Abstract
Diabetic gastroparesis is a disorder that predominantly affects women. However, the biological basis of this sex bias remains completely unknown. In this study we tested the hypothesis that a component of this effect may be mediated by the nitrergic inhibitory system of the enteric nervous system. Age-matched male and female Sprague-Dawley rats were studied 8 or 12 wk after streptozotocin (55 mg/kg body wt ip)-induced sustained hyperglycemia and compared with controls. Solid gastric emptying (GE) studies were performed in all the groups. Changes in gastric antrum neuronal nitric oxide synthase (nNOS) mRNA and protein levels were analyzed by real-time PCR and Western immunoblotting, respectively. nNOS dimerization studies were performed using low-temperature SDS-PAGE. In vitro nitrergic relaxation (area under curve/mg tissue wt) was studied after the application of electric field stimulation in an organ bath. Changes in intragastric pressure (mmHg•s) in freely moving rats in the presence or absence of NGnitro-l-arginine methyl ester (nitric oxide synthase inhibitor) were examined by an ambulatory telemetric method. After diabetes induction, GE is delayed in both male and female rats. However, diabetic females exhibited significant delayed GE than in diabetic males. Compared with male controls, gastric nNOS expression and nitrergic relaxation were substantially elevated in healthy female control rats, accompanied by significantly reduced intragastric pressure. The active dimeric form and dimer-to-monomer ratio of nNOSα were also higher in healthy females compared with male rats (P < 0.05). Diabetic females, but not males, showed significant (P < 0.05) impairment in both gastric nNOSα dimerization and nitrergic relaxation, accompanied by an increase in intragastric pressure. Our data provide evidence that females may have a greater dependency on the nitrergic mechanisms in health. Furthermore, diabetes seems to affect the nitrergic system to a greater extent in females than in males. Together, these changes may account for the greater vulnerability of females to diabetic gastric dysfunction.
Keywords: solid gastric emptying, nitrergic relaxation, intragastric pressure
Gastric dysmotility or gastropathy occurs in 20–55% of patients with Type 1 (insulin dependent) and up to 30% of patients with Type 2 diabetes (non-insulin dependent) (37). Symptoms of diabetic gastropathy can range from mild dyspepsia to recurrent vomiting and abdominal pain and are often associated with delayed or accelerated gastric emptying. Fundic tone abnormalities and/or poor antroduodenal coordination has been reported in diabetic patients (28). Although the pathogenesis of diabetic gastropathy is not completely understood, it appears to be much more common (up to four times) in women (46, 22). Such a sex bias is also seen in the idiopathic variety of gastric dysfunction, reinforcing the concept of women being more susceptible to this condition. It is not clear, however, whether this phenomenon suggests a unique biological vulnerability in females or simply an exaggeration of preexisting differences in gastric motility. Thus gastric antroduodenal motility is slower even in healthy women, perhaps owing to hormonal influence (2). Premenopausal women, pregnant women during labor, and postmenopausal women receiving hormone replacement therapy develop reversible gastrointestinal complications including delayed gastric emptying for both solids and liquids compared with age-matched men (3, 4, 27). Moreover, studies using male and female rats indicate that estradiol-17β, either alone or in combination with progesterone, may cause delayed gastric emptying (16).
Gastric motility is regulated in large part by neurons of the enteric nervous system located in the muscle wall (53). These neurons are either excitatory (releasing acetylcholine) or inhibitory [releasing nitric oxide (NO) and vasoactive intestinal peptide]. NO is the principal nonadrenergic noncholinergic (NANC) inhibitory neurotransmitter in the gastrointestinal tract and is produced by neuronal NO synthase (nNOS), expressed in inhibitory enteric neurons (48). NO activates soluble guanylate cyclase, producing an increase in the intracellular cGMP leading to muscle relaxation (14, 48). Nitrergic signaling (from NO-releasing neurons) plays a critical role in the control of gastric accommodation and pyloric relaxation in response to a meal. The importance of NO in gastric function was established by the findings of pyloric hypertrophy and gastric dilation in mice with a targeted genomic deletion of nNOS (nNOS−/− mice) (19, 32). Vagal modulation of enteric neuronal function (both inhibitory and excitatory) also plays an important role in gastric physiology and is predominantly cholinergic in character (29).
Unlike two other NO synthase (NOS) enzymes (endothelial and inducible NOS), both full-length and NH2-terminally truncated forms of nNOS exist because of alternative splicing of 5′ regions of the nNOS gene in both humans and rodents (36, 41, 42). The predominant form of nNOS in the enteric nerves of the stomach is nNOSα, which contains a PDZ/GLGF motif encoded by exon 2 in the NH2-terminal region (41). The PDZ/GLGF domain interacts with various proteins and determines nNOSα subcellular localization and function (9, 47). In contrast, the NH2-terminally truncated nNOSβ and nNOSγ lack the PDZ/GLGF motif for protein-protein interaction and possible membrane association. nNOS−/− mice lacking exon 2 and subsequent membrane-associated full-length nNOSα exhibit delayed gastric emptying of solids and liquids (19, 32). The catalytic activity of NOS depends on dimerization of two NOS polypeptides (25). Dimerization results in the creation of high-affinity binding sites for (6R)-tetrahydrobiopterin (BH4) and arginine in the oxygenase domain and enables electron transfer between flavin and heme groups (7, 17). Enzymatic uncoupling of NOS due to lack of BH4 may in part account for reduced NO production and increased oxidative stress factors such as superoxide (26, 51). The importance of dimerization for the catalytic activity of endothelial NOS and its disruption in diabetes has been reported by several independent investigators (13, 33). However, to the best of our knowledge, there have been no previous in vivo studies on gastrointestinal nNOS dimerization and function.
Although delayed or accelerated gastric emptying has long been taken as a hallmark of diabetes, in recent reports most experts concur that this correlates poorly if at all with clinical symptoms (37, 38). Nitrergic relaxation in gastric muscle preparations obtained from diabetic biobreeding/Worcestor (BB/W; an animal model of human Type 1 diabetes) rats is significantly impaired, along with a decrease in nNOS-immunoreactive cells in the gastric myenteric plexus and mRNA expression (49). Similar changes have also been shown in obese and streptozotocin (STZ)-induced diabetic mice and rats (21, 52). Generally, these changes in phenotypic expression have not been accompanied by neuronal loss and, indeed, have been shown to be reversible by insulin treatment (14, 52). It is noteworthy, however, that these studies have all been performed in male animals, despite the knowledge that both nitrergic tissue relaxation and nNOS in gastric fundus can be enhanced by estrogen treatment in female rats (45). Since irregular antral motility may be an important factor responsible for altered gastric emptying in patients, we focused on possible disturbances in nitrergic regulation of gastric motility in the diabetic state (18, 50). The results of our study suggest that nNOS expression, dimerization, and function are sex dependent and furthermore that diabetes affects these processes differentially in males and females.
MATERIALS AND METHODS
Experimental rats and induction of diabetes mellitus with STZ
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male and female nonpregnant rats (7 wk old) were purchased from Harlan Sprague Dawley (Houston, TX). After arrival at the animal care facility, all rats were maintained in the colony room with a 12:12-h fixed light-dark photoperiod. Animals were allowed free access to water and rodent chow.
Diabetes was induced in groups of male (n = 10) and female (n = 10) rats with a single STZ injection of 55 mg/kg body wt ip (Sigma Chemical, St. Louis, MO) prepared in 5 mM citrate buffer (vehicle), pH 4.0 (30). Control groups were injected with the vehicle only. Blood glucose levels from overnight fasting animals were obtained 48 h after STZ to confirm that diabetes was induced in treated animals. Similarly, blood glucose levels were also obtained from overnight fasting male and female diabetic as well as control rats at the time the experiment was performed (12 wk after STZ). Diabetes was confirmed if blood glucose levels ranged from 300–400 mg/dl in both male and female rats. No differences in blood glucose levels were noticed between male and female rats 12 wk after the induction of diabetes with STZ. Age-matched control male and female rats showed blood glucose levels between 80–95 mg/dl. Both control and diabetic rats were selected during the diestrous stage of the estrous cycle. As reported earlier, we noticed that 60–70% of diabetic rats show a persistent diestrous stage of the estrous cycle (data not shown) (8, 24).
Solid gastric emptying studies
Solid gastric emptying studies were performed in healthy male and female rats. Similarly, gastric emptying studies were performed in both male and female ras 8 wk after diabetes induction. The gastric emptying rate of a solid meal was measured as reported previously (31). Groups of healthy and diabetic rats from both sexes were fasted for 20–24 h. A known amount of solid meal with free access to water was supplied for a 3-h period. Food and water were then removed, and the gastric emptying rate of the ingested meal was assessed 4 h later. Following euthanization, the abdominal cavity was opened, the pylorus and cardia were clamped, and the stomach was removed. The amount of food contained in the stomach and solid food ingested by the animals were calculated. The rate of gastric emptying during the 4-h experimental time was calculated according to the following equation: gastric emptying (% in 4 h) = (1 − gastric content/food intake) × 100.
Organ bath studies
For studies on NANC activity, the gastrointestinal tract from the lower esophageal sphincter to the distal duodenum was removed from the body cavity and placed in Krebs bicarbonate solution gassed with a mixture of 95% O2 and 5% CO2. The Krebs (pH 7.4) buffer contains (in mM) 118.0 NaCl, 4.7 KCl, 25.0 NaHCO3, 1.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 11.5 glucose. Circular gastric antrum muscle strips were mounted between two L-shaped tissue hooks in 5-ml water-jacketed organ baths containing Krebs buffer at 37°C and continuously bubbled with 95% O2-5% CO2 (Radnoti Glass Technology, Monrovia, CA). Tension for each muscle strip was monitored with an isometric force transducer and analyzed by a digital recording system (Biopac Systems, Santa Barbara, CA). Tension was increased progressively, and contractile responses to potassium chloride (60 mM) were analyzed in Krebs bicarbonate solution measured in various resting conditions (45, 52). Gastric antrum neuromuscular strips were preincubated for 30 min with atropine (10 µM), phentolamine (10 µM), and propranolol (10 µM) to block cholinergic and adrenergic mediated responses. Tone was raised 60–80% of maximal contraction produced by 60 mM KCl by addition of 5-hydroxytryptamine (5-HT) to a final concentration of 100 µM. Specimens demonstrating sustained tonic contractions with 5-HT stimulation were used for the experiments. Nitrergic relaxations were induced for 60 s after contraction with 5-HT by electric field stimulation (EFS; 90 V, 2 Hz, 1-ms pulse for a duration of 1 min, as indicated) (Grass stimulator model SD9, Grass Instruments, Astro-Med, West Warrick, RI). The NO dependence of nitrergic relaxations was confirmed by preincubation with NG-nitro-l-arginine methyl ester (l-NAME, 100 µM; 30 min). All drugs used in this study were purchased from Sigma Chemical. To confirm the role of neuronal depolarization in evoking NANC relaxations, gastric tissues were preincubated for 30 min in the presence of TTX (1 µM; Calbiochem). At the end of each experimental protocol, the muscle strip was removed, blotted dry with filter paper, and weighed.
Comparisons between the groups were performed by measuring the area under the curve (AUC) of the EFS-induced relaxation (AUCR) for 1 min and the baseline for 1 min (AUCB) according to the formula (AUCR − AUCB)/weight of tissue (mg) = AUC/mg of tissue (34).
Ambulatory telemetric studies
Gastric contractility in control and diabetic rats was measured by using an ambulatory telemetric experimental apparatus previously reported for quantifying uterine contractility (10, 11).
Groups (n = 3–4) of diabetic and control rats were anesthetized with ketamine (45 mg/kg body wt; Fort Dodge Laboratory, Fort Dodge, IA) and xylazine (5 mg/kg body wt; Burns Veterinary Supply, New York, NY). A pressure telemetry transducer/transmitter (C50-PXT model, Data Sciences, Arden Hills, MN) was implanted into the abdomen of each animal. The transducer pressure catheter was introduced into the gastric antrum cavity 5 mm proximal to the pylorus to record intragastric pressure (IGP, mmHg•s). After the baseline IGP was recorded, l-NAME (200 mg•day−1•kg body wt−1 per rat) was administered subcutaneously in the same rats by osmotic minipumps (Alza, Palo Alto, CA, model 2ML1 with a pumping rate of 10 µl/h) for 4 days. All subsequent studies on these animals were performed 1 wk after surgery in overnight fasted rats that were awake and in a free-moving state. Pressure recordings were performed at least 2–3 h between 9:00 AM and 12:00 noon throughout the studies.
Data were transmitted by telemetry (RLA 1020 telemetry receivers, Data Sciences), multiplexed (BCM consolidation matrix, Data Sciences), and sent to an adapter where the signal was then demultiplexed, sampled at 1,024 Hz, and converted to analog (UA-10 universal adapter, Data Sciences). This output was band-pass filtered and amplified. The final sampling rate was 10 Hz (10, 11). The information was then fed to a recording system (MacLab 16/s, AD Instruments; Castle Hill, Australia) and stored for later analysis.
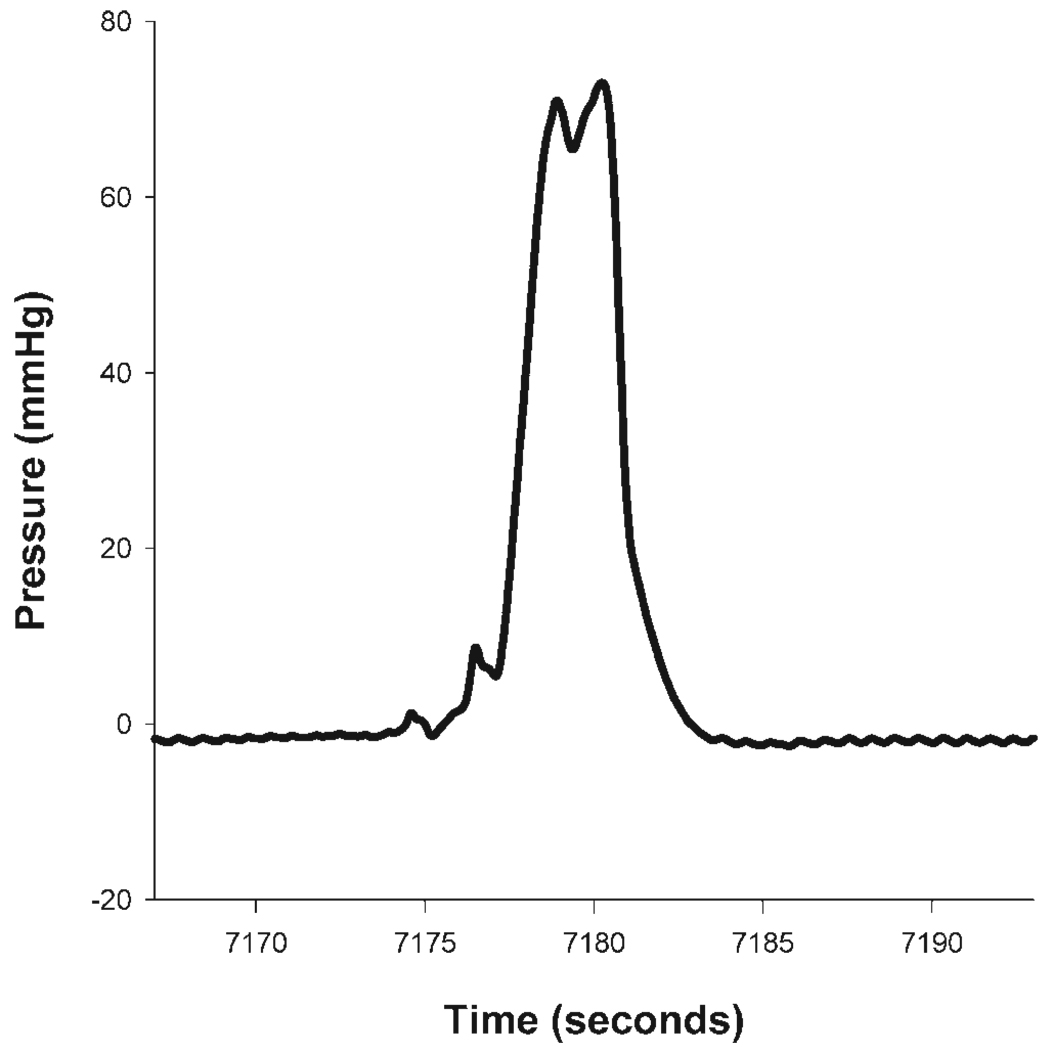
For each animal’s data analysis, pressure recordings for at least 10 consecutive contraction events were averaged at each time point. A contraction event was defined as a rapidly occurring increase in IGP of 5 mmHg or more, such that the increased IGP persisted for 5 s or longer (Fig. 1). The pressure integral (or area under the pressure curve) was found for each animal at each time point investigated.
Fig. 1.
Intragastric pressure (IGP) contractions were observed using implanted telemetric IGP devices. Contractions were defined as rapidly increasing pressure events, 5 mmHg or more in amplitude, with a duration of 5 s or more. These pressure curves were isolated and an integral from baseline was calculated. Ten such consecutive contractions were evaluated in this way to obtain a mean IGP for each animal for each time point. Means and SE of the IGP were then found for each group of animals at each time point.
Real-time RT-PCR
Total tissue RNA was isolated from the rat gastric antrum neuromuscular tissues by a single-step guanidine thiocyanate method using the reagent Trizol (BRL, Gaithersburg, MD). The quality of RNA was determined by NanoDrop (NanoDrop Technologies), and the quantity was estimated by an Agilent 2100 bioanalyzer (Agilent Technologies, Houston, TX). One microgram of total RNA was denatured at 65°C for 5 min and cDNA synthesis was then performed at 42°C for 1 h by using Superscript reverse transcriptase (BRL). An aliquot of generated cDNA was amplified with a pair of primers (accession number NM_052799) for nNOS (forward 2782–2800, 5′-ACGGACCCGACCTCAGAGA3′, reverse 2856–2837, 5′-CGAGGCCGAACACTGAGAAC-3′) and probe [2810 –2835, 5′-(FAM)-AAGTACTGGACCCCTGGCCAATGTGA-(TAMARA)]. Quantitative RT-PCR amplification was performed by using two-step TaqMan Universal PCR master mix and the ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA). Cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 repeats of 95°C for 0.15 min and 60°C for 1 min. Relative amounts of mRNA were normalized by 18S and calculated threshold cycle numbers (CT), i.e., 2−ΔΔCT, according to the manufacturer’s suggestion (Applied Biosystems). All studies were performed in the Molecular Genomics Core Laboratory, The University of Texas Medical Branch, Galveston, TX.
Western blot analysis
We examined nNOS protein expression and dimerization using COOH- and NH2-terminal antibodies, respectively. COOH-terminal antibody detects all (total) forms of nNOS (α, β, and γ) whereas NH2-terminal antibody detects only the full-length nNOSα protein (41). Under normal conditions, both dimers and monomers of nNOS protein are intensified at 155 kDa. However, low-temperature SDS-PAGE of nonboiled sample homogenates separates NOS dimers (310 kDa) and monomers (155 kDa) (23).
Equal amounts of total protein (40 µg each; Pierce Kit, Rockford, IL) from each preparation were resolved on a 6% SDS-polyacrylamide gel, transferred to a nitrocellulose membrane, and primed with primary COOH-terminal nNOS monoclonal antibody (1:1,000, Transduction Laboratory, San Jose, CA) overnight at 4°C (52). To investigate nNOS homodimer formation in gastric antrum tissues, low-temperature SDS-PAGE was employed with nonboiled samples by using specific NH2-terminal (1–195 amino acids of nNOSα, exon 2 region) nNOS polyclonal antibody (1:200, Zymed) as previously described (23). The membrane was washed three times with 20 mmol/l Tris•HCl, pH 7.6, 0.05% Tween 20, 100 mM NaCl and then incubated with a respective secondary antibody, goat anti-mouse and anti-rabbit coupled to horseradish peroxidase (Amersham Life Sciences, Arlington Heights, IL). After three washes, the membrane was developed by using the enhanced chemiluminescence system (ECL, Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer’s protocol. To verify equal loading of sample protein, the immunoblots were stripped in stripping solution (Pierce, Rockford, IL) and reprobed with monoclonal anti-γ-tubulin antibody (1: 10,000; Sigma Chemical). Densitometry analysis was performed in the linear range using a Fluorchem Analysis System (Alpha InfoTech; San Leandro, CA) and the amount of total nNOS (155 kDa) was normalized to the γ-tubulin (48 kDa) signal.
Statistical analysis
Results are expressed as means ± SE obtained from four to seven animals for solid gastric emptying and organ bath studies and three to four samples for both IGP and nNOS expression studies. Data were analyzed for statistical differences with Student’s t-test or two-way ANOVA followed by the Bonferroni t-test to verify the differences between individual groups. P < 0.05 was considered significant (n = 3–6).
RESULTS
Sex-dependent gastric emptying rate in control and diabetic rats
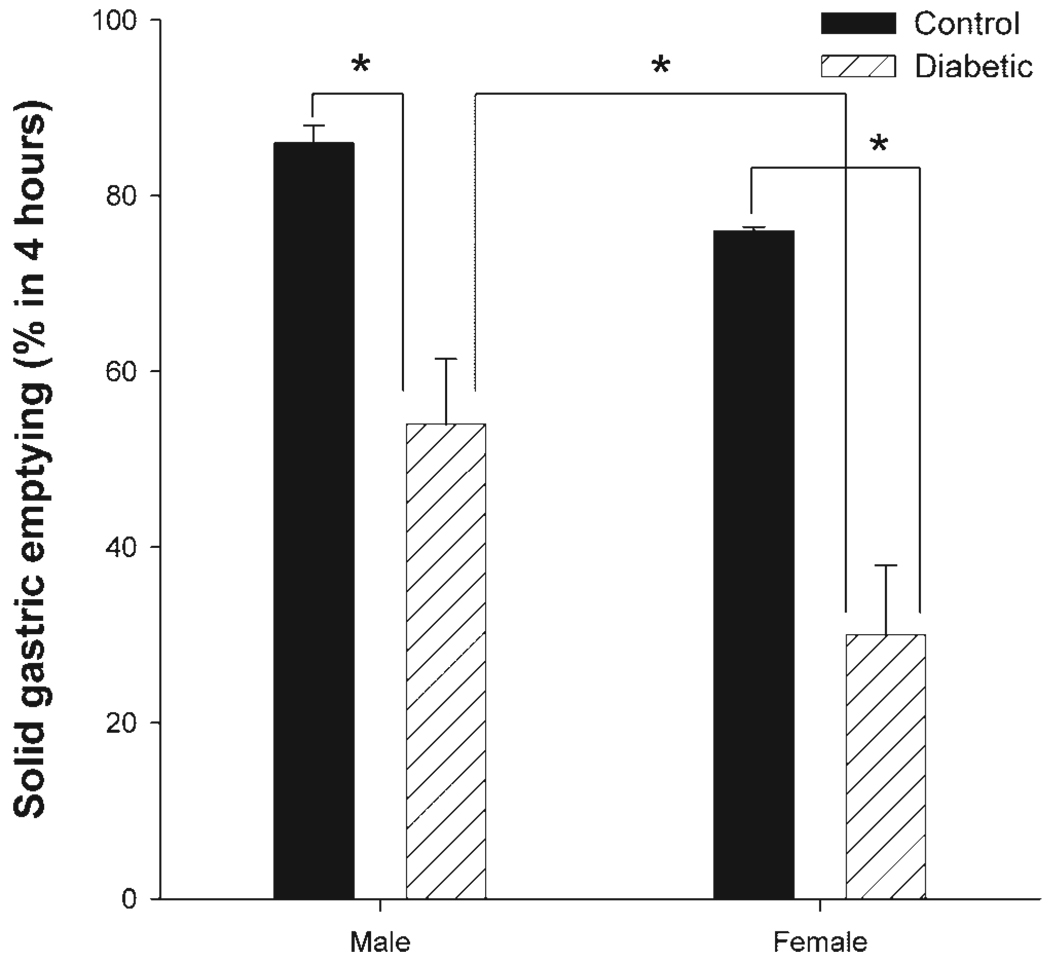
Figure 2 shows differences in solid gastric emptying rate in healthy and diabetic male and female rats. Healthy females during the diestrous stage of their estrous cycle (when circulatory estrogens are beginning to be elevated) exhibited a trend toward a delay in gastric emptying for solids compared with age-matched male rats. However, this was not statistically significant. Eight weeks after diabetic induction, a significant delay in gastric emptying rate was noticed in both male and female rats, compared with their respective controls (Fig. 2). Furthermore, gastric emptying was reduced to a significantly greater extent in diabetic females compared with diabetic males.
Fig. 2.
Sex-dependent changes in solid gastric emptying in healthy and diabetic rats. Diabetes was induced with a single injection of streptozotocin (STZ; 55 mg/kg body wt ip), and all studies were performed after 8 wk of diabetes induction. The control group received vehicle (citrate buffer). Fasted rats were exposed to normal rat chow ad libitum for a 3-h period and gastric emptying was monitored 4 h later. Data are means ± SE of 4–7 animals per group. *P < 0.05 (ANOVA).
Sex-dependent nitrergic relaxation in control and diabetic rats
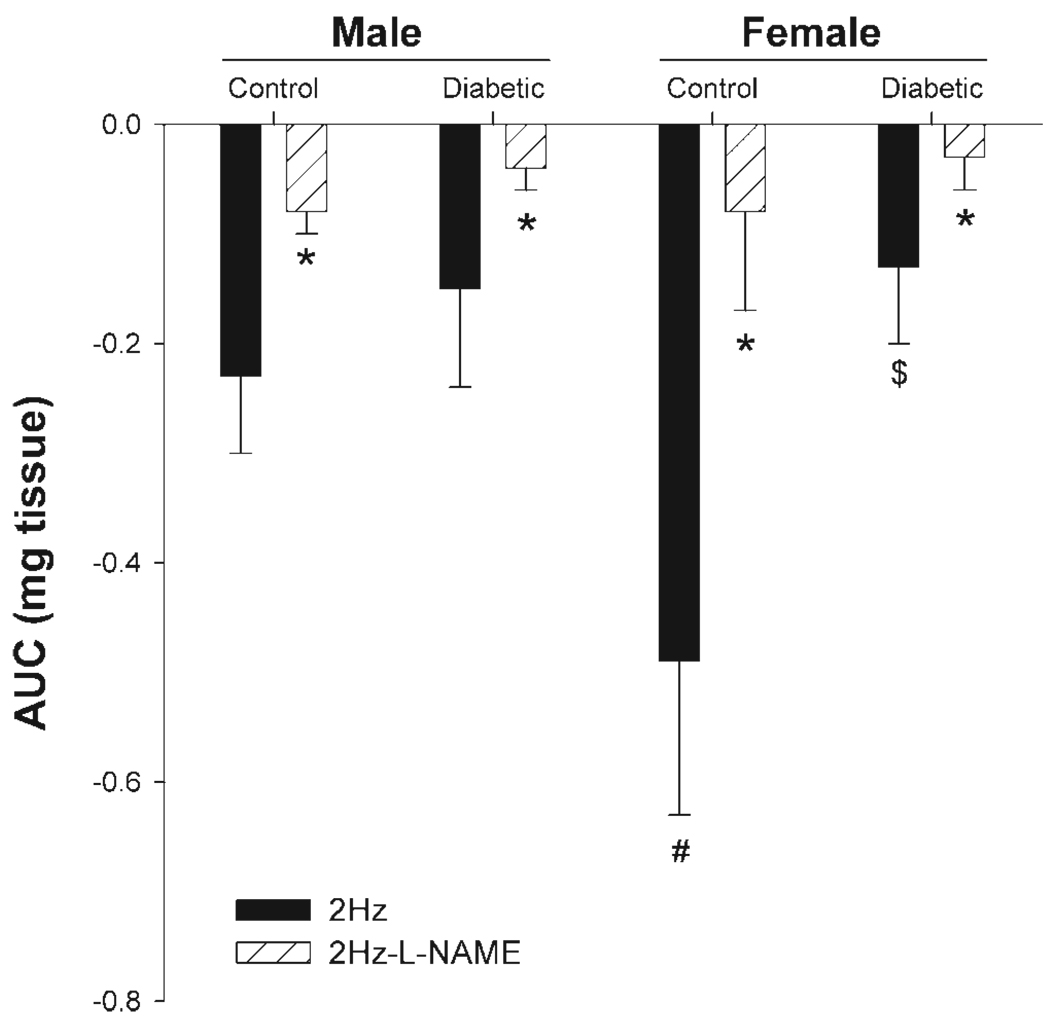
Circular muscle strips from gastric antrum from both control and diabetic rats showed nitrergic relaxation in response to EFS using a frequency of 2 Hz. This relaxation was significantly antagonized by preincubation with the NOS inhibitor l-NAME (100 µM) and completely abolished in the presence of TTX (data not shown), confirming its nitrergic nature and neuronal source, respectively (Fig. 3).
Fig. 3.
Effect of diabetes on gastric antrum nonadrenergic and noncholinergic (NANC) relaxation in response to transmural nerve stimulation (90 V, 2 Hz) in age-matched male and female rats. Gastric antrum muscle tissues obtained from 12-wk-old rats were preincubated with atropine, phentolamine, and propranolol (10 µM each) and active tone was induced initially with 100 µM 5-hydroxytryptamine. The nitric oxide (NO) dependence of the NANC relaxations in control and diabetic groups was confirmed by preincubation with the NO inhibitor nitro-l-arginine methyl ester (l-NAME; 100 µM). Each point represents means ± SE from 4–6 animals in each group. *Significant inhibition with l-NAME; #P < 0.05 for female control compared with male control; $P < 0.05 for female diabetic compared with female control.
Tissues obtained from female control showed substantially greater nitrergic relaxation compared with the male control group (P < 0.05). Furthermore, nitrergic relaxation was significantly impaired (P < 0.05) in female diabetic rats but not in male diabetics, compared with their respective control groups (Fig. 3).
Sex-dependent IGP in control and diabetic rats
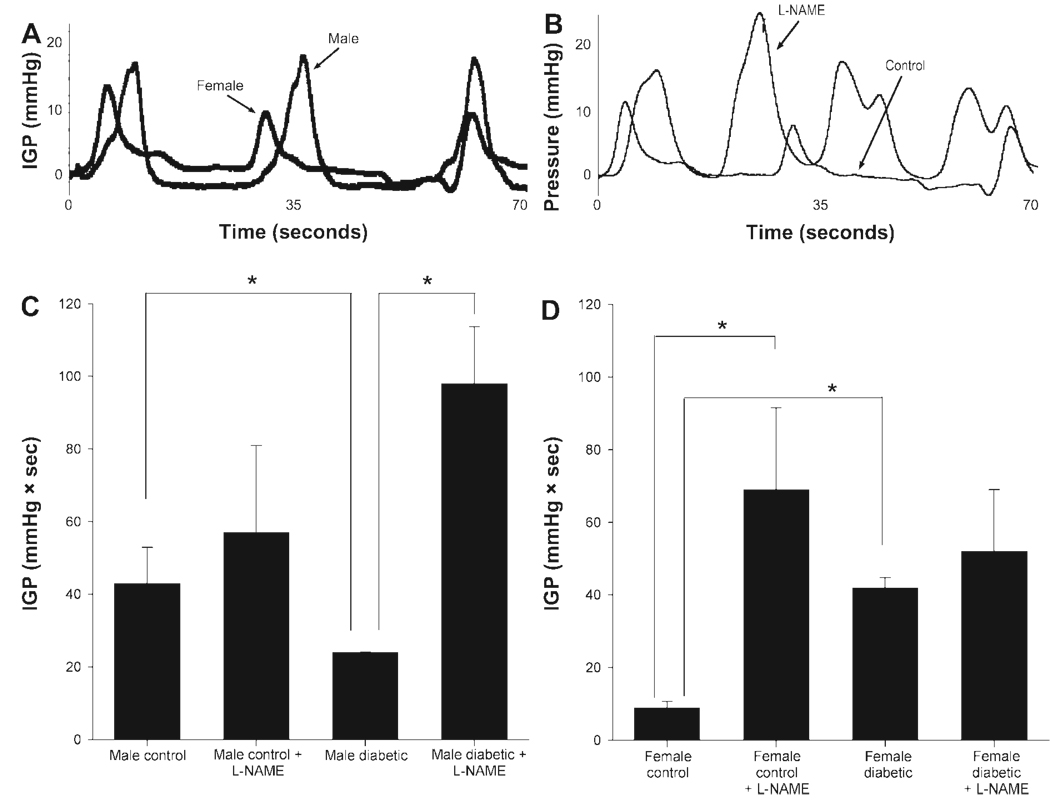
Intraluminal antral pressure was measured in age-matched male and female controls (Fig. 4A) and 12 wk after induction of diabetes in controls and in l-NAME-treated animals (Fig. 4B). As shown in Fig. 4C and Fig. 3D, the female control group showed a significant (P < 0.05) reduction in IGP (mmHg•s) compared with male controls. l-NAME infusion attenuated the lowered antral pressure in female controls, implicating a role for the nitrergic system in this phenomenon. Diabetes caused a significant (P < 0.05) IGP elevation in female diabetic rats compared with female controls (Fig. 4D), and this did not change after l-NAME infusion, suggesting loss of nitrergic tone. By contrast, IGP was reduced in male diabetic rats compared with male controls (Fig. 4C), an effect that was at least partly reversed by l-NAME administration (Fig. 4C), suggesting relative preservation of nitrergic tone.
Fig. 4.
A: comparison of IGP (mmHg•s) for male control vs. female control animals. We noticed that the male animals tend to have more powerful contractions in general, but roughly the same number of contractions per unit time. In fact, the integral of this activity is significantly higher for the male controls compared with female controls. B: comparison of control vs. nitric oxide synthase inhibitor, l-NAME-treated (200 mg/kg body wt sc per rat for 4 days) female animals. Notice that the l-NAME-treated animals tend to have generally more powerful and more frequent contractions. In fact, the integral of this activity is significantly higher for the l-NAME-treated animals compared with female controls. C and D: effect of diabetes on IGP in age-matched male (C) and female (D) rats. IGP were measured using ambulatory telemetric devices (see MATERIALS AND METHODS) in the presence or absence of l-NAME (200 mg/kg body wt sc per rat for 4 days) administration by osmotic minipumps in conscious, unrestrained animals. Each point represents means ± SE from 3–4 animals in each group. *P < 0.05.
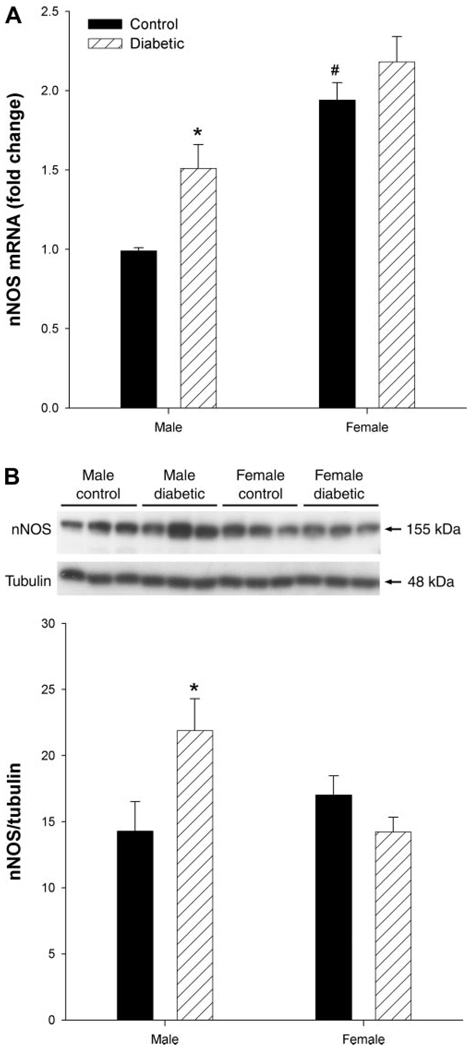
Sex-dependent nNOS expression in gastric tissues in control and diabetic rats
Compared with their male counterparts, female controls had higher nNOS mRNA (Fig. 5A), but not total protein levels (Fig. 5B). Diabetes did not appear to affect the expression of either mRNA or protein in females. However, both mRNA and total protein expression was significantly (P < 0.05) elevated in gastric antrum obtained from male diabetic rats compared with their respective controls (Fig. 5).
Fig. 5.
Sex- and diabetes-related changes in the expression of neuronal nitric oxide synthase (nNOS) in the gastric distal antrum of male control, female control, male diabetic, and female diabetic rats. nNOS mRNA (A) and protein (B) levels were measured from gastric antrum by quantitative RT-PCR and Western blot analysis, respectively. Diabetes was induced with a single injection of STZ (55 mg/kg body wt ip) and all studies were performed after 12 wk of diabetes induction. The control group received vehicle (citrate buffer). A: nNOS and 18S mRNA expressions were measured by real-time RT-PCR. Relative abundance of nNOS mRNA normalized by 18S was calculated from threshold cycles (CT), i.e., 2−ΔΔCT. B: representative immunoblot analysis of gastric distal antrum homogenates shows the expression of nNOS (155 kDa) and γ-tubulin (48 kDa) (top). Equal amounts of protein (40 µg) were loaded on each lane and detected with monoclonal antibody specific for the COOH-terminal domain of nNOS. Densitometric analysis followed by ratio of nNOS to γ-tubulin are calculated (bottom). Bars represent means ± SE (n = 3–4). Significant differences from control vs. diabetes are noted. *P < 0.05 for male diabetic compared with male control; #P < 0.05 for female control compared with male control.
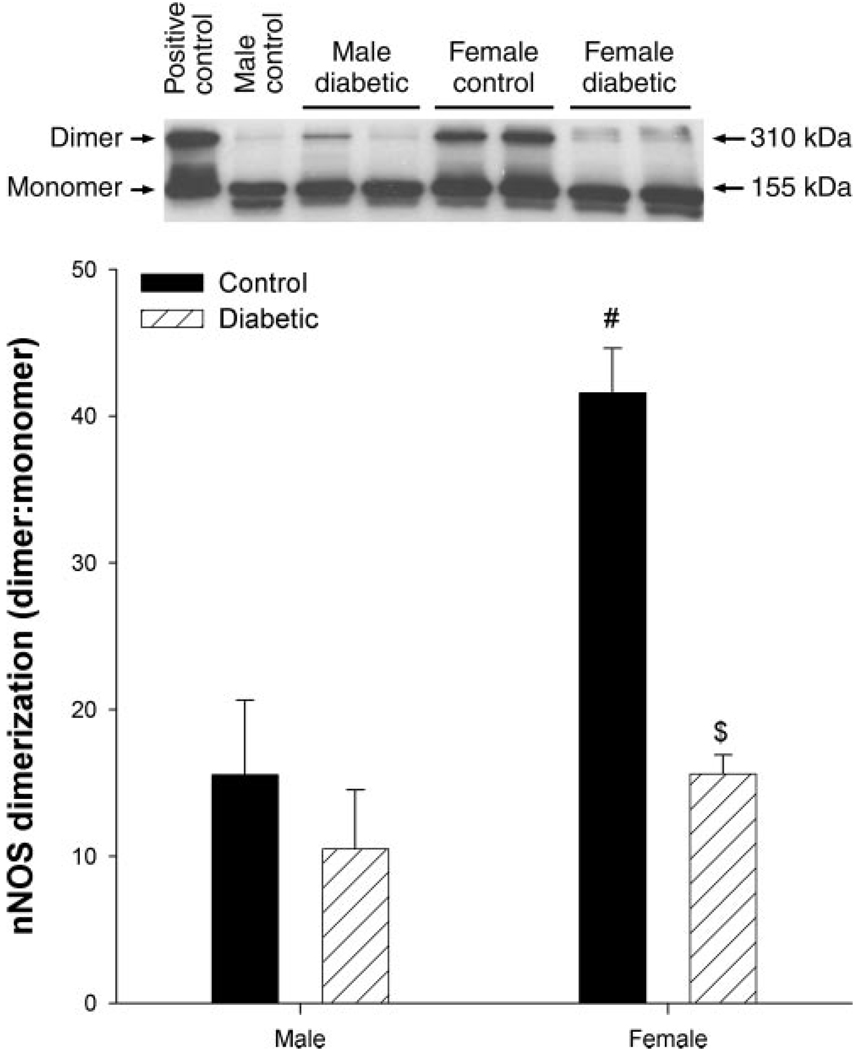
Dimerization of nNOSα in gastric antrum
We next set out to determine changes in dimerization of nNOSα in the various experimental states. We examined this by low-temperature SDS-PAGE so that the SDS-resistant dimeric form of nNOSα could be measured. This assay is not a measure of the absolute dimeric content under native conditions, but it is a measure of the amount of stable dimer that is not dissociated by SDS and thus somewhat underestimates the total dimeric content. Nonetheless, it is a convenient and reliable measure for studying relative changes in the dimeric state of nNOS. We found that the ratio of nNOSα dimers to monomers was significantly increased (P < 0.05) in female control rats compared with male controls in antrum (Fig. 6). Diabetes resulted in significant (P < 0.05) decreases in nNOSα dimerization in female antral tissue but not in males, compared with their respective controls (Fig. 6).
Fig. 6.
Sex- and diabetes-related changes in the expression of neuronal nitric oxide-α dimerization (nNOSα) in the gastric distal antrum of male control, female control, male diabetic, and female diabetic rats. Equal amounts of protein (40 µg) were loaded on each lane and detected with polyclonal antibody specific for the exon 2 encoded NH2-terminal domain of nNOSα. Top: representative immunoblot of gastric distal antrum homogenates (n = 3–4) showing the ratio of nNOSα dimers (310 kDa) to monomers (155 kDa). Bottom: densitometric analysis followed by a ratio of nNOSα dimerization to γ-tubulin is calculated. Bars represent means ± SE. Significant differences from control vs. diabetes are noted. #P < 0.05 for female control compared with male control and $P < 0.05 for female diabetic compared with female control.
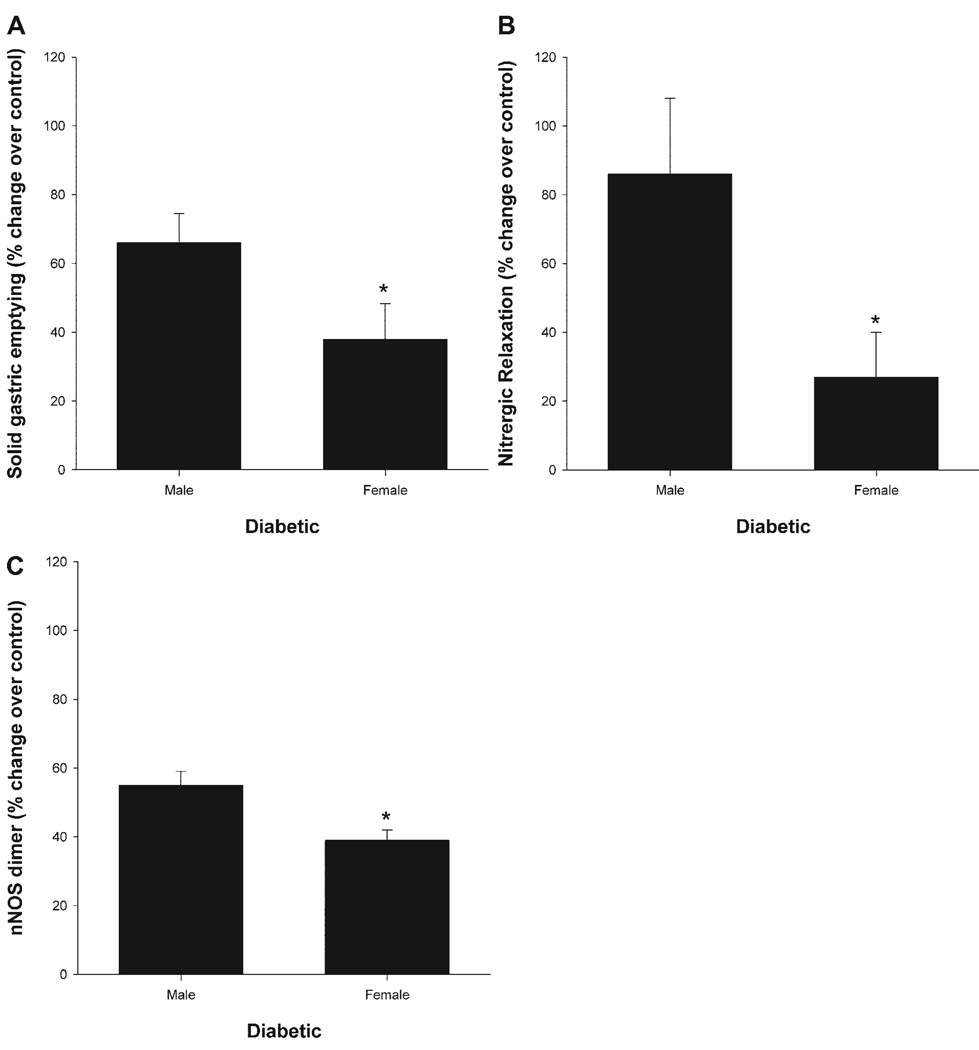
Figure 7 shows the changes in gastric emptying (A), gastric antrum nitrergic relaxation (B) and nNOSα protein dimer levels (C) in diabetic male and diabetic females after normalization to their own controls. As can be seen, diabetes significantly (P < 0.05) reduced gastric emptying, nitrergic relaxation, and nNOSα protein dimer levels in females compared with male rats (Fig. 7). The decline in gastric antrum nNOSα dimer levels correlated with changes in gastric antrum nitrergic relaxation and solid gastric emptying in diabetic female rats (Fig. 7).
Fig. 7.
Sex-dependent changes in solid gastric emptying (A), gastric antrum nitrergic relaxation (B) and gastric antrum nNOS dimer levels (C) in healthy and diabetic rats. Diabetes was induced with a single injection of STZ (55 mg/kg body wt ip). The control group received vehicle (citrate buffer). Diabetes reduced solid gastric emptying (A), nitrergic relaxation (B), and nNOSα protein dimer levels (C) in diabetic female gastric tissues compared with diabetic male rats. The changes in the above parameters were normalized with their respective control group. Data were presented as % change over control. *P < 0.05 (ANOVA).
DISCUSSION
Abnormal gastrointestinal motility in diabetes mellitus is likely multifactorial in origin, reflecting disturbances in enteric and vagal neural activity as well as interstitial cells of Cajal and smooth muscle function (20, 28, 33, 38). Of these, enteric neuropathy may be particularly important (5, 6, 48). Several studies of animal models of diabetes have convincingly shown disturbances in enteric nerves, particularly involving nitrergic nerves (14, 49, 52). Since these studies have used male rats exclusively, it has been difficult to correlate these findings with the clinical finding that 80% of all gastroparetic patients are female. It is possible that the pathogenetic mechanisms of diabetic gastric dysmotility are common to both men and women but the latter are disproportionately symptomatic because the motility of their stomachs is slower to begin with. On the other hand, it is also possible that there are fundamental sex-specific differences between how the gastric neuromuscular apparatus is affected by diabetes. The results of our study suggest that the latter explanation may be more likely.
In this study, we have demonstrated sex differences in solid gastric emptying, antral nNOS expression, nitrergic relaxation, IGP, and nNOSα protein dimerization in both diabetic and nondiabetic rats. Compared with control male rats, healthy females during the diestrous stage of the estrous cycle showed delayed gastric emptying (Fig. 2). However, this was not statistically significant. Previous studies have demonstrated that estradiol-17β administration delays gastric emptying for liquids in both male and female rats (16). Thus, more studies are warranted to address whether solid gastric emptying rate is different during various stages of the estrous cycle when estrogen levels are higher than in the diestrous phase. However, nitrergic relaxation of the antrum is clearly greater in healthy diestrous females (Fig. 3) compared with males, and this correlates with a corresponding increase in both total nNOS protein expression (Fig. 5) and nNOSα dimerization (Fig. 6). Our studies further demonstrate that IGP is significantly lower in females compared with male rats (Fig. 4, C and D). This is in keeping with human studies showing that gastric antral contractility and gastric emptying is slower in young women compared with men whereas circulatory estrogen levels are elevated (27). Their method for measuring antral contractility was not telemetric, but since the study was performed in humans, the externalized catheters posed little problem for implementation insofar as the integrity of the data is concerned. In animals this is not the case, where with externalized catheters, either the animal must be restrained (introducing undue stress) to prevent destruction of the measurement apparatus or the data must be acquired while the animals are unconscious. Both of these alternatives may lead to spurious data. By using a telemetric device in conscious, unrestrained animals, we obtained the most accurate measurement for intraluminal pressure. In our study, l-NAME infusion increased IGP significantly in female but not in male rats, suggesting that nonnitrergic mechanisms may be more important in the latter (Fig. 4, C and D). Thus it appears that females rely on nitrergic control of gastric motility to a greater extent than males and hence may be more vulnerable to alterations of this system induced by diabetes.
We next turned our attention to changes in these parameters after induction of diabetes. The rate of gastric emptying may vary depending upon the type of method employed, i.e., liquids vs. solids; type, duration, and severity of diabetes. Human studies have demonstrated that diabetic women have more delayed gastric emptying compared with diabetic men (22, 46). In the present study, we have demonstrated that solid gastric emptying rate is significantly slower in both male and female rats after diabetic induction compared with their respective controls. Furthermore, these studies suggested that diabetic females showed significant delayed gastric emptying compared with diabetic males. These studies together with clinical findings suggested that gastric emptying is slower in diabetic females than in diabetic males.
In diabetic gastric dysfunction, antral motility and the coordination of pressures between the antrum and duodenum are diminished (28, 46, 53). Antral hypomotility has been recorded with intraluminal pressure transducers in patients with diabetes mellitus. In this study, we have shown that nitrergic relaxation (Fig. 3) is significantly reduced with concomitant increases in IGP in female diabetic rats (Fig. 4D). Furthermore, nNOSα dimerization (Fig. 6) but not total (Fig. 5) nNOS expression is significantly reduced in these animals. After normalization with their own control group, diabetic females exhibited significant reduction in solid gastric emptying (Fig. 7A), gastric antrum nitrergic relaxation (Fig. 7B), and nNOSα protein dimer levels (Fig. 7C) compared with male rats. These data suggest that a decline in nNOSα dimerization correlates with changes in nitrergic relaxation and gastric emptying and may explain why females have a greater tendency to gastroparesis.
In this study, we also demonstrate an increase in gastric antrum nNOS expression in the male diabetic group. Although our results are similar to those reported by Adeghate et al. (1), they are in contrast to most of the previous literature (all of which deals exclusively with male rats) reporting a decrease in gastric nNOS expression in diabetic rats (48, 49, 52). We are unable to satisfactorily explain this discrepancy but do note that we have utilized a lower dose of STZ to induce diabetes compared with others (15, 49). Furthermore, by contrast to female diabetic rats, we were unable to show a significant reduction in nNOSα dimerization or nitrergic relaxation in male diabetic animals. Interestingly, diabetes resulted in reduced IGP in males (compared with an increase in females), again suggesting a potentially important sex difference in the control of gastric motility (Fig. 4, C and D). The mechanism underlying the reduction in IGP in diabetic males remains unknown but may be due to other factors such as impairment of interstitial cells of Cajal dysfunction and antral electrical pacemaking and neurotransmitter coupling. More studies are needed to understand the biological basis of this sex effect.
The mechanisms regulating changes in nNOS dimerization and nitrergic function in female diabetic animals are not addressed in the present investigation but may be related to alterations in female hormonal control. Circulatory estradiol levels are lower in diabetic women and rats (30, 39). Estradiol-17β increases both nNOS expression and GTP cyclohydrolase 1 expression (43, 44). The latter is a rate-limiting enzyme for the production of BH4, which is required for dimerization of nNOS (12). Therefore, we suggest that the decrease in estradiol in diabetic female gastric tissues results in a reduced BH4 synthesis with adverse effects on nNOS activity, thus leading to increased gastric dysmotility. Studies are in progress to address this issue.
In summary, this study demonstrates significant sex differences in gastric emptying, gastric antral nitrergic function, and nNOS expression and dimerization in both health and disease. Nitrergic relaxation is more pronounced in healthy females, accounted for, perhaps, by an increased expression of the active dimeric form of nNOSα. On the other hand, chronic hyperglycemia causes a greater reduction in active forms of nNOS in females associated with significantly greater impairment of nitrergic relaxation. This study also illustrates for the first time the importance of nNOSα dimerization in gastric physiology. These findings may provide a biological explanation for the greater vulnerability of females to develop diabetic gastric dysmotility problems.
ACKNOWLEDGMENTS
The authors thank Xiemin Cao for technical assistance and John Winston and Raj Kumar for thoughtful discussions. Also, we thank Dr. Shao-Qing Shi for help with the surgeries for telemetric studies.
REFERENCES
- 1.Adeghate E, al-Ramadi B, Saleh AM, Vijayarasathy C, Ponery AS, Arafat K, Howarth FC, El-Sharkawy T. Increase in neuronal nitric oxide synthase content of the gastroduodenal tract of diabetic rats. Cell Mol Life Sci. 2003;60:1172–1179. doi: 10.1007/s00018-003-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aytug N, Giral A, Imeryuz N, Enc FY, Bekiroglu N, Aktas G, Ulusoy NB. Gender influence on jejunal migrating motor complex. Am J Physiol Gastrointest Liver Physiol. 2001;280:G255–G263. doi: 10.1152/ajpgi.2001.280.2.G255. [DOI] [PubMed] [Google Scholar]
- 3.Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders during pregnancy. Ann Intern Med. 1993;118:366–375. doi: 10.7326/0003-4819-118-5-199303010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Baschetti R. Gender differences in gastric emptying. Eur J Nucl Med. 1999;26:295–296. doi: 10.1007/pl00006654. [DOI] [PubMed] [Google Scholar]
- 5.Belai A, Lefebvre RA, Burnstock G. Motor activity and neurotransmitter release in the gastric fundus of streptozotocin-diabetic rats. Eur J Pharmacol. 1991;194:225–234. doi: 10.1016/0014-2999(91)90109-4. [DOI] [PubMed] [Google Scholar]
- 6.Belai A, Lincoln J, Milner P, Crowe R, Loesch A, Burnstock G. Enteric nerves in diabetic rats: increase in vasoactive intestinal polypeptide but not substance P. Gastroenterology. 1985;89:967–976. doi: 10.1016/0016-5085(85)90195-7. [DOI] [PubMed] [Google Scholar]
- 7.Bender AT, Nakatsuka M, Osawa Y. Heme insertion, assembly, and activation of apo-neuronal nitric-oxide synthase in vitro. J Biol Chem. 2000;275:26018–26023. doi: 10.1074/jbc.275.34.26018. [DOI] [PubMed] [Google Scholar]
- 8.Blades RA, Bryant KR, Whitehead SA. Feedback effects of steroids and gonadotrophin control in adult rats with streptozotocin-induced diabetes mellitus. Diabetologia. 1985;28:348–354. doi: 10.1007/BF00283142. [DOI] [PubMed] [Google Scholar]
- 9.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 10.Buhimschi C, Boyle MB, Saade GR, Garfield RE. Uterine activity during pregnancy and labor assessed by simultaneous recordings from the myometrium and abdominal surface in the rat. Am J Obstet Gynecol. 1998;178:811–822. doi: 10.1016/s0002-9378(98)70498-3. [DOI] [PubMed] [Google Scholar]
- 11.Buhimschi CS, Saade GR, Buhimschi IA, Gokdeniz R, Boyle MB, Garfield RE. Effect of stimulatory and inhibitory drugs on uterine electrical activity measured noninvasively from the abdominal surface of pregnant rats. Am J Obstet Gynecol. 2000;183:68–75. doi: 10.1067/mob.2000.105348. [DOI] [PubMed] [Google Scholar]
- 12.Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res. 2005;65:823–831. doi: 10.1016/j.cardiores.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- 14.Cellek S. Point of NO return for nitrergic nerves in diabetes: a new insight into diabetic complications. Curr Pharm Des. 2004;10:3683–3695. doi: 10.2174/1381612043382792. [DOI] [PubMed] [Google Scholar]
- 15.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–2362. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 16.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol Gastrointest Liver Physiol. 1995;268:G171–G176. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- 17.Crane BR, Arvai AS, Ghosh DK, Wu C, Getzoff ED, Stuehr DJ, Tainer JA. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279:2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz M, Dent J, Fraser R, Sun W, Hebbard G. Role and integration of mechanisms controlling gastric emptying. Dig Dis Sci. 1994;39:7S–13S. doi: 10.1007/BF02300360. [DOI] [PubMed] [Google Scholar]
- 19.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 20.James AN, Ryan JP, Crowell MD, Parkman HP. Regional gastric contractility alterations in a diabetic gastroparesis mouse model: effects of cholinergic and serotoninergic stimulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G612–G619. doi: 10.1152/ajpgi.00431.2003. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson KM, Reid JJ. Effect of diabetes on relaxations to non-adrenergic, non-cholinergic nerve stimulation in longitudinal muscle of the rat gastric fundus. Br J Pharmacol. 1995;116:1551–1556. doi: 10.1111/j.1476-5381.1995.tb16372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–1269. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 23.Kamada Y, Jenkins GJ, Lau M, Dunbar AY, Lowe ER, Osawa Y. Tetrahydrobiopterin depletion and ubiquitylation of neuronal nitric oxide synthase. Brain Res Mol Brain Res. 2005;142:19–27. doi: 10.1016/j.molbrainres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Kirchick HJ, Keyes PL, Frye BE. An explanation for anovulation in immature alloxan-diabetic rats treated with pregnant mare’s serum gonadotropin: reduced pituitary response to gonadotropin-releasing hormone. Endocrinology. 1979;105:1343–1349. doi: 10.1210/endo-105-6-1343. [DOI] [PubMed] [Google Scholar]
- 25.Klatt P, Pfeiffer S, List BM, Lehner D, Glatter O, Bachinger HP, Werner ER, Schmidt K, Mayer B. Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J Biol Chem. 1996;271:7336–7342. doi: 10.1074/jbc.271.13.7336. [DOI] [PubMed] [Google Scholar]
- 26.Klatt P, Schmidt K, Uray G, Mayer B. Multiple catalytic functions of brain nitric oxide synthase. Biochemical characterization, cofactor-requirement, and the role of N omega-hydroxy-l-arginine as an intermediate. J Biol Chem. 1993;268:14781–14787. [PubMed] [Google Scholar]
- 27.Knight LC, Parkman HP, Brown KL, Miller MA, Trate DM, Maurer AH, Fisher RS. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol. 1997;92:968–975. [PubMed] [Google Scholar]
- 28.Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–1075. doi: 10.1023/a:1026647417465. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Owyang C. Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? V. Remodeling of vagus and enteric neural circuitry after vagal injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G461–G469. doi: 10.1152/ajpgi.00119.2003. [DOI] [PubMed] [Google Scholar]
- 30.Mankhey RW, Bhatti F, Maric C. 17β-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F399–F405. doi: 10.1152/ajprenal.00195.2004. [DOI] [PubMed] [Google Scholar]
- 31.Martinez V, Barquist E, Rivier J, Tache Y. Central CRF inhibits gastric emptying of a nutrient solid meal in rats: the role of CRF2 receptors. Am J Physiol Gastrointest Liver Physiol. 1998;274:G965–G970. doi: 10.1152/ajpgi.1998.274.5.G965. [DOI] [PubMed] [Google Scholar]
- 32.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119:766–773. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- 33.Meininger CJ, Cai S, Parker JL, Channon KM, Kelly KA, Becker EJ, Wood MK, Wade LA, Wu G. GTP cyclohydrolase I gene transfer reverses tetrahydrobiopterin deficiency and increases nitric oxide synthesis in endothelial cells and isolated vessels from diabetic rats. FASEB J. 2004;18:1900–1902. doi: 10.1096/fj.04-1702fje. [DOI] [PubMed] [Google Scholar]
- 34.Micci MA, Kahrig KM, Simmons RS, Sarna SK, Espejo-Navarro MR, Pasricha PJ. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology. 2005;129:1817–1824. doi: 10.1053/j.gastro.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 35.Owyang C, Hasler WL. Physiology and pathophysiology of the interstitial cells of Cajal: from bench to bedside. VI. Pathogenesis and therapeutic approaches to human gastric dysrhythmias. Am J Physiol Gastrointest Liver Physiol. 2002;283:G8–G15. doi: 10.1152/ajpgi.00095.2002. [DOI] [PubMed] [Google Scholar]
- 36.Panda K, Adak S, Aulak KS, Santolini J, McDonald JF, Stuehr DJ. Distinct influence of N-terminal elements on neuronal nitric-oxide synthase structure and catalysis. J Biol Chem. 2003;278:37122–37131. doi: 10.1074/jbc.M304456200. [DOI] [PubMed] [Google Scholar]
- 37.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 38.Rayner CK, Horowitz M. New management approaches for gastroparesis. Nat Clin Pract Gastroenterol Hepatol. 2005;2:454–462. doi: 10.1038/ncpgasthep0283. quiz 493. [DOI] [PubMed] [Google Scholar]
- 39.Resnick HE, Howard BV. Diabetes and cardiovascular disease. Annu Rev Med. 2002;53:245–267. doi: 10.1146/annurev.med.53.082901.103904. [DOI] [PubMed] [Google Scholar]
- 40.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 41.Saur D, Neuhuber WL, Gengenbach B, Huber A, Schusdziarra V, Allescher HD. Site-specific gene expression of nNOS variants in distinct functional regions of rat gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2002;282:G349–G358. doi: 10.1152/ajpgi.00226.2001. [DOI] [PubMed] [Google Scholar]
- 42.Saur D, Paehge H, Schusdziarra V, Allescher HD. Distinct expression of splice variants of neuronal nitric oxide synthase in the human gastrointestinal tract. Gastroenterology. 2000;118:849–858. doi: 10.1016/s0016-5085(00)70171-5. [DOI] [PubMed] [Google Scholar]
- 43.Serova LI, Filipenko M, Schilt N, Veerasirikul M, Sabban EL. Estrogen-triggered activation of GTP cyclohydrolase 1 gene expression: role of estrogen receptor subtypes and interaction with cyclic AMP. Neuroscience. 2006;140:1253–1263. doi: 10.1016/j.neuroscience.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res. 2004;1015:1–8. doi: 10.1016/j.brainres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: implications in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1546–R1554. doi: 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]
- 46.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–2404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 47.Stricker NL, Christopherson KS, Yi BA, Schatz PJ, Raab RW, Dawes G, Bassett DE, Jr, Bredt DS, Li M. PDZ domain of neuronal nitric oxide synthase recognizes novel C-terminal peptide sequences. Nat Biotechnol. 1997;15:336–342. doi: 10.1038/nbt0497-336. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421–430. doi: 10.1007/s00535-003-1094-y. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–1544. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 50.Tougas G, Anvari M, Dent J, Somers S, Richards D, Stevenson GW. Relation of pyloric motility to pyloric opening and closure in healthy subjects. Gut. 1992;33:466–471. doi: 10.1136/gut.33.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasquez-Vivar J, Hogg N, Martasek P, Karoui H, Pritchard KA, Jr, Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem. 1999;274:26736–26742. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 52.Watkins CC, Sawa A, Jaffrey S, Blackshaw S, Barrow RK, Snyder SH, Ferris CD. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373–384. doi: 10.1172/JCI8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood JD, Alpers DH, Andrews PL. Fundamentals of neurogastroenterology. Gut. 1999;45 Suppl 2:II6–II16. doi: 10.1136/gut.45.2008.ii6. [DOI] [PMC free article] [PubMed] [Google Scholar]