholar]
- Noumi T., Beltrán C., Nelson H., Nelson N. Mutational analysis of yeast vacuolar H(+)-ATPase. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1938–1942. doi: 10.1073/pnas.88.5.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelles D. A., Fey E. G., Penman S. Cytochalasin releases mRNA from the cytoskeletal framework and inhibits protein synthesis. Mol Cell Biol. 1986 May;6(5):1650–1662. doi: 10.1128/mcb.6.5.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. X., Gu H. H., Xu J., Dean G. E. Saccharomyces cerevisiae expression of exogenous vacuolar ATPase subunits B. Biochim Biophys Acta. 1993 Sep 19;1151(2):175–185. doi: 10.1016/0005-2736(93)90102-6. [DOI] [PubMed] [Google Scholar]
- Pan Y. X., Xu J., Strasser J. E., Howell M., Dean G. E. Structure and expression of subunit A from the bovine chromaffin cell vacuolar ATPase. FEBS Lett. 1991 Nov 18;293(1-2):89–92. doi: 10.1016/0014-5793(91)81158-5. [DOI] [PubMed] [Google Scholar]
- Papandrikopoulou A., Doll T., Tucker R. P., Garner C. C., Matus A. Embryonic MAP2 lacks the cross-linking sidearm sequences and dendritic targeting signal of adult MAP2. Nature. 1989 Aug 24;340(6235):650–652. doi: 10.1038/340650a0. [DOI] [PubMed] [Google Scholar]
- Puopolo K., Kumamoto C., Adachi I., Forgac M. A single gene encodes the catalytic "A" subunit of the bovine vacuolar H(+)-ATPase. J Biol Chem. 1991 Dec 25;266(36):24564–24572. [PubMed] [Google Scholar]
- Puopolo K., Kumamoto C., Adachi I., Magner R., Forgac M. Differential expression of the "B" subunit of the vacuolar H(+)-ATPase in bovine tissues. J Biol Chem. 1992 Feb 25;267(6):3696–3706. [PubMed] [Google Scholar]
- Rings E. H., Büller H. A., Neele A. M., Dekker J. Protein sorting versus messenger RNA sorting? Eur J Cell Biol. 1994 Apr;63(2):161–171. [PubMed] [Google Scholar]
- Selander K., Lehenkari P., Vänänen H. K. The effects of bisphosphonates on the resorption cycle of isolated osteoclasts. Calcif Tissue Int. 1994 Nov;55(5):368–375. doi: 10.1007/BF00299317. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Langevin G. L., Lawrence J. B. Ultrastructural visualization of cytoskeletal mRNAs and their associated proteins using double-label in situ hybridization. J Cell Biol. 1989 Jun;108(6):2343–2353. doi: 10.1083/jcb.108.6.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H. The cytoskeleton and mRNA localization. Curr Opin Cell Biol. 1992 Feb;4(1):15–19. doi: 10.1016/0955-0674(92)90053-f. [DOI] [PubMed] [Google Scholar]
- Sundell C. L., Singer R. H. Actin mRNA localizes in the absence of protein synthesis. J Cell Biol. 1990 Dec;111(6 Pt 1):2397–2403. doi: 10.1083/jcb.111.6.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundell C. L., Singer R. H. Requirement of microfilaments in sorting of actin messenger RNA. Science. 1991 Sep 13;253(5025):1275–1277. doi: 10.1126/science.1891715. [DOI] [PubMed] [Google Scholar]
- Sundquist K. T., Leppilampi M., Järvelin K., Kumpulainen T., Vänänen H. K. Carbonic anhydrase isoenzymes in isolated rat peripheral monocytes, tissue macrophages, and osteoclasts. Bone. 1987;8(1):33–38. doi: 10.1016/8756-3282(87)90129-3. [DOI] [PubMed] [Google Scholar]
- Sundquist K., Lakkakorpi P., Wallmark B., Vänänen K. Inhibition of osteoclast proton transport by bafilomycin A1 abolishes bone resorption. Biochem Biophys Res Commun. 1990 Apr 16;168(1):309–313. doi: 10.1016/0006-291x(90)91709-2. [DOI] [PubMed] [Google Scholar]
- Trapp B. D., Moench T., Pulley M., Barbosa E., Tennekoon G., Griffin J. Spatial segregation of mRNA encoding myelin-specific proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7773–7777. doi: 10.1073/pnas.84.21.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto N., Yoshihisa T., Hirata R., Anraku Y. Roles of the VMA3 gene product, subunit c of the vacuolar membrane H(+)-ATPase on vacuolar acidification and protein transport. A study with VMA3-disrupted mutants of Saccharomyces cerevisiae. J Biol Chem. 1990 Oct 25;265(30):18447–18453. [PubMed] [Google Scholar]
- Vänänen H. K., Karhukorpi E. K., Sundquist K., Wallmark B., Roininen I., Hentunen T., Tuukkanen J., Lakkakorpi P. Evidence for the presence of a proton pump of the vacuolar H(+)-ATPase type in the ruffled borders of osteoclasts. J Cell Biol. 1990 Sep;111(3):1305–1311. doi: 10.1083/jcb.111.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Cohen D. S., Palmer E., Sheppard D. Polarized regulation of fibronectin secretion and alternative splicing by transforming growth factor. J Biol Chem. 1991 Aug 25;266(24):15598–15601. [PubMed] [Google Scholar]
- Yisraeli J. K., Melton D. A. The material mRNA Vg1 is correctly localized following injection into Xenopus oocytes. Nature. 1988 Dec 8;336(6199):592–595. doi: 10.1038/336592a0. [DOI] [PubMed] [Google Scholar]
- Zimniak L., Dittrich P., Gogarten J. P., Kibak H., Taiz L. The cDNA sequence of the 69-kDa subunit of the carrot vacuolar H+-ATPase. Homology to the beta-chain of F0F1-ATPases. J Biol Chem. 1988 Jul 5;263(19):9102–9112. [PubMed] [Google Scholar]
- van Hille B., Richener H., Evans D. B., Green J. R., Bilbe G. Identification of two subunit A isoforms of the vacuolar H(+)-ATPase in human osteoclastoma. J Biol Chem. 1993 Apr 5;268(10):7075–7080. [PubMed] [Google Scholar]
- van Hille B., Richener H., Schmid P., Puettner I., Green J. R., Bilbe G. Heterogeneity of vacuolar H(+)-ATPase: differential expression of two human subunit B isoforms. Biochem J. 1994 Oct 1;303(Pt 1):191–198. doi: 10.1042/bj3030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hille B., Vanek M., Richener H., Green J. R., Bilbe G. Cloning and tissue distribution of subunits C, D, and E of the human vacuolar H(+)-ATPase. Biochem Biophys Res Commun. 1993 Nov 30;197(1):15–21. doi: 10.1006/bbrc.1993.2434. [DOI] [PubMed] [Google Scholar]