Abstract
CJ9-gD is a novel herpes simplex virus (HSV) type 1 recombinant virus that is completely replication-defective, expresses high-levels of HSV-1 major antigen glycoprotein D (gD), and can function in trans to inhibit replication of wild-type HSV-1 and HSV-2 in co-infected cells. Here, we show that immunization with CJ9-gD elicits strong and long-lasting humoral and Th1-like cellular immune responses against both HSV-1 and HSV-2. Mice immunized with CJ9-gD exhibited significant reductions in the extent and duration of intravaginal replication of challenge HSV-1 and HSV-2 compared with mock-immunized controls, and were completely protected from local or systemic herpetic disease after intravaginal challenge with wild-type HSV-1 or HSV-2.
INTRODUCTION
Genital herpes is a chronic, lifelong disease caused by herpes simplex virus (HSV) that is characterized by unpredictable recurrences. It is one of the most common sexually transmitted diseases worldwide, and the primary cause of genital ulcer disease in the developed and developing countries (Mertz et al., 1998; Paz-Bailey et al., 2007). HSV-2 has been the main cause for genital herpes, whereas HSV-1 has been predominantly associated with orofacial infections, herpetic keratitis, and herpes encephalitis (Liesegang, 1991; Whitley, 2006). However, more recently, it has been reported that in developed countries HSV-1 is now the main causative agent for primary genital herpes, especially among adolescents, women, and homosexual men (Roberts, 2005; Jin et al., 2006). The clinical signs of genital herpes are indistinguishable between HSV-1 and HSV-2 (Corey et al., 1983). After primary genital infection, both HSV subtypes establish latent infection in sacral dorsal root ganglia, subsequently giving rise to intermittent reactivation. However, the frequency of recurrent genital disease and asymptomatic viral shedding is much higher for HSV-2 (Lafferty et al., 1987; Benedetti et al., 1994). A potentially grave complication of genital herpes is the transmission of HSV to neonates, which is often associated with a high mortality rate and a high incidence of neurological sequelae in survivors (Jones and Cunningham, 2004). Moreover, genital herpes increases the risk of acquiring and transmitting HIV, which can be strongly reduced by HSV-preventive or suppressive therapy (Wald and Link, 2002; Freeman et al., 2007). To date, no medication can effectively prevent primary as well as latent infection with HSV, and the options for treatment and prevention of recurrences are very limited (Whitley and Roizman, 2001). Experimental vaccine approaches against genital herpes have mainly focused on HSV-2 and included peptides, proteins, killed virus, DNA vaccines, heterologous replicating viral vectors, replication-defective viruses, and attenuated replication-competent viruses (Bernstein and Stanberry, 1999; Koelle and Corey, 2003). However, in light of the general impact of HSV-1 and the rising number of primary genital infections caused by this subtype, a vaccine efficacious against both HSV-1 and HSV-2 is desirable.
The 155-kb genome for HSV-2 and the 152-kb genome for HSV-1 show approximately 80% sequence homology, and consist of a corresponding set of at least 74 genes (McGeoch et al., 1988; Dolan et al., 1998). Owing to their similarity HSV-1 and HSV-2 cross-react antigenically and elicit type-common immune responses. Although it is unclear whether pre-existing immunity to HSV-1 in humans protects against the HSV-2 infection (Bryson et al., 1993; Looker and Garnett, 2005), individuals seropositive for HSV-1 are reported to be more likely to have asymptomatic HSV-2 seroconversion than HSV-seronegative persons (Langenberg et al., 1999). In mouse and guinea pig models of genital herpes, it has been shown that several vaccine candidates, including whole virus, and replication-conditional or replication-defective HSV of one subtype, are also capable of eliciting protective type-common immune responses against the other subtype and vice versa (Erturk et al., 1989; McLean et al., 1994; Boursnell et al., 1997; Da Costa et al., 2001).
One of the main targets for subunit vaccines (viral as well as plasmid-based vaccines) has been the major HSV antigen glycoprotein D (gD). Although subunit vaccines containing gD in combination with an adjuvant seemed to be safe and effective in the animal models of genital herpes (Stanberry et al., 1987; Bourne et al., 2005), they failed to provide general protection in clinical trials (Corey et al., 1999; Stanberry et al., 2002). Replication-defective viruses lacking functions essential for viral replication or assembly of infectious progeny virus particles elicit a broad spectrum of protective immune responses against HSV in mice and guinea pigs without causing the disease (Dudek and Knipe, 2006). However, the use of replication-defective viruses, particularly when used in latently infected individuals, imposes certain risks, as they might regain replication competence in the presence of wild-type virus or reactivate latent wild-type virus infections.
In an effort to minimize these safety concerns, using the T-REx (Invitrogen, Carlsbad, CA) gene switch technology and the dominant-negative mutant polypeptide, UL9-C535C, of HSV-1 origin-binding protein, UL9, we developed a UL9-C535C-encoding HSV recombinant virus, CJ83193, capable of preventing its own viral DNA replication, as well as that of wild-type HSV-1 and HSV-2 in co-infected cells (Yao and Eriksson, 1999, 2002). Although infection of cells with CJ83193 can lead to expression of viral immediate-early (α), early (β), and certain early-late gene (γ1) products, little gD is detected in CJ83193-infected cells (Augustinova et al., 2004). Aiming to further increase its safety and improve its efficacy as a vaccine against HSV, we recently constructed a CJ83193-derivative viral recombinant, CJ9-gD, in which the essential UL9 gene is replaced by an extra copy of the HSV-1 gD (gD1) gene under the control of the tetracycline operator sequence-bearing human cytomegalovirus major immediate-early promoter (Brans et al., 2008; Lu et al., 2009). Thus, CJ9-gD is completely replication-defective, expresses high-level of gD, and causes no detectable latent infection after ocular or nasal infection in mice (Lu et al., 2009). In a mouse model of HSV-1 ocular infection, we showed that CJ9-gD is more effective than CJ83193 in inducing protective immune responses against wild-type HSV-1 challenge (Lu et al., 2009) and protects guinea pigs against HSV-1 skin disease (Brans et al., 2008). We further show that immunization with CJ9-gD elicits strong HSV-1 neutralization antibody (Ab) response, as well as HSV-1-specific CD4+ and CD8+ T-cell response at levels similar to that seen in wild-type HSV-1-immunized mice (Lu et al., 2009).
Here, we evaluate the efficacy of the HSV-1 recombinant virus, CJ9-gD, as a prophylactic vaccine against wild-type HSV-1 and HSV-2 in a mouse model of genital infection.
RESULTS
HSV-1
Dose-dependent induction of HSV-1-specific neutralizing Abs in mice immunized with CJ9-gD
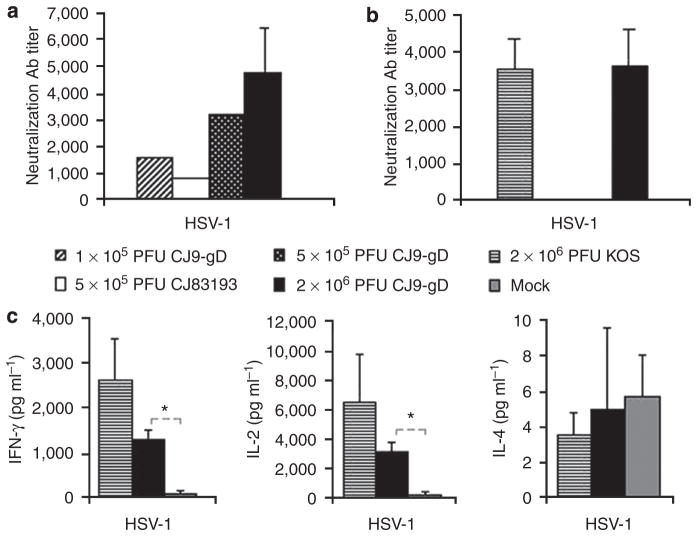
The ability of CJ9-gD to elicit anti-HSV-1-specific neutralizing Abs was first determined in mice immunized with CJ9-gD at a dose of 2 × 106 PFU (plaque-forming unit), and then at reduced doses of 5 × 105 and 1 × 105 PFU. As control, a group of mice was also immunized with CJ83193 at a dose of 5 × 105 PFU. The HSV-1-specific neutralization Ab titers in mice immunized with 2 × 106 PFU of CJ9-gD 2 weeks after the second immunization were on average 4,800 (Figure 1a). HSV-1-specific neutralization Ab titers after immunization with 5 × 105 PFU of CJ83193 were 800, twofold and fourfold lower than those of mice immunized with 1 × 105 or 5 × 105 PFU of CJ9-gD, respectively (Figure 1a). No specific Ab titers against HSV-1 were detected in the mock-vaccinated mice at a 1:10 dilution.
Figure 1. Induction of herpes simplex virus (HSV)-1-specific neutralizing antibodies (Abs) and T-cell responses in immunized mice.
(a) Three sets of BALB/c mice (n = 6; n = 17; n = 17) were injected with 2 × 106 PFU per mouse of CJ9-gD or DMEM. Two sets of 32 BALB/c mice (eight mice per group) were mock-vaccinated with DMEM, or vaccinated with CJ9-gD or CJ83193 at the indicated doses. At 4 weeks after primary immunization, the blood was collected and pooled for each group, and HSV-1-specific neutralizing Ab titers were determined. The results represent average titers ± SEM. (b) BALB/c mice were mock-immunized with DMEM (n = 2) or immunized with either KOS (n = 5) or CJ9-gD (n = 4) at 2 × 106 PFU per mouse. AT 6 months after the primary immunization, blood and splenocytes were collected individually from each mouse. Serum was assayed for HSV-1-specific neutralizing Ab titers. (c) Splenocytes from mice described in (b) were cultured in the presence or absence of UV-inactivated HSV-1 strain McKrae. Levels of IFN-γ, IL-2, and IL-4 in supernatants were determined by ELISA. The results represent average cytokine levels after subtraction of background values from mock-stimulated wells ± SEM. P-values were assessed by student’s t-test (*P<0.05).
The effectiveness of CJ9-gD in inducing long-term anti-HSV-specific neutralizing Ab response was evaluated at 6 months after primary immunization and compared with that elicited after immunization with the same dose of the wild-type HSV-1, strain KOS (Figure 1b). The titer of HSV-1-specific neutralization Abs in mice immunized with 2 × 106 PFU of CJ9-gD was 3,600, which is comparable with that after immunization with KOS, and that detected 2 weeks after the second immunization with 2 × 106 PFU of CJ9-gD.
Induction of HSV-1-specific long-term cytokine response in mice immunized with CJ9-gD
We earlier showed that immunization with CJ9-gD elicited strong HSV-1-specific Th1 T-cell response (Lu et al., 2009). To evaluate the long-term effect of cellular immune responses induced by CJ9-gD, mice were mock-immunized with DMEM or immunized with 2 × 106 PFU of KOS or CJ9-gD. After 6 months splenocytes were isolated and cultured for 72 hours in the presence or absence of UV-inactivated HSV-1. Figure 1c show that significantly higher levels of HSV-1-specific IFN-γ and IL-2 expression were detected in splenocytes from CJ9-gD-immunized mice than from the mock-immunized controls (P < 0.05). The degrees of IFN-γ and IL-2 response detected in KOS-immunized mice were higher than those of CJ9-gD-immunized mice after stimulation with UV-inactivated HSV-1, but these differences did not reach significance. Levels of IL-4 were low for all the groups.
Protection against HSV-1 genital infection in immunized mice
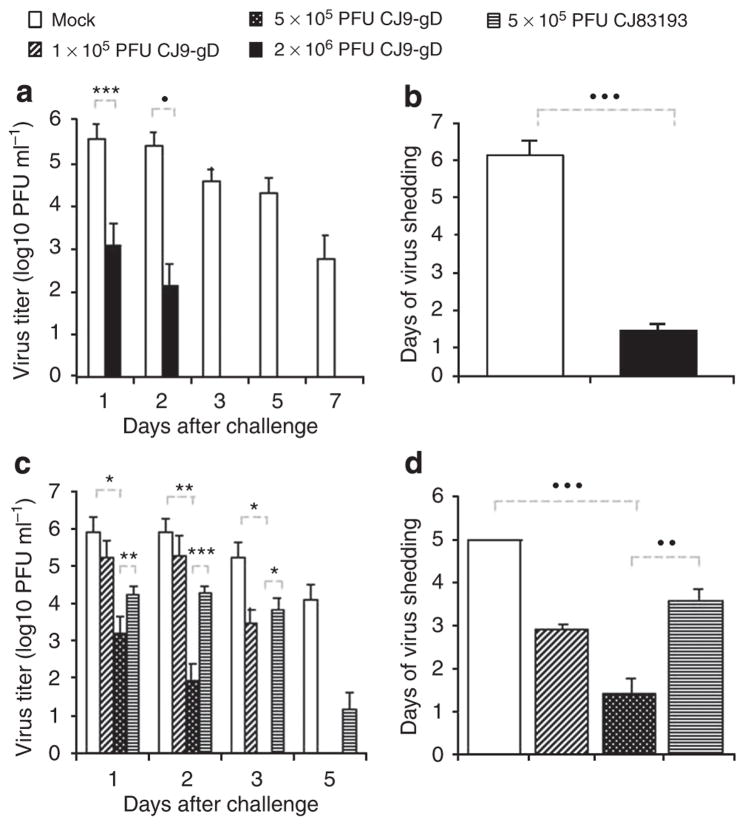
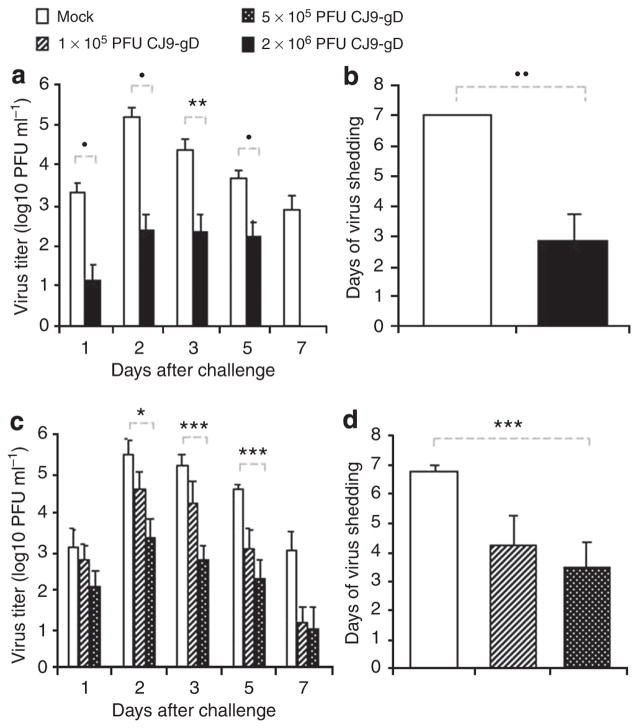
At 4 weeks after the initial immunization, two sets of mice (n = 6; n = 17), which were either mock-immunized or immunized with 2 × 106 PFU of CJ9-gD, and another set of mice (n = 32), which were mock-immunized or immunized with CJ9-gD at doses of 1 × 105 or 5 × 105 PFU, or CJ83193 at a dose of 5 × 105 PFU, were challenged intravaginally with HSV-1 strain, mP. Vaginal swabs were taken on days 1, 2, 3, 5, and 7 after challenge. The yields of challenge virus were significantly lower in mice immunized with 2 × 106 PFU of CJ9-gD compared with those in mock-immunized mice, with a reduction of more than 280-fold on day 1 (P < 0.005) and 1,800-fold on day 2 (P ≤ 0.001) (Figure 2a). By day 3 no challenge virus was detected in CJ9-gD-immunized mice, whereas all mock-vaccinated mice continued to shed virus at an average yield of more than 3.6 × 104 PFU ml−1. The average duration of viral shedding in immunized mice was 1.25 days as compared with 6.1 days in the mock-immunized mice (P < 0.0001) (Figure 2b).
Figure 2. Reduction of challenge herpes simplex virus (HSV)-1 vaginal replication in mice immunized with CJ9-gD.
One set of six and another set of 17, 6-week-old female BALB/c mice were subcutaneously (s.c.) inoculated with either 2 × 106 PFU per mouse of CJ9-gD or DMEM (a and b). BALB/c mice (eight mice per group) were inoculated s.c. with either 1 × 105 PFU of CJ9-gD, 5 × 105 PFU of CJ9-gD, 5 × 105 PFU of CJ83193, or DMEM (c and d). Mice were boosted after 2 weeks. At 4 weeks, mice were pretreated with medroxyprogesterone and challenged intravaginally with 5 × 105 PFU of HSV-1 strain mP. Vaginal swabs were taken on days 1, 2, 3, 5, and 7 post-challenge. Infectious viruses in swab materials were assessed by standard plaque assay on Vero cell monolayers. Viral titers are expressed as the mean ± SEM in the individual vaginal swabs (a and c). The duration of viral shedding is represented as the mean number of days during which infectious virus was detected in swab material after challenge ± SEM (b and d). P-values were assessed by student’s t-test (*P < 0.05, **P < 0.01, P < 0.005, •P < 0.001, ••P < 0.0005, •••P < 0.0001)
Immunization with the lower dose of 5 × 105 PFU of CJ9-gD also significantly reduced the viral titer of challenge virus recovered from vaginal swabs on day 1 (P < 0.05) and day 2 (P < 0.01) compared with those from mock-immunized mice (Figure 2c). No virus was detected in mice immunized with 5 × 105 PFU of CJ9-gD by day 3, whereas at the same time all mock-vaccinated mice continued to shed virus at an overall average yield of 1.7 × 105 PFU ml−1 (P < 0.05). Immunization with 5 × 105 PFU of CJ83193 also led to a significant reduction in challenge virus replication on days 1 (P ≤ 0.05) and 2 (P < 0.05), but the average titers of challenge virus was significantly higher than those found in mice immunized with 5 × 105 PFU of CJ9-gD (day 1: P < 0.01 and day 2: P < 0.001) (Figure 2c). The duration of viral shedding was decreased to an average of 1.4 days after immunization with 5 × 105 PFU of CJ9-gD, which was significantly shorter than the average of 3.6 days of viral shedding observed in mice immunized with 5 × 105 PFU of CJ83193 (P < 0.001) or in mock-immunized controls (P < 0.0001) (Figure 2d). Immunization with the lower dose of 1 × 105 PFU of CJ9-gD led to a significant reduction in challenge virus replication on day 3 (P < 0.05), whereas no challenge virus was detected by day 5 post-challenge with duration of virus shedding for 2.8 days in average.
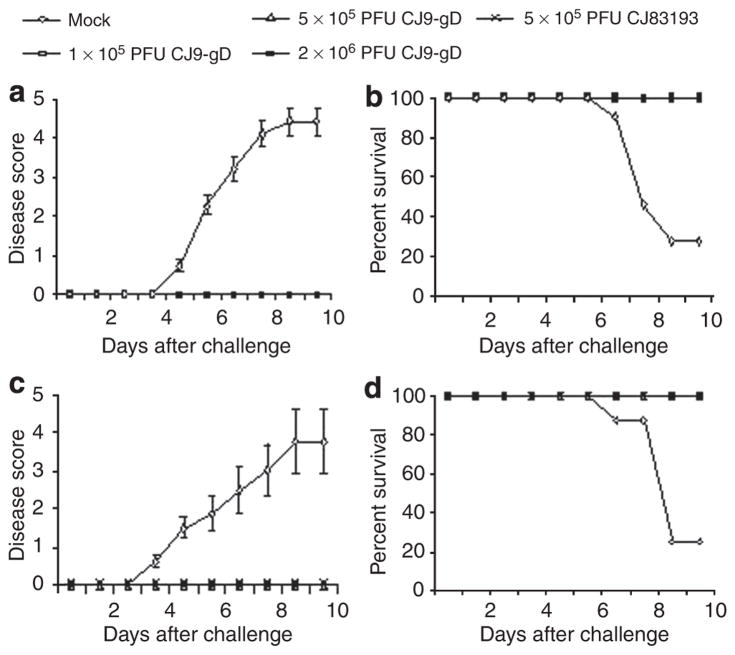
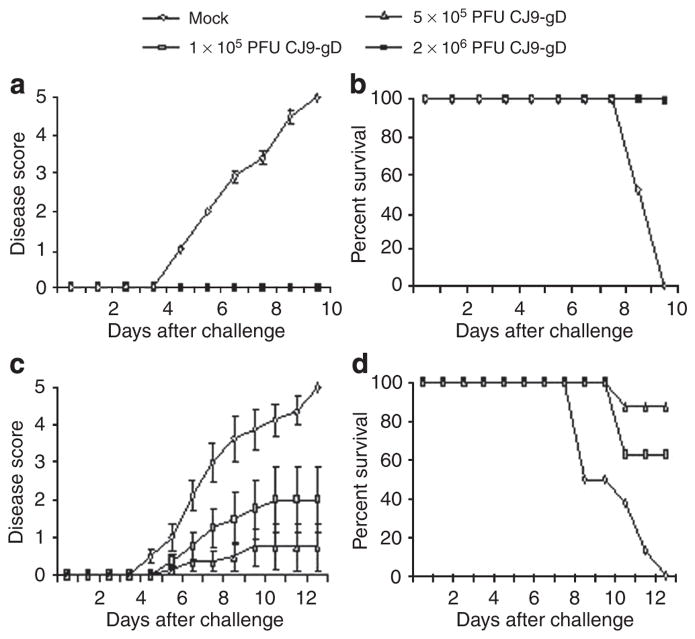
Mice immunized with 2 × 106 PFU of CJ9-gD were completely protected from the development of local genital lesions and exhibited no signs of systemic disease after challenge with wild-type HSV-1, whereas 100% of mock-vaccinated mice developed severe genital lesions after challenge with the wild-type HSV-1 (Figure 3a). A total of, 8 of 11 mice (73%) developed hind limb paralysis, and eventually succumbed to the challenge of infection (Figure 3b). After immunization with 1 × 105 and 5 × 105 PFU of CJ9-gD, or with 5 × 105 PFU of CJ83193, mice were again completely protected from signs of local or systemic HSV-1 disease. The control group of mock-immunized mice exhibited a morbidity and mortality comparable with those described above (Figure 3c and d).
Figure 3. Prevention of herpes simplex virus (HSV)-1 disease in mice immunized with CJ9-gD or CJ83193.
After challenge with wild-type HSV-1, individual mice described in the legend of Figure 2 were observed during a 30-day follow-up period for the incidence of genital and disseminated HSV-1 disease (a and c), and survival (b and d) using the following score: 0 = no sign, 1 = slight genital erythema and edema, 2 = moderate genital inflammation, 3 = purulent genital lesions, 4 = hind-limb paralysis, 5 = death.
Protection from latent HSV-1 infection
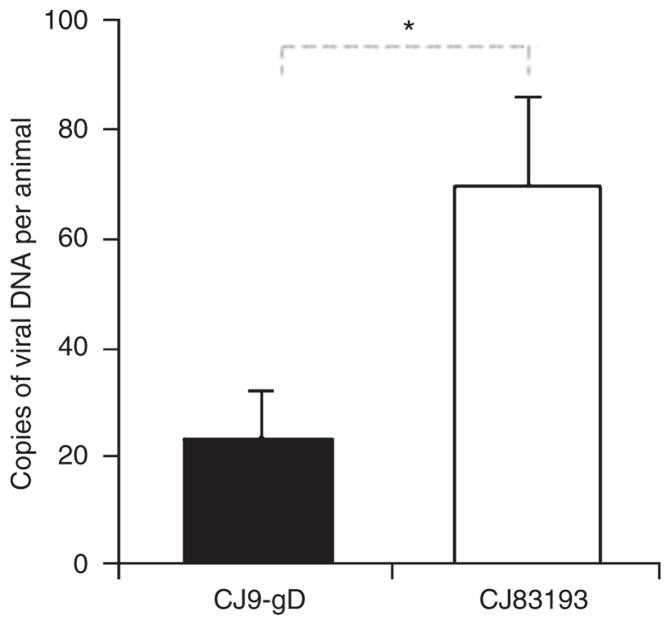
To compare the ability of CJ9-gD to prevent accumulation of latent challenge virus with that of CJ83193, the lower spinal column, including spinal cord and dorsal root ganglia, were harvested 30 days after intravaginal challenge with 5 × 105 PFU of HSV-1 strain mP from 16 mice immunized either with CJ9-gD or CJ83193 at a dose of 5 × 105 PFU. The whole DNA was extracted and tested for latent viral DNA using quantitative real-time PCR. The average amount of latent HSV DNA per mouse was significantly higher in mice vaccinated with CJ83193 than in CJ9-gD-immunized animals (69.8 vs 23.2 DNA copies, P < 0.05, Figure 4). As a control, the same procedure was carried out in eight mice 30 days after the second immunization with 2 × 106 PFU of either CJ9-gD or CJ83193, which were, however, not challenged with wildtype HSV. No latent viral DNA was detected in these two groups of mice, indicating that neither virus can establish detectable latent infection after s.c. (subcutaneous) injection, and that the viral DNA detected in dorsal root ganglia after challenge with wild-type HSV-1 was indeed the challenge wild-type HSV viral DNA.
Figure 4. CJ9-gD is more effective than CJ83193 in preventing latent herpes simplex virus (HSV)-1 infection.
One set of 16 female 6- to 8-week-old BALB/c mice was subcutaneously injected either with 5 × 105 PFU of CJ9gD or with 5 × 105 PFU of CJ83193, and boosted after 2 weeks. At 4 weeks, the mice were pretreated with medroxyprogesterone and challenged intravaginally with 5 × 105 PFU of HSV-1 strain mP. At 30 days after challenge, lower spinal column, including spinal cord and dorsal root ganglia, were harvested, the whole DNA was extracted and examined for the presence of latent viral DNA by quantitative real-time PCR. The results represent the mean amount of viral DNA per animal ± SEM. P-value was assessed by student’s t-test (*P < 0.05).
HSV-2
Induction of HSV-2-specific Ab and T-cell responses in mice immunized with CJ9-gD
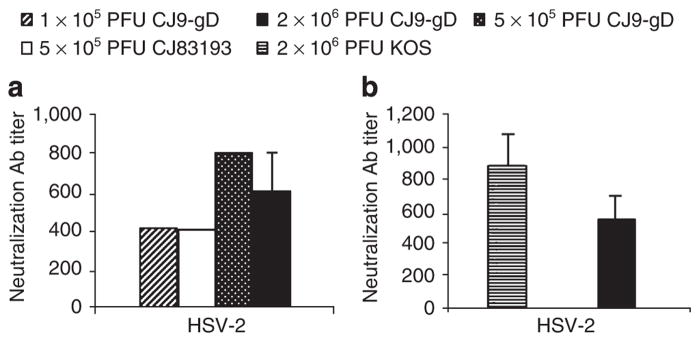
Given the effectiveness of CJ9-gD immunization in preventing HSV-1 infection, we next examine the ability of CJ9-gD to elicit anti-HSV-2 cross-specific neutralizing Abs. The HSV-2-specific neutralization Ab titers in mice immunized with 2 × 106 PFU of CJ9-gD 2 weeks after the second immunization were on average 600 (Figure 5a). The titer of HSV-2-specific neutralization Abs after immunization with 1 × 105 or 5 × 105 PFU of CJ9-gD was 400 and 800, respectively (Figure 5a). The HSV-2 cross-specific neutralizing Abs in mice immunized with 5 × 105 PFU of CJ83193 was 400, two fold lower than that in mice immunized with the same dose of CJ9-gD. No specific Ab titers against HSV-2 were detected in the mock-vaccinated mice at a 1:10 dilution.
Figure 5. Induction of herpes simplex virus (HSV)-2-specific neutralizing antibodies (Abs) in immunized mice.
Serum from BALB/c mice mock-immunized with DMEM, immunized with CJ9-gD at indicated doses, or with CJ83193 at 5 × 105 PFU per mouse as described in the legend of Figure 1a and b was assayed for short (a) and long-term (b) HSV-2-specific neutralizing Abs as described.
The long-term anti-HSV-2-specific neutralizing Ab response was evaluated at 6 months after primary immunization (Figure 5b). The titer of HSV-2-specific neutralization Abs in mice immunized with 2 × 106 PFU of CJ9-gD was 500, which is comparable with that after immunization with KOS.
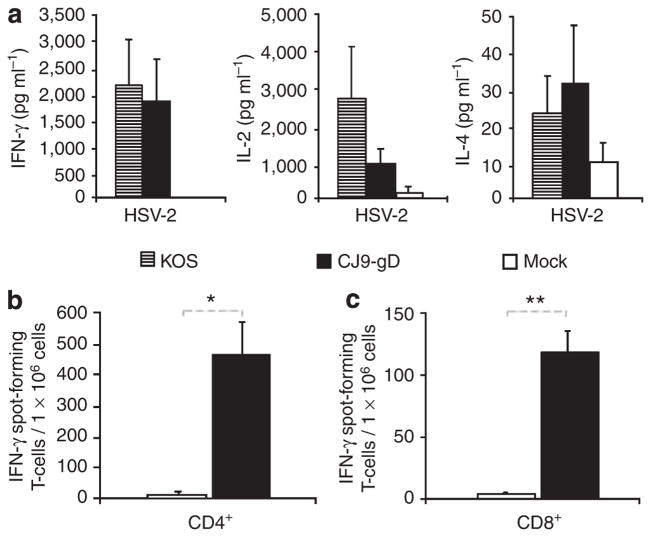
We next examined the effectiveness of CJ9-gD in the induction of HSV-2 cross-specific T-cell immune response with cytokine assays (Figure 6a), as well as CD4+ T cell (Figure 6b) and CD8+ T cell (Figure 6c) IFN-γ ELISPOT assays. Figure 6a shows that lymphocytes from mice immunized with KOS produced higher levels of anti-HSV-2-specific IL-2 and similar levels of IFN-γ responses compared with those detected in CJ9-gD-immunized mice. Levels of IL-4 were low and no significant difference was detected among the vaccinated and mock-vaccinated controls.
Figure 6. Induction of herpes simplex virus (HSV)-2 cross-specific cytokine response in immunized mice.
Cytokine assays (a). Splenocytes from BALB/c mice mock-immunized with DMEM (n = 4), or immunized with 2 × 106 PFU per mouse of KOS (n = 4) or with CJ9-gD (n = 4) were isolated from individual mice, and cultured in the presence or absence of UV-inactivated HSV-2 strain 186. Levels of IFN-γ, IL-2, and IL-4 in the supernatants were assessed by ELISA. The results represent average cytokine levels after subtraction of background values from mock-stimulated wells ± SEM (a). IFN-γ ELISPOT assays (b and c). Splenocytes from BALB/c mice mock-immunized with DMEM (n = 2) or immunized with 2 × 106 PFU per mouse of KOS (n = 4) or with CJ9-gD (n = 4) were prepared individually. (b) CD4+ T cells were seeded in plates precoated with IFN-γ-specific MAb and stimulated with mock-infected or UV-inactivated HSV-2-infected syngeneic CD11c+ bone marrow-derived dendritic cells. (c) CD8+ T cells were seeded in plates precoated with IFN-γ-specific MAb and stimulated with mock-infected or HSV-2-infected syngeneic CL7 cells. The HSV-2-specific IFN-γ spot-forming cells are expressed as the mean ± SEM per million CD4+ T cells (b) or splenocytes (c) from four mice per group. P-values were assessed by student’s t-test (*P < 0.005, **P ≤ 0.001).
IFN-γ ELISPOT assays show that immunization with CJ9-gD elicited an HSV-2-specific CD4+ T-cell response yielding 58-fold more IFN-γ spot-forming cells than the mock-immunized controls (Figure 6b, P = 0.002). An HSV-2 cross-specific CD8+ T-cell response is also elicited in CJ9-gD-immunized mice compared with mock-immunized controls (Figure 6c, P = 0.001). Taken together, the results show that immunization with CJ9-gD can effectively elicit HSV-2 cross-specific Th1 T-cell response.
Protection against HSV-2 genital infection in immunized mice
The results in Figure 7a show that the ability of challenge virus to replicate in vaginal mucosa was significantly reduced from day 1 to day 5 (P < 0.001, days 1, 2, and 5; P < 0.05, day 3) in mice immunized with 2 × 106 PFU of CJ9-gD compared with those in mock-immunized mice. The challenge virus infection had resolved by day 7 in the immunized mice, whereas at the same time all mock-immunized mice continued to shed virus at an average yield of more than 7.6 × 102 PFU ml−1 (Figure 7a). Compared with mock-immunized mice, the average duration of viral shedding in the immunized mice decreased significantly from 7 days to 2.8 days (P < 0.0005) (Figure 7b).
Figure 7. Reduction of challenge herpes simplex virus (HSV-2) vaginal replication in mice immunized with CJ9-gD.
One set of 17 female BALB/c mice were subcutaneously (s.c.) injected either with 2 × 106 PFU per mouse of CJ9-gD or with DMEM (a and b). One set of 24 female 6- to 8-week-old BALB/c mice was injected s.c. either with 1 × 105 PFU of CJ9-gD, 5 × 105 PFU of CJ9-gD, or with DMEM (c and d). Mice were boosted after 2 weeks. At 4 weeks, mice were pretreated with medroxyprogesterone and challenged intravaginally with 1 × 105 PFU of HSV-2 strain 186. Vaginal swabs were taken on days 1, 2, 3, 5, and 7 post-challenge. Infectious viruses in the swab materials were assessed by standard plaque assay on Vero cell monolayers. Viral titers are expressed as the mean ± SEM in the individual vaginal swabs (a and c). The duration of viral shedding is represented as the mean number of days during which injections virus was detected in swab material after challenge ± SEM (b and d). P was assessed by student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.005, •P < 0.001, ••P < 0.0005).
Immunization with a lower dose of 5 × 105 PFU of CJ9-gD also led to a significant reduction in the viral titer recovered from the vaginal swabs after challenge with wild-type HSV-2 on days 2 (P < 0.05), 3 (P < 0.005), and 5 (P < 0.001) compared with those of mock-immunized mice (Figure 7c). Although immunization with 1 × 105 PFU of CJ9-gD reduced viral titers significantly compared with mock-immunized mice on days 3 (P < 0.01) and 5 (P < 0.001), it was notably less effective than immunization with 5 × 105 PFU of CJ9-gD (Figure 7c). The average duration of viral shedding after immunization with 5 × 105 or 1 × 105 PFU of CJ9-gD was 3.5 days and 4.25 days, respectively, which was significantly shorter than that of mock-immunized controls (P < 0.005) (Figure 7d).
As shown in Figure 8, mice immunized with 2 × 106 PFU of CJ9-gD were completely protected from development of local genital lesions and exhibited no signs of systemic disease after challenge with wild-type HSV-2. All mock-vaccinated mice developed severe genital lesions followed by hind limb paralysis and eventually succumbed to the challenge of infection (Figure 8b). Among mice immunized with 5 × 105 PFU of CJ9-gD, two mice (25%) developed signs of clinical disease, of which one (12.5%) was killed due to systemic involvement (Figure 8c and d). Overall, mice immunized with 1 × 105 PFU of CJ9-gD had a higher disease score than mice immunized with 5 × 105 PFU of CJ9-gD and only 60% of mice survived the challenge with wild-type HSV-2.
Figure 8. Prevention of herpes simplex virus (HSV)-2 disease in mice immunized with CJ9-gD.
After challenge with wild-type HSV-2, individual mice described in the legend of Figure 7a and b (a and c) and mice described in the legend of Figure 7c and d (b and d), were observed during a 30-day follow-up period for the incidence of genital and disseminated HSV-1 disease (a and c), and survival (b and d) using the following scale: 0 = no sign, 1 = slight genital erythema and edema, 2 = moderate genital inflammation, 3 = purulent genital lesions, 4 = hind-limb paralysis, 5 = death.
DISCUSSION
Genital herpes is mainly caused by HSV-2, but recently, rising numbers of primary infections due to HSV-1 have indicated a great need for a vaccine against both HSV-1 and HSV-2 (Langenberg et al., 1999; Xu et al., 2006).
In the early phase after vaginal infection of mice and guinea pigs, HSV is targeted by specific Abs and cellular immunity, thereby limiting acute infection and spreading, whereas clearance of HSV from the genital mucosa as well as immune control of latency is primarily mediated by T cells and Th1-associated cytokines such as IFN-γ (Stanberry et al., 2000; Koelle and Corey, 2003). Therefore, the effective prevention of HSV genital disease may be accomplished by a vaccine inducing both humoral and cellular immune responses against HSV-1 and HSV-2. This study shows that immunization of mice with the HSV-1 recombinant virus, CJ9-gD, is capable of eliciting long-lasting and high-level neutralizing Abs and Th1-like cellular immune responses against HSV-1 and, to a lesser extent, against HSV-2. Notably, HSV-1- and HSV-2-specific neutralization Ab titers detected at 2 weeks (data not shown) and 6 months after the second immunization with 2 × 106 PFU of CJ9-gD were essentially similar to those achieved after immunization with HSV-1 wild-type strain KOS at the same dose, indicating that CJ9-gD is as efficient as wild-type HSV-1 in inducing humoral immune responses against HSV-1 and HSV-2. In addition, as assessed by IFN-γ-ELISPOT assays, immunization with CJ9-gD induced strong HSV-2-specific cellular immune responses by CD4+ and CD8+ T cells.
Compared with mock-immunization, immunization with 2 × 106 or 5 × 105 PFU of CJ9-gD significantly reduced the amount and duration of acute vaginal replication of wild-type HSV-1 after challenge and provided complete protection against genital herpes. Furthermore, immunization with CJ9-gD at these two doses also significantly reduced the amount and duration of acute replication of wild-type HSV-2 after vaginal challenge compared with mock-immunization. At an immunization dose of 2 × 106 PFU of CJ9-gD, mice were completely protected from HSV-2 disease, whereas at the lower immunization dose of 5 × 105 PFU of CJ9-gD 25% of mice developed signs of local disease, of which 50% (n = 1) progressed to disseminated disease. Taken together, these data indicate that in addition to its good protection against genital challenge with HSV-1, immunization with the HSV-1 recombinant virus, CJ9-gD, elicits a dose-dependent cross-immunity against genital challenge with HSV-2. The mouse model of genital HSV infection does not allow us to directly assess the effect of immunization with CJ9-gD on latent infection or recurrent disease because of the high mortality rate in the mock-vaccinated mice and the lack of spontaneous recurrences after genital challenge with wild-type HSV. We have recently investigated the efficacy of CJ9-gD as a vaccine against primary and recurrent HSV-2 genital disease in guinea pigs, showing that CJ9-gD immunization can prevent recurrent HSV-2 disease and significantly reduce the latent challenge viral DNA load in immunized animals compared with that of mock-vaccinated controls (Brans and Yao, unpublished data). This is consistent with our earlier data, showing that after skin challenge with wild-type HSV-1 the amount of latent HSV-1 challenge virus was significantly lower in dorsal root ganglia of guinea pigs immunized with CJ9-gD compared with those of mock-vaccinated controls (Brans et al., 2008).
CJ9-gD seems more effective in inducing protective immune responses against HSV-1 than against HSV-2. This finding is consistent with earlier reports that HSV vaccines are generally less effective in prevention of heterotypic HSV infection than homotypic infection (Erturk et al., 1989; McLean et al., 1994; Boursnell et al., 1997; Da Costa et al., 2001). It is reasonable to speculate that immunization with an HSV-2 recombinant virus containing the same features, as CJ9-gD will likely provide a greater effectiveness against challenge with wild-type HSV-2 than the current HSV-1 recombinant virus.
We earlier showed that CJ9-gD elicits stronger immune responses against HSV-1 keratitis in mice than CJ83193, and is more effective than CJ83193 in prevention of reactivation of challenge virus from latent infection (Lu et al., 2009). This study extents this finding, showing that although immunization with CJ9-gD or CJ83193 lead to a marked decrease in the amounts and duration of acute vaginal replication of HSV-1 challenge virus and prevents genital herpetic disease, CJ83193 was significantly less effective than CJ9-gD in limiting the acute vaginal replication of HSV-1 in the immunized mice. There are greater than 6,000-fold more challenge HSV-1 detected in vaginal swabs of mice immunized with CJ83193 than in swabs from CJ9-gD-immunized mice on day 3 post-challenge, and an average of a 2-day longer period of viral shedding was detected in CJ83193-immunized mice than mice immunized with CJ9-gD. Furthermore, CJ9-gD was significantly better in limiting latent infection of lumbosacral dorsal root ganglia by challenge HSV-1 than CJ83193. As reduction of latent viral load is associated with a lower risk of reactivation (Lekstrom-Himes et al., 1998; Sawtell, 1998), this observation further indicates that CJ9-gD is more effective than CJ83193 as a vaccine against HSV-1 infection. The expression of gD by CJ9-gD significantly enhances its ability to elicit anti-HSV-1-specific neutralizing Abs compared with CJ83193 (Lu et al., 2009), whereas similar levels of HSV-1-specific Th1 cytokine response were detected between mice immunized with CJ9-gD and CJ8319 (Lu et al., 2009), suggesting that induction of a stronger neutralizing Ab response may have a large impact on the observed superiority of CJ9-gD as a vaccine against HSV-1. It should be noted that we detected no significant difference between the efficacy of CJ9-gD and CJ83193 as a vaccine in prevention of HSV-2 genital infection at a dose of 5 × 105 PFU per mouse (data not shown), indicating that expression of gD1 by CJ9-gD is less important in eliciting overall protective immunity against HSV-2 than against HSV-1 in mice.
Taken together, our results show that CJ9-gD is capable of eliciting strong and long-lasting humoral and cellular immune responses against both HSV-1 and HSV-2 in mice. The level of immunity and cross-immunity is sufficient to significantly reduce the amount and duration of acute vaginal replication, and protects the mice from genital and disseminated disease caused by either HSV-1 or HSV-2. Given that immunization with CJ9-gD protects mice from HSV-1 ocular disease and guinea pigs from HSV-1 cutaneous infection, we conclude that CJ9-gD is a safe and effective vaccine against a range of HSV-associated diseases of both subtypes in rodents.
MATERIALS AND METHODS
Cells and viruses
African Green Monkey Kidney (Vero) cells were grown and maintained in DMEM (Sigma Aldrich, St Louis, MO) supplemented with 10% fetal bovine serum in the presence of 100U ml−1 penicillin G and 100 μg ml−1 streptomycin sulfate (Invitrogen, Carlsbad, CA) (Yao and Schaffer, 1995). RUL9-8 cells, U2OS cells expressing UL9 and tetracycline repressor (tetR), were grown and maintained in DMEM plus 10% fetal bovine serum supplemented with hygromycin B (50 μg ml−1) and G418 (400 μg ml−1) (Yao et al., 2006).
Wild-type HSV-1 strain mP and HSV-2 strain 186 were propagated and plaque-assayed on Vero cells. Both viruses were the kind gift of David M. Knipe (Harvard Medical School). mP is a virulent strain of HSV-1 that has been used earlier as a challenge virus for HSV-1 vaccine studies (Nguyen et al., 1992; Morrison and Knipe, 1994; Augustinova et al., 2004). CJ83193 is a dominant-negative and replication-impaired virus, in which both copies of the HSV-1 ICP0 gene in strain KOS are replaced by DNA sequences encoding the dominant-negative HSV-1 polypeptide, UL9-C535C, under the control of the tetracycline operator sequence-bearing human cytomegalovirus major immediate-early promoter (Yao and Eriksson, 1999). CJ9-gD was derived from CJ83193 by replacing the essential UL9 gene with the HSV-1 gD gene driven by the tetracycline operator sequence-containing human cytomegalovirus major immediate-early promoter (Brans et al., 2008; Lu et al., 2009). No infectious virus was detected in Vero cells when a total of 1.1 × 108 PFU of CJ9-gD virus was plaque-assayed. CJ9-gD was propagated and assayed in RUL9-8 cells.
Mice
Female BALB/c mice 6–8 weeks of age were purchased from Charles River Laboratories (Wilmington, MA). Mice were housed in metal cages at four mice per cage and maintained on a 12 hours light–dark cycle. Mice were allowed to acclimatize to the housing conditions for 1 week before experimentation. All animal experiments were conducted according to the protocols approved by Harvard Medical Area Standing Committee on Animals and the American Veterinary Medical Association.
Immunization and challenges
BALB/c mice were randomly divided into several groups and the hair on their left rear flank was trimmed. One set of six and two sets of 17 mice were either vaccinated with 2 × 106 PFU per mouse of CJ9-gD or mock-vaccinated with DMEM in a volume of 20 μl s.c. in the left rear flank using a 1-ml syringe fitted with a 27-gauge needle. Mice were boosted after 2 weeks and challenged with wild-type virus 4 weeks after primary immunization. At 5 days before challenge, mice were injected s.c. in the neck ruff with medroxyprogesterone (SICOR Pharmaceuticals Inc., Irvine, CA) at 3mg per mouse in a volume of 20 μl. For intravaginal challenge, mice in all groups were anesthetized and preswabbed with a calcium alginate swab (Fisher Scientific, Waltham, MA). One set of six mice and another set of 17 mice were inoculated intravaginally with 20 μl of culture medium containing 5 × 105 PFU of HSV-1 strain mP. Another set of 17 mice was inoculated intravaginally with 20 μl of culture medium containing 1 × 105 PFU of HSV-2 strain 186. Animals were kept on their backs with their rear part elevated under the influence of anesthesia for 30–45 minutes post-infection.
Comparison of CJ9-gD and CJ83193
Two sets of 32 BALB/c mice were either vaccinated with 1 × 105 PFU per mouse of CJ9-gD, 5 × 105 PFU per mouse of CJ9-gD, 5 × 105 PFU per mouse of CJ83193 or mock-vaccinated with DMEM, boosted after 2 weeks and challenged intravaginally with 5 × 105 PFU of HSV-1 strain mP, as described above.
Assay of acute infection
On days 1, 2, 3, 5, and 7 post-challenge, vaginal mucosae were swabbed with a moist calcium alginate swab (Fisher Scientific), which was kept in 1ml DMEM, and stored at −80°C until assayed for infectious virus by standard plaque assay on Vero cell monolayers.
Clinical observation
After challenge with wild-type HSV-1 and HSV-2, mice were observed daily during a 30-day follow-up period for signs of genital lesions and systemic illness. The severity of disease was scored as follows: 0 = no sign, 1 = slight genital erythema and edema, 2 = moderate genital inflammation, 3 = purulent genital lesions, 4 = hind-limb paralysis, 5 = death.
Detection of HSV-1- and HSV-2-specific neutralizing Abs
Blood was collected from tail veins of immunized and mock-immunized mice 4 weeks after primary immunization. Neutralizing serum Ab titers were determined as described earlier in the presence of complement (Bourne et al., 1996; Augustinova et al., 2004) with either 250 PFU of wild-type HSV-1 strain mP or 200 PFU of wild-type HSV-2 strain 186. The neutralizing titer was expressed as the final serum dilution required to achieve a 50% reduction in HSV PFU relative to the HSV PFU obtained in medium plus complement alone.
Long-term effect of vaccination
A total of, 11 female BALB/c mice 6–8 weeks of age were randomly divided into three groups, and immunized s.c. with either wild-type HSV-1 strain KOS (n = 5) or CJ9-gD (n = 4) at 2 × 106 PFU per mouse, or mock-vaccinated with DMEM (n = 2). Mice were boosted 2 weeks after primary immunization. At 6 months after the primary immunization, mice were sacrificed, blood was taken for neutralization Ab assays and spleens were harvested for cytokine assays.
Cytokine assays
A total of 12 female BALB/c mice 6–8 weeks of age were randomly divided into three groups, and immunized s.c. with either KOS or CJ9-gD at 2 × 106 PFU per mouse, or mock-vaccinated with DMEM. Mice were boosted 2 weeks after primary immunization. At 2 weeks after the second immunization, splenocytes were isolated and seeded into 24-well plates at 1.5 × 106 cells per well in a volume of 1ml (Lu et al., 2009). Cells were either mock-stimulated with medium or stimulated with UV-inactivated HSV-2 strain 186 at a multiplicity of infection of 5 PFU per cell, according to the titer before UV inactivation. For the long-term effect of vaccination, one set of cells was stimulated with UV-inactivated HSV-1 strain McKrae at a multiplicity of infection of 5 PFU per cell. After incubation at 37°C for 72 hours, supernatants were collected and stored at −80°C. Levels of IFN-γ, IL-2, and IL-4 expression were determined with the Endogen Mouse Colorimetric ELISA Kits (Fisher Scientific, Waltham, MA).
IFN-γ ELISPOT assays
Splenocytes were isolated from individual female Balb/c mice 10–12 weeks after mock-immunization or primary immunization with 2 × 106 PFU of KOS (n = 4) or 2 × 106 PFU of CJ9-gD (n = 4). The CD4+ T cell ELISPOT assay and the CD8+ T cell ELISPOT assay were carried out as described earlier (Lu et al., 2009). For the CD4+ T cell ELISPOT assay, T cells, isolated from splenocytes using Dynal mouse CD4-negative isolation kit (Invitrogen, Carlsbad, CA), in triplicates were stimulated either with mock-infected or with UV-inactivated HSV-2 strain 186-infected (5 PFU per cell), and with mitomycin C-treated (50 μg ml−1) syngeneic CD11c+ bone marrow-derived dendritic cells at responder-to-stimulator ratios of 5:1 and 10:1, respectively. For the CD8+ T cell ELISPOT assay, cells in triplicate were stimulated with either mock-infected or HSV-2 strain 186-infected (1 PFU per cell), and mitomycin C-treated (50 μg ml−1) syngeneic CL7 cells (ATCC, Manassas, VA) at responder-to-stimulator ratios of 2:1 and 5:1, respectively. Spots were counted in a dissecting microscope and the number of HSV-1-specific IFN-γ spot-forming cells was expressed as the mean ± SEM per million splenocytes minus the spot-forming cells detected in the corresponding control wells stimulated with mock-infected bone marrow-derived dendritic cells or CL7 cells.
Quantitative real-time PCR
The lower lumbar and sacral part of the spinal column, including spinal cord and dorsal root ganglia, were collected 30 days after intravaginal challenge with 5 × 105 PFU of HSV-1 strain mP from 16 mice that had been either immunized with 5 × 105 PFU of CJ9-gD or with 5 × 105 PFU of CJ83193. The spinal column was cut into four pieces and each piece was kept separately in 0.5 ml of normal growth medium and stored at −80°C for further processing. Total DNA was isolated from each dorsal root ganglion using the DNeasy tissue kit (Qiagen, Santa Clarita, CA), and suspended in 60 μl AE buffer. The presence of HSV-1 DNA was quantified by real-time PCR with a pair of primers specific to the HSV-1 DNA polymerase (Forward: 5′-GCT CGA GTG CGA AAA AAC GTT C, Reverse: 5′-CGG GGC GCT CGG CTA AC) as described earlier (Brans et al., 2008). The minimal copies of HSV-1 viral DNA that can be reliably detected were five copies per reaction.
Statistical analysis
For statistical analysis, Student’s t-tests were carried out.
Acknowledgments
This work was supported by the Public Health Service Grant 5RO1AI05088 from the National Institutes of Health.
Abbreviations
- Ab
antibody
- gD
glycoprotein D
- HSV
herpes simplex virus
- PFU
plaque-forming unit
- s.c
subcutaneous
Footnotes
The work was done in Boston, MA, USA
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Augustinova H, Hoeller D, Yao F. The dominant-negative herpes simplex virus type 1 (HSV-1) recombinant CJ83193 can serve as an effective vaccine against wild-type HSV-1 infection in mice. J Virol. 2004;78:5756–65. doi: 10.1128/JVI.78.11.5756-5765.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847–54. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- Bernstein DI, Stanberry LR. Herpes simplex virus vaccines. Vaccine. 1999;17:1681–9. doi: 10.1016/s0264-410x(98)00434-4. [DOI] [PubMed] [Google Scholar]
- Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J Infect Dis. 2005;192:2117–23. doi: 10.1086/498247. [DOI] [PubMed] [Google Scholar]
- Bourne N, Stanberry LR, Bernstein DI, Lew D. DNA immunization against experimental genital herpes simplex virus infection. J Infect Dis. 1996;173:800–7. doi: 10.1093/infdis/173.4.800. [DOI] [PubMed] [Google Scholar]
- Boursnell ME, Entwisle C, Blakeley D, Roberts C, Duncan IA, Chisholm SE, et al. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J Infect Dis. 1997;175:16–25. doi: 10.1093/infdis/175.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans R, Eriksson E, Yao F. Immunization with a dominant-negative herpes simplex virus (HSV) type 1 protects against HSV-1 skin disease in guinea pigs. J Invest Dermatol. 2008;128:2825–32. doi: 10.1038/jid.2008.142. [DOI] [PubMed] [Google Scholar]
- Bryson Y, Dillon M, Bernstein DI, Radolf J, Zakowski P, Garratty E. Risk of acquisition of genital herpes simplex virus type 2 in sex partners of persons with genital herpes: a prospective couple study. J Infect Dis. 1993;167:942–6. doi: 10.1093/infdis/167.4.942. [DOI] [PubMed] [Google Scholar]
- Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–72. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA. 1999;282:331–40. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- Da Costa XJ, Morrison LA, Knipe DM. Comparison of different forms of herpes simplex replication-defective mutant viruses as vaccines in a mouse model of HSV-2 genital infection. Virology. 2001;288:256–63. doi: 10.1006/viro.2001.1094. [DOI] [PubMed] [Google Scholar]
- Dolan A, Jamieson FE, Cunningham C, Barnett BC, McGeoch DJ. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72:2010–21. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344:230–9. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Erturk M, Welch MJ, Phillpotts RJ, Jennings R. Protection and serum antibody responses in guinea-pigs and mice immunized with HSV-1 antigen preparations obtained using different detergents. Vaccine. 1989;7:431–6. doi: 10.1016/0264-410x(89)90158-8. [DOI] [PubMed] [Google Scholar]
- Freeman EE, Orroth KK, White RG, Glynn JR, Bakker R, Boily MC, et al. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect. 2007;83(Suppl 1):i17–24. doi: 10.1136/sti.2006.023549. [DOI] [PubMed] [Google Scholar]
- Jin F, Prestage GP, Mao L, Kippax SC, Pell CM, Donovan B, et al. Transmission of herpes simplex virus types 1 and 2 in a prospective cohort of HIV-negative gay men: the health in men study. J Infect Dis. 2006;194:561–70. doi: 10.1086/506455. [DOI] [PubMed] [Google Scholar]
- Jones CA, Cunningham AL. Vaccination strategies to prevent genital herpes and neonatal herpes simplex virus (HSV) disease. Herpes. 2004;11:12–7. [PubMed] [Google Scholar]
- Koelle DM, Corey L. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin Microbiol Rev. 2003;16:96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med. 1987;316:1444–9. doi: 10.1056/NEJM198706043162304. [DOI] [PubMed] [Google Scholar]
- Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med. 1999;341:1432–8. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- Lekstrom-Himes JA, Pesnicak L, Straus SE. The quantity of latent viral DNA correlates with the relative rates at which herpes simplex virus types 1 and 2 cause recurrent genital herpes outbreaks. J Virol. 1998;72:2760–4. doi: 10.1128/jvi.72.4.2760-2764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang TJ. A community study of ocular herpes simplex. Curr Eye Res. 1991;10(Suppl):111–5. doi: 10.3109/02713689109020366. [DOI] [PubMed] [Google Scholar]
- Looker KJ, Garnett GP. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex Transm Infect. 2005;81:103–7. doi: 10.1136/sti.2004.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Brans R, Akhrameyeva NV, Murakami N, Xu X, Yao F. High-level expression of glycoprotein D by a dominant-negative HSV-1 augments its efficacy as a vaccine against HSV-1 infection. J Invest Dermatol. 2009;129:1174–84. doi: 10.1038/jid.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, et al. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69(Part 7):1531–74. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McLean CS, Erturk M, Jennings R, Challanain DN, Minson AC, Duncan I, et al. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J Infect Dis. 1994;170:1100–9. doi: 10.1093/infdis/170.5.1100. [DOI] [PubMed] [Google Scholar]
- Mertz KJ, Trees D, Levine WC, Lewis JS, Litchfield B, Pettus KS, et al. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis. 1998;178:1795–8. doi: 10.1086/314502. [DOI] [PubMed] [Google Scholar]
- Morrison LA, Knipe DM. Immunization with replication-defective mutants of herpes simplex virus type 1: sites of immune intervention in pathogenesis of challenge virus infection. J Virol. 1994;68:689–96. doi: 10.1128/jvi.68.2.689-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Knipe DM, Finberg RW. Replication-defective mutants of herpes simplex virus (HSV) induce cellular immunity and protect against lethal HSV infection. J Virol. 1992;66:7067–72. doi: 10.1128/jvi.66.12.7067-7072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Bailey G, Ramaswamy M, Hawkes SJ, Geretti AM. Herpes simplex virus type 2: epidemiology and management options in developing countries. Sex Transm Infect. 2007;83:16–22. doi: 10.1136/sti.2006.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. Genital herpes in young adults: changing sexual behaviours, epidemiology and management. Herpes. 2005;12:10–4. [PubMed] [Google Scholar]
- Sawtell NM. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol. 1998;72:6888–92. doi: 10.1128/jvi.72.8.6888-6892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry LR, Bernstein DI, Burke RL, Pachl C, Myers MG. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987;155:914–20. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- Stanberry LR, Cunningham AL, Mindel A, Scott LL, Spruance SL, Aoki FY, et al. Prospects for control of herpes simplex virus disease through immunization. Clin Infect Dis. 2000;30:549–66. doi: 10.1086/313687. [DOI] [PubMed] [Google Scholar]
- Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–8. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–8. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Yao F, Eriksson E. A novel anti-herpes simplex virus type 1-specific herpes simplex virus type 1 recombinant. Hum Gene Ther. 1999;10:1811–8. doi: 10.1089/10430349950017491. [DOI] [PubMed] [Google Scholar]
- Yao F, Eriksson E. Inhibition of herpes simplex virus type 2 (HSV-2) viral replication by the dominant negative mutant polypeptide of HSV-1 origin binding protein. Antiviral Res. 2002;53:127–33. doi: 10.1016/s0166-3542(01)00207-8. [DOI] [PubMed] [Google Scholar]
- Yao F, Schaffer PA. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol. 1995;69:6249–58. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F, Theopold C, Hoeller D, Bleiziffer O, Lu Z. Highly efficient regulation of gene expression by tetracycline in a replication-defective herpes simplex viral vector. Mol Ther. 2006;13:1133–41. doi: 10.1016/j.ymthe.2006.01.009. [DOI] [PubMed] [Google Scholar]