Abstract
Purpose
To present a unified description of reticular macular disease (RMD), a common clinical entity that includes reticular pseudodrusen and confers high risk of progression to advanced age-related macular degeneration (AMD).
Design
Population-based, retrospective, cross-sectional study.
Participants
Forty-two patients with reticular findings in at least one imaging modality, of whom 21 had follow-up.
Methods
RMD was defined as reticular pseudodrusen (RPD) in color or red-free (RF) photography and/or a reticular pattern on scanning laser ophthalmoscope (SLO) imaging (autofluorescence (AF) scans, infrared (IR) photographs or indocyanine green (ICG) angiography). Color and RF images were contrast-enhanced, and color photos were examined in green and blue channels. Image registration in different modalities allowed comparison of areas involved and assessment of lesion co-localization.
Results
RMD was generally present in both photography and SLO imaging. When present in two image modalities, areas of RMD either largely overlapped, or one fell within the other. Individual lesions had high spatial correspondence. Serial imaging showed faded to absent findings in eyes that developed choroidal neovascularization (CNV).
Conclusions
RMD is a single disease entity with stereotypical presentations in multiple imaging modalities, of which reticular pseudodrusen is one. AF, IR, and ICG suggest that it involves the retinal pigment epithelium and choriocapillaris, while photographic patterns implicate the inner choroid. IR imaging, unlike other modalities, can demonstrate RMD in the central macula. RMD is associated with progression to advanced AMD, perhaps on an inflammatory basis. RMD deserves wider recognition among clinicians caring for our elderly patients.
Introduction
Reticular pseudodrusen (RPD) were first characterized by their distinctive appearance in blue light photography.1 They were identified on color fundus photographs as indistinct, interlacing, yellowish lesions occurring in the outer macula,2,3 sometimes seen more clearly on red-free (RF) fundus photographs.1,3,4 Their distribution is typically along the arcades, particularly the superior arcade, occasionally reaching the mid-periphery.5 They were included as a separate entity in the Wisconsin grading system on the basis of their characteristic appearance.2 The choroidal changes on the histopathology of an eye with RPD were atypical of age-related macular degeneration (AMD)-associated drusen,3 but no other pathology has been available. RPD have been postulated to result from an inflammatory process,6 and subsequent studies have linked them to an increased risk of choroidal neovascularization (CNV).3,4,6–9 The epidemiology and significance of RPD, also called retinal reticular drusen, were recently clarified by Klein et al.5 Retinal reticular drusen and reticular pseudodrusen are the same entity, with the choice of terminology dependent on whether or not one considers them to be true drusen. Their overall prevalence was 0.7% in the general population and their 15-year incidence increased with age parallel to that of AMD, from 0.4% to 6.6% in patients aged 43 to 54 to patients aged 75 to 86, respectively.5 Furthermore, they were a strong risk factor for progression to both geographic atrophy (GA) and CNV and were associated with decreased longevity, a finding shared by no other AMD lesion.5
Lois and Bird used the term reticular autofluorescence (AF) to describe a grouping of ill-defined, hypo-autofluorescent lesions against a background of mildly elevated AF,7 but these lesions were not ascribed any particular significance. We subsequently showed that these lesions corresponded to RPD on color photographs.6 Both RPD and reticular AF are notable for being relatively low contrast as compared to the usual soft drusen and the typical hyper- or hypo-autofluorescent lesions of AMD, respectively. Both lesions occur in rather regular patterns and in well-defined domains as compared to the often random scattering of soft drusen and the AF lesions of AMD. Additionally, these lesions were observed to be hypofluorescent on mid- to late-phase indocyanine green (ICG) angiography.8 RPD are not well-visualized on fluorescein angiography (FA) and have not been reported as hyperfluorescent, contrary to what is commonly observed with soft drusen.8 As a result of the challenging visualization on traditional color photos, a recent study by Cohen et al. suggested that examinations for RPD should use additional modalities (red-free, blue-light and AF images) for improved diagnosis.4
In this study, we present a unified imaging description of reticular macular disease, a clinical entity that includes RPD and reticular AF, is not uncommon, and confers high risk of progression to advanced AMD. Specifically, we demonstrate the characteristic appearances of reticular macular disease in color fundus, red free, infrared (IR), ICG, and AF images, noting improved identification and visualization of these lesions when using multiple imaging modalities. We also observe fading of these lesions as CNV develops.
Methods
Study Population
The Macular Genetics Study at Columbia University began in 1999, adhering to a standard color photographic protocol for the classification of AMD status. With the availability of SLO imaging, some new and returning patients also had AF and IR images obtained. Imaging data from 625 patients enrolled in this study with grade 2 age-related maculopathy or higher10 was reviewed for evidence of reticular macular disease. Standard photographs of typical presentations of reticular macular disease were used to aid diagnosis. Each image series (photographic or SLO) was deemed positive, negative, or ungradeable for reticular disease by two graders. All photographic image series were also contrast-enhanced and viewed in the blue and green channels using Adobe Photoshop (version 9.0.2, Adobe Systems Inc. San Jose, CA). Both green and blue channel photographs demonstrated reticular findings as light, interlacing networks, often with improved resolution as compared to standard color fundus photographs. Disagreements were resolved by a senior grader. All eyes with at least one positive image were re-graded by two senior graders for positive or negative findings in each image type. Disagreements were resolved by consensus. If available, an early FA frame was added to each positive image set for comparison. The final study population, 65 eyes of 42 patients, included only images confirmed by senior graders as having evidence of reticular disease. One such patient had no evidence of classic age-related maculopathy, with only bilateral reticular findings (Figure 5).
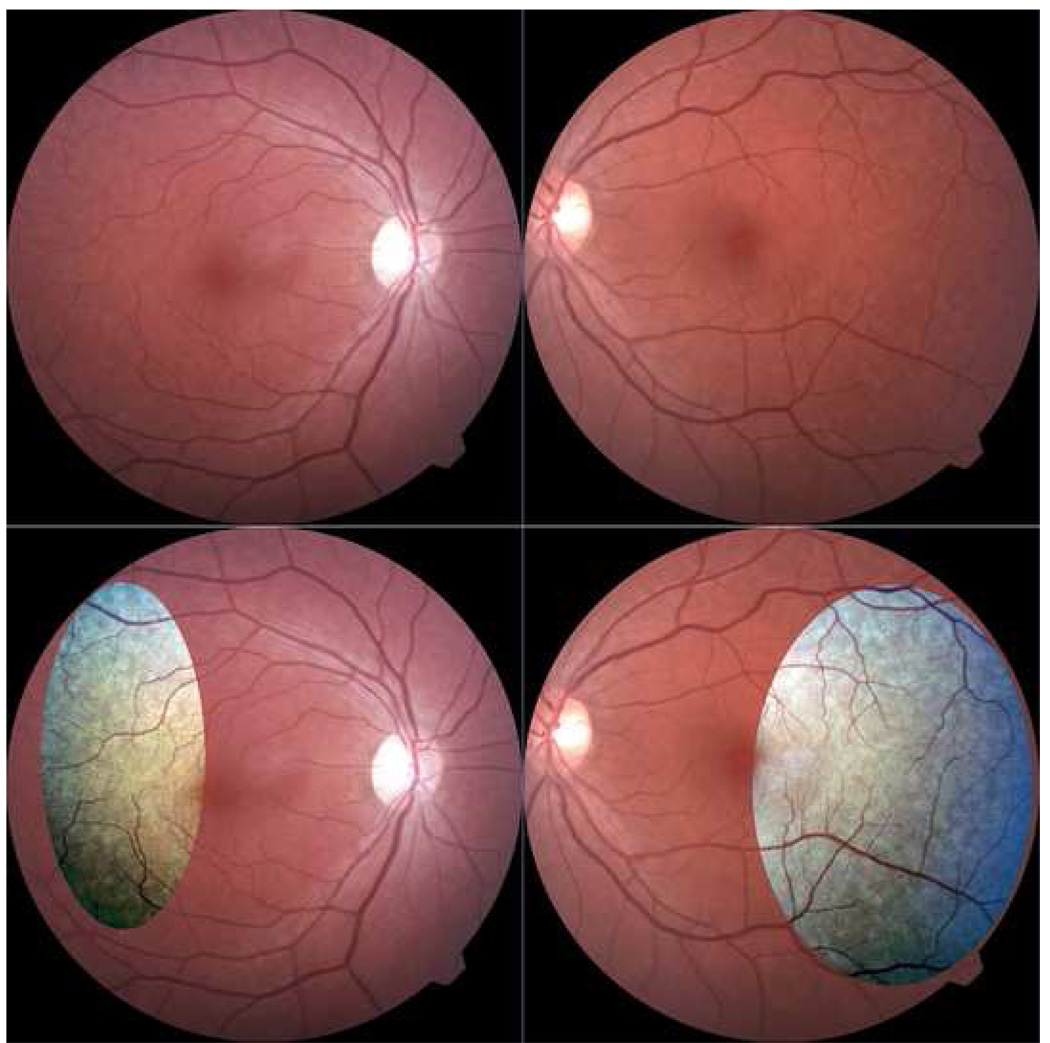
Figure 5. Reticular pseudodrusen without maculopathy in a patient with reticular macular disease.
Contrast-enhanced color photos. This 50-year-old female has significant reticular pseudodrusen peripherally bilaterally in the absence of other age-related maculopathy. The pale spot centrally in the right eye is artifact.
Of the total eyes included, 75% (49/65) had gradable images in both photographic and SLO categories on at least one exam date. If more than one date qualified, the first such date was chosen for detailed image analysis and correlation between photo and SLO positivity. Correlation was also made between the imaging subtypes. For the remaining 16 eyes (color fundus without SLO or vice versa) a similar analysis was made of the available imaging subtypes. Twenty-one patients had follow-up imaging ranging from 1 to 5 years (median 2 years) after the initial visit, of whom 7 had images from 1 follow-up visit, 9 from 2 visits, and 5 from 3 to 6 visits, respectively. Follow-up images were examined for any changes in the appearance of reticular disease or the appearance of late-stage macular degeneration.
Image Acquisition
Color fundus and RF photographs were either film-based photographs acquired on the Topcon TRC-50EX (Topcon Medical Systems, Inc., Paramus, NJ) and digitized on the Nikon CoolScan V (Nikon Corp., Tokyo, Japan) or acquired digitally on the Zeiss FF 450 Plus Fundus Camera (Carl Zeiss Meditec, Inc., Jena, Germany). AF and IR images were obtained using the Heidelberg model HRA/HRA2 confocal scanning laser ophthalmoscope (SLO; Heidelberg Engineering Inc., Dossenheim, Germany). For AF images, this instrument used blue laser light at 488 nm for illumination and a barrier filter at 500 nm to limit the captured light to autofluorescent structures. Each image was an average of 3 to 6 scans composed by the SLO software. IR reflectance images were obtained at 810 nm. Simultaneous ICG angiography with the HRA/HRA2 was performed on 9 patients.
Definitions
For color fundus or corresponding RF photographs, RPD were identified as yellow or light interlacing networks ranging from 125 to 250 microns in width.3 We also required that the lesions be relatively low-contrast and occur in regular patterns and well-defined domains (Figure 1 and Figure 2). Reticular AF was defined as a grouping of ill-defined, hypofluorescent lesions against a background of mildly elevated AF.6,7 As with RPD, we required that the lesions be relatively low-contrast and occur in regular patterns and well-defined domains (Figure 3 and Figure 4). Reticular IR was defined as groups of hyporeflectant lesions against a background of mild hyperreflectance with analogous characteristics (Figure 4). For ICG angiography, a reticular pattern was identified as a distinctive grouping of hypofluorescent dots present in the mid-to-late phases of the angiogram (Figure 3).8 Reticular macular disease was defined as RPD in color or RF photography and/or a reticular pattern on SLO imaging (AF, IR or ICG). Early phase FA images, where available, were examined for supplemental information only (defects in choriocapillaris filling), but were not used to define the disease because such defects are common to other disease processes, making them non-specific for reticular macular disease.
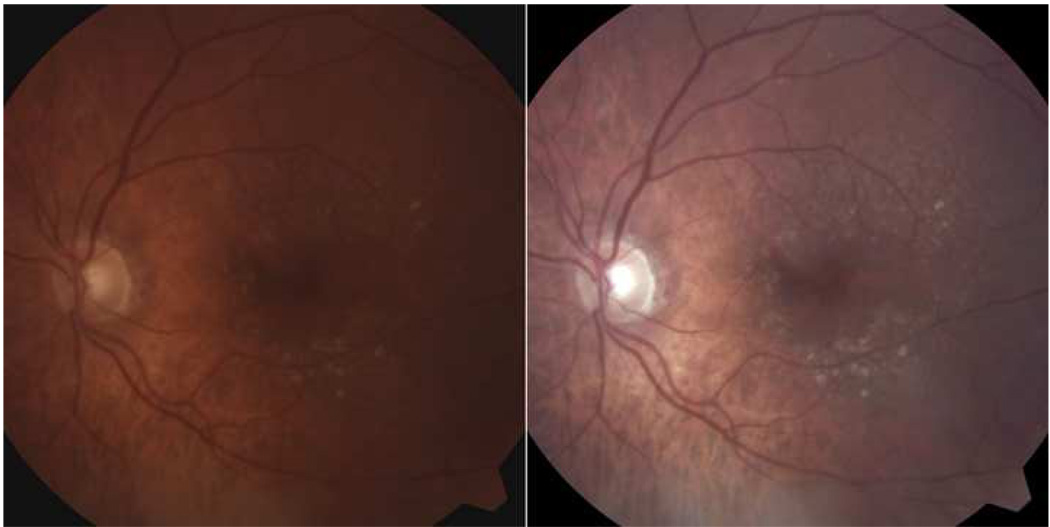
Figure 1. Color image of patient with reticular macular disease before and after contrast-enhancement.
(Left) Color photo of the left eye showing large soft drusen centrally. Careful inspection reveals reticular pseudodrusen (RPD) in the superior macula. (Right) Contrast-enhanced version of image on left. The RPD in a uniform, interlacing pattern are easily seen.
Figure 2. Blue-channel visualization of reticular pseudodrusen (RPD) in a patient with reticular macular disease.
The contrast-enhanced color photo (left) and red-free photo (center) are negative for RPD. However, the blue channel of the contrast-enhanced color photo (right) shows a definite reticular pattern superiorly.
Figure 3. Overall spatial correspondence of reticular lesions between imaging types, characteristic of reticular macular disease.
(Top left). Enhanced color photo with reticular pseudodrusen superiorly. (Top right) Blue channel of color photo shows a reticular pattern superiorly. (Bottom left) Mid-phase indocyanine green (ICG) angiography showing a reticular pattern extending from the superior macula to the arcade and above the disc. (Bottom right) Autofluorescence scan with reticular pattern in exactly the same distribution as the patterns on the ICG and color images.
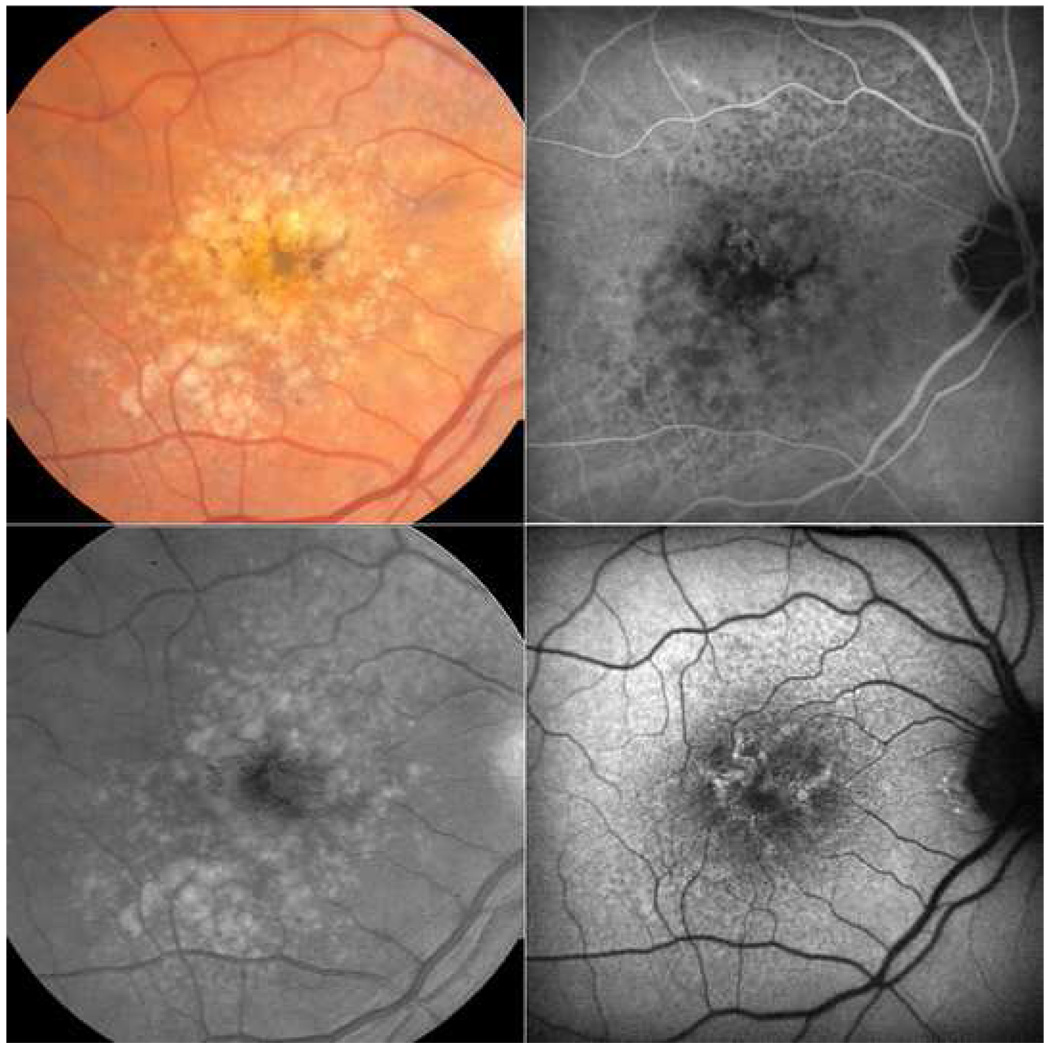
Figure 4. Spatial correspondence between autofluorescence (AF) and infrared (IR) reticular patterns, characteristic of reticular macular disease.
(Left) AF scan of the left eye (OS) showing reticular pattern temporal and superiorly to the more typical AF lesions of age-related macular degeneration (dark areas of impending atrophy and hyper-AF overlying soft drusen). (Right) IR scan OS showing a reticular pattern of hypo-reflectant lesions with hyper-reflectant borders mostly temporally and falling within the areas of reticular AF.
Image Analysis
Color fundus and RF photographs were viewed in Adobe Photoshop (version 9.0.2, Adobe Systems Inc. San Jose, CA), using the standardized Auto Contrast tool to verify the presence of RPD. Contrast-enhanced color photos were also examined in the green and blue channels to aid in RPD visualization.3,4 The advantages of these techniques are seen in Figure 1 and Figure 2. Monochromatic blue light photographs have been reported to improve visualization of RPD1 and as such, have traditionally been recommended for their study. This modality was not used in the Columbia Macular Genetics Study, so as a substitute, we examined the blue channel of color photographs. This is not strictly equivalent, but assisted in some cases.
For eyes with positive reticular findings on more than one imaging modality, representative images were precisely registered (MatLab ver. 7.0; The MathWorks, Natick, MA) through an automated method described previously (Chen J, et al. A Novel Registration Method for Retinal Images. 30th Annual International Conference of IEEE EMBS, 2008; Vancouver). Registered images were cropped using an auto-crop tool (Invest Ophthalmol Vis Sci 47:E-Abstract 5715, 2005), then layered in Photoshop. Our custom automated software was used only for convenience. Image registration and cropping can be performed completely within Photoshop and Matlab.13 All areas with reticular disease were outlined manually within Photoshop using the pencil tool (Figure 6) and, in 20 pairs and triples of registered images, markings were placed over individual lesions in a new layer for each image type. These outlines were then superimposed to compare areas of involvement and to assess co-localization (Figure 7). Segmentation of RPD was performed in the 6000-micron region by the automated mathematical model-based digital technique previously described, tested for accuracy, and implemented in AF images.6,11–14 Results were used to compare the distribution of RPD on color photographs to the distribution of reticular lesions on AF and IR (Figure 8).
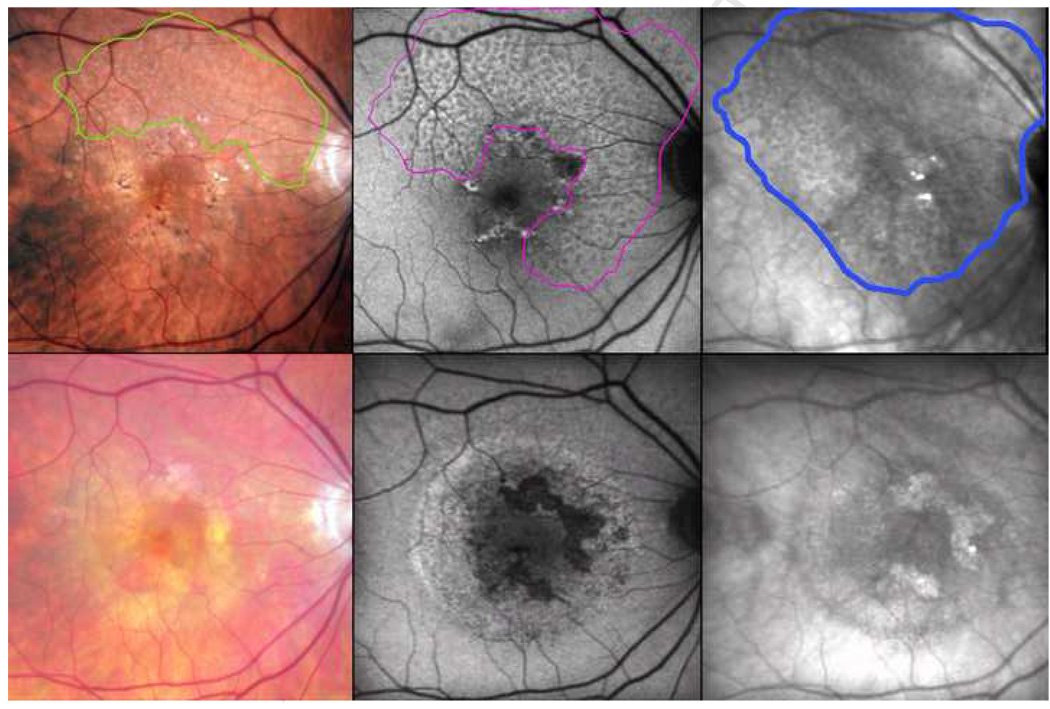
Figure 6. Fading reticular patterns across imaging modalities after choroidal neovascularization, following the pattern seen in patients with reticular macular disease.
The contrast-enhanced color photo shows reticular pseudodrusen (RPD) in the superior quadrants, outlined in green (top left). The autofluorescence (AF) scan, registered to the color photo, shows a reticular pattern in 3 quadrants, outlined in magenta (top center). The infrared (IR) scan, also registered, shows a reticular pattern in all 4 quadrants extending through the central macula, outlined in blue (top right). The area involved on IR includes that involved on AF, which in turn includes the area of RPD. (Bottom row) Follow-up images were obtained after two years, during which time choroidal neovascularization developed. The enhanced color photo no longer shows RPD (bottom left), nor did the red-free or the blue channel photos (not shown). The reticular AF and IR patterns are faint, even in the areas not involved by exudation (bottom center and right, respectively).
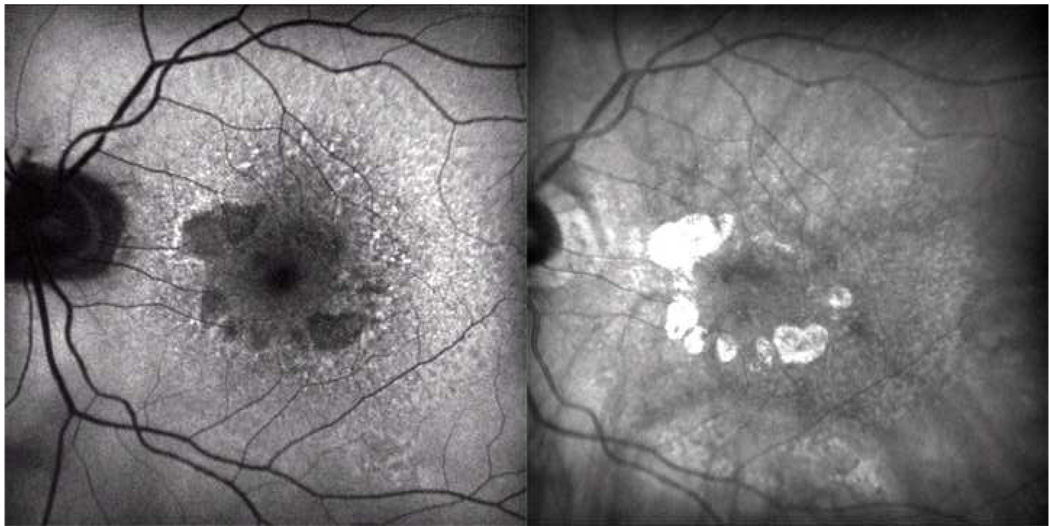
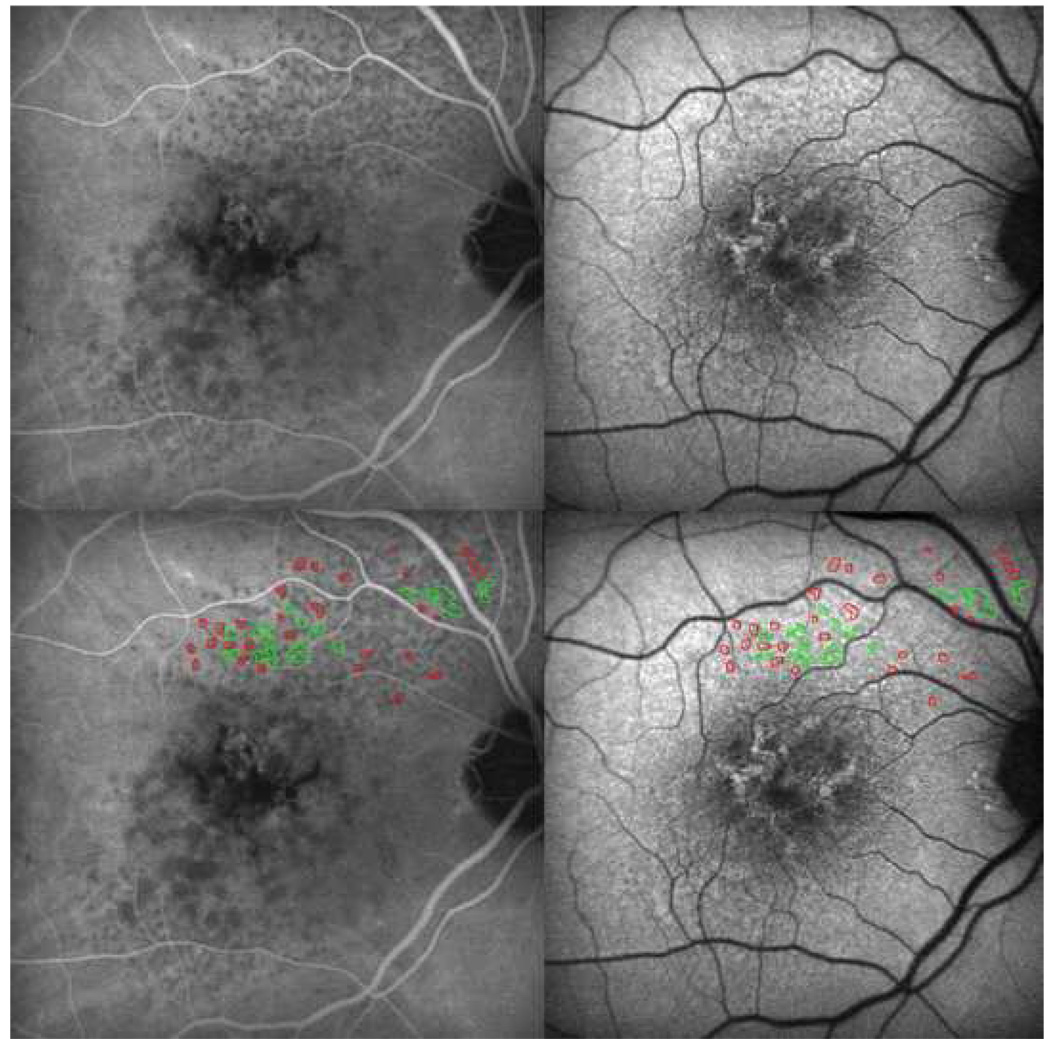
Figure 7. Marked spatial correspondence between reticular lesions on autofluorescence and indocyanine green angiography, characteristic of reticular macular disease.
Late-phase indocyanine green angiography (ICG, top left) and autofluorescence (AF, top right) images for the same patient are shown. Tracings of reticular pattern on the ICG photograph (red) and the autofluorescence photograph (green) were done on a separate layer using the pencil tool in Photoshop. The mask was then super-imposed onto the same ICG (bottom left) and AF (bottom right) photographs. Tracings correspond to reticular findings on both photographs, regardless of the image used to create the outlines.
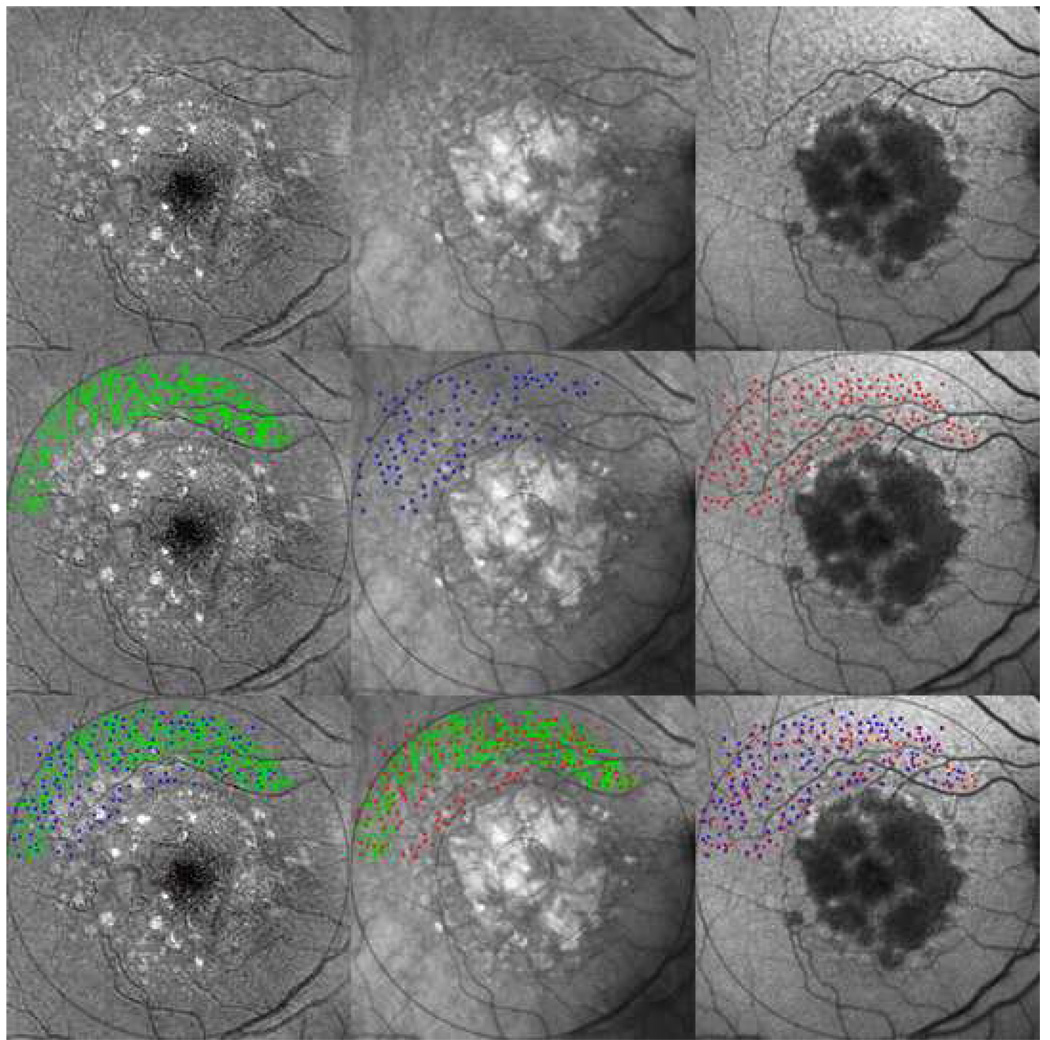
Figure 8. Marked spatial correspondence between reticular pseudodrusen (RPD) and reticular findings on autofluorescence (AF) and infrared (IR) images, characteristic of reticular macular disease.
Reticular lesions extend peripherally with visible geographic atrophy and soft drusen centrally in the contrast-enhanced blue channel color fundus photo (top left), IR (top center), and AF (top right) images of the same eye, all of which were registered and cropped. (Center left) The automated drusen segmentation tool applied in the 6000-micron region created a segmentation mask for RPD (green). (Center middle) IR reticular lesions manually marked as dots (blue) (Center right) AF reticular lesions manually marked as dots (red). These segmentations in turn are superimposed pairwise to give: (Bottom left) IR lesions (blue) overlaid on RPD (green). (Bottom center) AF lesions (red) overlaid on RPD (green). (Bottom right) AF lesions (red) overlaid on IR lesions (blue). The final results show striking co-localizations between the reticular lesions from each image modality.
Results
Inter-observer concordance for initial gradings for reticular disease was 92% for SLO and 90% for photographic image sets. The remaining 8% of SLO and 10% of photographic image sets with disagreements between graders were reviewed by senior graders who determined their eligibility for inclusion in the study. Of the 42 patients identified with reticular macular disease, 74% (31/42) had advanced AMD (GA or CNV) in one or both eyes, as classified by fundus photography per the International grading system.10 Of the 31 patients with advanced AMD (GA or CNV), 29% (9/31) had GA, 61% (19/31) had CNV, and 10% (3/31) had both GA and CNV. The gender distribution was 79% female and 21% male. Ages ranged from 50 to 95 years old, with a mean age of 80. Of the study patients, 37 were Caucasian, 2 were Hispanic, 2 were Asian, and 1 was Middle Eastern. Of the eyes with both SLO and photographic images, 12% (5/42) had reticular patterns only on SLO images, and 5% (2/42) with marginal AF scan quality had RPD only on color fundus photographs. Where RPD were not present, the SLO reticular lesions had no particular association with other fundus abnormalities (Figure 10). All 9 patients with ICG imaging had positive reticular AF or IR findings as well. Of the eyes with both AF and IR imaging, 82% (35/43) had reticular findings on both AF and IR images, 5% (2/43) had reticular AF only, and 14% (6/43) had reticular IR only. Of the 6 eyes with reticular IR, 5 had documented cataracts. When RPD were present and early FA frames were available, areas of decreased choriocapillaris filling were found to lie in register with the RPD lesions. In general, however, such areas were difficult to distinguish from normal variation and were not used to diagnose reticular macular disease.
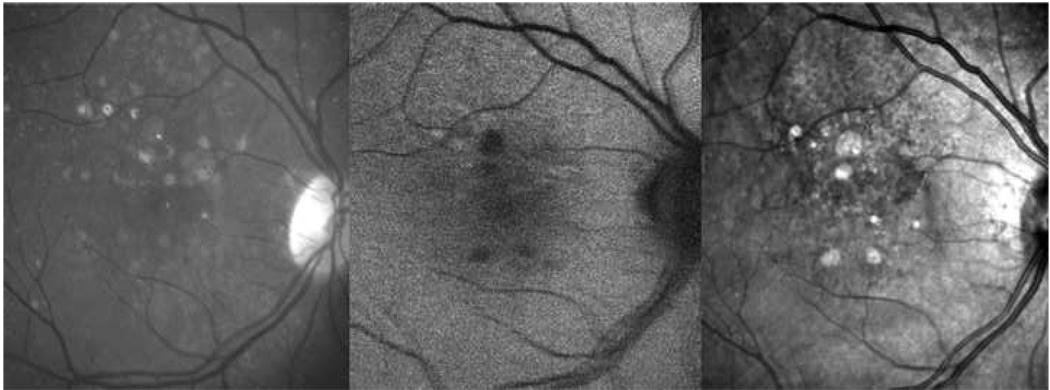
Figure 10. Negative reticular findings in color and autofluorescence (AF) images with positive findings on infrared (IR) imaging in a patient with reticular macular disease.
Green-channel color fundus photograph (left), AF (center), and IR (right) images are shown. Color and AF images were graded negative for reticular findings, but IR shows a clear reticular pattern supero-nasally.
Distribution of the reticular lesions in color fundus, AF, and IR imaging can be seen in Figure 8. Within the 6000-micron diameter circle, all lesions had a predilection for the superior outer macula, although infrared lesions were more evenly distributed. Outside this circle, all lesions had virtually 100% representation in and near the superior arcades, with significant representations also occurring temporally, nasally, and inferiorly, in that order. When reticular AF and RPD were both present (36 eyes), RPD were present in the same (20 eyes) or fewer (16 eyes) quadrants as the reticular AF pattern. When AF and IR reticular patterns were both present (35 eyes), the distributions always overlapped, but in 2 eyes, the AF involved more quadrants, and in 21 eyes, the IR involved more quadrants. Significantly, the region involved by the IR lesions in 17 eyes covered the central 1000-micron diameter zone. By contrast, the AF lesions and the RPD never involved the central zone.
Image registration showed high spatial correspondence of the individual lesions from different modalities (Figure 8). When present in two image modalities, areas of reticular macular disease either largely overlapped, or one fell within the other (Figure 6–Figure 8). Serial imaging demonstrated that reticular patterns tended to be nearly constant in time, with the notable exception of eyes that had or developed CNV. In the case of 6 eyes with reticular macular disease that went on to develop new CNV, subsequent images in each modality showed faded to absent reticular findings in comparison with the original images (Figure 6) Images from years prior to CNV demonstrated constant reticular findings. We found one example of partial regression of photographic reticular disease after 5 years without CNV.
Discussion
Reticular macular disease, as explored herein, appears to be a single entity presenting in a stereotyped fashion on multiple imaging modalities, of which the classic photographic entity of RPD is only one manifestation. Our results provide strong evidence that a single disease is responsible for each of these presentations. First, when present in the same eye, individual lesions of different modalities have high spatial correspondence. Second, the total areas of the macula involved, though not necessarily identical, either largely overlap or fall within one another. Finally, for any given modality, the individual lesions and their pattern of arrangement in all of the eyes examined are remarkably similar. Reticular disease appears to involve multiple layers where tissue damage may in turn be preferentially imaged by different acquisition methods. The most involved layer and other intraocular structures, such as macular pigment or crystalline lens, may determine the predominant imaging presentation. Thus, damage occurring in the RPE may be more prominent on AF imaging since AF specifically images the RPE. On the other hand, AF imaging with blue laser light could be more hampered by lens opacity than other modalities, whereas IR would be least affected. Although reticular macular disease may still be a form of soft drusen,5 the imaging data points to other possibilities, with AF and IR suggesting RPE involvement. While soft drusen have a highly reflectant or neutral appearance on infrared imaging, IR imaging of reticular disease uniformly reveals dark, hyporeflectant lesions, suggesting a separate pathogenic process. FA and ICG show filling defects that implicate the choriocapillaris without apparent defects in the retinal circulation. Color fundus photography might imply deposits in the similarly intertwined vessels of the inner choroid. The sensitivies of each imaging technique in the detection of reticular macular disease, as well as the stereotypical appearance of reticular findings on each imaging modality, can be seen in Table 1. Indeed, reticular macular disease may present fully developed in the absence of any other classic lesions of age-related maculopathy,5 suggesting a distinct pathological process (Figure 5). However, when combined with AMD, the prognosis for progression of AMD is strong.
Table 1.
Reticular Macular Disease – Different Imaging Patterns and Sensitivities
| Image Type | Pattern Description | Sensitivity |
|---|---|---|
| Color Fundus | Yellow, interlacing networks ranging from 125–250 microns in width | 88% |
| RF | Light, interlacing networks ranging from 125–250 microns in width | 88% |
| AF | Groupings of ill-defined, hypofluorescent lesions against a background of mildly elevated AF | 86% |
| IR | Groupings of hyporeflectant lesions against a background of mild hyperreflectance with analogous characteristics | 95% |
| FA | Defects in choriocapillaris filling in the early-phase images | 17% |
| ICG Angiography | Distinctive groupings of hypofluorescent dots present in the mid-to-late phases of the angiogram | 100% |
CI = 95% Confidence Interval; RF = Red-Free; AF = Autofluorescence; IR = Infrared; FA = Fluorescein Angiography; ICG = Indocyanine Green
It is important to distinguish the appearance of reticular patterns, which in our data were often found intermingling with hard and soft drusen.2 We found 42 (6.7%) patients out of 625 with existing maculopathy to have reticular macular disease as well, in line with other published data.3–5 A summary of the differences between the distinct appearances of each of the types of drusen on imaging can be found in Table 2, along with the associated clinical picture and recommendations. The interlacing pattern characteristic of reticular macular disease is clearly distinguishable from more common drusen, more so because the reticular pattern often extends peripherally while large, soft drusen that can be mistaken for RPD are often restricted to the central region. Reticular pseudodrusen also tend to form a single, well-defined group with a close-knit, uniform pattern of lesions within the group, as opposed to soft drusen, which are typically more scattered and heterogeneous (Figure 3). Despite the similar nomenclature, cuticular or basal laminar drusen (BLD), in contrast to RPD, are tiny (30 to 50 microns), have been associated with pseudovitelliform macular detachment, and exhibit a characteristic “starry sky” pattern of multifocal window defects on fluorescein angiography.16 AF images also demonstrate the presence of BLD through hypo-autofluorescent lesions in a similar pattern of distribution.16 Reticular pseudodrusen have also been called retinal reticular drusen,2,5 but these terms are interchangeable and refer to the same findings on color fundus photographs.
Table 2.
Reticular Macular Disease – Distinguishing Reticular Findings from Other Drusen Types
| Drusen Type | Clinical Pattern | Imaging Pattern | Recommendations |
|---|---|---|---|
| Hard Drusen | Characteristic of earlier stages of macular degeneration, can be normal finding in older patients | Yellow, discrete appearance on color fundus photos, between 1–63 microns2 | Monitor with follow-up. |
| Soft Drusen | Found in early and late-stage macular degeneration, typically associated with pigmentary changes as disease progresses | Yellow, fuzzy appearance on color fundus photos, > 125 microns or from 63–125 microns with visible thickness2 | Check for pigmentary changes, monitor with follow-up. AREDS supplements if at least one large or multiple intermediate. |
| Reticular Pseudodrusen | Often associated with macular degeneration and associated drusenoid/pigmentary changes | Yellow, interlacing network ranging from 125–250 microns on color fundus photos,3 best seen on RF, IR, AF, or ICG angiography images | If suspected, order additional imaging including RF photos. If available, also check SLO images as well. Monitor closely for advanced AMD. AREDS supplements if soft drusen also present. |
| Basal Laminar Drusen | Associated with pseudovitelliform detachments | Shows “starry sky” pattern on FA or multifocal areas of hypofluorescence on AF16 | Order AF or FA, per availability. |
SLO = scanning laser ophthalmoscope; AF = autofluorescence; IR = infrared; RF = red-free; ICG = indocyanine green; FA = fluorescein angiography; AMD = age-related macular degeneration; AREDS = age-related eye disease study
IR demonstrated reticular disease in the central macula of 17 eyes, which was not seen in other modalities in our series or in the literature.3,5 The reason that reticular AF does not involve the central zone is likely due to blocking of the SLO blue laser light by luteal pigment. Likewise, the reason that RPD do not involve the central zone may also be suppression of blue light, particularly scattering. Conversely, while recognizing that light will necessarily be multiply scattered at several layers, we suggest that a single construct, wavelength dependence of scattering, which would be most pronounced for blue light, the most highly scattered wavelength. This might explain the improved visualization of RPD in blue light photos1 and the suppression of their visualization centrally by luteal pigment. An analogous but better understood phenomenon is that blue irides are apparently explained by excess scatter of blue light, even though there are multiple layers and potential scatterers in the iris itself. With regards to the question of how the peripheral lesions of pseudodrusen or reticular AF can be so strongly tied to CNV, which almost invariably arises in the central macula, the answer in some cases may then be that IR imaging is necessary for the visualization of reticular disease in the central macula.
Although RPD have been particularly associated with progression to CNV,3,4,6–9 the Beaver Dam study found an increase in both CNV and GA in such eyes.5 In our study, we confirmed a high prevalence of advanced AMD of both types in patients with reticular macular disease. We also found that patients who had RPD and subsequently developed CNV in the same eye displayed significant fading or loss of the pattern, while those who progressed to GA retained their patterns. Thus, while we herein and previously6 commonly found reticular patterns in the fellow eyes of unilateral CNV but not in the CNV-affected eye itself, this trend can now be explained by prior diminishment of the reticular lesions in the CNV eye. Large soft drusen may of course regress in areas of active CNV, but the regression of reticular findings at the arcades, which are generally beyond the areas clinically involved by exudation, requires elucidation (e.g. sub-clinical tracking of fluid over a larger area than expected)
An inflammatory basis for RPD has been suggested,6 consistent with the finding of increased heterozygosity and homozygosity for the Y402H CFH variant in patients with reticular findings.5 In accordance with the study by Klein et al.,5 we noticed a female predominance in our reticular patient population, with 79% female to 21% male patients.5 Being female has been associated with an increased prevalence of systemic autoimmune inflammatory disease. It remains to be seen whether there will be inflammatory markers or pathogens associated with reticular macular disease.
This study has several limitations, the first being that it was a retrospective study without complete imaging data on every patient. Likewise, the serial imaging studies were only available on a subset of subjects, the follow-up periods were relatively short, and the imaging data was not complete at each serial exam. This was largely due to the fact that we drew data from the Macular Genetics Study, which required only color photography on every patient, leading to partial availability of other image types. In particular, the total number of eyes with ICG data was small. However, the findings of the study were specifically restricted to conclusions that were supportable by this type of data. For example, we found that when both SLO and photographic imaging were available (48 eyes of 31 patients), the concordance of positive findings of reticular disease was quite high. In such cases, the spatial correspondence of individual lesions was also quite high. Regarding the short follow-up periods, it should be noted that they strengthen the case for a cause and effect relationship in the most important longitudinal finding, that of the disappearance of reticular patterns with incident CNV. However, it may be that longer follow-up would have shown further instances of fading or increasing reticular macular disease with or without CNV.
The main strength of the study is the remarkable consistency of findings between modalities in a given eye and the similarity of patterns in eyes from different patients in each modality. This result was reinforced by the rigor of image analysis techniques. For example, although the observation of ICG hypofluorescence of RPD has been reported,8 we have now verified that the ICG lesions are in precise registration with the RPD and with the lesions of reticular AF.
We have presented evidence that RPD and corresponding reticular patterns on AF/IR/ICG are hallmarks of a single but complex disease process, reticular disease of the macula, which involves the RPE, choriocapillaris, and inner choroid. IR and ICG now implicate the choriocapillaris in addition to the RPE damage suggested by AF, and the photographic data further implicate inner choroid. We conclude that reticular macular disease, which confers high risk of progression to advanced AMD, both GA and CNV, deserves wider recognition among clinicians caring for our elderly patients. Although research tools like AF and IR imaging may not always be available, reticular macular disease may be better diagnosed on standard photography. We recommend careful inspection of color and RF photographs from AMD patients, which generally reveal these subtle, low-contrast lesions in the outer macular area. As demonstrated herein, widely available contrast-enhancement techniques can assist in the diagnosis. Fellow eyes of unilateral CNV eyes with reticular macular disease appear to be at special risk, perhaps warranting greater efforts at identification and, if present, more meticulous follow-up. The increased visual morbidity associated with reticular macular disease, as well as the findings of decreased longevity in such patients, make identification of these lesions and research into their underlying cause imperative.
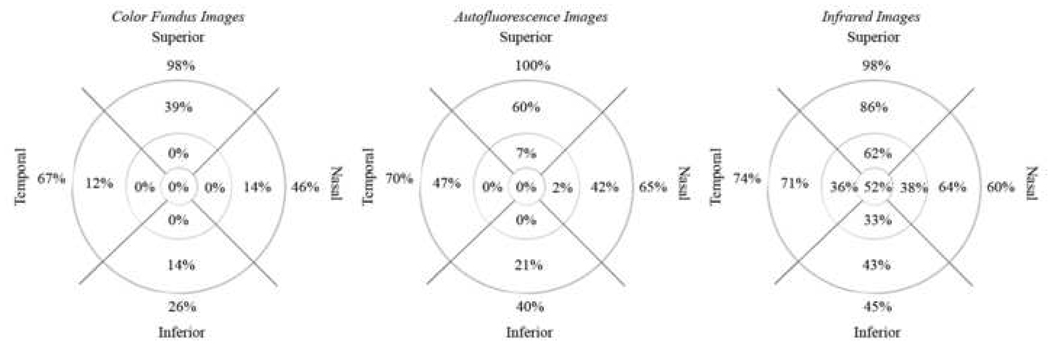
Figure 9. Percent distributions of reticular lesions in different imaging modalities for study patients with reticular macular disease.
Percent distributions of reticular pattern, when positive, in color fundus, autofluorescence, and infrared images, displayed from left to right, respectively. The reticular pattern was most frequently noted in the outer macula and outside of the grid, near the arcades, and was notably absent in the inner and central macula of color fundus images, likely secondary to pre-existing macular pathology from moderate-to-late stage age-related macular degeneration.
Acknowledgements
a. Funding/Support: Supported by grants from The New York Community Trust (New York, NY), National Eye Institute Grant R01 EY015520 (Bethesda, MD), and unrestricted funds from Research to Prevent Blindness (New York, NY). The funding organizations had no role in the design or conduct of this research.
b. Financial Disclosures: None.
c. Author Contributions: Study design and conduct (RTS, MAS, MB, GB); collection, management, analysis, and interpretation of data (RTS, MAS, MB, GB); and preparation, review, or approval of the manuscript (RTS, MAS, MB, GB).
d. Conformity with Author Information: The Macular Genetics Study at Columbia University was approved by the IRB of Columbia University and adhered to the tenets of the Declaration of Helsinki. All participants provided signed, informed consent for participation in the study and for the publication of the data obtained.
e. Other Acknowledgements: None.
Biographies

Gaetano R. Barile, M.D. is Associate Professor of Clinical Ophthalmology and the Glaubinger Scholar in Retinal Research at Columbia University in New York. Along with Dr. Smith, he is clinical co-investigator of the NEI-supported Columbia Macular Genetics Study.

R. Theodore Smith, M.D., Ph.D. is Professor of Clinical Ophthalmology and Biomedical Engineering and director of the NIH supported Retinal Image Analysis Lab at Columbia. Along with Dr. Barile, he is clinical co-investigator of the NEI-supported Columbia Macular Genetics Study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mimoun G, Soubrane G, Coscas G. Macular Drusen. J Fr Ophtalmol. 1990;13:511–530. Medline. [PubMed] [Google Scholar]
- 2.Klein R, Davis MD, Magli YL, Segal P, Klein BEK, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. Medline. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular Pseudodrusen: A Risk Factor in Age-Related Maculopathy. Retina. 1995;15:183–191. Medline. doi:10.1097/00006982-199515030-00001. [PubMed] [Google Scholar]
- 4.Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G. Prevalence of Reticular Pseudodrusen in Age-Related Macular Degeneration with Newly Diagnosed Choroidal Neovascularization. Br J Ophthalmol. 2007;91:354–359. doi: 10.1136/bjo.2006.101022. Medline. doi:10.1136/bjo.2006.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BEK. The Epidemiology of Retinal Reticular Drusen. Am J Ophthalmol. 2008;145:317–326. doi: 10.1016/j.ajo.2007.09.008. Medline. doi:10.1016/j.ajo.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RT, Chan JK, Busuoic M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence Characteristics of Early, Atrophic, and High-Risk Fellow Eyes in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2006;47:5495–5504. doi: 10.1167/iovs.05-1318. Medline. doi:10.1167/iovs.05-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lois NL, Owens SL, Coco R, Hopkins J, Fitzke FW, Bird AC. Fundus autofluorescence in patients with age-related macular degeneration and high risk of visual loss. Am J Ophthalmol. 2002;133:341–349. doi: 10.1016/s0002-9394(01)01404-0. Medline. doi:10.1016/S0002-9394(01)01404-0. [DOI] [PubMed] [Google Scholar]
- 8.Arnold JJ, Quaranta M, Soubrane G, Sarks SH, Coscas G. Indocyanine Green Angiography of Drusen. Am J Ophthalmol. 1997;124:344–356. doi: 10.1016/s0002-9394(14)70826-8. Medline. [DOI] [PubMed] [Google Scholar]
- 9.Prenner JL, Rosenblatt BJ, Tolentino MJ, et al. Risk Factors for Choroidal Neovascularization and Vision Loss in the Fellow Eye Study of CNVPT. Retina. 2003;23:307–314. doi: 10.1097/00006982-200306000-00004. Medline. doi:10.1097/00006982-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Bird AC, Bressler NM, Bressler SB, et al. An International Classification and Grading System for Age-Related Maculopathy and Age-Related Macular Degeneration. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. Medline. doi:10.1016/S0039-6257(05)80092-X. [DOI] [PubMed] [Google Scholar]
- 11.Smith RT, Chan JK, Nagasaki T, Sparrow JR, Barbazetto I. A method of drusen measurement based on reconstruction of fundus background reflectance. Br J Ophthalmol. 2005;89:87–91. doi: 10.1136/bjo.2004.042937. Medline. doi:10.1136/bjo.2004.042937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith RT, Chan JK, Nagasaki T, et al. Automated detection of macular drusen using geometric background leveling and threshold selection. Arch Ophthalmol. 2005;123:200–207. doi: 10.1001/archopht.123.2.200. Medline. doi:10.1001/archopht.123.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith RT, Koniarek JP, Chan J, Nagasaki T, Sparrow JR, Langton K. Autofluorescence characteristics of normal foveas and reconstruction of foveal autofluorescence from limited data subsets. Invest Ophthalmol Vis Sci. 2005;46:2940–2946. doi: 10.1167/iovs.04-0778. Medline. doi:10.1167/iovs.04-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang JC, Chan JWK, Chang S, Smith RT. Predictive value of fundus autofluorescence for development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:2655–2661. doi: 10.1167/iovs.05-1027. Medline. doi:10.1167/iovs.05-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein R, Knudtson MD, Klein BEK, et al. Inflammation, Complement Factor H, and Age-Related Macular Degeneration. Ophthalmology. 2008;115:1742–1749. doi: 10.1016/j.ophtha.2008.03.021. Medline. doi:10.1016/j.ophtha.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyerle CB, Smith RT, Barbazetto IA, Yannuzzi LA. Autofluorescence of Basal Laminar Drusen. Retina. 2007;27:1101–1106. doi: 10.1097/IAE.0b013e3181451617. Medline. doi:10.1097/IAE.0b013e3181451617. [DOI] [PMC free article] [PubMed] [Google Scholar]