Abstract
Pro-inflammatory CC chemokines control leukocyte recruitment and function during inflammation by engaging chemokine receptors expressed on circulating leukocytes. The D6 chemokine receptor can bind several of these chemokines but appears unable to couple to signal transduction pathways or direct cell migration. Instead, D6 has been proposed to act as a chemokine scavenger, removing pro-inflammatory chemokines to dampen leukocyte responses. In this report, we have examined the role of D6 in the colon using the dextran sodium sulphate-induced model of colitis. We show that D6 is expressed in the resting colon, predominantly by stromal cells and B cells, and is up-regulated during colitis. Unexpectedly, D6-deficient mice showed reduced susceptibility to colitis and had less pronounced clinical symptoms associated with this model. D6 deletion had no impact on the level of pro-inflammatory CC chemokines released from cultured colon explants, or on the balance of leukocyte subsets recruited to the inflamed colon. However, late in colitis, inflamed D6-deficient colons showed enhanced production of several pro-inflammatory cytokines, including IFNγ and IL-17A, and there was a marked increase in IL-17A-secreting γδ T cells in the lamina propria. Moreover, antibody-mediated neutralisation of IL-17A worsened the clinical symptoms of colitis at these later stages of the response in D6-deficient, but not wild-type, mice. Thus, D6 can contribute to the development of colitis by regulating IL-17A secretion by γδ T cells in the inflamed colon.
Keywords: Chemokines, inflammation, mucosa
Introduction
The chemokines are a family of small, secreted proteins that trigger leukocyte migration and activation via G-protein coupled receptors (1). They are divided into four subgroups (CXC, CC, XC and CX3C) based on variations in a cysteine motif in the amino-terminus of the protein (2). The CC family contains a subset of ~20 pro-inflammatory chemokines that can stimulate leukocyte recruitment and migration though one, or more, of the receptors CCR1 to 5. These chemokines play a critical role in orchestrating tissue inflammation.
D6 is structurally-related to CCR1 to 5, and can bind to at least ten of the CC chemokines capable of activating these receptors. However, in transfected cell lines, this receptor neither couples to signalling pathways used by CCR1 to 5, nor directs cell migration. Instead, D6 is proposed to be a ‘decoy’ receptor, because when it is expressed in cell lines in vitro, it progressively scavenges large quantities of its chemokine ligands by virtue of its ability to constitutively traffic to and from the cell surface (3-5). In support of this, D6 deficiency in mice results in increased inflammatory responses in the skin, lung and placenta, often accompanied by higher than usual levels of local chemokines (6-9), and D6-deficient mice also show a marked increase in susceptibility to inflammation-associated skin tumour formation (10).
In humans, D6 is expressed strongly throughout the gastrointestinal tract by lymphatic endothelial cells (LECs)4 and some resident leukocytes (11), but its role in intestinal inflammation has yet to be explored. Many D6 ligands have been implicated in the pathophysiology of both human and murine inflammatory bowel disease (IBD) and there is considerable interest in targeting chemokine receptors therapeutically in human IBD (12). Increased levels of CCL2, 3, 4, 5, 7 and 8 are found in the colonic mucosa of patients with ulcerative colitis and Crohn's disease (13-15), with a strong correlation between CCL7 expression and the extent of epithelial destruction in patient biopsies (15). Additionally, mice lacking CCR5 or CCR2 are protected from experimental colitis induced by administration of dextran sodium sulphate (DSS) (16). In this report we show that D6 is expressed in the mouse colon by stromal cells and leukocytes, and is up-regulated during the induction of colitis with DSS. Unexpectedly, compared to wild-type (WT) animals, D6-deficient mice show reduced tissue damage in response to acute colitis induced with DSS. D6 had no effect on the abundance of chemokine released from explants of inflamed colon, but D6-deficient mice showed a marked increase in the production of several inflammatory cytokines, notably IL-17A and IFNγ, and an increased abundance of IL-17A-secreting γδ T cells in the lamina propria (LP). Moreover, antibody-mediated neutralisation of IL-17A led to a worsening of disease during the recovery phase post-DSS treatment. Our work reveals that the atypical chemokine receptor D6 impacts upon the development of intestinal inflammation by regulating γδ T cells, and identifies it as a potential therapeutic target in IBD.
Materials and Methods
Animals
Colitis experiments were performed on age-matched male mice that were between 8-12 weeks of age at the start of the experiment. D6-deficient animals were generated and maintained along with WT counterparts as previously described (6, 10). Mice were housed under specific pathogen-free conditions in the Central Research Facility, University of Glasgow. All procedures had received local ethical approval and were performed in accordance with UK Home Office regulations.
Induction and assessment of colitis
To induce acute colitis, mice received DSS (molecular weight 36-50 kDa; ICN Biomedicals) dissolved to 2% in sterile drinking water, ad libitum, for 5 days followed by water alone for 2-4 days. Chronic colitis was induced by repeated rounds of 2% DSS (3 days), alternating with periods on normal water (7-10 days). Control mice received water without DSS. Animals were monitored daily and scored for clinical disease based on the following parameters: (a) weight loss (0-3); (b) diarrhoea (0-3); (c) rectal bleeding (0-3). Weight change was calculated as percent change in weight compared with body weight at start of experiment. Any animal that lost >20% of its original body weight was sacrificed immediately by cervical dislocation according to UK Home Office recommendations. At the end of the experiment, colons were excised and their lengths recorded. Histological scoring of colitis was performed on samples of colon, which were fixed in 10% formalin, embedded in paraffin, sectioned (5μm), and stained with haematoxylin and eosin. Scoring was performed blind using a combined score of inflammatory cell infiltration (0-5) and tissue pathology (0-5). For anti-IL-17 blocking experiments, DSS colitis was induced as described above and mice were injected i.p. with 100μg of neutralising rat anti-IL-17A monoclonal antibody (17) (a kind gift from Prof J Van Snick and Dr C Uyttenhove, Ludwig Institute for Cancer Research, Brussels, Belgium) or isotype control antibody (Sigma) at the start of the DSS protocol and every 48h thereafter.
Isolation of RNA, and RT-PCR
Colonic RNA was extracted with TRIzol reagent (Invitrogen) and cleaned on RNeasy Mini columns (Qiagen) with on-column DNase digestion performed using the RNAse-Free DNase kit (Qiagen) according to manufacturer's instructions. RNA was extracted from FACS-purified cell populations using RNeasy Mini columns with on-column DNase digestion as described above. RNA from purified colonic epithelial cells was kindly provided by Dr Sansom (Beatson Institute for Cancer Research, Glasgow). First-strand cDNA synthesis (Superscript II; Invitrogen) with oligo(dT) was carried out according to the manufacturer's instructions and PCR amplifications were performed using Prealiquoted ReddyMix PCR Master Mix tubes (ABgene) and primers for D6 (AGCTTTACCTGCTGAACCTGG and AAGAAGATCATCGCCAAGAGTG) or GAPDH (AACTCCCACTCTTCCACCTT and GCCCCTCCTGTTATTATGG). Tubes were incubated in a thermocycler under the following condition: 34 cycles of 94°C (20s), 57°C (45s) and 72°C (1min). Products were electrophoresed on a 1.5% agarose gel containing ethidium bromide and visualised under UV light. A 7900HT Sequence Detector (Applied Biosystems) was used for quantitative real-time PCR on cDNA (prepared as described above) using Taqman Universal PCR Master Mix (Applied Biosystems) and primers specific for murine GAPDH (internal control; Applied Biosystems) or murine D6 (Applied Biosystems). Results were normalised to GAPDH and presented relative to expression of D6 in resting unfractionated colon.
Lamina propria cell isolation and flow cytometry
Whole colons were washed in CMF-HBSS (Gibco), opened longitudinally, and cut into segments of ~1cm. Epithelial cells were removed by incubating the colon segments three times in 2mM EDTA/CMF-HBSS for 15min at 37°C, with shaking, after which the tissue was washed thoroughly in sterile PBS and digested in CMF-HBSS containing 20% FCS, 100 U/ml collagenase-A (Sigma), and 30μg/ml DNase (Roche) for 3 × 45min incubations at 37°C. At the end of each of the incubations the cells in the supernatants were stored in RPMI/10% FCS on ice until use. For flow cytometry, aliquots of cells were washed in FACS buffer (PBS/2%FCS/5mM EDTA) and incubated with anti-CD16/CD32 in FACS buffer for 15min at 4°C to prevent non-specific binding of antibody. Subsequently, cells were washed and stained for 45min with FITC-, PE-, allophycoerythrin-, or biotin-conjugated antibodies specific for the surface markers of interest. Biotinylated antibodies were detected using allophycoerythrin-coupled streptavidin (BD Pharmingen). All antibodies were purchased from BD Pharmingen, except for allophycoerythrin- and biotin-coupled anti-F4/80, which were obtained from Caltag. Cellular fluorescence data was acquired using a dual-laser (488-nm argon laser, 635-nm red diode laser) Becton Dickinson FACSCalibur flow cytometer and CELLQuest software (BD Biosciences). Dead cells were excluded by addition of propidium iodide solution (Sigma-Aldrich) to each tube immediately before analysis. Following acquisition, data files were analysed using FlowJo software (Tree Star Inc).
Intracellular IL-17A staining
To assess expression of IL-17A, isolated LP leukocytes were stimulated for 4.5h with 0.1μM PMA, 1μM ionomycin, 1μM Monensin and 10μg/ml Brefeldin A, in complete RPMI. Cells were then washed in FACS buffer and incubated with ethidium monoazide to exclude dead cells. After another wash, cells were stained for 15 min with FITC- or PE- conjugated antibodies (BD Pharmingen) specific for the surface markers of interest, then fixed, permeabilised, and incubated with APC-conjugated anti-IL-17A antibody or isotype control antibody (BD Pharmingen) for 40 min. Cellular fluorescence data was acquired as above.
Assessment of D6 expression by uptake of fluorescently-labelled CCL22
Alexa647-labelled CCL22, a chemically-synthesised variant of CCL22 which carries a carboxy terminal, fluorescently-labelled lysine residue, was from Almac Sciences. This reagent has bioactivity comparable to commercially-available unlabelled recombinant CCL22 (data not shown). For uptake experiments, isolated leukocytes were pre-incubated in polypropylene tubes at 37°C in RPMI/10% FCS/50μM HEPES for 30mins. Fluorescent CCL22 was added to 25nM, and the cells incubated for a further 100mins with regular agitation to maintain cells in solution. The cells were then washed, immunostained, and analysed by flow cytometry, as described above. Samples to which PBS had been added in place of fluorescent CCL22 were used to set appropriate gates on the flow cytometer.
Measurement of cytokines and chemokines in colon cultures
Colons were opened longitudinally and washed in sterile PBS (Gibco) supplemented with 1% penicillin/streptomycin (Gibco). Three segments from the distal colon of approximately 1cm in length were placed in 24 flat-bottom well culture plates (Costar) containing fresh RPMI 1640 (Gibco) supplemented with 1% penicillin/streptomycin and incubated at 37°C for 24h. Culture supernatants were then harvested, centrifuged at 13,000g and stored at −20°C. Subsequently, the following cytokines and chemokines were analysed simultaneously using Luminex Multiplex Bead Assay (BioSource) according to manufacturer's instructions: CCL2, CCL3, CCL4, CCL5, CCL19, CXCL1, CXCL9, CXCL10, FGF, GM-CSF, IFNγ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p40 and p70), IL-13, IL-17A, and TNFα.
Statistics
Data were compared using Student's t tests or Mann-Whitney U tests as appropriate using GraphPad Prism software (GraphPad Software Inc).
Results
D6 is expressed in the resting murine colon and up-regulated during colitis
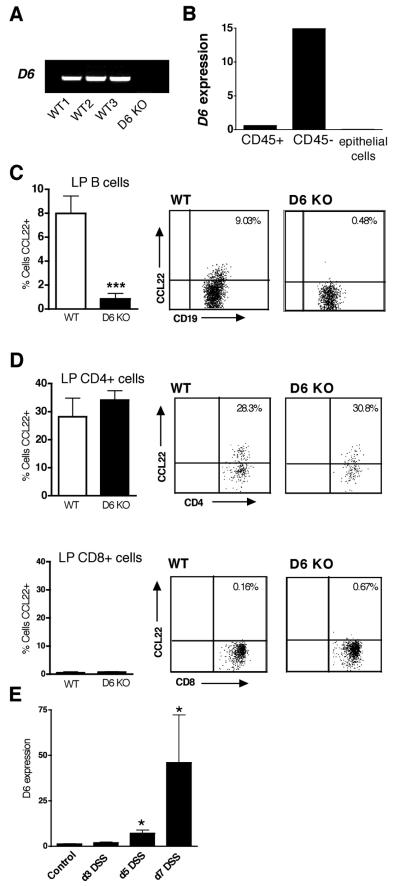
Analysis of murine intestinal tissue by RT-PCR showed robust expression of D6 mRNA in resting WT mouse colon, and this was absent in D6-deficient mice (Figure 1A). The lack of a suitable antibody against mouse D6 prevented the localisation of D6 protein by flow cytometry or immunohistochemistry. However, using Q-PCR to analyse D6 expression in cell fractions isolated from resting colon (Figure 1B), we found that D6 mRNA was not detectable in colonic epithelial cells, and was expressed mainly by the CD45− non-leukocytic compartment of the LP. However, lower levels of D6 mRNA were also evident in CD45+ leukocytes from the LP (Figure 1B), and to determine which populations express D6, we compared the uptake of fluorescently-labelled CCL22, a D6 ligand, by isolated LP leukocytes from WT and D6-deficient mice. Interestingly, D6-dependent internalisation of fluorescent CCL22 was only observed in a small population of colonic B cells (Figure 1C). WT and D6-deficient CD4+ T cells demonstrated substantial but equivalent uptake of CCL22 (~30% of cells were CCL22+), suggesting they express CCR4, the conventional CCL22 receptor (Figure 1D). We observed no significant CCL22 uptake by colonic CD8+ cells (Figure 1D) or other LP leukocyte populations, including F4/80+ macrophages (data not shown), indicating that they do not express D6 or CCR4 capable of detectably internalising CCL22. Thus, D6 is expressed by some colonic B cells, but is primarily expressed by non-leukocytic cells in the colon.
Figure 1. D6 expression in murine colon.
(A) RT-PCR amplification of D6 from cDNA generated from RNA isolated from resting murine colon (3 WT; 1 D6-deficient (D6 KO)). (B) Quantitative real-time PCR of D6 from cDNA prepared from RNA isolated from pooled FACS-sorted CD45+ leukocytes and CD45− cells from colonic LP, and purified colonic epithelial cell extracts, from 3 individual mice, compared to expression of D6 in resting unfractionated colon (set to 1). (C) Detection of functional D6 on colonic LP B cells by uptake of fluorescent CCL22. LP cells were isolated from colons of WT and D6-deficient (D6 KO) animals and cultured at 37°C with fluorescently-labelled CCL22. Uptake of labelled chemokine by CD19+ LP cells was assessed by flow cytometry. Bar graphs represent the mean + SD for 3-4 mice per group and representative dot plots are shown on the right. ***p< 0.0001 (D6 KO vs WT). (D) Uptake of CCL22 by CD4+ and CD8+ T cells from resting WT and D6-deficient (D6 KO) mice. Data represent the mean + SD for 3-4 animals per group. (E) Acute colitis was induced in WT animals by DSS administration, RNA prepared from whole colons at days 3, 5 and 7, and levels of D6 mRNA assessed by quantitative real-time PCR and compared to resting control colon (set to 1). Data represent the mean + SD for 3-4 animals per time-point. *p<0.05 (DSS-treated vs control). Data are representative of at least three repeats.
Gross examination, histological analysis and flow cytometry of intestinal tissue from WT and D6-deficient mice revealed no significant differences in the mucosal architecture or resident leukocyte populations between D6-deficient and WT mice under resting conditions (data not shown). Therefore, we went on to explore whether D6 expression is altered during DSS-induced colitis, in which administration of DSS in drinking water results in the development of severe colonic inflammation with some characteristics of human ulcerative colitis (18). Analysis of total colonic RNA at days 3, 5 and 7 following DSS administration revealed that D6 expression increased progressively during the course of disease, with maximum levels detected at day 7 (Figure 1E). Thus, D6 is significantly up-regulated during colitis, particularly late in the disease.
D6-deficient mice show reduced clinical symptoms and tissue pathology in response to DSS
To explore the functional relevance of D6 expression during intestinal inflammation, we compared the development of acute DSS colitis in WT and D6-deficient mice, a model that is partly dependent on CCR2 and CCR5 (16, 19-21). Due to the proposed scavenging of the ligands for these receptors by D6, we hypothesised that D6-deficient mice would show increased susceptibility to colitis induction. Surprisingly however, in many repeat experiments, D6-deficient mice were consistently less susceptible to the development of colitis than their WT counterparts (Figure 2). As expected, DSS-treated WT mice developed colitis, with most mice displaying clinical signs of diarrhoea and rectal bleeding within 4-5 days (Figure 2A). DSS-treated WT mice demonstrated marked weight loss by day 5, were severely ill by day 7 (Figure 2B), and upon sacrifice on day 8, had significantly shorter colons than controls (Figure 2C). DSS-treated D6-deficient mice also became ill, but diarrhoea and rectal bleeding only became evident later and their clinical scores were significantly lower than those in WT mice from day 5 onwards (Figure 2A). D6-deficient mice also showed significantly less weight loss and colonic shortening than WT mice (Figure 2B-C). Histological examination confirmed these results: although all WT and D6-deficient animals demonstrated colonic pathology and inflammation, this was significantly reduced in D6-deficient mice, as determined by the combined scoring of mucosal damage and inflammation (Figure 2D). Reflecting these differences, WT colons frequently showed areas of complete epithelial necrosis and crypt loss, whereas mucosal integrity was retained in most D6-deficient colons, despite the presence of inflammation and some epithelial damage (Figure 2D). The reduced disease observed in the D6-deficient mice was not the result of decreased DSS intake, as water consumption was equivalent between WT and D6-deficient groups throughout the course of the experiment (data not shown). The decreased susceptibility of D6-deficient mice was also seen during chronic colitis when mice were exposed to three rounds of DSS treatment separated by recovery periods on normal water (Supplementary Figure S1). These data clearly demonstrate that D6 augments colitis.
Figure 2. D6-deficient mice are less susceptible to DSS-induced colitis.
Colitis was induced in WT and D6-deficient (D6 KO) mice by addition of 2% DSS to their drinking water for 5 days, followed by up to 3 days with normal water. Animals were monitored daily for clinical disease signs (A) and weight loss (B), and colon length was determined on sacrifice at day 8 (C). Data in (A) and (C) are means + SD, while in (B) are means ± SEM with 13 or more animals per group. (D) Histological scoring (left panel) was performed blind using a combined score of inflammatory cell infiltration (0-5) and tissue pathology (0-5). The data are the mean + SD for 4-8 animals per group. The images, captured from H&E-stained sections of colon 7 days after start of DSS administration, show a region of a WT colon (left panel) in which the epithelium has suffered complete ulceration and crypt loss. By contrast, the pathology observed in the D6-deficient colonic mucosa is less severe (right panel), although crypt hyperplasia, goblet cell loss, and inflammatory cell infiltration are still evident. Original magnification, ×200. ***p<0.001, *p<0.05. The data are representative of experiments repeated on at least five occasions.
Composition of the LP leukocyte infiltrate, expression of chemokine receptors, and release of chemokines from colon explants
From our histological analysis it was clear that despite evident leukocyte infiltration in both WT and D6-deficient animals, less ulceration and architectural destruction was apparent in the colons of DSS-treated D6-deficient mice compared with WT counterparts. Amelioration of DSS colitis in mice deficient in CCR2 or CCR5 mice is associated with a modified, rather than a reduced, leukocytic infiltration (16). Therefore we considered whether the decreased tissue damage in colitic D6-deficient mice might be due to an altered balance of cell populations in the DSS-induced inflammatory infiltrate. To this end, we isolated LP cells from the inflamed colons at days 2 and 7 by collagenase digestion, and determined the relative proportions of CD4+ T cells, CD8+ T cells, B220+ B cells, Ly6G+ neutrophils, F4/80+ macrophages and CD11c+ dendritic cells by flow cytometry (Figure 3A-B). Increased numbers of all these cell types were observed in WT and D6-deficient colons as early as 2 days following DSS treatment, and the numbers of each population were increased further by day 7 (p<0.05 (controls vs day 2; day 2 vs day 7) for all cell populations analysed). However, no significant differences were observed between WT and D6-deficient LP infiltrates in terms of the total number of leukocytes retrieved, or in the abundance of individual cell types, at either day 2 or day 7 of colitis.
Figure 3. D6 deficiency has no effect on the balance of leukocyte populations infiltrating the lamina propria during DSS-induced colitis.
WT and D6-deficient mice (KO) received 2% DSS in the drinking water for 5 days, and normal water thereafter. At days 2 and 7 after commencing DSS administration, LP cells were isolated, (A) the total number of cells retrieved were counted, and (B) F4/80+, CD11c+, CD4+, CD8+, B220+ and Ly6G+ cells quantified by flow cytometry. Statistical analyses are discussed in the text of the Results. (C) Uptake of fluorescent CCL22 by WT and D6-deficient B220+ B cells (left) and CD4+ T cells (right) isolated from the LP 5 or 7 days after starting DSS treatment. *p<0.01, **p<0.001. (D) Expression of CCR5 by B220+ cells from isolated from colitic mice at day 7 after starting DSS treatment protocol. ***p<0.0001 WT vs KO. All graphs show means + SD, with 3 or more mice per group, and at least two repeat experiments yielded similar results.
To assess leukocytic D6 expression, we compared uptake of fluorescent CCL22 by WT and D6-deficient LP leukocytes isolated from colitic colons (Figure 3C). On day 5, there was a small increase in the proportion of WT LP B cells showing D6-dependent CCL22 uptake (up to ~13% from ~8% in resting colon). By day 7, this had returned to normal levels. The proportion of CD4+ T cells showing D6-independent CCL22 uptake did not differ between WT and D6-deficient colons (Figure 3C) suggesting no preferential recruitment of CCR4-expressing T cells to DSS-inflamed colons in either strain. Specific CCL22 uptake, D6-dependent or –independent, was not observed by any other cell types (data not shown). We also examined surface expression of CCR2 and CCR5 by LP leukocytes isolated from colitic mice, as both of these receptors are known to be involved in leukocyte recruitment to the LP during DSS-induced colitis (16, 22). In WT mice, a large proportion of the various T cell and innate leukocyte populations defined in Figure 3B showed robust expression of CCR2 or CCR5, and this was similar in equivalent populations isolated from D6-deficient colons (data not shown). Surprisingly however, compared to WT, there was a significant increase in the proportion of D6-deficient LP B cells which expressed CCR5 (Figure 3D). Further investigation indicated that this was not a result of colonic inflammation as B cells from the colon of untreated D6-deficient mice also showed increased levels of CCR5 compared to WT controls (data not shown).
In the skin, we and others have shown that D6 deficiency is associated with increased release of D6 ligands from inflamed tissue (6, 7). To assess this in the colon, we measured the levels of CCL2, 3, 4 and 5 in supernatants from cultured colon segments taken from WT and D6-deficient mice before or after the induction of colitis (Figure 4). These chemokines operate through one or more of the receptors CCR1, 2, 3 and 5, and all bind with high affinity to D6. Only CCL2 was detectable in WT or D6-deficient control cultures, but CCL3, and particularly CCL4 and 5, were present after 3 days of DSS treatment. On day 7, CCL2, CCL5, and notably CCL3, were even more abundant (p<0.02 (WT control vs WT day 7; D6 KO control vs D6 KO day 7) for CCL2, 3, 4 and 5). Strikingly, at no time-point did D6-deficient mice show elevated abundance of any of these four chemokines. The non-D6-binding inflammatory chemokines CXCL9 and CXCL10 were also measured. Like CCL2-5, they increased during colitis and were found at comparable levels in supernatants from WT and D6-deficient colon explants (Figure 4).
Figure 4. Release of pro-inflammatory chemokines from colon explants from WT and D6-deficient mice.
WT and D6-deficient mice (D6 KO) received 2% DSS in the drinking water for 5 days, and normal water thereafter. At days 3 and 7, explants of colon were cultured for 24 hours and culture supernatants harvested. The levels of the D6 ligands CCL2, CCL3, CCL4 and CCL5, and non D6-binding chemokines CXCL9 and CXCL10 in these supernatants were determined by Luminex multiplex bead assays. Data are the mean + SD for 5 mice per group. Statistical analysis is described in the text of the Results. Two repeat experiments gave similar results.
D6-deficient mice produce elevated levels of inflammatory cytokines during DSS-induced colitis
Although there were no obvious quantitative differences in the leukocyte infiltrate between DSS-treated WT and D6-deficient colons, the milder disease observed in D6-deficient mice could reflect more subtle qualitative changes in the inflammatory process. Thus, we measured the levels of a range of cytokines in supernatants from colon explants taken from control and colitic mice 3 and 7 days after the initiation of DSS treatment (Figure 5). Consistent with previous studies (23), supernatants prepared from colitic WT mice contained significantly increased quantities of IL-α, IL-1β, and IL-6 on day 7 (p<0.05 (WT control vs WT day 7; D6 KO control vs D6 KO day 7)). The abundance of these cytokines was unaffected by D6 deletion and there were also no significant differences in the levels of IL-2, IL-10, IL-12 or IL-23 found in WT and D6-deficient supernatants (data not shown). On the other hand, despite similar levels of TNFα, IFNγ and IL-17A after 3 days of DSS treatment, there was a substantial elevation in these cytokines, particularly IFNγ and IL-17A, in supernatants from D6-deficient colons isolated at day 7 (Figure 5), while release of IFNγ and IL-17A from inflamed WT colons showed no significant increase at day 7 above untreated controls (p>0.05). Within the D6-deficient group, there were significant inverse correlations between weight loss and the abundance of IFNγ and IL-17A released from day 7 DSS-inflamed colons (IFN-γ: R2=0.55, p<0.001; IL-17A: R2=0.39, p<0.005).
Figure 5. Production of inflammatory cytokines by colon explants from WT and D6-deficient mice during DSS colitis.
WT and D6-deficient mice (D6 KO) received 2% DSS in the drinking water for 5 days, and normal water thereafter. At days 3 and 7, explants of colon were cultured for 24 hours and the levels of IL-1α, IL-1β, IL-6, TNFα, IFNγ and IL-17A in culture supernatants were determined using Luminex multiplex bead assays. Data show the mean + SD for 3-5 mice per group. *p<0.05, **p<0.005 - other statistical analysis is described in the text of the Results. Two repeat experiments produced similar datasets.
In vivo blockade of IL-17A increases the severity of colitis in D6-deficient mice
IFNγ-deficient mice are resistant to DSS colitis (24), suggesting that the increased levels of IFNγ generated by D6-deficient colons are unlikely to be responsible for the relative protection of these mice against disease. However, antibody-mediated blockade of IL-17A, or genetic deficiency of IL-17A, has been reported to enhance the severity of DSS-induced colitis in some mouse strains (25, 26). The abundance of IL-17A in D6-deficient mice at day 7 might therefore contribute to their increased resistance to colitis and this may be particularly important late in the course of the disease. To examine this, WT and D6-deficient animals were treated with neutralising antibodies to IL-17A before and during colitis (Figure 6). In our hands, and contrary to previous work (25), WT mice treated with anti-IL-17 developed disease at the same rate as those receiving isotype control antibody. Similarly, up until day 7, anti-IL17 had no impact on disease course in D6-deficient mice. On days 8 and 9 however, colitis in D6-deficient animals treated with anti-IL17 worsened compared with that in D6-deficient mice treated with isotype control, shown by greater weight loss and increased clinical signs (Figure 6). On these days, isotype-treated D6-deficient mice remained significantly healthier than isotype-treated WT animals, but, in the anti-IL17-treated group, D6-deficient mice now had similar disease activity to their WT counterparts. These observations suggest that IL-17A contributes to the suppression or resolution of colitis at later time-points in D6-deficient mice, but that other factors are involved in the earlier resistance to colitis evident in these animals.
Figure 6. Anti-IL-17 antibodies increase the severity of colitis in D6-deficient mice.
Colitis was induced in WT and D6-deficient mice by addition of 2% DSS to their drinking water for 5 days, replaced with normal water thereafter. Mice were injected i.p with 100μg of anti-IL-17A or isotype control antibody on days 0, 2, 4 and 6, as shown at the base of the graphs. Animals were monitored daily for weight loss (upper panel) and clinical disease (lower panel). Data are the mean + SEM for 6-12 mice per group. **p<0.005 and ***p<0.001 where indicated for WT control IgG vs KO control IgG, and WT anti-IL-17 vs KO anti-IL-17. *p<0.05 where indicated for WT control IgG vs KO control IgG only. There were no significant differences between WT anti-IL-17 vs KO anti-IL-17 on day 8 and 9, between WT anti-IL-17 vs WT control IgG throughout the experiment, or between KO anti-IL-17 vs KO control IgG up to day 7.
D6-deficiency is associated with an increase in IL-17A-secreting LP γδ T cells during colitis
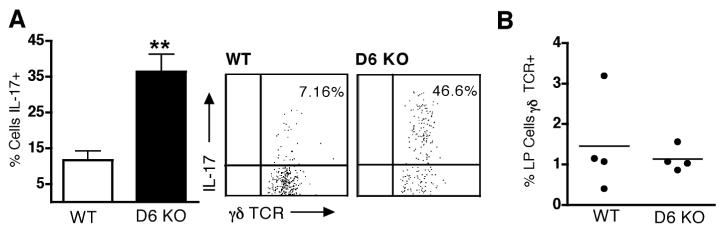
To identify the source of IL-17A in D6 deficient mice, we undertook intracellular IL-17A staining of LP leukocytes isolated from DSS-inflamed colons of WT and D6-deficient mice. Interestingly, there was an approximately three-fold increase in the percentage of γδ T cells capable of producing IL-17A in D6-deficient mice during the late stages of the colitic response (Figure 7A). This was not due to an overall increase in the number of γδ T cells retrieved from the LP of D6-deficient mice compared with WT mice (Figure 7B), but rather that many more of those isolated from D6-deficient animals could secrete IL-17A. ~1% of retrieved CD4+ T cells showed evidence of IL-17A production, as did a very small population of non-T cells, but their frequency was not significantly different between WT and D6-deficient mice (data not shown). Thus, the IL-17A-mediated protection of D6-deficient mice during the late stage of colitis is likely due to the increased abundance of IL-17A-producing γδ T cells in the LP of these animals.
Figure 7. Increased abundance of IL-17A-producing γδ T cells in the colons of colitic D6-deficient mice.
(A) Colonic LP cells were isolated from DSS-treated WT and D6-deficient animals on day 9 of DSS colitis, and IL-17 production assessed by intracellular cytokine staining. The graph shows the mean proportion (+ SD) of γδ T cells expressing intracellular IL-17 in WT and D6-deficient mice (n=4). ** p<0.01. Representative dot plots are shown adjacent to the graph. (B) Frequency of γδ T cells in leukocyte suspensions retrieved from the colonic LP of WT and D6-deficient on day 9 of DSS colitis. Data represent mean + SD for 4 mice per group. Repeat experiments produced similar datasets.
Discussion
The atypical chemokine receptor D6 is expressed in the resting murine colon, is up-regulated during DSS-induced colitis, and contributes to the development of the pathology associated with this model. Deletion of D6 leads to no detectable quantitative change in the composition of the leukocyte infiltrate in the LP during DSS colitis, but does result in marked increases in the production of some pro-inflammatory cytokines. In particular, much higher levels of IL-17A are detectable in D6-deficient colons during the late stages of the response to DSS, and this provides protection from colitis at these times. The elevated production of IL-17A appears to be due mainly to an increased frequency of γδ T cells capable of making this cytokine in the LP of D6-deficient colons.
In other tissues, D6 is clearly anti-inflammatory. In D6-deficient mice, cutaneous inflammatory responses to phorbol esters and CFA are more vigorous and prolonged (6, 7, 10), leukocyte recruitment to the lung after intranasal allergen challenge is enhanced (8), and lung infection with Mycobacterium tuberculosis leads to exaggerated local and systemic inflammatory responses with increased leukocyte infiltration of the lungs and live (27). Similarly, increased abortion of D6-deficient foetuses from D6-deficient mothers challenged with LPS or anti-phospholipid antibodies has been attributed to excessive placental inflammation (9). Thus, at first glance, the reduced weight loss and lower clinical scores of colitic D6-deficient mice may seem unexpected. However, DSS colitis is driven by bacterial penetration of the LP secondary to DSS-induced damage to the colonic epithelial barrier. This induces inflammation intended to protect the host by limiting bacterial invasion and promoting epithelial repair, and any suppression of these responses may allow more pronounced immunopathology to develop. Indeed it is now appreciated that human IBD may be associated with a failure to mount an effective acute inflammatory response which allows persistent luminal bacteria to trigger the development of adaptive immune responses that drive chronic immunopathology (28). Thus, the overall outcome of DSS exposure will depend on the precise nature of the inflammatory response and its impact on protection/repair versus immunopathology. As a result, gene deficient mice often have unpredictable responses in the DSS colitis model. For example, while IL-17F- or IFNγ-deficient mice are relatively resistant (24, 26), increased susceptibility is seen in animals lacking IL-17A, TNFα, TLR family members, or components of the TLR signalling pathway (26, 29-33), presumably because defective inflammatory responses fail to limit bacterial colonisation and/or promote epithelial repair. Therefore our findings of reduced susceptibility to DSS colitis in D6-deficient mice may be because the anti-inflammatory activity of D6 normally suppresses anti-bacterial or epithelial repair responses, and that this, over time, perpetuates the development of immunopathology.
In support of this, the presence of D6 clearly suppressed the production of pro-inflammatory cytokines during the later stages of DSS colitis. These cytokines included IL-17A and neutralisation of IL-17A in vivo abolished the protective effects of D6-deficiency at these later stages. IL-17A is known to provide critical protection against bacteria by inducing the expression of genes encoding neutrophil activators and anti-microbial peptides, as well as of the ligands for CXCR2 (34, 35), the principal chemokine receptor controlling neutrophils. Although we did not find an increase in neutrophil recruitment to the LP of colitic D6-deficient mice, preliminary Q-PCR studies have revealed increased levels of mRNA for the CXCR2 ligand CXCL2 in the colon of D6-deficient mice at times when IL-17A is elevated (data not shown). As CXCR2 ligands can stimulate neutrophil activation in addition to recruitment, IL-17A-induced chemokine release could enhance local anti-microbial responses in D6-deficient animals by increasing neutrophil activity. In addition, IL-17A has been shown to enhance intestinal epithelial integrity by promoting claudin-dependent tight junction formation, and through COX-2 induction, which generates cytoprotective prostaglandins (36-38). Interestingly, we have also found increases in COX-2 mRNA levels in the inflamed colon of D6 deficient mice compared with WT colons at times when IL-17A production is high (data not shown). These putative effects of IL-17A on host antibacterial responses and intestinal integrity, along with the induction of other IL-17A target genes, could combine to protect D6-deficient mice during the late stages of colitis. Experiments are underway to explore this in more detail. Notably, in WT mice, IL-17A was induced to a much lesser extent, and fewer IL-17A-producing γδ T cells were present. In addition, and contrary to a previous report (25), anti-IL-17A antibodies did not suppress colitis in WT animals. There may be many explanations for this difference in sensitivity to anti-IL-17A antibodies, but it is tempting to speculate that variation in D6 expression between WT animals (caused by differences in genetic background or environment for example) could contribute by influencing the extent of IL-17A production by LP γδ T cells.
Our results add a striking insight into the possible mechanisms by which γδ T cells may contribute to innate protective functions at epithelial surfaces. There is considerable evidence that γδ T cells can regulate immunopathology and promote epithelial integrity in the gut and skin, via interactions with conventional αβ T cells and by production of cytoprotective mediators such as keratinocyte growth factor (39-43). To our knowledge, our demonstration that γδ T cells appear to be a key source of IL-17A in the inflamed colon is novel, but is consistent with recent work showing that γδ T cells from various mucosal sites are capable of significant IL-17A production (44-48). The role of γδ T cell derived IL-17A in protective immunity and immunopathology clearly warrants further investigation.
Identifying individual cells expressing D6 in mice has been hampered by lack of antibodies, but we show here that colonic CD45+ leukocytes express D6 mRNA, and that a subset of B cells exhibits D6-dependent uptake of fluorescent chemokine. This adds to our recent work showing D6 expression by populations of B cells in mouse lymphoid tissue and human peripheral blood (49), and a separate report of D6 expression by murine splenic marginal zone B cells (50). Colonic B cell numbers increased during DSS colitis, and at day 5, a greater proportion showed evidence of D6 expression, accounting, at least in part, for the rise in D6 mRNA seen in the colon during colitis. However, most D6 mRNA in the colon is found in non-haemopoietic CD45- cells and, by extension from our work in humans (11), we consider that LECs will be a major contributor to this expression.
The individual contribution of colonic D6-expressing populations to the development of colitis remains to be determined, but current evidence suggests that D6 will act as a non-signalling scavenger of pro-inflammatory CC chemokines on these cells (3, 51). Although we did not detect any difference in chemokine release from colon explants from WT and D6-deficient mice, it should be noted that we compared samples at equivalent times after initiation of disease, when there were already marked differences in disease severity. These are likely to impact on chemokine abundance at multiple levels including production, consumption and degradation, which together may have masked detection of D6-mediated chemokine scavenging. Thus we would propose that local alterations in chemokine bioavailability in D6-deficient colons produce subtle changes in leukocyte localisation or function that modify the host inflammatory response and lead to increased production of IL-17A by γδ T cells. γδ T cells express conventional chemokine receptors, such as CCR2 and CCR5, which stimulate their migration in response to ligands that are targets for D6-mediated scavenging (52-54). Our own recent experiments have shown that LP γδ T cells from WT and D6-deficient colons internalise fluorescent CCL2, consistent with CCR2 expression by these cells (data not shown). Thus, loss of D6-mediated regulation of chemokine bioavailability could alter the localisation and/or activation of intestinal γδ T cells, leading to increased IL-17A production. This idea needs confirmed directly, but our results clearly demonstrate that D6 is a novel regulator of cytokine production by γδ T cells.
In summary, our studies demonstrate that deletion of D6 leads to the suppression of pathology caused by feeding DSS, and that in the late stages of the response this is caused by increased production of IL-17A by γδ T cells. They highlight that the impact of D6 deficiency on tissue pathology is dependent on the precise nature of the challenge, extend our knowledge of how γδ T cells may contribute to host immune regulation and mucosal defence, and offer the possibility of targeting D6 for treatment of chronic inflammatory bowel diseases.
Supplementary Material
As indicated under the under panel of the figure, WT and D6-deficient mice were subjected to repeated cycles of 2% DSS, interspersed with normal water, to induce chronic colonic inflammation. Compared with their WT counterparts, D6-deficient animals were less susceptibility to chronic DSS colitis throughout the course of the experiment, as shown by decreased weight loss (A), less clinical signs of disease (B) and better survival (C). * p<0.05, ** p<0.01.
Acknowledgements
We thank Prof J Van Snick and Dr C Uyttenhove (Ludwig Institute for Cancer Research, Brussels, Belgium) for anti-IL-17A antibodies, and Dr M Mack (University Hospital Regensburg, Germany) for antibodies against mouse CCR2 and CCR5. Thanks also to A Gilmour for assistance with FACS sorting. RJBN thanks Dr Wilson for support services.
Footnotes
This work was supported by research grants from the Medical Research Council (to YB, AMM and RJBN), Arthritis Research Campaign (to CAHH and RJBN), and Cancer Research-UK (to MC and RJBN). DPS was the recipient of a CJ Martin Fellowship (252924) from the National Health and Medical Research Council of Australia.
Abbreviations used: DSS, dextran sodium sulphate; IBD, inflammatory bowel disease; LEC, lymphatic endothelial cell; LP, lamina propria; WT, wild-type
References
- 1.Mackay CR. Chemokines: immunology's high impact factors. Nat Immunol. 2001;2:95–101. doi: 10.1038/84298. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 3.Nibbs R, Graham G, Rot A. Chemokines on the move: control by the chemokine “interceptors” Duffy blood group antigen and D6. Semin Immunol. 2003;15:287–294. doi: 10.1016/j.smim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Fra AM, Locati M, Otero K, Sironi M, Signorelli P, Massardi ML, Gobbi M, Vecchi A, Sozzani S, Mantovani A. Cutting edge: scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J Immunol. 2003;170:2279–2282. doi: 10.4049/jimmunol.170.5.2279. [DOI] [PubMed] [Google Scholar]
- 5.Bonecchi R, Locati M, Galliera E, Vulcano M, Sironi M, Fra AM, Gobbi M, Vecchi A, Sozzani S, Haribabu B, Van Damme J, Mantovani A. Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J Immunol. 2004;172:4972–4976. doi: 10.4049/jimmunol.172.8.4972. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 7.Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, Bonecchi R, Nebuloni M, Rukavina D, Vago L, Vecchi A, Lira SA, Mantovani A. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur J Immunol. 2005;35:1342–1346. doi: 10.1002/eji.200526114. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead GS, Wang T, Degraff LM, Card JW, Lira SA, Graham GJ, Cook DN. The Chemokine Receptor D6 has Opposing Effects on Allergic Inflammation and Airway Reactivity. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200606-839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez de la Torre Y, Buracchi C, Borroni EM, Dupor J, Bonecchi R, Nebuloni M, Pasqualini F, Doni A, Lauri E, Agostinis C, Bulla R, Cook DN, Haribabu B, Meroni P, Rukavina D, Vago L, Tedesco F, Vecchi A, Lira SA, Locati M, Mantovani A. Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc Natl Acad Sci U S A. 2007;104:2319–2324. doi: 10.1073/pnas.0607514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nibbs RJ, Gilchrist DS, King V, Ferra A, Forrow S, Hunter KD, Graham GJ. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J Clin Invest. 2007;117:1884–1892. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham GJ, Rot A. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158:867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gijsbers K, Geboes K, Van Damme J. Chemokines in gastrointestinal disorders. Curr Drug Targets. 2006;7:47–64. doi: 10.2174/138945006775270222. [DOI] [PubMed] [Google Scholar]
- 13.Reinecker HC, Loh EY, Ringler DJ, Mehta A, Rombeau JL, MacDermott RP. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995;108:40–50. doi: 10.1016/0016-5085(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 14.Grimm MC, Elsbury SK, Pavli P, Doe WF. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol. 1996;59:804–812. doi: 10.1002/jlb.59.6.804. [DOI] [PubMed] [Google Scholar]
- 15.Wedemeyer J, Lorentz A, Goke M, Meier PN, Flemming P, Dahinden CA, Manns MP, Bischoff SC. Enhanced production of monocyte chemotactic protein 3 in inflammatory bowel disease mucosa. Gut. 1999;44:629–635. doi: 10.1136/gut.44.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, Dawson T, Kuziel WA, Maeda N, MacDermott RP, Podolsky DK, Reinecker HC. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303–6312. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 17.Uyttenhove C, Van Snick J. Development of an anti-IL-17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. Eur J Immunol. 2006;36:2868–2874. doi: 10.1002/eji.200636662. [DOI] [PubMed] [Google Scholar]
- 18.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 19.Yang SK, C. M, Kim OH, Myung SJ, Jung HY, Hong WS, Kim JH, Min YI. The increased expression of an array of C-X-C and C-C chemokines in the colonic mucosa of patients with ulcerative colitis: regulation by corticosteroids. Am J Gastroenterol. 2002;97:126–132. doi: 10.1111/j.1572-0241.2002.05431.x. [DOI] [PubMed] [Google Scholar]
- 20.Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- 21.McCormack G, Moriarty D, O'Donoghue DP, McCormick PA, Sheahan K, Baird AW. Tissue cytokine and chemokine expression in inflammatory bowel disease. Inflamm Res. 2001;50:491–495. doi: 10.1007/PL00000223. [DOI] [PubMed] [Google Scholar]
- 22.Tokuyama H, Ueha S, Kurachi M, Matsushima K, Moriyasu F, Blumberg RS, Kakimi K. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int Immunol. 2005;17:1023–1034. doi: 10.1093/intimm/dxh284. [DOI] [PubMed] [Google Scholar]
- 23.Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 24.Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Kita M, Ueda Y, Iwakura Y, Kataoka K, Okanoue T, Mazda O. Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol. 2006;146:330–338. doi: 10.1111/j.1365-2249.2006.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Liberto D, Locati M, Caccamo N, Vecchi A, Meraviglia S, Salerno A, Sireci G, Nebuloni M, Caceres N, Cardona PJ, Dieli F, Mantovani A. Role of the chemokine decoy receptor D6 in balancing inflammation, immune activation, and antimicrobial resistance in Mycobacterium tuberculosis infection. J Exp Med. 2008;205:2075–2084. doi: 10.1084/jem.20070608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn's disease. Immunol Rev. 2005;206:277–295. doi: 10.1111/j.0105-2896.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 29.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Araki A, Kanai T, Ishikura T, Makita S, Uraushihara K, Iiyama R, Totsuka T, Takeda K, Akira S, Watanabe M. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 31.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 32.Cario EL, Gerken GU, Podolsky DA. Toll-Like Receptor 2 Controls Mucosal Inflammation by Regulating Epithelial Barrier Function. Gastroenterology. 2007 doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 33.Naito Y, Takagi T, Handa O, Ishikawa T, Nakagawa S, Yamaguchi T, Yoshida N, Minami M, Kita M, Imanishi J, Yoshikawa T. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J Gastroenterol Hepatol. 2003;18:560–569. doi: 10.1046/j.1440-1746.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 34.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 37.Kanda N, Koike S, Watanabe S. IL-17 suppresses TNF-alpha-induced CCL27 production through induction of COX-2 in human keratinocytes. J Allergy Clin Immunol. 2005;116:1144–1150. doi: 10.1016/j.jaci.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Morteau O, Morham SG, Sellon R, Dieleman LA, Langenbach R, Smithies O, Sartor RB. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest. 2000;105:469–478. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–4158. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- 42.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 43.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 44.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 45.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 46.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 47.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ γδ cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 49.McKimmie CS, Fraser AR, Hansell C, Gutierrez L, Philipsen S, Connell L, Rot A, Kurowska-Stolarska M, Carreno P, Pruenster M, Chu CC, Lombardi G, Halsey C, McInnes IB, Liew FY, Nibbs RJ, Graham GJ. Hemopoietic cell expression of the chemokine decoy receptor D6 is dynamic and regulated by GATA1. J Immunol. 2008;181:8171–8181. doi: 10.4049/jimmunol.181.11.8170-a. [DOI] [PubMed] [Google Scholar]
- 50.Kin NW, Crawford DM, Liu J, Behrens TW, Kearney JF. DNA microarray gene expression profile of marginal zone versus follicular B cells and idiotype positive marginal zone B cells before and after immunization with Streptococcus pneumoniae. J Immunol. 2008;180:6663–6674. doi: 10.4049/jimmunol.180.10.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 52.Penido C, Vieira-de-Abreu A, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Role of monocyte chemotactic protein-1/CC chemokine ligand 2 on gamma delta T lymphocyte trafficking during inflammation induced by lipopolysaccharide or Mycobacterium bovis bacille Calmette-Guerin. J Immunol. 2003;171:6788–6794. doi: 10.4049/jimmunol.171.12.6788. [DOI] [PubMed] [Google Scholar]
- 53.Roth SJ, Diacovo TG, Brenner MB, Rosat JP, Buccola J, Morita CT, Springer TA. Transendothelial chemotaxis of human alpha/beta and gamma/delta T lymphocytes to chemokines. Eur J Immunol. 1998;28:104–113. doi: 10.1002/(SICI)1521-4141(199801)28:01<104::AID-IMMU104>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 54.Glatzel A, Wesch D, Schiemann F, Brandt E, Janssen O, Kabelitz D. Patterns of chemokine receptor expression on peripheral blood gamma delta T lymphocytes: strong expression of CCR5 is a selective feature of V delta 2/V gamma 9 gamma delta T cells. J Immunol. 2002;168:4920–4929. doi: 10.4049/jimmunol.168.10.4920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As indicated under the under panel of the figure, WT and D6-deficient mice were subjected to repeated cycles of 2% DSS, interspersed with normal water, to induce chronic colonic inflammation. Compared with their WT counterparts, D6-deficient animals were less susceptibility to chronic DSS colitis throughout the course of the experiment, as shown by decreased weight loss (A), less clinical signs of disease (B) and better survival (C). * p<0.05, ** p<0.01.