Abstract
Bakuchiol was prepared from commercial (−)-citronellol using the diazosulfonate C-H insertion to control the regioselectivity and install the quaternary center.
We recently reported a modification of C-H insertion that permits assembly of six-membered sulfur-containing heterocycles,1 effectively introducing substitution at a remote position to an existing functionality. The synthetic potential of this reaction has yet to be demonstrated. In this report we describe an application of this methodology for construction of the quaternary center in the structure of natural product Bakuchiol, which was isolated from seeds of Psoralea corylifolia L,2 and had a variety of applications. The two features of C-H insertion that make it particularly useful for this purpose are ease of insertion into a tertiary C-H bonds, and complete retention of configuration at the insertion site. Use of the sulfonate modification in this case appropriately controls the regioselectivity of insertion.
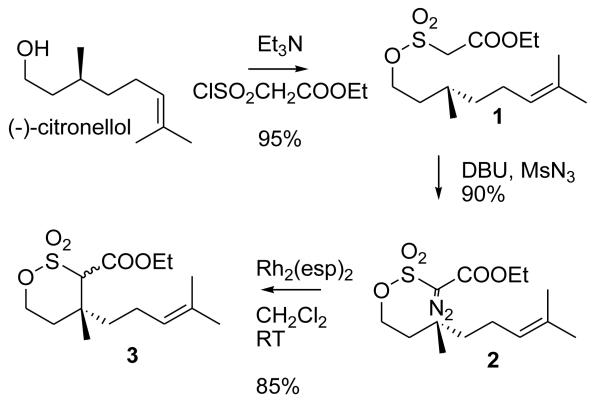
Thus, commercially available (−)-citronellol was converted to δ-sultone 3 using diazosulfonate C-H insertion in a manner identical to that previously reported by Du Bois (Scheme 1).3 Our materials matched the reported compounds by spectral data.12
Scheme 1.
Preparation of δ-sultone from citronellol by C-H insertion
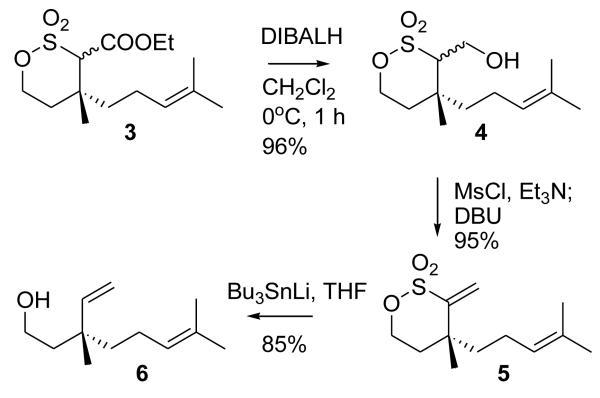
Initially, we attempted to reductively cleave the C-S bond in δ-sultone 3 using SmI2/DMPU.4 However, poor results were obtained. The expected reductive desulfonation product did appear to form, but in minor quantities, and contaminated by several byproducts (transformation of the double bond appeared to have taken place). Therefore, an alternative route was attempted (Scheme 2). DIBALH reduction of the ester in 3 to alcohol13 proceeded cleanly. While it appeared to be possible to transform it to alcohol 6 by conversion to alkyl halide and treatment with zinc in DME, both of these reactions were riddled with difficulties. Reaction of alcohol 4 with Ph3P/NBS or Ph3P/I2/ImH proceeded only at high temperatures (Toluene, 90°C), and in modest yields. Treatment with zinc tended to produce, along with 6, elimination product 5 and other byproducts. This elimination product, 5,14 was easily available from 4 by mesylation and elimination, so we explored the possibility of its conversion to 6.
Scheme 2.
Conversion of the sultone to the key intermediate
Only a single example of reductive desulfonation of vinylic sulfonates appears to have been reported, using lithium in ammonia.5 Under these conditions we saw formation of only small amounts (~5%) of the desired product. The major product, obtained as an apparent mixture of diastereomers, was not identified, but it appeared to retain the sulfonate moiety and lack the exomethylene double bond according to 1H NMR spectrum. Several methods, reported for desulfonation of vinylic sulfones, also had limited success (decomposition was observed with Na2S2O4,6 low yields with BuMgCl-Ni(acac)2 or BuMgCl-Pd(acac)2,7 no reaction with SmI2 or SmI2-DMPU, moderate yields with Na-EtOH-THF8). Eventually, we found that the procedure for desulfonation of vinylsulfones using Bu3SnLi9 was suitable in this case. Although it was somewhat touchy, this method would produce the desired alcohol 615 in high yield, using two modifications to the reported procedure: a) use of greater excess of the tin reagent (5 equiv or more) proved beneficial 2) use of TBAF (THF, reflux, 5 h) instead of silica gel for elimination of the tin adduct was more effective; also, nearly complete elimination was happening if the reaction mixture was warmed up to 0°C before workup. Thus, effectively, a “remote vinylation” of citronellol was achieved by this sequence. NMR study of R-acetylmandelic ester of alcohol 6 (and its independently prepared racemic form) confirmed that no detectable loss of optical purity took place.
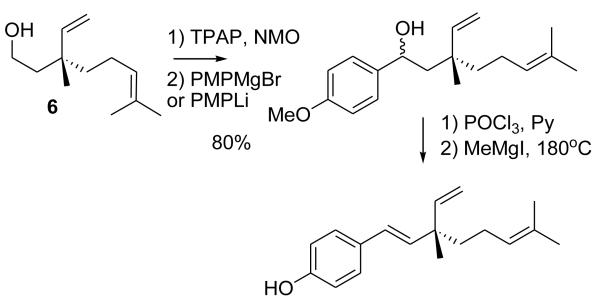
The obtained alcohol was oxidized to the aldehyde,16 and converted to Bakuchiol using the described method (Scheme 3),10,11 via addition of p-methoxyphenyl magnesium bromide or lithium reagents (the latter was obtained by metal-halogen exchange between p-bromomethoxybenzene and n-butyllithium), elimination and demethylation. The synthetic compound matched the natural product by spectral data and specific rotation.
Scheme 3.
Preparation of Bakuchiol
Thus, synthesis of Bakuchiol was achieved using diazosulfonate C-H insertion to install the quaternary center, demonstrating the utility of this methodology. Further studies on synthetic applications of this modification of C-H insertion are being performed and will be reported in due course.
Supplementary Material
Acknowledgements
This work was supported by the American Chemical Society Petroleum Research Fund under grant No. PRF#46278-G1 and National Institutes of Health under grant No. GM085645. We thank Alena Kubatova for HRMS analyses. The work on TOF MS was supported by the National Foundation under grant No. CHE-0216038.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.John JP, Novikov AV. Org. Lett. 2007;9:61. doi: 10.1021/ol062592h. [DOI] [PubMed] [Google Scholar]
- 2.Mehta G, Nayak UR, Dev S. Tetrahedron Lett. 1966:4561. [Google Scholar]
- 3.Wolckenhauer SA, Devlin AS, Du Bois J. Org. Lett. 2007;9:4363. doi: 10.1021/ol701950d. [DOI] [PubMed] [Google Scholar]
- 4.Jungong CS, John JP, Bequette JB, Novikov AV. Heterocycles. 2009:78. in press. [Google Scholar]
- 5.Metz P, Cramer Tetrahedron Lett. 1993;34:6371. [Google Scholar]
- 6.Bremner J, Julia M, Launay M, Stacino J-P. Tetrahedron Lett. 1982;23:3265. [Google Scholar]
- 7.Fabre J-L, Julia M. Tetrahedron Lett. 1983;40:4311. [Google Scholar]
- 8.Masaski Y, Iwata I, Imaeda T, Oda H, Nagashima H. Chem. Pharm. Bull. 1988;36:1241. doi: 10.1248/cpb.36.1241. [DOI] [PubMed] [Google Scholar]
- 9.Ochiai M, Ukita T, Fujita E. J. Chem. Soc. Chem. Commun. 1983:619. [Google Scholar]
- 10.Chen H, Du X, Tang W, Zhou Y, Zuo J, Feng H, Li Y. Bioorg. Med. Chem. 2008;16:2403. doi: 10.1016/j.bmc.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 11.Candruff J, Miller JA. J. Chem. Soc. (C) 1968:2671. [Google Scholar]
- 12.Specific rotations for optically pure compounds: Ethyl (3S),2-[[(3,7-dimethyl-6-octen-1-yl)oxy]sulfonyl]-acetate (1): [α]D20 = −2.0 (1.2, CHCl3).Ethyl (3S)-2-diazo-2-[[(3,7-dimethyl-6-octen-1-yl)oxy]sulfonyl]-acetate (2): [α]D20 = −35.6-44.6 (1.2, CHCl3). Constant rapid oscillation in the specified range was observed for undetermined reasons.(4R)-3-carbethoxy-4-methyl-4-(4-methylpent-3-enyl)-1,2-oxathiane-2,2-dioxide (3): Less polar isomer: [α]D20 = +48.3 (1.1, CHCl3). More polar isomer: [α]D20 = −52.7 (1.2, CHCl3).
- 13.Physcal data for (4R)-3-hydroxymethyl-4-methyl-4-(4-methylpent-3-enyl)-1,2-oxathiane-2,2-dioxide (4).Less polar isomer: colorless oil. Rf 0.44 (1:2 EtOAc:Hexanes). [α]D20 = −11.6 (1.0, CHCl3). 1H NMR (500 MHz, CDCl3): δ 5.07 (t, J = 7 Hz, 1H), 4.66 (td, J = 11.5, 2.5 Hz, 1H), 4.50 (dt, J = 11.5, 4 Hz, 1H), 4.16-4.22 (m, 1H), 4.01-4.07 (m, 1H), 3.21 (dd, J = 8, 3 Hz, 1H), 2.34 (dd, J = 9.5, 4.5 Hz, 1H), 1.95-2.05 (m, 3H), 1.70 (s, 3H), 1.63-1.69 (m, 2H), 1.62 (s, 3H), 1.44-1.52 (m, 1H), 1.22 (s, 3H). 13C NMR (CDCl3, 125Mhz): δ 133.0 (C), 123.1 (CH), 69.5 (CH2), 68.0 (CH), 58.2 (CH2), 41.3 (CH2), 38.4 (C), 35.8 (CH2), 25.9 (CH3), 22.0 (CH2), 20.5 (CH3), 17.9 (CH3). HRMS (ESI) calcd for C12H26NO4S [M+NH4] 280.1583, found 280.1583. IR (neat, cm−1)): 3527, 2971, 2919, 1461, 1348, 1172.More polar isomer: colorless oil. Rf 0.38 (1:2 EtOAc:Hexanes). [α]D20 = +23.4 (0.55, CHCl3). 1H NMR (500 MHz, CDCl3): δ 5.07 (t, J = 7 Hz, 1H), 4.55 (t, J = 5.5 Hz, 2H), 4.14-4.21 (m, 1H), 4.02-4.07 (m, 1H), 3.11 (dd, J = 8, 2,5 Hz, 1H), 2.52 (dd, J = 10, 4 Hz, 1H), 1.84-1.96 (m, 4H), 1.69 (s, 3H), 1.62-1.66 (m, 1H), 1.61 (s, 3H), 1.34-1.40 (m, 1H), 1.33 (s, 3H). 13C NMR (CDCl3, 125Mhz): δ 132.8 (C), 123.3 (CH), 70.3 (CH), 69.5 (CH2), 58.5 (CH2), 38.7 (C), 35.7 (CH2), 34.8 (CH2), 25.9 (CH3), 25.6 (CH3), 21.9 (CH2), 17.9 (CH3). HRMS (ESI) calcd for C12H26NO4S [M+NH4] 280.1583, found 280.1584. IR (neat, cm−1)): 3527, 2970, 2924, 1459, 1344, 1172.
- 14.Physical data for 3-methylene-4-methyl-4-(4-methylpent-3-enyl)-1,2-oxathiane-2,2-dioxide (5):Colorless oil. Rf 0.49 (1:5 EtOAc:Hexanes). [α]D20 = −52.7 (0.70, CHCl3). 1H NMR (500 MHz, CDCl3): δ 6.29 (s, 1H), 5.68 (s, 1H), 5.11 (t, J = 7 Hz, 1H), 4.79 (td, J = 11.5, 2.5 Hz, 1H), 4.45 (dt, J = 11.5, 4 Hz, 1H), 2.12-2.18 (m, 1H), 2.00 (ddd, J = 15.5, 11, 4.5 Hz, 1H), 1.89-1.95 (m, 2H), 1.73 (ddd, J = 14, 4, 2.5 Hz, 1H), 1.69 (s, 3H), 1.61 (s, 3H), 1.38-1.45 (m, 1H), 1.33 (s, 3H). 13C NMR (CDCl3, 125Mhz): δ 150.5 (C), 132.6 (C), 123.5 (CH), 121.4 (CH2), 68.8 (CH2), 41.8 (C), 39.0 (CH2), 37.5 (CH2), 25.9 (CH3), 23.1 (CH2), 17.8 (CH3). 13C NMR (C6D6, 125Mhz): δ 151.1 (C), 132.4 (C), 124.5 (CH), 121.0 (CH2), 68.4 (CH2), 41.7 (C), 39.2 (CH2), 37.8 (CH2), 26.2 (CH3), 25.7 (CH3), 23.7 (CH2), 18.0 (CH3). HRMS (ESI) calcd for C12H24NO3S [M+NH4]+ 262.1477, found 262.1490. IR (neat, cm−1)): 2970, 2918, 2857 1456, 1353, 1185. There is 1 less signal in 13C spectrum of the compound due to accidental equivalence of chemical shifts of two methyl signals. It is consistent with the increased height of the signal and confirmed by a 13C spectrum in C6D6, where these two carbons show separately.
- 15.Although alcohol 6 was previously reported in the literature (in racemic form), no complete set of physical data was provided. Data for (R)-3,7-dimethyl-3-vinyloct-6-en-1-ol (6):Colorless oil. Rf 0.42 (1:5 EtOAc:Hexanes). [α]D20 = −20.6 (0.35, CHCl3). 1H NMR (500 MHz, CDCl3): δ 5.79 (dd, J = 17.5, 10.5 Hz, 1H), 5.09 (t, J = 7 Hz, 1H), 5.04 (dd, J = 10.5, 1Hz, 1H), 4.96 (dd, J = 17.5, 1 Hz, 1H), 3.62-3.70 (m, 2H), 1.86-1.94 (m, 2H), 1.57-1.70 (m, 8H), 1.68 (s), 1.59 (s), 1.42 (br s, 1H), 1.32 (dd, J = 9.5, 8 Hz, 2H), 1.03 (s, 3H). 13C NMR (CDCl3, 125Mhz): δ 147.2 (CH), 131.5 (C), 124.9 (CH), 112.3 (CH2), 60.1 (CH2), 43.6 (CH2), 41.7 (CH2), 38.9 (C), 25.9 (CH3), 22.8 (CH2), 22.6 (CH3), 17.8 (CH3). HRMS (ESI) calcd for C12H26NO [M+NH4]+ 200.2014, found 200.2018. IR (neat, cm−1): 3337, 3082, 2967, 2926, 1636 1453, 1376, 1048.
- 16.(R)-3,7-dimethyl-3-vinyloct-6-enal: [α]D20 = −25.0 (0.5, CHCl3).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.