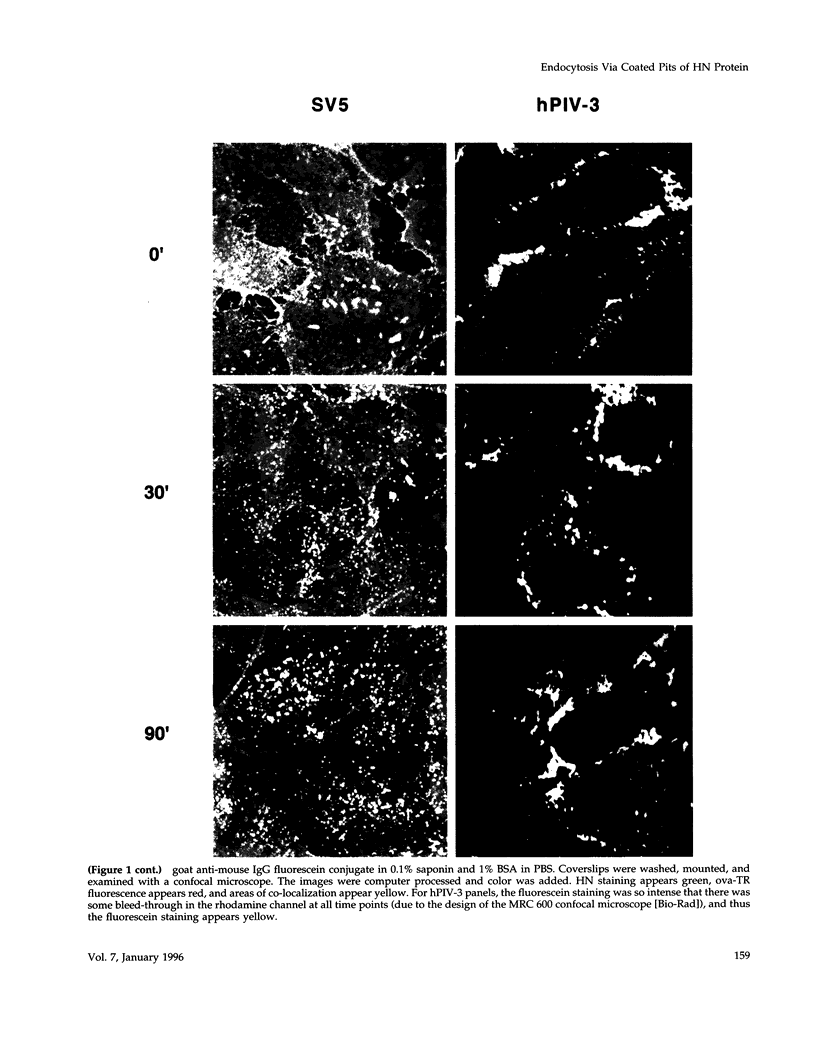
Abstract
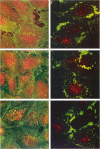
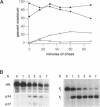
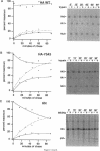
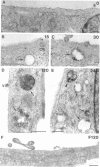
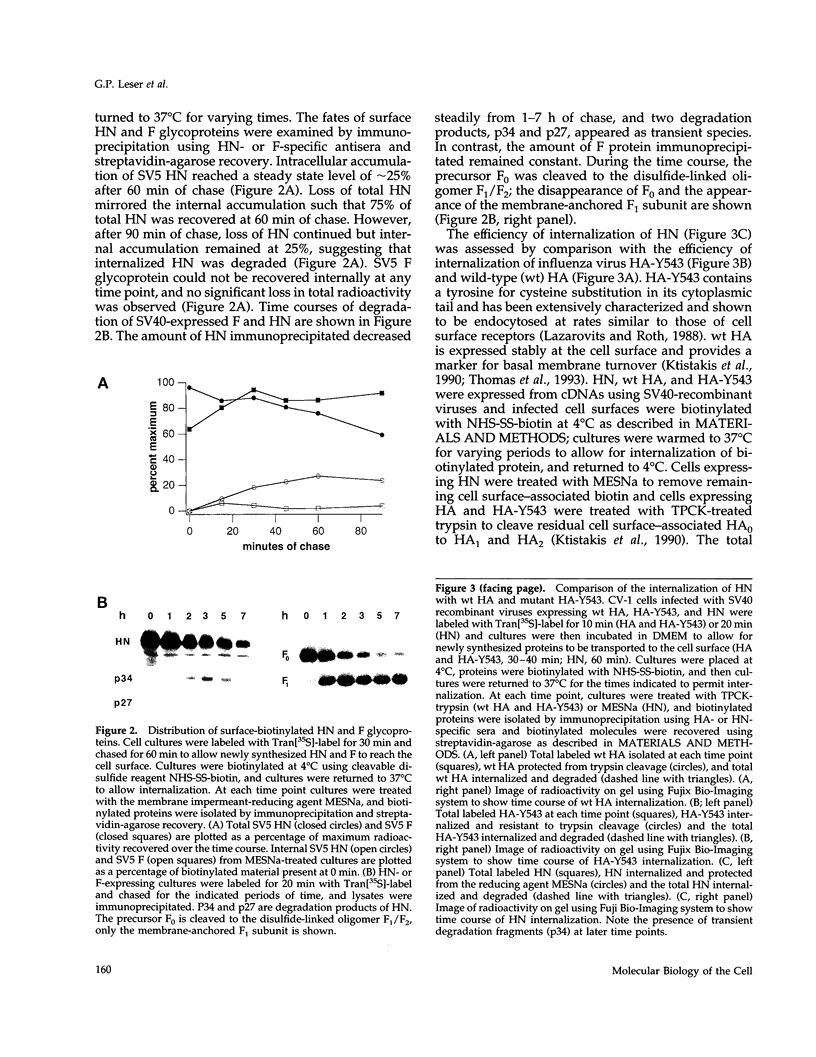
The hemagglutinin-neuraminidase (HN) and fusion (F) glycoproteins of the paramyxovirus simian virus 5 (SV5) are expressed on the surface of virus-infected cells. Although the F protein was found to be expressed stably, the HN protein was internalized from the plasma membrane. HN protein lacks known internalization signals in its cytoplasmic domain that are common to many integral membrane proteins that are internalized via clathrin-coated pits. Thus, the cellular pathway of HN protein internalization was examined. Biochemical analysis indicated that HN was lost from the cell surface with a t1/2 of approximately 45-50 min and turned over with a t1/2 of approximately 2 h. Immunofluorescent analysis showed internalized SV5 HN in vesicle-like structures in a juxtanuclear pattern coincident with the localization of ovalbumin. In contrast the SV5 F glycoprotein and the HN glycoprotein of the highly related parainfluenza virus 3 (hPIV-3) were found only on the cell surface. Immunogold staining of HN on the surface of SV5-infected CV-1 cells and examination using electron microscopy, showed heavy surface labeling that gradually decreased with time. Concomitantly, gold particles were detected in the endosomal system and with increasing time, gold-labeled structures having the morphology of lysosomes were observed. On the plasma membrane approximately 5% of the gold-labeled HN was found in coated pits. The inhibition of the pinching-off of coated pits from the plasma membrane by cytosol acidification significantly reduced HN internalization. Internalized HN was co-localized with gold-conjugated transferrin, a marker for the early endosomal compartments, and with gold-conjugated bovine serum albumin, a marker for late endosomal compartments. Taken together, these data strongly suggest that the HN glycoprotein is internalized via clathrin-coated pits and delivered to the endocytic pathway.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond B. D., Eidels L. The cytoplasmic domain of the diphtheria toxin receptor (HB-EGF precursor) is not required for receptor-mediated endocytosis. J Biol Chem. 1994 Oct 28;269(43):26635–26641. [PubMed] [Google Scholar]
- Alvarez E., Gironès N., Davis R. J. A point mutation in the cytoplasmic domain of the transferrin receptor inhibits endocytosis. Biochem J. 1990 Apr 1;267(1):31–35. doi: 10.1042/bj2670031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G., Brown M. S., Goldstein J. L. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977 Mar;10(3):351–364. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Goldstein J. L., Brown M. S. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977 Dec 22;270(5639):695–699. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- Balch W. E., McCaffery J. M., Plutner H., Farquhar M. G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994 Mar 11;76(5):841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Bansal A., Gierasch L. M. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991 Dec 20;67(6):1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986 Sep 5;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., Casanova J. E., McKinnon W. C., Mostov K. E. Deletions in the cytoplasmic domain of the polymeric immunoglobulin receptor differentially affect endocytotic rate and postendocytotic traffic. J Biol Chem. 1990 Aug 15;265(23):13750–13757. [PubMed] [Google Scholar]
- Bretscher M. S. Circulating integrins: alpha 5 beta 1, alpha 6 beta 4 and Mac-1, but not alpha 3 beta 1, alpha 4 beta 1 or LFA-1. EMBO J. 1992 Feb;11(2):405–410. doi: 10.1002/j.1460-2075.1992.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S. Q., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990 Nov 30;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Davis C. G., Lehrman M. A., Russell D. W., Anderson R. G., Brown M. S., Goldstein J. L. The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell. 1986 Apr 11;45(1):15–24. doi: 10.1016/0092-8674(86)90533-7. [DOI] [PubMed] [Google Scholar]
- Davis C. G., van Driel I. R., Russell D. W., Brown M. S., Goldstein J. L. The low density lipoprotein receptor. Identification of amino acids in cytoplasmic domain required for rapid endocytosis. J Biol Chem. 1987 Mar 25;262(9):4075–4082. [PubMed] [Google Scholar]
- Davis J. E., Cresswell P. Lack of detectable endocytosis of B lymphocyte MHC class II antigens using an antibody-independent technique. J Immunol. 1990 Feb 1;144(3):990–997. [PubMed] [Google Scholar]
- Doms R. W., Lamb R. A., Rose J. K., Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993 Apr;193(2):545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Draye J. P., Courtoy P. J., Quintart J., Baudhuin P. A quantitative model of traffic between plasma membrane and secondary lysosomes: evaluation of inflow, lateral diffusion, and degradation. J Cell Biol. 1988 Dec;107(6 Pt 1):2109–2115. doi: 10.1083/jcb.107.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez V., Dautry-Varsat A. Receptor-mediated endocytosis of interleukin 2 in a human tumor T cell line. Degradation of interleukin 2 and evidence for the absence of recycling of interleukin receptors. J Biol Chem. 1986 Nov 25;261(33):15450–15454. [PubMed] [Google Scholar]
- Eskelinen S., Kok J. W., Sormunen R., Hoekstra D. Coated endosomal vesicles: sorting and recycling compartment for transferrin in BHK cells. Eur J Cell Biol. 1991 Dec;56(2):210–222. [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990 May 18;61(4):623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Fuhrer C., Geffen I., Spiess M. Endocytosis of the ASGP receptor H1 is reduced by mutation of tyrosine-5 but still occurs via coated pits. J Cell Biol. 1991 Aug;114(3):423–431. doi: 10.1083/jcb.114.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993 Mar;120(5):1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C. E., Felder S., Schlessinger J., Ullrich A., Hopkins C. R. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol. 1993 Jan;120(1):77–83. doi: 10.1083/jcb.120.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Fixman A., Simionescu M., Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986 Apr;102(4):1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J. N., Conibear E., Pearse B. M. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989 Apr;8(4):1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodinsky A., Harris D. A. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995 May;129(3):619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Back R., Marsh M. A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J Cell Biol. 1989 Dec;109(6 Pt 1):2703–2720. doi: 10.1083/jcb.109.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. H., Sandvig K., van Deurs B. Internalization efficiency of the transferrin receptor. Exp Cell Res. 1992 Mar;199(1):19–28. doi: 10.1016/0014-4827(92)90457-j. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Lamb R. A. Cell surface expression of glycosylated, nonglycosylated, and truncated forms of a cytoplasmic protein pyruvate kinase. J Cell Biol. 1988 Sep;107(3):865–876. doi: 10.1083/jcb.107.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert S. W., Paterson R. G., Lamb R. A. Hemagglutinin-neuraminidase protein of the paramyxovirus simian virus 5: nucleotide sequence of the mRNA predicts an N-terminal membrane anchor. J Virol. 1985 Apr;54(1):1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E., Cohn Z. A. Vesicle fusion and formation at the surface of pinocytic vacuoles in macrophages. J Cell Biol. 1968 Sep;38(3):629–632. doi: 10.1083/jcb.38.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobman T. C., Woodward L., Farquhar M. G. The rubella virus E2 and E1 spike glycoproteins are targeted to the Golgi complex. J Cell Biol. 1993 Apr;121(2):269–281. doi: 10.1083/jcb.121.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L. Endocytosis. Curr Opin Cell Biol. 1989 Aug;1(4):675–683. doi: 10.1016/0955-0674(89)90033-1. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Harter C., Matter K., Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991 Sep 6;66(5):907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Iacopetta B. J., Rothenberger S., Kühn L. C. A role for the cytoplasmic domain in transferrin receptor sorting and coated pit formation during endocytosis. Cell. 1988 Aug 12;54(4):485–489. doi: 10.1016/0092-8674(88)90069-4. [DOI] [PubMed] [Google Scholar]
- Jing S. Q., Spencer T., Miller K., Hopkins C., Trowbridge I. S. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990 Feb;110(2):283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. A., Siegel M. W., Caras I. W. Endocytosis of glycophospholipid-anchored and transmembrane forms of CD4 by different endocytic pathways. EMBO J. 1992 Mar;11(3):863–874. doi: 10.1002/j.1460-2075.1992.tb05124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J., Ericsson M., Rottier P. J., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994 Jan;124(1-2):55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis N. T., Thomas D., Roth M. G. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990 Oct;111(4):1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovits J., Roth M. A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell. 1988 Jun 3;53(5):743–752. doi: 10.1016/0092-8674(88)90092-x. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann L. E., Eberle W., Krull S., Prill V., Schmidt B., Sander C., von Figura K., Peters C. The internalization signal in the cytoplasmic tail of lysosomal acid phosphatase consists of the hexapeptide PGYRHV. EMBO J. 1992 Dec;11(12):4391–4399. doi: 10.1002/j.1460-2075.1992.tb05539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Brown M. S., Russell D. W., Schneider W. J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985 Jul;41(3):735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987 Jun 5;49(5):669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- Lobel P., Fujimoto K., Ye R. D., Griffiths G., Kornfeld S. Mutations in the cytoplasmic domain of the 275 kd mannose 6-phosphate receptor differentially alter lysosomal enzyme sorting and endocytosis. Cell. 1989 Jun 2;57(5):787–796. doi: 10.1016/0092-8674(89)90793-9. [DOI] [PubMed] [Google Scholar]
- Masui H., Castro L., Mendelsohn J. Consumption of EGF by A431 cells: evidence for receptor recycling. J Cell Biol. 1993 Jan;120(1):85–93. doi: 10.1083/jcb.120.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K., Bainton D. F., Pesonen M., Louvard D., Genty N., Simons K. Transepithelial transport of a viral membrane glycoprotein implanted into the apical plasma membrane of Madin-Darby canine kidney cells. I. Morphological evidence. J Cell Biol. 1983 Sep;97(3):627–637. doi: 10.1083/jcb.97.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw T. E., Maxfield F. R. Human transferrin receptor internalization is partially dependent upon an aromatic amino acid on the cytoplasmic domain. Cell Regul. 1990 Mar;1(4):369–377. doi: 10.1091/mbc.1.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw T. E., Pytowski B., Arzt J., Ferrone C. Mutagenesis of the human transferrin receptor: two cytoplasmic phenylalanines are required for efficient internalization and a second-site mutation is capable of reverting an internalization-defective phenotype. J Cell Biol. 1991 Mar;112(5):853–861. doi: 10.1083/jcb.112.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Miettinen H. M., Rose J. K., Mellman I. Fc receptor isoforms exhibit distinct abilities for coated pit localization as a result of cytoplasmic domain heterogeneity. Cell. 1989 Jul 28;58(2):317–327. doi: 10.1016/0092-8674(89)90846-5. [DOI] [PubMed] [Google Scholar]
- Miller K., Shipman M., Trowbridge I. S., Hopkins C. R. Transferrin receptors promote the formation of clathrin lattices. Cell. 1991 May 17;65(4):621–632. doi: 10.1016/0092-8674(91)90094-f. [DOI] [PubMed] [Google Scholar]
- Montesano R., Roth J., Robert A., Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982 Apr 15;296(5858):651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Ciechanover A., Owen L. S., Lodish H. F. Intracellular transport of transferrin- and asialoorosomucoid-colloidal gold conjugates to lysosomes after receptor-mediated endocytosis. J Histochem Cytochem. 1985 Nov;33(11):1134–1144. doi: 10.1177/33.11.2997327. [DOI] [PubMed] [Google Scholar]
- Ng D. T., Randall R. E., Lamb R. A. Intracellular maturation and transport of the SV5 type II glycoprotein hemagglutinin-neuraminidase: specific and transient association with GRP78-BiP in the endoplasmic reticulum and extensive internalization from the cell surface. J Cell Biol. 1989 Dec;109(6 Pt 2):3273–3289. doi: 10.1083/jcb.109.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G., Joggerst B., Simons K. Regulated internalization of caveolae. J Cell Biol. 1994 Dec;127(5):1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6706–6710. doi: 10.1073/pnas.81.21.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988 Nov;7(11):3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Armes J. E., Griffiths G., Marsh M. Differential endocytosis of CD4 in lymphocytic and nonlymphocytic cells. J Exp Med. 1991 Mar 1;173(3):575–587. doi: 10.1084/jem.173.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Boulet I., Littman D. R., Fagard R., Marsh M. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J Cell Biol. 1992 Apr;117(2):279–290. doi: 10.1083/jcb.117.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racoosin E. L., Swanson J. A. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992 Aug;102(Pt 4):867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Young D. F., Goswami K. K., Russell W. C. Isolation and characterization of monoclonal antibodies to simian virus 5 and their use in revealing antigenic differences between human, canine and simian isolates. J Gen Virol. 1987 Nov;68(Pt 11):2769–2780. doi: 10.1099/0022-1317-68-11-2769. [DOI] [PubMed] [Google Scholar]
- Roettger B. F., Rentsch R. U., Pinon D., Holicky E., Hadac E., Larkin J. M., Miller L. J. Dual pathways of internalization of the cholecystokinin receptor. J Cell Biol. 1995 Mar;128(6):1029–1041. doi: 10.1083/jcb.128.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J., Bénédetti H., Zanolari B., Riezman H. Identification of a novel sequence mediating regulated endocytosis of the G protein-coupled alpha-pheromone receptor in yeast. Mol Biol Cell. 1993 May;4(5):511–521. doi: 10.1091/mbc.4.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Doyle C., Sambrook J., Gething M. J. Heterologous transmembrane and cytoplasmic domains direct functional chimeric influenza virus hemagglutinins into the endocytic pathway. J Cell Biol. 1986 Apr;102(4):1271–1283. doi: 10.1083/jcb.102.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kolhouse J. F., Kamen B. A., Anderson R. G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990 Mar;110(3):637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberger S., Iacopetta B. J., Kühn L. C. Endocytosis of the transferrin receptor requires the cytoplasmic domain but not its phosphorylation site. Cell. 1987 May 8;49(3):423–431. doi: 10.1016/0092-8674(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer P. E., Lisanti M. P., Baldini G., Sargiacomo M., Mastick C. C., Lodish H. F. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol. 1994 Dec;127(5):1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Smythe E. Stage-specific assays for coated pit formation and coated vesicle budding in vitro. J Cell Biol. 1991 Sep;114(5):869–880. doi: 10.1083/jcb.114.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng S. L., Heuser J. E., Harris D. A. A glycolipid-anchored prion protein is endocytosed via clathrin-coated pits. J Cell Biol. 1994 Jun;125(6):1239–1250. doi: 10.1083/jcb.125.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Smythe E., Carter L. L., Schmid S. L. Cytosol- and clathrin-dependent stimulation of endocytosis in vitro by purified adaptors. J Cell Biol. 1992 Dec;119(5):1163–1171. doi: 10.1083/jcb.119.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P. K., Davis N. G., Sprague G. F., Payne G. S. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993 Dec;123(6 Pt 2):1707–1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Brewer C. B., Roth M. G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993 Feb 15;268(5):3313–3320. [PubMed] [Google Scholar]
- Thomas D. C., Roth M. G. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994 Jun 3;269(22):15732–15739. [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Kuriyama H., Sone H., Neda H., Yamauchi N., Maeda M., Niitsu Y. Continuous internalization of tumor necrosis factor receptors in a human myosarcoma cell line. J Biol Chem. 1988 Jul 25;263(21):10262–10266. [PubMed] [Google Scholar]
- Watts C., Marsh M. Endocytosis: what goes in and how? J Cell Sci. 1992 Sep;103(Pt 1):1–8. doi: 10.1242/jcs.103.1.1a. [DOI] [PubMed] [Google Scholar]
- West M. A., Bretscher M. S., Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989 Dec;109(6 Pt 1):2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. W., Goodhouse J., Farquhar M. G. Transferrin receptors and cation-independent mannose-6-phosphate receptors deliver their ligands to two distinct subpopulations of multivesicular endosomes. Eur J Cell Biol. 1989 Oct;50(1):132–143. [PubMed] [Google Scholar]
- van Deurs B., Holm P. K., Kayser L., Sandvig K., Hansen S. H. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur J Cell Biol. 1993 Aug;61(2):208–224. [PubMed] [Google Scholar]
- van Deurs B., Holm P. K., Sandvig K., Hansen S. H. Are caveolae involved in clathrin-independent endocytosis? Trends Cell Biol. 1993 Aug;3(8):249–251. doi: 10.1016/0962-8924(93)90045-3. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K., Tierney E. L. Antigenic and functional organization of human parainfluenza virus type 3 fusion glycoprotein. J Virol. 1989 Jan;63(1):375–382. doi: 10.1128/jvi.63.1.375-382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]