Abstract
The KCl cotransporter (KCC) is a major determinant of osmotic homeostasis and plays an emerging role in tumor biology. This study stresses the important role of KCC4 in tumor malignant behavior. Real-time reverse transcription polymerase chain reaction on samples collected by laser microdissection and immunofluorescent stainings with different KCC isoform antibodies indicate that KCC4 is abundant in metastatic cervical and ovarian cancer tissues. Insulin-like growth factor 1 (IGF-1) and epidermal growth factor (EGF) stimulate KCC4 recruitment from a presumably inactive cytoplasmic pool of endoplasmic reticulum and Golgi to plasma membrane along actin cytoskeleton that is significantly inhibited by LY294002 and wortmannin. Throughout the trafficking process, KCC4 is incorporated into lipid rafts that function as a platform for the association between KCC4 and myosin Va, an actin-dependent motor protein. KCC4 and ezrin, a membrane cytoskeleton linker, colocalize at lamellipodia of migratory cancer cells. Interference with KCC activity by either the inhibitor or a dominant-negative loss-of-function mutant profoundly suppressed the IGF-1-induced membrane trafficking of KCC4 and structural interaction between KCC4 and ezrin near surface. Endogenous cancer cell invasiveness was significantly attenuated by small interfering RNA targeting KCC4 and the residual invasiveness was much less sensitive to IGF-1 or EGF stimulation. In the metastatic cancer tissues, KCC4 colocalizes with IGF-1 or EGF, indicating a likely in vivo stimulation of KCC4 function by growth factors. Thus, blockade of KCC4 trafficking and surface expression may provide a potential target for the prevention of IGF-1 or EGF-dependent cancer spread.
Keywords: IGF-1, KCl cotransport, membrane trafficking, metastasis, invasion
Introduction
K-Cl cotransport is the coupled electroneutral movement of K and Cl ions carried out by four protein isoforms, KCC1-4. These transporters play an important role in epithelial ion transport and osmotic homeostasis (1). The activities of KCC1, KCC3 and KCC4 are osmotically-sensitive and involved in cell volume regulation (2). The neuron-specific KCC2 is critical for the maturation of inhibitory GABA responses in the central nervous system by the control of intracellular Cl− concentration (3, 4). KCC activity in red blood cells was undiminished in KCC1 knockout mice, decreased in KCC3 knockout mice, and almost abolished in mice lacking both isoforms (5). Loss of KCC3 caused deafness, neurodegeneration and reduced seizure threshold (6). KCC3 also plays an important role in regulating cell proliferation (7). Deafness and renal tubular acidosis were noted in mice lacking KCC4 (8).
Our previous studies highlight the important role of KCC in tumor development and progression. The malignant transformation of cervical epithelial cells is associated with the differential expression of volume-sensitive KCC activities (9). The loss-of-function KCC mutant cervical cancer cells exhibit inhibited cell growth accompanied by decreased activities of the cell cycle gene products and matrix metalloproteinase (MMP) (10). In addition, KCC activation by IGF-1 stimulation plays an important role in IGF-1 signaling to promote the growth and spread of gynecological cancers (11). However, most functional studies were done on KCl cotransport fluxes without knowing the molecular details. By overexpression or knockdown of specific KCC isoforms, we demonstrated that IGF-1 upregulates KCC3 and KCC4 which are differentially required for breast cancer cell proliferation and invasiveness (12). In addition, KCC3 overexpression downregulates E-cadherin/β-catenin complex formation by inhibiting transcription of the E-cadherin gene and accelerating degradation of β-catenin protein, thereby promoting epithelial-mesenchymal transition of cervical cancer cells (13).
IGF-1 and EGF are known to be overexpressed in most types of cancer tissues and notably contribute to cancer resistance to existing treatments (14–16). Our previous study also demonstrated that IGF-1 and EGF are two most potent stimulators for gynecological cancer cell invasiveness (17). This study aims to investigate the contribution of individual KCC isoforms in cancer metastasis. The results indicate that metastatic cancer tissues express abundant KCC4 which benefits cancer cells in invasiveness. IGF-1 and EGF stimulate the membrane recruitment of KCC4, in which KCC4 interacts with ezrin, an actin-binding protein, at lamellipodia. We thus propose that, in addition to ion transport, KCC4 can function as a membrane scaffold in the assembly of signal complexes.
Materials and Methods
Primary antibodies and reagents
Antibodies against KCC1, mannosidase II,β1 and αvβ3 integrin, and functional-blocking antibodies against α1, α4, β1 and αvβ3 integrin were purchased from Chemicon. KCC3 and KCC4 polyclonal antibodies were generated with the epitopes of KKARNAYLNNSNYEEGDEY and AERTEEPESPESVDQTSPT, respectively (18, 19). KCC1 polyclonal antibody was also generated against KCC1 C-terminal amino acids 1074–1085 (10). Antibodies against KCC4, EGF, calnexin, myosin Ib, Va, and VI were obtained from Santa Cruz Biotechnology. Antibody against IGF-1 was from Upstate Biotechnology. Antibodies against E-cadherin and ezrin were from BD Transduction Laboratories. Antibodies against phospho-p44/42 MAP kinase (Thr202/Tyr204) and phospho-Akt (Ser473) were from Cell Signaling Technology. IGF-1, EGF, MβCD, PD98059, LY294002, wortmannin, DIOA and antibody against β-actin were from Sigma-Aldrich.
Cell cultures, invasion assay and MMP zymography
Human cervical cancer SiHa cell line, ovarian cancer OVCAR-3 cell line, lung cancer AS-2 cell line and breast cancer T47D cell line were prepared as previously described (11, 20, 21). The clones of cervical cancer SiHa cell lines with KCC1, KCC3 or KCC4 overexpression were established previously (13). Invasive migration was done in the BD Matrigel invasion chamber (BD Biosciences) for 6 hours for cervical cancer SiHa cells and 12 hours for ovarian cancer OVCAR-3 cells and lung cancer AS-2 cells in serum-free culture medium at 37°C, as an index of invasive activity of tumor cells (17, 22). Conditioned media from the invasion assays was cleared of cells and debris by centrifugation at 3,000g for 10 min. MMP-2 activity was measured in conditioned media by gelatin zymography (23).
Surgical specimens, laser microdissection and real-time RT-PCR
From January 1999 to November 2001, 150 cervical cancer patients with FIGO staging Ib-IIa were scheduled for radical hysterectomy and pelvic lymphadenectomy at National Cheng Kung University Hospital, Taiwan. Patients who had undergone the loop electro-surgical excision for the transformation zone of uterine cervix before radical hysterectomy and who had unusual histopathology such as small cell and adenosquamous carcinoma were excluded. Paraffin sections of surgical specimens were immunostained with polyclonal KCC4 antibody, followed by Alexa 488-labeled secondary antibody and Hoechst 33258 (Molecular Probes). At least five sections of each specimen were analyzed for histology and graded for KCC4 expression over 15–20 high-power fields, examined by two investigators trained in gynecological pathology. Low grade indicates the distribution of KCC4 staining is less than 50% of tumor area, whereas high grade indicates the distribution of KCC4 staining is more than 50% of tumor area. For laser microdissection, frozen specimens of cervical squamous carcinoma or ovarian serous adenocarcinoma were obtained at the same hospital. These cases contained the following tissues representing cancer metastatic process: [1] normal tissues; [2] primary tumor tissues; and [3] metastatic tumor tissues. The preparation protocol for laser microdissection and the detail of amplification primers and probes were described elsewhere (13). The RNA levels of individual KCC isoforms in the surgical specimens were examined by real-time quantitative RT-PCR with the use of ABI Prism 7900 Analyzer and TaqMan probes (Applied Biosystems). cDNA was normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
siRNA
Cells were transfected with 100 nM of either targeting or control non-targeting siRNA using Lipofectamine 2000 (Invitrogen) for 48 hours. The functional assays were subsequently performed after validation of KCC4 or myosin Va knockdown. The siRNA targeting exon 24 of human KCC4 (NM_006598) (sense: 5′-GGUUGUCCAUAAAGAGGUUtt-3′; antisense: 5′-AACCUCUUUAUGGACAACCtt-3′) was from Ambion. The myosin Va siRNA pool of three duplexes (1, sense: 5′-GCAAGAAUGUGUUAGAGAAtt-3′; antisense: 5′-UUCUCUAACA CAUUCUUGCtt-3′; 2, s e n s e 5′-CAAGUGGCCUAUCUAGAAAtt-3′; antisense: 5′-UUUCUAGAUAGGCCACUUGtt-3′; 3, sense: 5′-CAAGCGUCAAGAACUAGAAtt-3′; antisense: 5′-UUCUAGUUCUUGACGCUUGtt-3′) targeting exon 18, 19 and 28 of human myosin Va (NM_000259) were from Santa Cruz Biotechnology.
Immunofluorescent images and quantitative analyses
Immunofluorescent studies were done by affinity-purified antibodies against various molecules. The fluorophores were excited by laser at 405, 488, or 543 nm and detected by a scanning confocal microscope (FV-1000, Olympus). Positive KCC4 membrane staining was defined as the distribution of KCC4 staining more than one third of the plasma membrane area. A pixel-by-pixel analysis by FV-1000 software was used to assess the colocalization of KCC4 with other molecules in confocal images.
Immunoblotting, surface biotinylation, raft fractionation and dot blot
For Western immunoblotting, the preparation of cell lysate was described in detail elsewhere (13). Plasma membrane proteins for surface biotinylation assay were isolated with the cell surface protein isolation kit (Pierce Biotechnology) according to the manufacturer’s instructions. Bands in the Western blots were quantified using ImageJ 1.30 software (NIH, USA) and normalized to the total protein fraction. For raft fractionation and dot blot, cells were harvested in the ice-cold protein lysis solution (50 mM Tris pH7.5, 150 mM NaCl) containing 1% Triton X-100 and a protease inhibitor mixture (Roche Diagnostics), and adjusted lysates to contain 40% iodixanol gradient medium Opti-prep™ (Sigma Aldrich). The lysates (2 ml) were placed in an ultracentrifuge tube and overlaid with 4 ml of lysis solution containing 30% iodixanol gradient medium and 2 ml of lysis solution containing 5% iodixanol gradient medium. The lysates were subjected to ultracentrifugation in a SW40Ti rotor (Beckman) for 18 hours at 40,000 rpm at 4°C. Eight fractions were collected. Proteins in each fraction were concentrated and precipitated with trichloroacetic acid, re-dissolved in lysis solution and subjected for immunoblotting.
Statistics
All values were reported as mean ± S.E.M. (standard error of the mean). Student’s paired, unpaired t test or Chi-square test was used for statistical analyses. Survival data were assessed by Kaplan Meier methods and differences were compared by the log-rank statistic. Differences between values were considered significantly when P< 0.05.
Results
KCC4 is abundant in metastatic tumor tissues
We first analyzed the expression patterns of KCC isoforms in the surgical specimens of cervical and ovarian carcinoma. We selected the cases of cervical cancer containing the following tissues for laser microdissection: [1] normal or non-cancerous squamous epithelia; [2] primary tumor tissues; [3] metastatic tumor tissues in the parametrium and pelvic lymph nodes (Fig. 1A). These tissues represented the progression of cervical carcinoma. Compared with normal or non-cancerous squamous epithelia, mRNA levels of KCC3 and KCC4 were significantly increased 25±3 fold and 7±3 fold in the primary tumor (n=8), respectively (Fig. 1A). In contrast, KCC4 was the most abundant KCC isoform in the metastatic tumor tissues, such as invaded parametrium or metastatic pelvic lymph nodes. Similar results were observed in the cases of ovarian cancer (Fig. 1B). The immunofluorescent stainings by different KCC isoform antibodies confirmed that KCC4 is abundant in metastatic tumor tissues (Fig. 1C).
Figure 1. KCC4 expression is associated with cancer metastasis and clinical outcome.
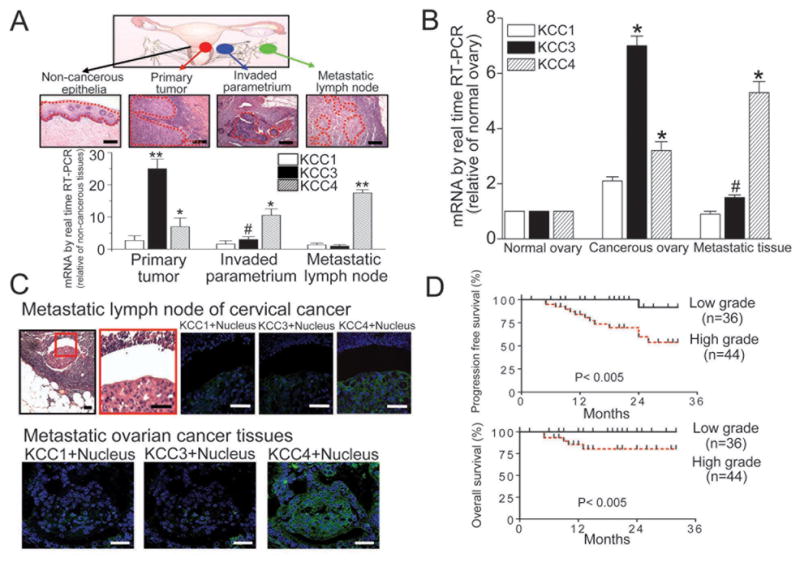
(A) & (B) KCC4 is abundant in metastatic tumor tissues. Laser microdissection with microscopic observation was utilized to sample the targeted tissues precisely (dashed line). The KCC mRNA levels were normalized against GAPDH. The KCC expression levels in normal squamous epithelia and normal ovaries were used as the control for the cases of cervical cancer and ovarian cancer, respectively. Each column represents mean ± S.E.M. (n=8 for cervical cancer; n=6 for ovarian cancer). #P<0.05; *P<0.01; **P<0.001 by paired t test. Scale bar, 10μm. (C) The expression pattern of KCC family in metastatic pelvic lymph nodes of cervical cancer (representative images of 6 different cases; scale bar, 5μm) and metastatic tissues of ovarian cancer (representative images of 6 different cases; scale bar, 10μm). (D) The association between KCC4 expression and clinical outcome at early stage cervical cancer. Cervical cancer patients were grouped by KCC4 grading and the survival data analyzed accordingly.
The association between KCC4 expression and clinical outcome was studied. KCC4 protein was scanty in non-cancerous cervical squamous epithelial tissues (n=80). In contrast, primary cancerous tissues of cervix clearly expressed KCC4 protein at different levels (Supplementary Fig. 1). The patients were grouped by KCC4 grading and the survival data were analyzed accordingly. Compared with low grade KCC4 expression, primary tumor with high grade KCC4 expression presented the significantly higher percentage of parametrium invasion and pelvic lymph node metastasis, which are two major poor prognostic factors for early-stage cervical cancer (Table 1). Consistently, increased KCC4 expression was associated with the poor clinical outcome (Fig. 1D).
(A) Table 1.
Clinical characteristics of cervical cancer patients grouped by KCC4 grading
| Characteristic | Low grade (N=36) | High grade (N=44) | P value |
|---|---|---|---|
| Age – year | |||
| Mean | 51 | 49 | 0.65 |
| Range | 31 – 66 | 37 – 69 | |
| Histology - no. (%) | |||
| Squamous cell carcinoma | 30 (83%) | 37 (84%) | 1.0 |
| Adenocarcinoma | 6 (17%) | 7 (16%) | |
| FIGO staging - no. (%) | |||
| Ib | 36 (100%) | 41 (93%) | 0.25 |
| IIa | 0 (0%) | 3 (7%) | |
| Parametrium invasion - no. (%) | 2 (6%) | 22 (50%) | P<0.0001 |
| Lymph node metastasis - no. (%) | 3 (8%) | 23 (52%) | P<0.0001 |
Low grade indicates that the distribution of KCC4 staining is less than 50% of tumor area, whereas high grade indicates that the distribution of KCC4 staining is more than 50% of tumor area. Unpaired t test or Chi-square test was used for statistical analyses.
To study KCC-mediated tumor behavior, we have established previously various clones of cervical cancer SiHa cell lines with KCC1, KCC3 or KCC4 overexpression (13). Among these clones, KCC4 was dominant in enhancing cancer cell invasiveness (Supplementary Fig. 2A). In addition, KCC4 knockdown by siRNA reduced the invasive migration of ovarian cancer OVCAR-3 cells in parallel with decreased MMP-2 activity (Supplementary Fig. 2B).
IGF-1 and EGF stimulate KCC4 membrane trafficking
Interestingly, in 80% of surgical specimens we examined, KCC4 protein colocalized with IGF-1 or EGF in metastatic ovarian cancer tissues and metastatic lymph nodes of cervical cancer (Supplementary Fig. 3A & B). We studied whether KCC4 expression is important for IGF-1 or EGF-stimulated cancer cell invasiveness. As shown in Supplementary Fig. 3C, endogenous invasiveness of ovarian cancer OVCAR-3 cells was significantly attenuated by KCC4 knockdown and residual invasiveness was much less sensitive to either IGF-1 or EGF stimulation.
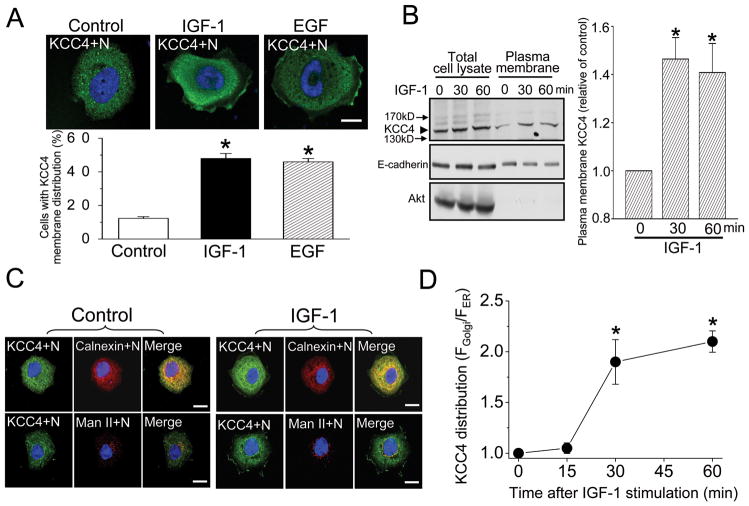
We have previously demonstrated that the chronic treatment (>12 hours) of IGF-1 increased KCC4 biosynthesis in cancer cells (11, 12). Here we studied the dynamic process of KCC4 in the acute response to growth factor. Most ovarian cancer OVCAR-3 cells (>85%) displayed the quiescent phenotype in the absence of growth factor, in which KCC4 randomly scattered in the cytosol with punctuate spots representing KCC4-containing vesicles (Fig. 2A). Upon IGF-1 or EGF stimulation for 30 minutes, more than 45% of OVCAR-3 cells exhibited pronounced KCC4 staining with continuous distribution or punctuate clusters in the juxta-membrane region of lamellipodia (Fig. 2A). IGF-1 and EGF also induced the membrane recruitment of KCC4 in migratory lung, cervical and breast cancer cells (Supplementary Fig. 4). In a surface biotinylation experiment, IGF-1 increased membrane KCC4 abundance by 50%, but total KCC4 amount was not changed (Fig. 2B). These results indicate that IGF-1 and EGF induce KCC4 membrane trafficking.
Figure 2. Membrane trafficking of KCC4.
(A) EGF and IGF-1 stimulate the membrane recruitment of KCC4. Ovarian cancer OVCAR-3 cells were incubated in the absence (control group) or presence of EGF (100 ng/ml) or IGF-1 (100 ng/ml) for 30 min. Upper panel: representative pictures from 6 different experiments. Lower panel: the quantitative analyses of KCC4 membrane trafficking in response to growth-factor stimulation. Each column represents mean ± S.E.M. of at least 250 cells. *P<0.01 by unpaired t test. N:nucleus. Scale bar, 10 μm. (B) IGF-1 increases KCC4 surface expression, detected by surface biotinylation assay. Total cell lysates were prepared in parallel for comparison. Akt: a marker of cytosolic protein; E-cadherin: an internal control of membrane protein. Left panel: a representative immunoblot. Right panel: the densitometric quantification of KCC4 surface expression. Each column represents mean ± S.E.M. (n=3). *P<0.01 by paired t test (C) & (D) IGF-1 stimulates KCC4 redistribution between ER and Golgi. (C) Representative confocal images of ovarian cancer OVCAR-3 cells incubated with or without 100 ng/ml IGF-1 for 30 min. Calnexin: ER marker protein; Man II: mannosidase II, Golgi marker protein. Scale bar, 10 μm. (D) The ratio of mean fluorescence intensity of KCC4 in the Golgi (FGolgi) over its mean fluorescence intensity in the ER (FER) was calculated at different time points and used as a measurement of KCC4 redistribution. Each points represents mean ± S.E.M. of at least 60 cells. *P<0.01 by paired t test
We analyzed the juxta-nuclear location of KCC4 aggregation. A large proportion of juxta-nuclear KCC4 colocalized with ER marker protein calnexin in the quiescent cells (Fig. 2C). A small proportion of juxta-nuclear KCC4 was located at the Golgi, as shown by the colocalization of KCC4 with Golgi marker protein mannosidase II. IGF-1 increased a large proportion of juxta-nuclear KCC4 accumulated at the Golgi. The redistribution of KCC4 between ER and Golgi became apparent in a time-dependent manner (Fig. 2D), which was consistent with the increased KCC4 abundance at the plasma membrane. This suggests that IGF-1 stimulation of KCC4 membrane trafficking is via a direct transport pathway, i.e. from ER to Golgi, then targeting to the plasma membrane.
The regulatory mechanisms of KCC4 membrane trafficking
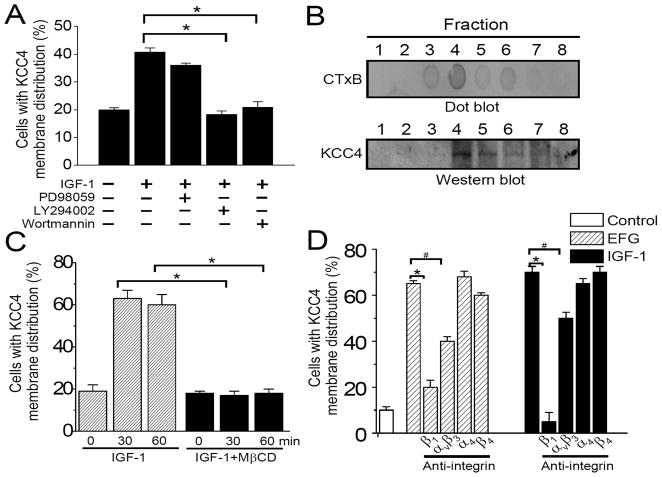
PI3K and mitogen-activated protein kinase represent the distinct signaling pathways that mediate the biological functions of IGF-1 (24). In ovarian cancer OVCAR-3 cells, IGF-1 treatment resulted in Erk1/2 and Akt phosphorylation that were abolished by specific inhibitors (Supplementary Fig. 5). These specific inhibitors decreased IGF-1 stimulation of KCC4 trafficking to different extents (Fig. 3A), indicating that PI3K activation is the dominant signalings controlling KCC4 recruitment.
Figure 3. The regulatory mechanisms of KCC4 recruitment.
(A) Signals regulating KCC4 surface expression. OVCAR-3 cells were pre-incubated with different inhibitors (20 μM LY294002, 100 nM wortmannin or 50 μM PD98059) for 30 min and then exposed to IGF-1 (100 ng/ml) stimulation for 30 min. The confocal imaging analyses were performed after immunofluorescent staining with KCC4. (B) KCC4 mainly distributes in the fractions of lipid rafts after IGF-1 stimulation. Lipid rafts were isolated as described in Materials and Methods. CT×B: cholera toxin subunit B, the lipid raft marker. (C) Lipid rafts are necessary for KCC4 membrane trafficking. OVCAR-3 cells were pre-incubated without or with MβCD (10 mM) for 30 min to disrupt lipid rafts prior to IGF-1 stimulation. (D) Integrin signaling is involved in KCC4 recruitment. Before IGF-1 or EGF stimulation, OVCAR-3 cells were pretreated with different functional-blocking monoclonal antibodies (15 μg/ml) against integrin for 30 min. Each column in (C) & (D) represents mean ± S.E.M. of at least 150 cells. #P<0.05; *P<0.01 by unpaired t test.
Lipid rafts, the membrane microdomain rich in cholesterol and sphingolipid, have been implicated in recruiting several ion transport systems to form signaling complexes (25, 26). KCC4 mainly distributed in the lipid raft fractions after IGF-1 treatment (Fig. 3B). Moreover, membrane recruitment of KCC4 was markedly reduced by MβCD-induced cholesterol depletion (Fig. 3C). Integrin signals may regulate the location of lipid rafts and thereby control domain-specific signaling events in anchorage-dependent cells (27, 28). Integrin expressions such as αvβ3,β1, α4 and β4 have been reported in human ovarian cancer cells (29). Functional-blocking monoclonal antibody against integrin αvβ3 or β1, but not α4, orβ4, inhibited significantly IGF-1 or EGF-stimulated KCC4 membrane trafficking (Fig. 3D). Thus, in addition to EGF and IGF-1 receptor signaling, integrin signaling participates in regulating membrane recruitment of KCC4.
KCC4 trafficking depends on actin-associated myosin
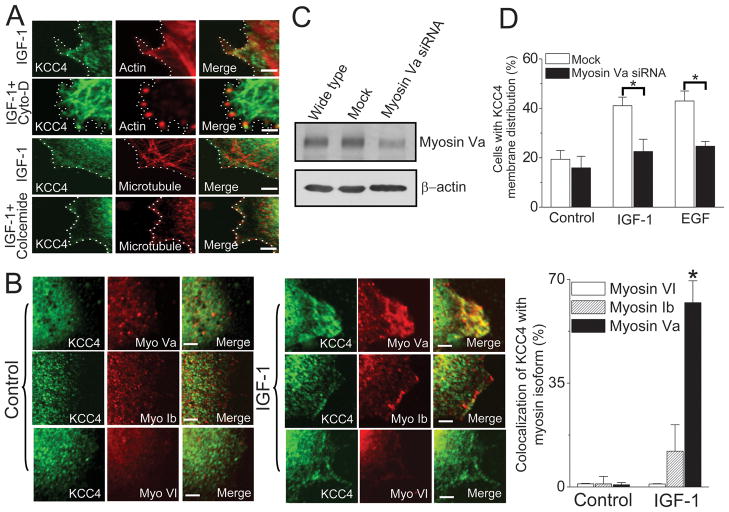
Protein trafficking between organelles usually depends on actin filaments with myosin motors or microtubules with associated motors (30). Upon IGF-1 stimulation, the complex networks of actin filament and microtubule were apparent at juxta-membrane area, where KCC4 seemed to colocalize with actin filament (Fig. 4A). Cytochalasin D disrupted actin filaments into discontinuous spots and, as a consequence, KCC4 aggregated in the cytosol (Fig. 4A). This indicates that KCC4 membrane trafficking was abolished by cytochalasin D. In contrast, the collapse of microtubule complex induced by colcemide showed little effect on KCC4 membrane expression.
Figure 4. Motor protein-dependent KCC4 trafficking.
(A) KCC4 recruitment is along actin cytoskeleton. Representative confocal images of KCC4 and cytoskeleton in IGF-1-stimulated ovarian cancer OVCAR-3 cells. Cells were pre-incubated with cytochalasin D (cyto-D, 1μg/ml) or colcemide (10 μg/ml) for 60 min to disrupt actin filaments or microtubule, respectively, prior to 100 ng/ml IGF-1 stimulation. Dashed line: cell periphery. Scale bar, 2μm. (B) Myosin Va motor protein powers KCC4 membrane trafficking. Left panel: Confocal images of KCC4 and actin-associated motors, such as myosin Va (myo Va), myosin Ib (myo Ib) and myosin VI (myo VI), in the absence (control) or presence of IGF-1 stimulation. Right panel: the colocalization ratio between KCC4 and actin-associated motors at juxta-plasma membrane area with the pixel-by-pixel analyses. Scale bar, 2μm. (C) & (D): The effects of Myosin Va knockdown on growth factor (100 ng/ml)-stimulated KCC4 surface expression. Each column represents mean ± S.E.M. of at least 150 cells. *P<0.01 by unpaired t test.
We dissected the actin-associated motors in charge of KCC4 membrane trafficking. Myosin Ib, Va and VI are well known for powering various intracellular trafficking among actin-associated motors (30). The dynamics of myosin Ib, Va and VI were sensitive to IGF-1 stimulation (Fig. 4B). Among them, KCC4 apparently colocalized with myosin Va at lamellipodia of migratory OVCAR-3 cells (Fig. 4B). The EGF and IGF-1 effect on increasing the surface expression of KCC4 was significantly attenuated in the presence of myosin Va-specific siRNA (Fig. 4C & 4D), indicating that myosin Va motor protein powers dominantly KCC4 membrane trafficking along actin cytoskeleton.
KCC4 functions as a plasma membrane scaffold
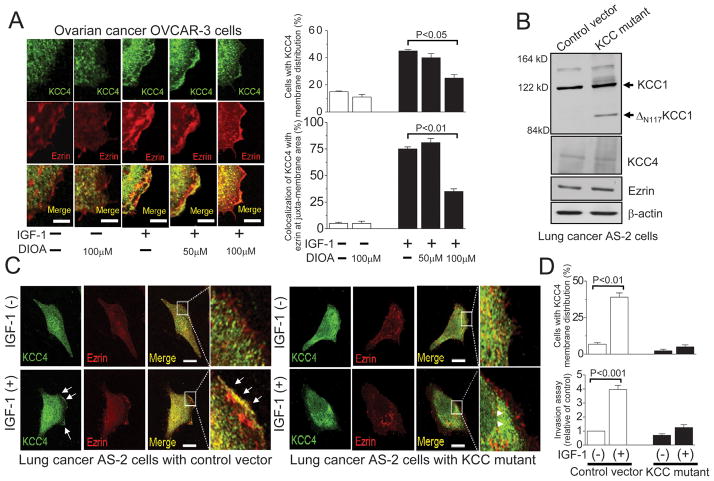
We are interested in knowing whether KCC4 functions as a membrane scaffold protein to facilitate the cytoskeletal reorganization that is required for the invasive migration. The neuron-specific KCC2 has been demonstrated to have a structural interaction with actin-binding ezrin/radixin/moesin (ERM) proteins that is important for the maturation of dendritic spines (31). The ERM family is associated with a number of membrane proteins at the cytoplasmic face of the plasma membrane and is involved in epithelial cell migration and adhesion (31). As shown in Fig. 5A, IGF-1 induced the extensive formation of lamellipodia, where KCC4 was associated with ezrin, as demonstrated by quantitative pixel-by-pixel analyses of confocal images. DIOA, a KCC inhibitor, inhibited the membrane recruitment of KCC4 as well as ezrin in a concentration-dependent manner (Fig. 5A). We also overexpressed the loss-of-function KCC mutant in lung cancer AS-2 cells to study the functional role of KCC4 as a membrane scaffold. The previous studies have demonstrated that removal of the N-terminal 117 amino acids (ΔN117KCC1) from KCC1 produced a dominant-negative loss-of-function phenotype for KCl cotransport in different types of cells (10–12, 32). Overexpression of ΔN117KCC1 in AS-2 cells did not change the cellular levels of KCC4 and ezrin (Fig. 5B). Upon IGF-1 stimulation, more than 40% of AS-2 cells exhibited pronounced KCC4 distribution at or near surface area, where ezrin apparently colocalized with KCC4 at lamellipodia (arrows in Fig. 5C). The loss-of-function KCC mutant cells presented a striking contrast that KCC4 membrane trafficking and ezrin recruitment were almost abolished regardless of IGF-1 stimulation (arrowheads in Fig. 5C & 5D). More importantly, endogenous invasiveness of AS-2 cells was significantly attenuated in loss-of-function KCC mutant cells and the residual invasiveness was much less sensitive to IGF-1 stimulation. These results indicate that KCC activity is necessary for the membrane recruitment of KCC4 as well as ezrin.
Figure 5. KCC activity is necessary for the membrane recruitment of KCC4 and ezrin.
(A) IGF-1 induced the extensive formation of lamellipodia, where KCC4 was associated with ezrin. DIOA, the KCC inhibitor, inhibited the colocalization of KCC4 and ezrin in a concentration-dependent manner. Ovarian cancer OVCAR-3 cells were pre-incubated with DIOA for 30 min, prior to 100 ng/ml IGF-1 stimulation. Each column for image analysis represents mean ± S.E.M. of at least 60 cells. Scale bar, 2μm. (B) Overexpression of the loss-of-function KCC mutant (ΔN117KCC1) in lung cancer AS-2 cells. ΔN117KCC1: removal of the N-terminal 117 amino acids from KCC1. (C) The membrane recruitments of KCC4 and ezrin were almost abolished in loss-of-function KCC mutant cancer cells regardless of IGF-1 stimulation. Arrow: the association of KCC4 with ezrin at lamellipodia; Arrowhead: the cytosolic aggregation of KCC4. Scale bar, 5 μm. (D) The invasion assays and quantitative analyses of KCC4 recruitment were performed in different clones of lung cancer AS-2 cells. Each column in invasion assay represents mean ± S.E.M. from at least 5 different experiments. The invasive ability of cells with empty vector was used as the control. For image analysis, each column represents mean ± S.E.M. of at least 50 cells. Statistics were done by unpaired t test.
Discussion
This study stresses the important role of KCC4 in tumor malignant behavior. We show that ovarian and cervical cancer cells benefit from KCC4 overexpression in the invasive migration. This conclusion is supported by the following evidence: (i) KCC4 is overexpressed in the metastatic ovarian and cervical cancer tissues, at both mRNA and protein levels; (ii) KCC4 overexpression enhances cancer cell invasiveness, whereas KCC4 knockdown attenuates that. IGF-1 and EGF are two potent growth factors which stimulate cancer invasiveness and metastasis (17). Our previous studies showed that the chronic treatment with IGF-1 upregulates KCC biosynthesis which plays an important role in IGF-1 signaling to promote growth and spread of gynecological cancers (11, 12). Here we highlight the novel mechanisms by which IGF-1 and EGF increase KCC4 surface expression. IGF-1 and EGF stimulate KCC4 recruitment from a presumably inactive cytoplasmic pool such as ER and Golgi, to the active membranous target pool along the actin cytoskeleton. PI3K activation is the dominant signal controlling KCC4 trafficking. Throughout the trafficking process, KCC4 is incorporated into lipid rafts which function as a platform for the association between KCC4 and lipid raft-associated myosin motor. The high correlation of KCC4 expression with growth factors in metastatic cancer tissues suggests a likely in vivo stimulation of KCC4 function and biosynthesis by IGF-1 or EGF which contributes to cancer invasiveness and metastasis. Taken together with our previous studies, the mechanisms by which growth factors increase KCC4 surface expression could be summarized as follows. The increased cellular functions of KCC4 are highly dependent on the upregulation of de novo protein synthesis which occurs within hours, as well as the regulation of dynamic protein trafficking from inactive cytoplasmic pools to the active membranous ones which mostly takes several minutes and can respond to stimulation immediately. The membrane dynamics of KCC abundance have been described in neurons and lens. Oxidative stress and seizure attack induced a rapid loss of tyrosine phosphorylation of KCC2 that resulted in translocation of the protein and functional loss of transport activity (33). Osmotic stress could modulate the subcellular localization of KCC isoforms to different extents in peripheral fiber cells of lens (34).
We considered three possible explanations for the potent effect of KCC4 overexpression on cancer invasion and metastasis. First and most simply, KCC4 abundance changes the regulatory mechanisms of cell volume control. Cancer invasion is a complex process of cell adhesion, migration, and secretion of different classes of enzymes (35). Cell migration involves substantial alterations of cell volume and thus volume regulatory mechanisms are certainly activated (36, 37). Volume-sensitive KCC activity significantly contributes to the volume regulation of cervical and ovarian cancer cells (10, 11). KCC4 is one of the KCC isoforms critically involved in cell volume regulation (6). Cellular invasiveness is thus affected by KCC4 overexpression. Second, KCC activity appears to be associated with MMP activation (10). An important event in cancer invasiveness is the activation of MMP cascade to dissolve the basement membrane matrix (38, 39). We previously demonstrated that the loss-of-function KCC mutant cervical cancer cells exhibited inhibited cellular invasiveness accompanied by decreased MMP activity (10). Here we show that KCC4-specific siRNA reduced cellular invasiveness in parallel by decreased MMP-2 activity. Third, and perhaps more importantly, KCC4 may function as a plasma membrane scaffold with ezrin in the assembly of cytoskeletal reorganization complex. The association of actin cytoskeleton with the plasma membrane regulates the integrity of the cortical cytoskeleton and maintains cell shape and adhesion that are required for cellular invasive migration. The cytoskeletal and signaling functions of plasma membrane ion transporters have been described for KCC2 (31) and NHE1 (40–42). Further work will be required to determine whether the KCC4-associated changes in intracellular Cl−and K+ concentration and ion transporter-independent function of KCC4 act cooperatively to regulate cell invasion.
In conclusion, we show that IGF-1 and EGF stimulate the recruitment of KCC4 from the inactive storage pool in the cytosol to the active membranous target pool at lamellipodia through a mechanism involving PI3K activation and myosin Va-actin trafficking routes. KCC4 is incorporated into lipid rafts throughout the trafficking process. Furthermore, KCC4 functions as a plasma membrane scaffold protein to facilitate the modulation of cytoskeletal reorganization via the association with ezrin that is required for cellular invasive migration (summarized in supplementary Fig. 6). Blockade of KCC4 recruitment to the plasma membrane may provide a potential target for the prevention of IGF-1 and EGF-dependent invasive metastasis in cancers of epithelial origin.
Supplementary Material
Acknowledgments
This work was partly supported by National Science Council, Taiwan, Center of Excellence for Clinical Trial & Research (DOH-TD-B-111-004), Department of Health, Executive Yuan, Taiwan, Center for Gene Regulation and Signal Transduction Research, Center for Micro/Nano Science and Technology, National Cheng Kung University, Taiwan and Medical Research Council (MRC grant G0700759), UK.
Abbreviations
- DIOA
[(dihydroindenyl)oxy]alkanoic acid
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- Erk1/2
extracellular signal-related protein kinases 1/2
- IGF-1
insulin-like growth factor 1
- KCC
potassium chloride cotransporter
- MβCD
methyl-β-cyclodextrin
- MMP
matrix metalloproteinase
- PI3K
phosphatidylinositol 3′-kinase
- RT-PCR
reverse transcription-polymerase chain reaction
- siRNA
small interfering RNA
Footnotes
The authors declare no conflict of interest.
References
- 1.Adragna NC, Di Fulvio M, Lauf PK. Regulation of K-Cl cotransport: from function to genes. J Membr Biol. 2004;201:109–37. doi: 10.1007/s00232-004-0695-6. [DOI] [PubMed] [Google Scholar]
- 2.Mercado A, Song L, Vazquez N, Mount DB, Gamba G. Functional comparison of the K+-Cl− cotransporters KCC1 and KCC4. J Biol Chem. 2000;275:30326–34. doi: 10.1074/jbc.M003112200. [DOI] [PubMed] [Google Scholar]
- 3.Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–24. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 4.Song L, Mercado A, Vazquez N, et al. Molecular, functional, and genomic characterization of human KCC2, the neuronal K-Cl cotransporter. Brain Res Mol Brain Res. 2002;103:91–105. doi: 10.1016/s0169-328x(02)00190-0. [DOI] [PubMed] [Google Scholar]
- 5.Rust MB, Alper SL, Rudhard Y, et al. Disruption of erythroid K-Cl cotransporters alters erythrocyte volume and partially rescues erythrocyte dehydration in SAD mice. J Clin Invest. 2007;117:1708–17. doi: 10.1172/JCI30630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boettger T, Rust MB, Maier H, et al. Loss of K-Cl cotransporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. EMBO J. 2003;22:5422–34. doi: 10.1093/emboj/cdg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen MR, Chou CY, Hsu KF, et al. The KCl cotransporter isoform KCC3 can play an important role in cell growth regulation. Proc Natl Acad Sci USA. 2001;98:14714–9. doi: 10.1073/pnas.251388798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter KCC4. Nature. 2002;416:874–8. doi: 10.1038/416874a. [DOI] [PubMed] [Google Scholar]
- 9.Shen MR, Chou CY, Ellory JC. Volume-sensitive KCl cotransport associated with human cervical carcinogenesis. Pflugers Archiv. 2000;440:751–60. doi: 10.1007/s004240000338. [DOI] [PubMed] [Google Scholar]
- 10.Shen MR, Chou CY, Hsu KF, et al. KCl cotransport is an important modulator of human cervical cancer growth and invasion. J Biol Chem. 2003;278:39941–50. doi: 10.1074/jbc.M308232200. [DOI] [PubMed] [Google Scholar]
- 11.Shen MR, Lin AC, Hsu YM, et al. Insulin-like growth factor 1 stimulates KCl cotransport, which is necessary for invasion and proliferation of cervical cancer and ovarian cancer cells. J Biol Chem. 2004;279:40017–25. doi: 10.1074/jbc.M406706200. [DOI] [PubMed] [Google Scholar]
- 12.Hsu YM, Chou CY, Chen HH, et al. IGF-1 upregulates electroneutral K-Cl cotransporter KCC3 and KCC4 which are differentially required for breast cancer cell proliferation and invasiveness. J Cell Physiol. 2007;210:626–36. doi: 10.1002/jcp.20859. [DOI] [PubMed] [Google Scholar]
- 13.Hsu YM, Chen YF, Chou CY, et al. KCl cotransporter-3 down-regulates E-cadherin/beta-catenin complex to promote epithelial-mesenchymal transition. Cancer Res. 2007;67:11064–73. doi: 10.1158/0008-5472.CAN-07-2443. [DOI] [PubMed] [Google Scholar]
- 14.Aznavorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumour cell invasion and metastasis. Cancer. 1993;71:1368–83. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 16.Brand FX, Ravanel N, Gauchez AS, et al. Prospect for anti-HER2 receptor therapy in breast cancer. Anticancer Res. 2006;26:463–70. [PubMed] [Google Scholar]
- 17.Shen MR, Hsu YM, Hsu KF, et al. Insulin-like growth factor 1 is a potent stimulator of cervical cancer cell invasiveness and proliferation that is modulated by αvβ3 integrin signaling. Carcinogenesis. 2006;27:962–71. doi: 10.1093/carcin/bgi336. [DOI] [PubMed] [Google Scholar]
- 18.Howard HC, Mount DB, Rochefort D, et al. The K-Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet. 2002;32:384–92. doi: 10.1038/ng1002. [DOI] [PubMed] [Google Scholar]
- 19.Karadsheh MF, Byun N, Mount DB, Delpire E. Localization of the KCC4 potassium-chloride cotransporter in the nervous system. Neuroscience. 2004;123:381–91. doi: 10.1016/j.neuroscience.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Yeh HH, Lai WW, Chen HH, Liu HS, Su WC. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene. 2006;25:4300–9. doi: 10.1038/sj.onc.1209464. [DOI] [PubMed] [Google Scholar]
- 21.Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14:4743–50. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- 22.Albini A, Iwamoto Y, Kleinman HK, et al. Chemotaxis of 3T3 and SV3T3 cells to fibronectin is mediated through the cell-attachment site in fibronectin and a fibronectin cell surface receptor. J Cell Biol. 1987;105:1867–72. doi: 10.1083/jcb.105.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamani MH, Tuzcu EM, Starling RC, et al. Myocardial ischemic injury after heart transplantation is associated with upregulation of vitronectin receptor (αvβ3), activation of the matrix metalloproteinase induction system, and subsequent development of coronary vasculopathy. Circulation. 2002;105:1955–61. doi: 10.1161/01.cir.0000014971.09169.bc. [DOI] [PubMed] [Google Scholar]
- 24.Kim B, van Golen CM, Feldman EL. Insulin-like growth factor-I signaling in human neuroblastoma cells. Oncogene. 2004;23:130–41. doi: 10.1038/sj.onc.1206924. [DOI] [PubMed] [Google Scholar]
- 25.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 26.Martens JR, O’Connell K, Tamkun M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends Pharmacol Sci. 2004;25:16–21. doi: 10.1016/j.tips.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 27.del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RGW, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–42. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 28.Gaus K, Le Lay S, Balasubramanian N, Schwartz MA. Integrin-mediated adhesion regulates membrane order. J Cell Biol. 2006;174:725–34. doi: 10.1083/jcb.200603034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of β1 and αvβ3 integrins in ovarian cancer. Gynecol Oncol. 1995;58:216–25. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- 30.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Khirug S, Cai C, et al. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56:1019–33. doi: 10.1016/j.neuron.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 32.Casula S, Shmukler BE, Wilhelm S, et al. A dominant negative mutant of the KCC1 K-Cl cotransporter: both N- and C-terminal cytoplasmic domains are required for K-Cl cotransport activity. J Biol Chem. 2001;276:41870–8. doi: 10.1074/jbc.M107155200. [DOI] [PubMed] [Google Scholar]
- 33.Wake H, Watanabe M, Moorhouse AJ, et al. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J Neurosci. 2007;27:1642–50. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chee KS, Kistler J, Donaldson PJ. Roles for KCC transporters in the maintenance of lens transparency. Invest Ophthalmol Vis Sci. 2006;47:673–82. doi: 10.1167/iovs.05-0336. [DOI] [PubMed] [Google Scholar]
- 35.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 36.Lang F, Busch GL, Ritter M, et al. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 37.Ritter M. Cell volume regulatory ion transport in cell migration. Contrib Nephrol. 1998;123:135–57. doi: 10.1159/000059910. [DOI] [PubMed] [Google Scholar]
- 38.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 39.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 40.Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL. Direct binding of the Na--H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol Cell. 2000;6:1425–36. doi: 10.1016/s1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 41.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–96. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang Y, Chou CY, Hsu KF, Huang YF, Shen MR. EGF upregulates Na+/H+ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J Cell Physiol. 2008;214:810–9. doi: 10.1002/jcp.21277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.