Abstract
Signaling proteins driving the proliferation of stem and progenitor cells are often encoded by proto-oncogenes. EphB receptors represent a rare exception; they promote cell proliferation in the intestinal epithelium and function as tumor suppressors by controlling cell migration and inhibiting invasive growth. We show that cell migration and proliferation are controlled independently by the receptor EphB2. EphB2 regulated cell positioning is kinase-independent and mediated via phosphatidylinositol 3-kinase, whereas EphB2 tyrosine kinase activity regulates cell proliferation through an Abl-cyclin D1 pathway. Cyclin D1 regulation becomes uncoupled from EphB signaling during the progression from adenoma to colon carcinoma in humans, allowing continued proliferation with invasive growth. The dissociation of EphB2 signaling pathways enables the selective inhibition of the mitogenic effect without affecting the tumor suppressor function and identifies a pharmacological strategy to suppress adenoma growth.
Introduction
Essential pathways regulating cell proliferation are often shared between stem/progenitor cells and cancer cells. This poses a problem as these pathways cannot be targeted to specifically eliminate tumor cells without simultaneously risking the depletion of untransformed cells, which is often a limiting factor in chemotherapy when doses that may eradicate tumor cells give unacceptable side effects. EphB receptors represent a rare exception in that they promote proliferation in the normal intestinal epithelium but, paradoxically, act as tumor suppressors in colon cancer development (Batlle et al., 2005; Holmberg et al., 2006). How can the same protein drive proliferation in the normal situation and function as a tumor suppressor in the same tissue?
Eph receptors constitute the largest subgroup of tyrosine kinase receptors. Their ephrin ligands, which are either transmembrane proteins or attached to the cell membrane with a GPI anchor, are also capable of signaling. Eph receptors and ephrins are best known for their roles in controlling cell migration and axon guidance (Pasquale, 2008), but have more recently been identified as regulators of stem and progenitor cell proliferation (Chumley et al., 2007; Depaepe et al., 2005; Holmberg et al., 2005; Holmberg et al., 2006; Jiao et al., 2008; Ricard et al., 2006). The molecular mechanisms for Eph/ephrin mediated regulation of stem/progenitor cell proliferation are unknown. In the intestinal epithelium, EphB receptors regulate both cell migration and progenitor cell proliferation (Batlle et al., 2002; Holmberg et al., 2006). Cell migration is deranged in the intestinal epithelium in mice lacking EphB2 and EphB3 and absence of EphB signaling results in up to 50% reduction in the number of proliferating cells (Batlle et al., 2002; Holmberg et al., 2006).
EphB receptor expression is highly increased in intestinal adenomas (Batlle et al., 2002). EphB signaling regulates adherens junction formation and promotes compartmentalization of colorectal cancer cells, and in this way suppresses cancer progression by inhibiting invasive growth (Cortina et al., 2007). EphB expression is almost invariably lost during progression to carcinoma and initiation of invasive growth (Batlle et al., 2005; Guo et al., 2005; Jubb et al., 2005), and the tumor suppressor effect of EphB signaling is a consequence of its capacity to regulate cell migration (Cortina et al., 2007). It was unknown whether EphB receptors employ the same signaling pathways to control cell migration and mitosis, or if these functions are separate.
We here show that EphB2 regulates two separate signaling pathways in the intestinal epithelium to control cell proliferation and migration. The identification of distinct EphB signaling pathways provides a pharmacological strategy to inhibit adenoma growth.
Results
Separate transcriptional programs for EphB mediated proliferation and migration
To first gain a global view of the signaling pathways engaged by EphB receptors in the intestinal epithelium, we analyzed transcriptional alterations after acute inhibition of EphB signaling in vivo. Eph receptors need to be clustered to signal and soluble ephrins act as antagonists (Vearing and Lackmann, 2005). An intravenous injection of ephrin-B2-Fc decreases EphB2 tyrosine phosphorylation and results in a reduction in cell proliferation to a similar extent as in EphB2; EphB3 double null mutant mice (Holmberg et al., 2006). Moreover, cells become mislocalized along the crypt-villus axis, as in EphB2 −/−; EphB3 −/− mice, although this is not apparent until several days after injection of the antagonist (Holmberg et al., 2006). By analyzing the transcriptome within the first day after blocking EphB signaling, it is possible to study the effects of blocked EphB signaling without the potential secondary effects caused by distorted cell positioning. Since ephrin-B2-Fc binds exclusively to EphB expressing cells in the crypts in the intestine (Holmberg et al., 2006), it is possible to assess transcriptional alterations in these cells in response to the EphB antagonist in whole preparations of colon.
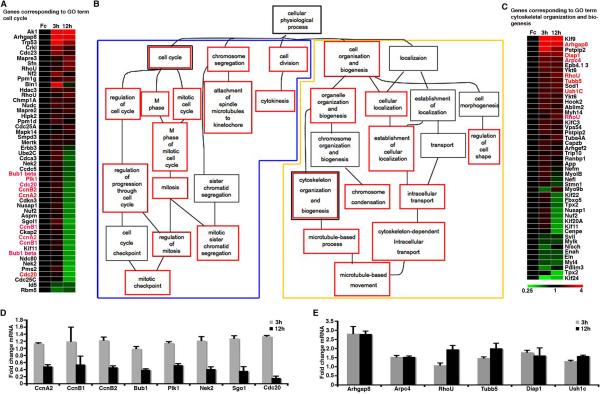
An injection of ephrin-B2-Fc resulted in significant (p<0.001) differential expression of 739 transcripts after 3 hours and 910 transcripts after 12 hours, as compared to Fc injected control animals. Pair-wise analyses followed by gene ontology classification identified alterations in two distinct transcriptional programs (Figure 1 and Table S1, S2). The majority of differentially expressed mRNAs clustered either under themes related to cell cycle regulation and progression or cell localization and cytoskeletal regulation (Figure 1B and Table S2). The differentially expressed mRNAs that did not cluster under these themes were related to general cellular metabolism (Table S2). Although EphB forward signaling mediates both proliferation and cell positioning (Holmberg et al., 2006), some transcriptional changes may be caused by blocked ephrin-B reverse signaling.
Figure 1. EphB Signaling Regulates Two Separate Transcriptional Programs.
(A–C) Differentially expressed mRNAs 12h after inhibiting EphB signaling by an ephrin-B2-Fc injection cluster into two separate programs, cell cycle (blue frame in B) and cell organization (yellow frame in B), by gene ontology classification. Significant gene ontology terms are indicated by red frame. Genes listed in (A) and (C) belong to the indicated gene ontology terms. n=4 mice in each group.
(D and E) Quantitative representation of expression levels (mean+SEM) of selected genes (names marked red in A and C) implicated in cell cycle (D) or cytoskeletal (E) regulation relative to Fc injected animals.
Many of the transcripts involved in cell cycle progression were reduced by approximately 50% at 12h after ephrin-B2-Fc injection (Figure 1A and 1D), quantitatively corresponding to the reduction in the number of proliferating cells in the crypts in the absence of EphB signaling (Holmberg et al., 2006). The expression of many of these genes was largely unaffected 3h after blocking EphB signaling, suggesting that their lower expression at 12h may be an effect of a reduced number of cells in cycle rather than direct regulation by EphB signaling.
The transcriptional regulation of many migration- and cytoskeleton-associated genes tended to be deregulated earlier, where inhibition of EphB signaling resulted in an altered expression at 3h that was sustained at 12h after injection of ephrin-B2-Fc (Figure 1C and 1E). The segregation of transcriptional changes after inhibition of EphB signaling into two distinct sets, one associated with cell migration and one with mitosis, provides a first indication that EphB receptors engage distinct signaling pathways to control cell positioning and proliferation.
EphB2 kinase activity is necessary for its mitogenic effect but is dispensable for cell positioning
We have generated a series of mutant mice carrying different substitutions in the EphB2 intracellular domain in an attempt to identify domains in the EphB2 receptor that regulate migration and proliferation. See Figure 2 for a schematic depiction of EphB2 variants and Figures S1–3 for targeting strategies. Since EphB2 and EphB3 are partly redundant in regulating proliferation and migration in the intestine (Batlle et al., 2002; Holmberg et al., 2006), the analyses were performed on an EphB3 null background.
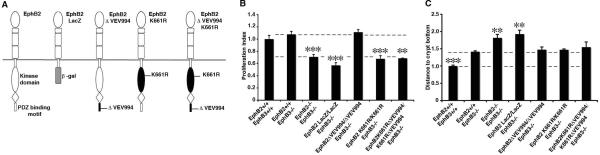
Figure 2. EphB2 kinase activity is required for its mitogenic effect but is dispensable for cell positioning.
(A) Schematic depiction of wild type EphB2 and modified receptors.
(B) Quantification of cell proliferation in colon crypts in adult mice with different EphB receptor modifications. The number of proliferating cells in each mouse line was normalized to its wild type control, giving the proliferation index. Kinase activity is required for the mitogenic effect of EphB2, whereas the PDZ domain-binding C-terminus is redundant. Broken lines indicate the levels for wild type and EphB2: EphB3 double null mice.
(C) Quantification of the distance of Paneth cells to the crypt base relative to wild type mice. The intracellular domain of EphB2 is required, but both kinase activity and the PDZ domain-binding C-terminus are redundant, for cell positioning. Broken lines indicate the levels for wild type and EphB2 +/+; EphB3 −/− mice. Representative images for the cell proliferation and migration analyses are shown in the supplemental material. n=3–8 mice in each group, except for the EphB2 K661RΔVEV994/ K661RΔVEV994; EphB3 −/− mice where n=2. Data are represented as mean+SEM. * = p≤ 0.05, ** = p≤ 0.01, *** = p≤ 0.001, compared to EphB2 +/+; EphB3−/−, Student's t-test.
Exchanging the intracellular part of the EphB2 receptor with β-galactosidase (Figure 2A), results in a receptor that is still capable of binding and activating ligands expressed by neighboring cells, but without being able to signal itself, thus allowing for discrimination between forward and reverse signaling (Henkemeyer et al., 1996). We previously demonstrated that substitution of the intracellular domain with β-galactosidase leads to a reduction in proliferation and mispositioning of cells, establishing that both the mitogenic and migration effects of EphB2 are mediated through forward signaling (Holmberg et al., 2006) (Figure 2B). To assess cell migration and positioning in a quantitative manner, we first focused on the differentiated Paneth cells, which normally are situated exclusively at the bottom of the crypts in the small intestine, but are mispositioned and spread throughout the crypt-villus axis in the absence of EphB signaling (Batlle et al., 2002; Holmberg et al., 2006). Paneth cells in EphB2 LacZ/LacZ; EphB3 −/− mice were displaced to the same extent as in the EphB2; EphB3 double null mutant mice (Figure 2C and S4).
Kinase activity and binding of PDZ domain-containing proteins to the Eph receptor PDZ binding motif located at the extreme C-terminus represent two distinct aspects of forward signaling. We generated mice carrying mutations disrupting EphB2 kinase activity (K661R) or its PDZ domain-binding motif (ΔVEV994), or both in combination. Deletion of the last three amino acids of EphB2 (ΔVEV994, see Figure S1A and S1B for targeting strategy) renders the receptor incapable of binding PDZ domain-containing proteins. The ΔVEV994 deletion does not alter the level of EphB2 tyrosine phosphorylation, the distribution or the membrane localization of the EphB2 receptor in the intestine (Figure S1C and S1D). Quantification of cell proliferation and Paneth cell displacement revealed no significant difference between EphB2 ΔVEV994/ΔVEV994; EphB3 −/− mice compared to EphB2 +/+; EphB3 −/− littermates (Figure 2B and C). The number of proliferating cells in each mouse line was normalized to its wild type control, giving the proliferation index.
Proliferation of intestinal progenitor cells is increased in mice that express a constitutively active EphB2 receptor (Holmberg et al., 2006), indicating that tyrosine kinase activity may be important for the mitogenic effect. We introduced a point mutation in the EphB2 gene (K661R) to express a kinase dead receptor that cannot convey kinase-dependent forward signals. Analysis of colon tissue from EphB2 K661R/K661R homozygote animals revealed an absence of EphB2 tyrosine phosphorylation, without any alteration in the expression level, membrane localization or distribution of EphB2 protein (Figure S2C and S2D). The number of mitotic cells in intestinal crypts in EphB2 K661R/K661R; EphB3 −/− mice was reduced to a similar extent as in EphB2 −/−; EphB3 −/− mice. However, EphB2 K661R/K661R; EphB3 −/− mice displayed no additional displacement of Paneth, neuroendocrine, goblet or progenitor cells compared to EphB3 −/− mice (Figure 2B and 2C and Figure S4). This indicates that EphB2 catalytic activity is important for conveying mitogenic, but not positional, cues in the intestinal epithelium. We also generated an EphB2 mutant mouse that combines the K661R and ΔVEV994 modifications (Figure 2A, see Figure S3A and S3B for targeting strategy). The intestinal phenotype in these mice was indistinguishable from mice that carry only the K661R kinase inactivating mutation (Figure 2B, C). In total, analysis of these three new mutations in EphB2 indicates that the mitogenic effect of EphB2 is kinase dependent, whereas regulation of cell migration is mediated by kinase- and PDZ-independent forward signaling.
EphB mediated cell positioning of intestinal cells is conveyed by phosphatidylinositol 3-kinase
Kinase-independent functions of Eph receptor signaling have previously been described in cell lines and were found to be conveyed by phosphatidylinositol 3-kinase (PI3K) (Gu and Park, 2001). As PI3K mediates cell migration signals by Eph receptors in some contexts (Brantley-Sieders et al., 2004; Maekawa et al., 2003), we asked whether EphB mediated cell positioning in the intestinal epithelium may be mediated by PI3K.
We first assessed the role of PI3K in conveying repulsive signals in intestinal cells in an in vitro assay. Ls174t colon carcinoma cells, which express high levels of EphB2 and EphB3 (Batlle et al., 2002), were plated on a confluent mosaic of wild type 293t cells (which do not endogenously express ephrins or Eph receptors) and 293t cells stably expressing ephrin-B2 and GFP. Ls174t cells avoided the ephrin-B2 expressing cells and were preferentially found on the wild type 293t cells 12 hours after plating (Figure 3A and 3B). However, the PI3K inhibitor LY294002 reduced this response in a dose-dependent manner, and at 5μM the cells did not show any avoidance of ephrin-B2 expressing cells (Figure 3A and 3B). This indicates that PI3K activity is important for conveying positional information in response to ephrin-B2 in intestinal cells.
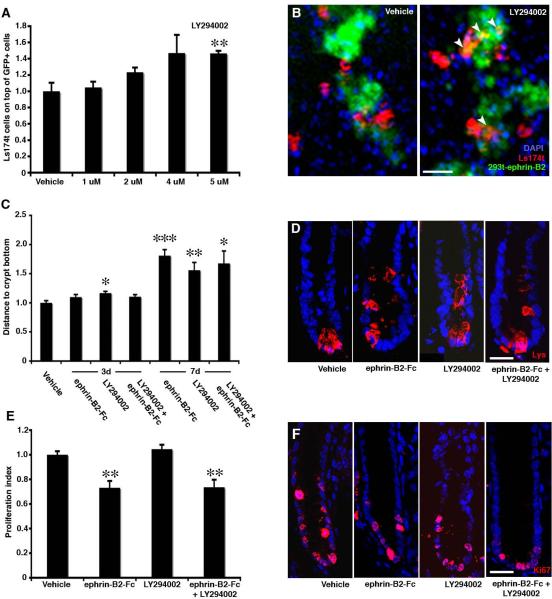
Figure 3. EphB mediated cell positioning is conveyed by phosphatidylinositol 3-kinase.
(A and B) Ls174t cells plated on a confluent mosaic of 293t cells and 293t cells expressing ephrin-B2 and GFP are preferentially found on the 293t wild type cells. Ls174t cells exposed to increasing concentrations of the PI3K inhibitor LY294002 loose their preference for wild type 293t cells in a dose-dependent manner.
(C and D) In vivo administration of LY294002 results in increasing displacement of Paneth cells from 3 to 7 days compared to animals administered with vehicle. PI3K inhibitor results in cell displacement to a similar degree as ephrin-B2-Fc, and administration of both LY294002 and ephrin-B2-Fc together does not further accentuate this phenotype.
(E and F) Injection of ephrin-B2-Fc significantly decreases proliferation in colon crypts, where as LY294002 administration does not. Treatment of the two inhibitors together does not further reduce proliferation as compared to the ephrin-B2-Fc only treatment. n=3 mice in each group. Data are represented as mean+SEM. * = p≤ 0.05, ** = p≤ 0.01, *** = p≤ 0.001, Student's t-test. Scale bars=60 μm in B, 10 μm in D and 15μm in F.
We next studied the role of PI3K in cell positioning in the intestinal epithelium in vivo. We administered the PI3K inhibitor LY294002, ephrin-B2-Fc or both in combination to adult mice and assessed cell positioning in the intestinal epithelium 3 or 7 days later. Paneth cells showed a quantitatively similar displacement after inhibiting PI3K or EphB signaling, and there was no additive or synergistic effect of blocking both PI3K and EphB signaling simultaneously (Figure 3C and 3D). Importantly, inhibiting PI3K activity had no effect on cell proliferation in the intestinal epithelium (Figure 3E and 3F).
The microarray analysis of gene expression in the intestine (Figure 1) revealed expression of both isoforms of the regulatory PI3K subunit, p85α and p85β, and two (out of four known) isoforms of the catalytic subunit, p110α and p110δ (Figure S5). p85α showed an approximately four-fold higher expression than p85β and p110α was expressed approximately three-fold higher than p110δ (Figure S5). Inhibiting EphB signaling by an injection of ephrin-B2-Fc resulted in decreased expression of both catalytic subunit isoforms, with the most dramatic deregulation of p110α mRNA levels showing a 37% reduction after 3h (p=0.0004) and 44% after 12h (p=0.0006) (Figure S5). It is difficult to formally exclude that LY294002 may modulate additional substrates to PI3K, but the EphB mediated regulation of expression of PI3K catalytic subunits corroborates the role for PI3K downstream of EphB signaling in the intestine.
EphB signaling does not affect β-catenin mediated transcription
To explore how EphB kinase activity promotes cell proliferation in intestinal crypts we first asked if it acts by modulating the β-catenin pathway, which is a pivotal regulator of proliferation in the intestinal epithelium (Clevers, 2006). β-catenin displaces the transcriptional repressor Groucho from the Tcf/Lef complex to drive the expression of target genes in crypt stem/progenitor cells (Clevers, 2006). We used TOPGAL reporter mice, in which the expression of lacZ is under the control of Tcf/Lef binding sites (DasGupta and Fuchs, 1999), to assess whether EphB signaling influences the activity of the β-catenin pathway. Inhibition of EphB signaling by an injection of ephrin-B2-Fc in TOPGAL mice resulted in a significant decrease in mRNA for the cell proliferation marker Ki67, whereas neither lacZ mRNA levels nor the number of β-galactosidase positive cells were altered (Figure 4A–D).
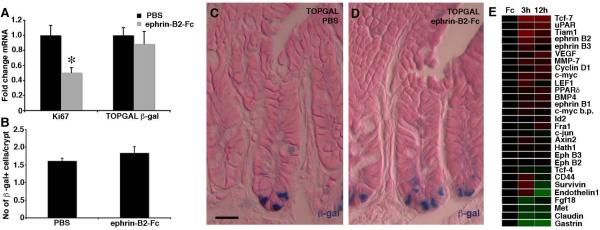
Figure 4. EphB signaling does not affect β-catenin mediated transcription.
(A–D) Inhibition of EphB signaling by an injection of ephrin-B2-Fc in TOPGAL mice results in a significant reduction in Ki67 mRNA levels (p=0.02, Student's t-test), whereas β-gal mRNA levels (p=0.59) and the number of β-gal expressing cells (p=0.30) are unaltered. Data are represented as mean+SEM. n=3 mice in each group.
(E) Microarray analysis reveals little effect on the expression levels of β-catenin target genes in colon 3 or 12 hours after injection of ephrin-B2-Fc compared to control Fc protein. * = p≤ 0.05. Scale bar = 10 μm.
Analysis of our microarray data for genes identified as being under β-catenin mediated transcriptional regulation in the colon (http://www.stanford.edu/~rnusse/pathways/targets.html), revealed that the level of most of these transcripts remained unaltered after inhibiting EphB signaling by ephrin-B2-Fc injection when compared to control Fc protein (Figure 4E). The microarray data was confirmed using qRT-PCR for selected genes (Figure S6). Thus, EphB signaling does not appear to have any major influence on β-catenin mediated transcription. These data are in line with the previous observation that the β-catenin pathway and EphB signaling drive cell proliferation in different domains of the crypt (Holmberg et al., 2006).
EphB regulates cyclin D1 levels in the intestinal epithelium
EphB signaling drives proliferation in intestinal crypts by promoting cell cycle entry (Holmberg et al., 2006). Cyclin D1 is a regulator of cell cycle entry and cyclin D1 null mice display reduced cell proliferation in intestinal crypts (Hulit et al., 2004). Several reports suggested that cyclin D1 is a β-catenin target gene in colon carcinoma cells in vitro (Shtutman et al., 1999; Tetsu and McCormick, 1999). However, cyclin D1 is expressed independently of β-catenin in vivo (Sansom et al., 2005), making cyclin D1 a candidate target of EphB receptor signaling in the intestinal epithelium.
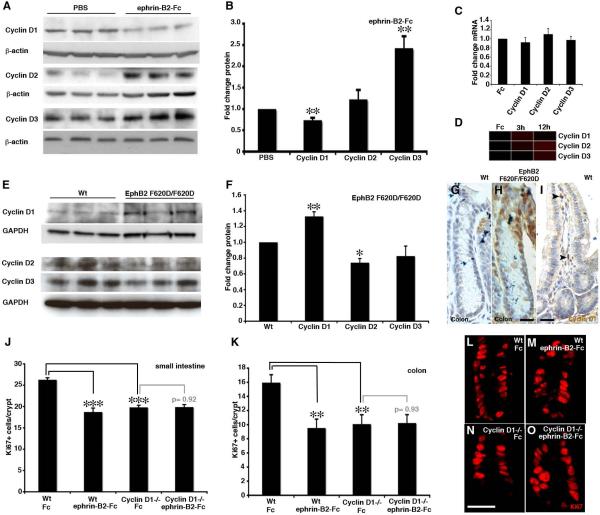
Inhibition of EphB signaling by an injection of ephrin-B2-Fc resulted in significantly reduced cyclin D1 levels in the intestine (Figure 5A and 5B). In contrast, cyclin D2 and cyclin D3 levels were increased (Figure 5A and 5B), which is likely to be a result of the well-documented compensatory upregulation when one family member is decreased (Ciemerych et al., 2002). The alterations detected in cyclin D protein levels could not be correlated to changes in mRNA levels 12 hours after ephrin-B2-Fc injection (Figure 5C and 5D), indicating that the EphB mediated regulation of cyclin D levels is posttranscriptional.
Figure 5. Cyclin D1 is required for the mitogenic effect of EphB.
(A and B) Western blot analysis of cyclin D levels in the colon after inhibition of EphB signaling with ephrin-B2-Fc compared to vehicle (PBS).
(C and D) mRNA levels for cyclin D family members are not altered after an injection of ephrin-B2-Fc as assayed by qRT-PCR (C, at 12 hours after ephrin-B2-Fc injection) or microarray analysis (D).
(E and F) Western blot analysis of cyclin D levels in the colon in wild type (Wt) mice and in mice with a modified (F620D/F620D) constitutively active EphB2 receptor.
(G and H) Cyclin D1 levels are too low to be detected by immunohistochemistry in wild type mice, but are readily detected in cell nuclei in colon crypts in EphB2 F620D/F620D mice. (I) Cyclin D1 positive cells are detected in the stroma of wild type small intestine (arrowheads).
(J and K) Quantification of Ki67-immunoreative cells in crypts of the small intestine and colon reveals a similar degree of reduction in the number of proliferating cells in cyclin D1 −/− mice and in animals receiving an ephrin-B2-Fc injection (24h prior to analysis) compared to wild type animals receiving control Fc protein. Administration of ephrin-B2-Fc does not reduce proliferation further in cyclin D1 −/− mice.
(L–O) Ki67-immiunoreactive cells in the crypts of the small intestine.
n=3 mice in each group, except for n=2 in some cyclin D1 −/− groups. Data are represented as mean+SEM. Scale bar=10 μm in G and 30μm in I. ** = p≤0.01, *** = p≤0.001, Student's t test.
To gain further insights into the regulation of cyclin D1, we turned to EphB2 F620D/F620D mice. The F620D substitution renders the catalytic domain constitutively active, independent of ligand binding, and these mice display increased progenitor proliferation in intestinal crypts (Holmberg et al., 2006). Cyclin D1 protein levels were significantly increased in the intestine of EphB2 F620D/ F620D mice, whereas the level of cyclin D2 and cyclin D3 were reduced as compared to wild type littermates (Figure 5E and 5F).
Although cyclin D1 in the intestine is easily detected by Western blot, it is difficult to localize by immunohistochemistry in the intestinal epithelium in wild type mice (Figure 5G). The elevated cyclin D1 levels in the intestine of EphB2 F620D/F620D mice were, however, readily detected and localized in nuclei of cells in crypts (Figure 5H). As cyclin D1 is present also in non-epithelial cells in the stroma of the intestine (Figure 5I), which lack EphB receptor expression, the altered levels detected in whole intestine lysates (Figure 5B and 5F) likely underestimates the effect of modulating EphB signaling on cyclin D1 levels in the epithelium.
The mitogenic effect of EphB signaling is mediated by cyclin D1
To assess the relative role of cyclin D1 in EphB mediated proliferation we inhibited EphB signaling in cyclin D1 −/− mice. Injection of ephrin-B2-Fc in wild type animals significantly reduced proliferation as compared to animals receiving control protein (Fc) in both the small intestine and colon (Figure 5J–M) (Holmberg et al., 2006). Administration of ephrin-B2-Fc to cyclin D1 null animals, however, did not reduce proliferation in either the colon or small intestine compared to cyclin D1 −/− mice receiving Fc (Figure 5J–O). Thus, EphB signaling does not have any detectable effect on proliferation of intestinal progenitor cells in the absence of cyclin D1, establishing cyclin D1 as a key EphB regulated mediator of proliferation in the intestinal epithelium.
Although cyclin D1 is best known for its role in cell cycle regulation, it has also been implicated in influencing cell migration (Li et al., 2006a; Li et al., 2006b). We asked whether cyclin D1 also participates in EphB-mediated cell positioning in intestinal crypts. However, no displaced Paneth cells were found in cyclin D1 null mice and EphB2-immunoreactive progenitor cells were organized as in wild type mice (Figure S7). Thus, cyclin D1 mediates the effects of EphB signaling on proliferation, but not migration, in the intestinal epithelium.
EphB regulates cyclin D1 levels and proliferation via Abl
Abl binds to EphB4 in an activity-dependent manner and regulates proliferation and tumorigenicity in breast cancer cell lines (Noren et al., 2006). We asked whether Abl is involved in EphB mediated regulation of cyclin D1 and cell proliferation in the intestinal epithelium.
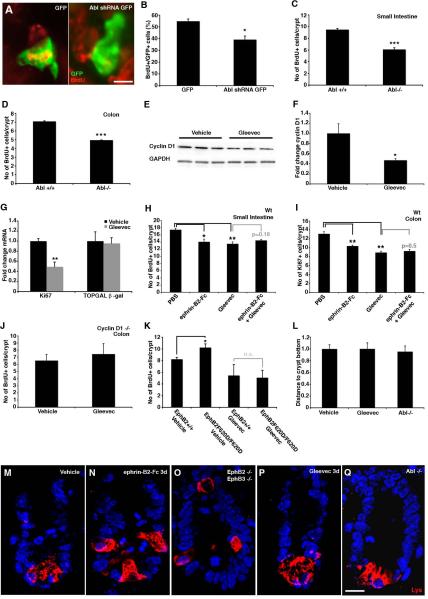
We first suppressed Abl levels by transfecting epithelial cells with Abl shRNA in intestinal explants by electroporation. This resulted in a significant reduction in the number of proliferating cells (Figure 6A and 6B). We next assessed cell proliferation in the intestinal epithelium in Abl −/− mice. The number of BrdU labeled proliferating cells was reduced to a similar degree as after blocking EphB signaling or deleting cyclin D1 in both the small intestine and colon (Figure 6C and 6D). Administration of the Abl kinase inhibitor Gleevec (imatinib mesylate) significantly reduced cyclin D1 protein levels compared to animals injected with vehicle (Figure 6E and 6F), without affecting EphB2 phosphorylation (Figure S8). TOPGAL mice showed an approximate 50% decrease in Ki67 mRNA whereas the level of lacZ mRNA was unaffected after Gleevec injection (Figure 6G). Thus, similarly to EphB signaling, Abl regulates cyclin D1 levels and cell proliferation in the intestinal epithelium, without significantly affecting the β-catenin signaling pathway.
Figure 6. Proliferation, but not migration, is mediated by Abl.
(A and B) Electroporation of Abl shRNA in intestinal explants leads to a reduction in the number of electroporated cells (GFP expressing) incorporating BrdU. (C and D) Proliferation in the Abl mutant mouse is reduced in both small intestine and colon. (E and F) Gleevec (100mg/kg) leads to a decrease in cyclin D1 protein levels in the colon detected by Western blot analysis.
(G) Administration of Gleevec to TOPGAL mice leads to a 50% reduction in Ki67 transcript, without affecting the expression of β-galactosidase (p=0.87).
(H and I) Inhibiting Abl with Gleevec or ephrin-B2-Fc reduces cell proliferation in the small intestine and colon to a similar extent, but there is no additive effect.
(J) Gleevec does not affect cell proliferation in colon crypts in cyclin D1 −/− mice.
(K) Analysis of EphB2 F620D/F620D mice with constitutively active EphB2 receptors reveal an increase in the number of BrdU positive cells when compared to wild type littermates. Administration of Gleevec to wild type and EphB2 F620D/F620D mice results in a suppression of proliferation to the same level in both genotypes. (L–Q) Paneth cells, visualized by lysozyme (Lys)-immunoreactivity, are displaced in EphB2; EphB3 double null mutant animals. Inhibition of EphB signaling with ephrin-B2-Fc also results in displaced Paneth cells 3 days after an injection. In contrast, animals receiving Gleevec for three consecutive days as well as Abl −/− mice show no evidence of Paneth cell displacement.
n=3–6 mice in each group, except for n=2 cyclin D1 −/− mice receiving Gleevec. Data are represented as mean+SEM. * = p≤ 0.05, ** = p≤ 0.01, *** = p≤ 0.001, Student's t-test. Scale bar = 10 μm.
We next asked whether Abl conveys the mitogenic signaling of EphB receptors. Ephrin-B2-Fc and Gleevec reduced the number of dividing progenitor cells in both the small intestine and colon to a similar extent (Figure 6H and 6I). However, administration of ephrin-B2-Fc and Gleevec together had no additive effect and did not further reduce proliferation (Figure 6H and 6I). Furthermore, Gleevec had no effect on proliferation in cyclin D1 −/− mice (Figure 6J), establishing that cyclin D1 is required for Abl to mediate mitogenic signals. We quantified the number of Tunel-positive dying cells, but could not detect any significant differences in apoptosis that could explain the observed reduction in proliferation in response to ephrin-B2-Fc, Gleevec or ephrin-B2-Fc and Gleevec when compared to control animals (Figure S9).
Cell proliferation is increased in the intestinal epithelium of mice with a modified (F620D) constitutively active EphB2 receptor ((Holmberg et al., 2006) and Figure 6K). Administering Gleevec to EphB2F620D/F620D mice reduced the proliferation of intestinal epithelial cells and, most importantly, there was no difference in the number of BrdU labeled cells compared to wild type mice receiving Gleevec (Figure 6K). Thus, the effect of increased EphB2 kinase activity on cell proliferation can be abolished by Gleevec.
Abl has been implicated in conveying EphA-mediated growth cone collapse (Harbott and Nobes, 2005). Does Abl, in addition to mediating the mitogenic effects of EphB signaling, control cell positioning in the intestinal epithelium? The Paneth cells of the small intestine are displaced three days after an ephrin-B2-Fc injection, mimicking the phenotype of EphB2 −/−; EphB3 −/− mice (Figure 6L–O). Administration of Gleevec for three consecutive days resulted in sustained reduced proliferation (Figure S10), but did not cause defects in cell positioning (Figure 6L and 6P). Moreover, analysis of Paneth cell distribution in Abl −/− mice did not reveal any evidence for cell displacement (Figure 6L and 6Q). Thus, the Abl pathway transduces the EphB mediated regulation of cyclin D1 and cell proliferation, but does not affect cell positioning.
Dissociation of EphB signaling from cyclin D1 during tumor progression
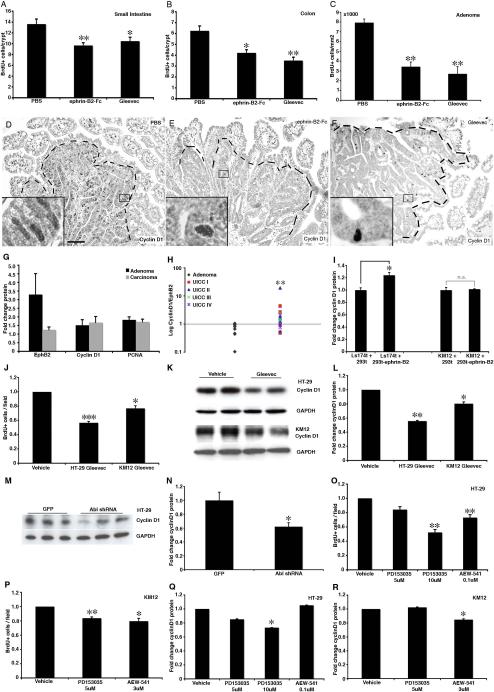
A common initial step in human colon cancer formation is gain-of-function mutations in the β-catenin pathway and adenoma formation (Clevers, 2006). EphB2 and EphB3 are targets of β-catenin-mediated transcription, resulting in highly proliferative and EphB expressing cells in adenomas (Batlle et al., 2002). We turned to the APCmin mouse model of adenomatous polyposis to study the role of EphB signaling in adenomas. Untransformed epithelial crypt cells in APCmin/+ mice are heterozygous for mutations rendering the β-catenin pathway constitutively active, resulting in increased proliferation compared to wild type mice. We detected a significant reduction in proliferation in the intact crypts in both the small intestine and colon 24 hours after an ephrin-B2-Fc or Gleevec injection in APCmin/+ mice (Figure 7A and 7B). Adenomas develop in both the colon and small intestine in APCmin/+ mice as cells lose APC heterozygosity. Both ephrin-B2-Fc and Gleevec reduced proliferation in adenomas by more than 50% (Figure 7C) and resulted in a depletion of cyclin D1 in adenoma cells (Figure 7D–F).
Figure 7. Dissociation of EphB signaling pathways during tumor progression.
(A–C) Inhibition of EphB signaling with ephrin-B2-Fc or administration of Gleevec in APCmin mice reduces the number of BrdU-incorporating cells in untransformed crypts in the small intestine (A) and colon (B) as well as in adenomas (C).
(D–F) Adenomas in APCmin mice display high levels of cyclin D1 compared to the surrounding tissue (D). Inhibition of EphB signaling with ephrin-B2-Fc (E) or Gleevec (F) in APCmin mice leads to reduced cyclin D1 levels in adenomas. Boxed areas are shown in higher magnification.
(G) Analysis of protein levels by Western blot in human colon adenoma and carcinoma samples. All values were normalized to neighboring untransformed tissue from the same patient. EphB2 receptor expression is high in adenomas. At the transition to carcinoma, EphB2 is down regulated and the proliferation marker PCNA and cyclin D1 are maintained independently of EphB expression.
(H) The ratio of cyclin D1/EphB2 protein changes at the transition from colon adenoma to carcinoma and there is no significant further change during the progression to more advanced UICC cancer stages.
(I) Ls174t cells expressing EphB2 and EphB3 receptors display a significant increase in cyclin D1 levels after being plated together with 293t cells expressing ephrin-B2, as compared to Ls174t cells plated together with wild type 293t cells. KM12 cells, expressing no or low levels of EphB receptors, do not up regulate cyclin D1 when plated together with ephrin-B2 expressing cells.
(J–L) HT-29 and KM12 cells treated with Gleevec for 48 hours display a reduced number of BrdU positive cells as compared to cells treated with vehicle. Gleevec administration also results in reduced levels of cyclin D1 in both cell lines.
(M and N) HT-29 cells transfected with shRNA against Abl with GFP display significantly reduced levels of cyclin D1 after 24 hours, as compared to cells transfected with GFP only.
(O and P) Administration of EGF (PD153035) or IGF (AEW-541) receptor inhibitor to HT-29 or KM12 colon carcinoma cell lines results in reduced proliferation in both cell lines, whereas PD135035 only affects cyclin D1 in HT-29 cells and AEW-541 only in KM12 cells.
Data are represented as mean+SEM. n=3–4 mice in each group. All in vitro experiments were made in triplicate. Scale bar=50 μm. * = p≤0.05, ** = p≤0.01, *** = p≤0.001, Student's t test.
Ephrin-B expressing cells in the intact tissue encapsulate the EphB expressing adenoma cells and form a repulsive barrier preventing the transformed cells from entering the surrounding normal tissue. The expression of EphB receptors is frequently lost during the progression of colorectal cancer in humans, and this correlates with a poor prognosis (Batlle et al., 2005; Guo et al., 2005; Jubb et al., 2005). Loss of EphB signaling allows invasive growth, but how can proliferation be maintained at a high level when EphB receptor expression is lost? To understand more about the adenoma to carcinoma transition, we analyzed EphB2, cyclin D1 and PCNA protein levels in human tumor samples (Figure 7G, Table S3). EphB2 levels were significantly reduced in carcinomas compared to adenomas, as previously reported (Batlle et al., 2005; Guo et al., 2005; Jubb et al., 2005). In spite of this, both the proliferation marker PCNA and cyclin D1 remained at a similar level in carcinomas when compared to adenomas. Cyclin D1 levels, and proliferation, correlate strongly with EphB signaling in untransformed intestinal epithelial progenitor cells and in adenoma cells. This correlation is lost at the stage of transformation from adenoma to carcinoma, indicating that cyclin D1 expression becomes independent of EphB2 in human colon carcinoma (Figure 7H).
To more directly study the role of EphB signaling in regulating cyclin D1 levels in human transformed intestinal cells we assessed the regulation of cyclin D1 in response to ephrin-B2 in Ls174t cells (which express high levels of EphB2 and EphB3, like adenoma cells) and KM12 cells (with little or no expression of EphB receptors, like carcinoma cells). We found that mixing Ls174t cells with 293t cells expressing ephrin-B2 resulted in significantly elevated cyclin D1 levels compared to cells mixed with wild type 293t cells, whereas there was no significant change in the cyclin D1 levels in KM12 cells in response to ephrin-B2 (Figure 7I and Figure S11). Thus, cyclin D1 regulation becomes dissociated from EphB signaling during the transition from adenoma to carcinoma, allowing continued high proliferation with the additional capacity for invasive growth.
To gain insight into the regulation of cyclin D1 in the absence of EphB receptor expression in colon carcinoma, we first asked whether Gleevec may affect cyclin D1 levels and cell proliferation in HT-29 and KM12 human colon carcinoma cells. HT-29 cells lack EphB2 and EphB3 receptor expression but express EphB4 and KM12 cells have little or no expression of EphB receptors (Batlle et al., 2005; Davalos et al., 2006). Gleevec significantly reduced BrdU incorporation and cyclin D1 levels in HT-29 and KM12 cells (Figure 7J–L). Moreover, Abl shRNA reduced cyclin D1 levels in HT-29 cells (Figure 7M, N and Figure S12). Thus, in contrast to in the untransformed intestinal epithelium, where Abl activity is strictly under the control of EphB signaling (Fig 6), Abl appears independent of EphB signaling in human colon carcinoma cells.
Many reports have demonstrated mitogenic effects and overactivation of the EGF and IGF receptor pathways in human colon carcinoma, and monoclonal blocking antibodies to the EGF receptor are used to treat colon cancer in humans (Ciardiello and Tortora, 2008; Donovan and Kummar, 2008). Both EGF and IGF receptors activate Abl in other contexts (Srinivasan and Plattner, 2006; Srinivasan et al., 2008). We found that the EGF receptor inhibitor PD153035 significantly reduced cyclin D1 levels and proliferation of HT-29 cells and that the IGF receptor inhibitor AEW-541 reduced cyclin D1 levels and proliferation in KM12 cells (Figure 7O–R and Figure S13). This indicates that EGF and IGF receptor signaling, at least in part, can compensate for the loss of EphB expression to maintain cyclin D1 levels and proliferation after the progression from adenoma to carcinoma.
Discussion
We show here that EphB receptors engage distinct signaling pathways in the intestinal stem cell niche to regulate cell proliferation and migration. EphB receptors regulate cell positioning in the intestinal epithelium via PI3K, independently of kinase activity. In contrast, intrinsic EphB tyrosine kinase activity drives proliferation in crypt progenitor cells through Abl, resulting in posttranscriptional regulation of cyclin D1 protein levels. EphB signaling promotes cell proliferation in adenomas and simultaneously inhibits invasive growth. At the progression from adenoma to carcinoma, cyclin D1 expression becomes independent of EphB signaling, explaining how high proliferation can be maintained and accompanied by invasive growth after loss of EphB expression.
Pathways regulating proliferation and migration diverge at the EphB2 receptor
The analysis of the intestinal transcriptome after acute inhibition of EphB signaling provided a first indication that cell migration and proliferation may be regulated independently. The EphB mediated regulation of proliferation appears to be mainly posttranscriptional, whereas the transcription of key components regulating cell positioning are rapidly altered following inhibition of EphB signaling. The generation of mutant mice with different EphB2 modifications established that these pathways diverge at the level of the receptor protein.
We find that whereas EphB intrinsic kinase activity conveys mitogenic signals, cell positioning is regulated by kinase-independent forward signaling. There are naturally occurring Eph receptors that lack kinase activity, as well as kinase-inactive spliced versions of some receptors that can modulate the kinase activity of full-length catalytically functional receptors (Holmberg et al., 2000). There are previous examples of kinase-dependent and -independent signaling by the same Eph receptor in vitro (Gu and Park, 2001; Miao et al., 2005). It is clear that Eph receptors convey directional signals to regulate cell migration or axon guidance in divergent ways in different contexts. For example, in contrast to kinase-independent cell positioning in the intestinal epithelium, the EphA4 kinase domain is required for the correct guidance of certain axons (Dufour et al., 2006; Kullander et al., 2001).
The EphB paradox is explained by the independent regulation of migration and proliferation
The recent recognition of the similarities between untransformed stem/progenitor cells and cancer cells has provided a mechanistic explanation for the inherent difficulty in developing strategies to eradicate tumor cells without interfering with tissue homeostasis. Identification of signaling pathways that have divergent effects in tissue stem/progenitor cells and cancer cells may offer insights into cancer development as well as offer novel therapeutic targets. EphB receptors are interesting in this context since they promote cell proliferation in the intact intestinal epithelium as well as in adenomas, but still act as tumor suppressors for colon carcinoma development. The tumor suppressor function of EphB receptors is a result of their regulation of cell migration and compartmentalization of tumor cells (Cortina et al., 2007). These dual functions, regulation of cell proliferation and migration, result in EphB receptors driving proliferation but still acting as tumor suppressors. The loss of EphB expression during carcinogenesis enables invasive growth.
In parallel, cyclin D1, which is regulated by EphB kinase activity in intestinal progenitors and is the effector of EphB mitogenic signaling, becomes independent of EphB signaling and continues to promote proliferation in colon carcinoma cells. Thus, the fact that EphB receptors engage separate signaling pathways to regulate proliferation and migration is the basis for the paradoxical proliferative and tumor suppressor functions of the same protein.
A pharmacological strategy to inhibit adenoma growth
The dissociation of signaling pathways for EphB mediated migration and proliferation enabled the identification of Gleevec as an inhibitor specifically of EphB mitogenic signaling, without affecting cell migration. Cyclin D1 is the effector of EphB-mediated proliferation and inhibiting EphB signaling with soluble ephrin or Gleevec was equally efficient in reducing cyclin D1 levels and cell proliferation. Analysis of APCmin mice hetero- or homozygous for cyclin D1 null alleles has established that the level of Cyclin D1 is an important determinant of tumor number and adenoma cell proliferation (Hulit et al., 2004).
Familial adenomatous polyposis (FAP) patients develop large numbers of adenomas and have highly increased risk of developing colon carcinoma (Lynch, 2007). The colon is often removed in FAP patients to avoid carcinoma development, and pharmacological alternatives to reduce adenoma growth in FAP and other patients with high risk of developing colon carcinoma would be very attractive (Lynch, 2007). The strong influence of reduced cyclin D1 levels on tumor number in APCmin mice, which is an animal model of FAP, lead to the suggestion that cyclin D1 may be an attractive target for pharmacological intervention (Hulit et al., 2004). We show that administration of the Abl inhibitor Gleevec reduces cyclin D1 levels and decreases proliferation in the intestinal epithelium to the same degree as in cyclin D1−/− mice. Gleevec is rather well tolerated and is given chronically to patients, although it remains to be studied whether a similar Gleevec dose would be sufficient to suppress proliferation in adenomas. The development of inhibitors restricted to inhibiting Abl's interaction with EphB receptors would likely reduce the side effects substantially compared to Gleevec. Inhibiting EphB-mediated proliferation may offer a pharmacological alternative to removal of the colon in patients with FAP and other similar conditions to reduce adenoma growth and potentially cancer progression.
Experimental Procedures
Animals
Tissues from all EphB2 mutant mice on CD1 genetic background were dissected and coded by genotype in the M.H. laboratory and all analyses were done blind to genotype in the J.F. laboratory. The generation of new EphB2 mouse mutants, analysis of TOPGAL mice and administration of compounds to animals are described in the Supplemental Data.
Expression analyses
Microarray, quantitative PCR, immunohistochemal and Western blot analyses are described in detail in the Supplemental Data.
Cell culture
HT-29 and Ls174t cells were obtained from the American Type Culture Collection (ATCC) and KM12 cells from NCI/NIH and cultured as described in the Supplemental Data.
Cell positioning assays
The in vitro cell positioning assay and the quantitative assessment of in vivo cell positioning is described in the Supplemental Data.
Transfections
Electroporation of intestinal explants and shRNA transfections are described in the Supplemental Data.
Human tumor tissue
Adenoma and adenocarcinoma (UICC Stage I–IV) tissue samples were collected during colonoscopy or surgery, frozen in liquid nitrogen and stored in −70°C until analysis.
Supplementary Material
Acknowledgements
We thank F. Granath, J. Holmberg, L. Huminiecki, J. Lindholm, A. Simon and members of the Frisén lab for valuable discussions, M.-L. Spångberg and M. Toro for technical assistance and Novartis for providing Gleevec and AEW-541. This study was supported by grants from the National Institutes of Health (R01CA70896, R01CA75503, and R01CA86072 to R.G.P. and 2R01 MH66332 to M.H.), Dr. Ralph and Marian C. Falk Medical Research Trust, Pennsylvania Department of Health (which specifically disclaims responsibility for analyses, interpretations or conclusions) (R.G.P.), the Swedish Cancer Society, the Swedish Research Council, Knut och Alice Wallenbergs Stiftelse, the Karolinska Institute and the Tobias Foundation (J.F.). The Kimmel Cancer Center was supported by the NIH Cancer Center Core Grant P30CA56036 (R.G.P).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batlle E, Bacani J, Begthel H, Jonkeer S, Gregorieff A, van de Born M, Malats N, Sancho E, Boon E, Pawson T, et al. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MMW, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, et al. ß-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/EphrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell Sci. 2004;117:2037–2049. doi: 10.1242/jcs.01061. [DOI] [PubMed] [Google Scholar]
- Chumley MJ, Catchpole T, Silvany RE, Kernie SG, Henkemeyer M. EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis. J Neurosci. 2007;27:13481–13490. doi: 10.1523/JNEUROSCI.4158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- Ciemerych MA, Kenney AM, Sicinska E, Kalaszczynska I, Bronson RT, Rowitch DH, Gardner H, Sicinski P. Development of mice expressing a single D-type cyclin. Genes Dev. 2002;16:3277–3289. doi: 10.1101/gad.1023602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cortina C, Palomo-Ponce S, Iglesias M, Fernandez-Masip JL, Vivancos A, Whissell G, Huma M, Peiro N, Gallego L, Jonkheer S, et al. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Davalos V, Dopeso H, Castano J, Wilson AJ, Vilardell F, Romero-Gimenez J, Espin E, Armengol M, Capella G, Mariadason JM, et al. EPHB4 and survival of colorectal cancer patients. Cancer Res. 2006;66:8943–8948. doi: 10.1158/0008-5472.CAN-05-4640. [DOI] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Donovan EA, Kummar S. Role of insulin-like growth factor-1R system in colorectal carcinogenesis. Crit Rev Oncol Hematol. 2008;66:91–98. doi: 10.1016/j.critrevonc.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour A, Egea J, Kullander K, Klein R, Vanderhaeghen P. Genetic analysis of EphA-dependent signaling mechanisms controlling topographic mapping in vivo. Development. 2006;133:4415–4420. doi: 10.1242/dev.02623. [DOI] [PubMed] [Google Scholar]
- Gu C, Park S. The EphA8 receptor regulates integrin activity through p110gamma phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Molecular and cellular biology. 2001;21:4579–4597. doi: 10.1128/MCB.21.14.4579-4597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo DL, Zhang J, Yuen ST, Tsui WY, Chan AS, Ho C, Ji J, Leung SY, Chen X. Reduced expression of EphB2 that parallels invasion and metastasis in colorectal tumors. Carcinogenesis. 2005;27:454–464. doi: 10.1093/carcin/bgi259. [DOI] [PubMed] [Google Scholar]
- Harbott LK, Nobes CD. A key role for Abl family kinases in EphA receptor-mediated growth cone collapse. Mol Cell Neurosci. 2005;30:1–11. doi: 10.1016/j.mcn.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Armulik A, Senti K-A, Edoff K, Spalding K, Momma S, Cassidy R, Flanagan JG, Frisén J. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes and Dev. 2005;19:462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J, Clarke DL, Frisén J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisen J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Hulit J, Wang C, Li Z, Albanese C, Rao M, Di Vizio D, Shah S, Byers SW, Mahmood R, Augenlicht LH, et al. Cyclin D1 genetic heterozygosity regulates colonic epithelial cell differentiation and tumor number in ApcMin mice. Molecular and cellular biology. 2004;24:7598–7611. doi: 10.1128/MCB.24.17.7598-7611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao JW, Feldheim DA, Chen DF. Ephrins as negative regulators of adult neurogenesis in diverse regions of the central nervous system. Proc Natl Acad Sci U S A. 2008;105:8778–8783. doi: 10.1073/pnas.0708861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubb AM, Zhong F, Bheddah S, Grabsch HI, Frantz GD, Mueller W, Kavi V, Quirke P, Polakis P, Koeppen H. EphB2 is a prognostic factor in colorectal cancer. Clin Cancer Res. 2005;11:5181–5187. doi: 10.1158/1078-0432.CCR-05-0143. [DOI] [PubMed] [Google Scholar]
- Kullander K, Mather NK, Diella F, Dottori M, Boyd AW, Klein R. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron. 2001;29:73–84. doi: 10.1016/s0896-6273(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Jiao X, Wang C, Ju X, Lu Y, Yuan L, Lisanti MP, Katiyar S, Pestell RG. Cyclin D1 induction of cellular migration requires p27(KIP1) Cancer Res. 2006a;66:9986–9994. doi: 10.1158/0008-5472.CAN-06-1596. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang C, Jiao X, Lu Y, Fu M, Quong AA, Dye C, Yang J, Dai M, Ju X, et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Molecular and cellular biology. 2006b;26:4240–4256. doi: 10.1128/MCB.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch PM. Prevention of colorectal cancer in high-risk populations: the increasing role for endoscopy and chemoprevention in FAP and HNPCC. Digestion. 2007;76:68–76. doi: 10.1159/000108395. [DOI] [PubMed] [Google Scholar]
- Maekawa H, Oike Y, Kanda S, Ito Y, Yamada Y, Kurihara H, Nagai R, Suda T. Ephrin-B2 induces migration of endothelial cells through the phosphatidylinositol-3 kinase pathway and promotes angiogenesis in adult vasculature. Arterioscler Thromb Vasc Biol. 2003;23:2008–2014. doi: 10.1161/01.ATV.0000096655.56262.56. [DOI] [PubMed] [Google Scholar]
- Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL, Wang B. Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase. Role of Rho family small GTPases. J Biol Chem. 2005;280:923–932. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Ricard J, Salinas J, Garcia L, Liebl DJ. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol Cell Neurosci. 2006;31:713–722. doi: 10.1016/j.mcn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, van de Wetering M, Muncan V, Winton DJ, Clevers H, Clarke AR. Cyclin D1 is not an immediate target of beta-catenin following Apc loss in the intestine. J Biol Chem. 2005;280:28463–28467. doi: 10.1074/jbc.M500191200. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648–5655. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Sims JT, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2008;27:1095–1105. doi: 10.1038/sj.onc.1210714. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Vearing CJ, Lackmann M. Eph receptor signalling; dimerisation just isn't enough. Growth Factors. 2005;23:67–76. doi: 10.1080/08977190500055869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.