Abstract
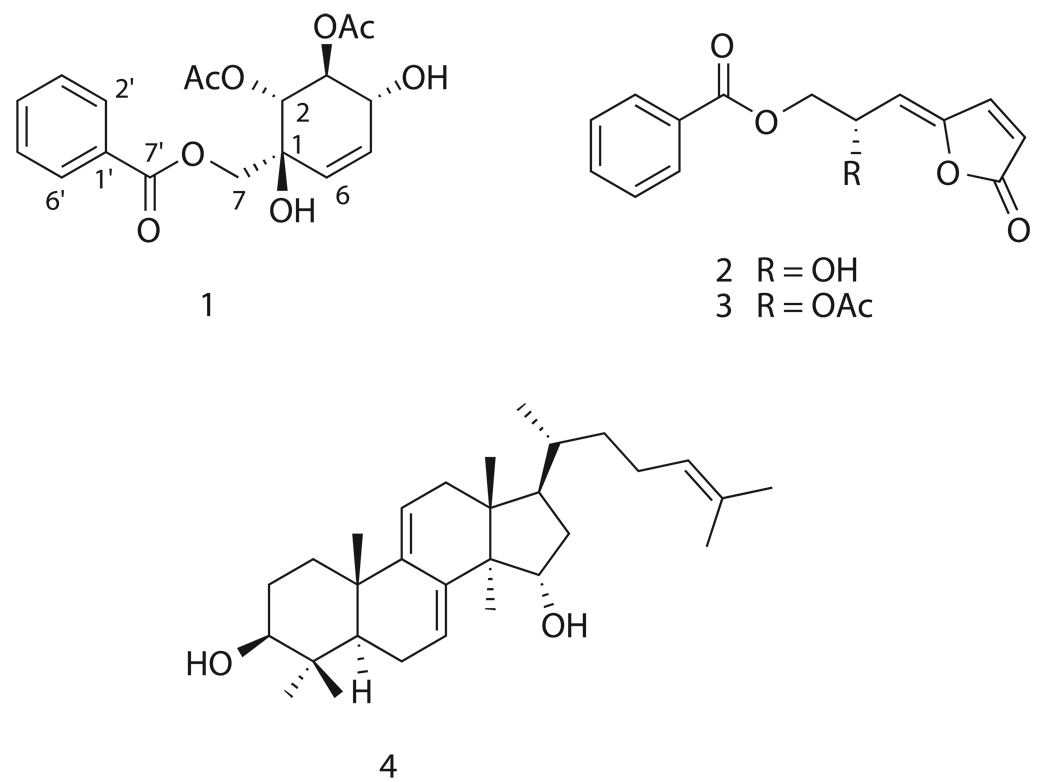
Bioassay-guided fractionation of an ethanol extract of Artabotrys madagascariensis led to the isolation of the new compound artabotrol A (1), two butenolides (2 and 3), and the tetracyclic triterpene polycarpol (4). Structure elucidation was determined on the basis of one and two-dimensional NMR, and absolute configuration of compounds 2–4 was verified by analysis of CD and optical rotation spectra. Two of the isolates, melodorinol (2) and acetylmelodorinol (3), were found to display antiproliferative activity against five different tumor cell lines with IC50 values ranging from 2.4 to 12 µM.
Keywords: butenolides, Artabotrys, Annonaceae, antiproliferative activity, NMR, HPLC
1. Introduction
In our continuing search for biologically active natural products from tropical rainforests as part of an International Cooperative Biodiversity Group (ICBG) program, we obtained an ethanol extract from Artabotrys madagascariensis Miq (family Annonaceae). Often referred to as the custard-apple family, Annonaceae is the largest family of Magnoliales, consisting of approximately 2300 species in 130 genera. To our knowledge, there are no known published reports of A. madagascariensis, but a survey of the literature on the genus Artabotrys revealed a multitude of chemical investigations. Artabotrys contains approximately 100 species of lianas situated throughout the tropical areas of Africa, Asia, and the western Pacific [2]. From it have been isolated isoquinoline [ 3 ] and cytotoxic aporphine alkaloids [ 4 ], butyrolactones [ 5 ], sesquiterpenes [6], flavonol glycosides [7], and the antimalarial peroxides yingzhaosus A–D [8–10], thus affirming the truly diverse structural repertoire of this genus.
2. Results and discussion
Bioassay-guided fractionation of an ethanol extract of the leaves and fruit of A. madagascariensis led to the isolation of the novel compound artabotrol A (1), two bioactive butenolides, melodorinol (2) and acetylmelodorinol (3), and the tetracyclic triterpene polycarpol (4).
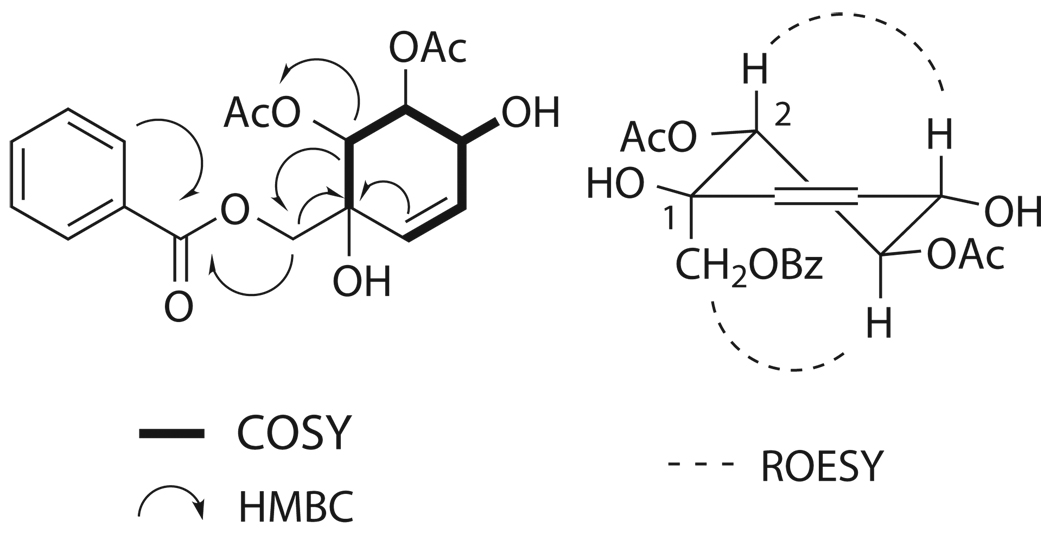
Compound 1 was obtained as colorless oil. Positive-ion HRFABMS analysis gave a pseudomolecular ion at m/z 365.12430 ([M+H]+), which suggested a molecular formula of C18H20O8. The 13C NMR spectrum of 1 in CDCl3 displayed signals for three ester carbonyls (δC 168.7, C-7'; 170.3, 2-OCOCH3; and 170.4, 3-OCOCH3) as well as two acetyl methyls (δC 20.6, 2-OCOCH3; and 20.9, 3-OCOCH3), five sp3-oxygenated carbons (δC 76.3, C-1; 74.7, C-2; 71.2, C-3; 73.8, C-4; and 65.1, C-7), and eight sp2-carbons (δC 125.4, C-5; 131.6, C-6; 129.3, C-1'; 129.9, C-2', -6'; 128.6, C-3', -5'; and 133.7, C-4'). The 1H NMR spectrum of 1 in CDCl3 suggested the existence of a mono-substituted benzene ring due to characteristic signals in the aromatic region (δH 8.08, d, J = 8.0 Hz, H-2', -6'; 7.48, t, J = 8.0 Hz, H-3', -5'; and 7.61, t, J = 7.6 Hz, H-4'). This left two sp2-carbons unaccounted for (C-5 and C-6), and analysis of the COSY spectrum identified them as being part of a six-membered ring via connectivities of H-6 (δH 5.83, ddd, J = 10.4, 2.0, 2.0 Hz) with H-5 (δH 5.62, ddd, J = 10.4, 2.8, 2.8 Hz), H-5 with H-4 (δH 4.53, m), H-4 with H-3 (δH 5.77, m), and H-3 with H-2 (δH 5.33, d, J = 8.0 Hz). The contour between H-4 and H-3 was weak, thus the linkage was confirmed by the employment of 1D TOCSY. The HMBC correlation of H-2 to the carbonyl signal at δC 170.3 proved the location of one acetoxy group. The placement of a hydroxyl moiety (δH 2.92, br s) at the 4-position was also confirmed by COSY. An additional hydroxyl group was placed at the 1-position based on the molecular formula and the 13C chemical shift of C-1. The location of the second acetoxy moiety at the 3-position was supported by the deshielded nature of H-3, in comparison with typical hydroxylated protons. Key HMBC correlations of C-1 with hydrogens H-2, -6, and -7 (δH 4.72, d, J = 12.8 Hz, Hα-7 and 4.65, d, J = 12.8 Hz, Hβ-7), were observed. Finally, the flat structure was confirmed through HMBC correlations of H2-7, H-2', and H-6' with C-7', thus placing the carbonyl directly adjacent to the aromatic ring. The relative configuration of 1 was determined by analysis of its ROESY spectrum. H-2 showed a through space correlation with H-4, representing axial-axial proton interactions, and the same type of interaction was observed between H-3 and H2-7. This half-chair conformation along with critical 2D correlations is shown in Figure 1. A compound with an identical flat structure was previously reported as a synthetic intermediate, but the orientation of the C-2 and C-3 acetoxy groups was opposite to that of 1 [11]. The absolute configuration of 1 was determined by comparison of its CD spectrum with that of a known polyoxygenated cyclohexene derivative with identical relative and known absolute configuration from Uvaria rufa [12]. This analog, uvarirufol B, contained an OBz moiety at the 3-position as opposed to the acetoxy present in 1. Artabotrol A produced a trend in its CD spectrum opposite to that afforded by uvarirufol B, indicating an enantiomeric difference between the two. In addition, the sign of optical rotation for 1 in CHCl3 was opposite to that of its counterpart uvarirufol B, [α]D + 89.4° and [α]D − 178°, respectively. Hence the absolute configuration of artabotrol A (1) was determined to be (1S, 2R, 3S, 4R).
Figure 1.
Key 2D NMR correlations of 1.
The butenolides melodorinol (2) and acetylmelodorinol (3) were identified on the basis of their one- and two-dimensional NMR spectra, optical rotation data, and subsequent comparison to literature values [13,14]. The existence of benzoyl derivatives in Annonaceous plants is not uncommon, and a brief summary of biosynthetic origins of these compounds has been published [13]. However, there have been few reports involving the isolation of such C-7 benzoyl derivatives [13–15], and total synthesis of these compounds by various methods has been reported [16–20].
The triterpene polycarpol (4) was originally isolated from the leaves and stem barks of Polyalthia oliveri (Annonaceae) [21]. It has been reported to be an agonist of liver X receptors, thus increasing cellular cholesterol efflux, lowering LDL, and raising HDL levels [22]. Other unique bioactive properties of polycarpol include antineoplastic [23] and antifilarial [24] activities, and cytotoxicity [25]. In the current study, its flat structure was identified on the basis of one- and two dimensional NMR data, while analysis of its ROESY spectrum confirmed the configuration.
All of the isolates were tested against the A2780 human ovarian cancer cell line, and compounds 2 and 3 were tested against four additional cell lines. The results are shown in Table 2. Of the four compounds, 2 and 3 displayed moderate antiproliferative activity, with the additional acetyl moiety of 3 apparently accounting for the increase in activity. Overall, 2 and 3 exhibited general antiproliferative activity toward tumor cells and further exploration of their bioactive potential was stopped. It was postulated that the γ-(Z)-alkyldienebutenolide moiety was responsible for the general bioactivity of the compounds [14].
Table 2.
Antiproliferative activities of compounds 1–4.
| compound | IC50 (µM) | ||||
|---|---|---|---|---|---|
| A2780a | MDA-MB-435b | HT-29b | H522-T1b | U937b | |
| 1 | 55 | - | - | - | - |
| 2 | 12 | 8.6 | 5.8 | 7.3 | 6.2 |
| 3 | 6.9 | 6.8 | 2.6 | 2.4 | 5.6 |
| 4 | 41 | - | - | - | - |
Concentration of each compound that inhibited 50% of the growth of the A2780 human ovarian cell line according to the procedure described [26, 27], with actinomycin D (IC50 0.8–2.4 nM) as the positive control.
Concentration of a compound which inhibited cell growth by 50% compared to untreated cell populations, with vinblastine as the positive control (average IC50 0.27 nM (MDA-MB-435), 0.53 nM (HT-29), 1.38 nM (H522-T1) and 0.49 nM( U937).
3. Experimental
3.1. General experimental procedures
IR and UV spectra were measured on MIDAC M-series FTIR and Shimadzu UV-1201 spectrophotometers, respectively. CD analysis was performed on a Jasco J-720 spectropolarimeter, and data is expressed in terms of circular-dichroic absorption, Δε(cm2 × mmole−1). Optical rotations were recorded on a Perkin-Elmer 241 polarimeter. Mass spectra were obtained on a JEOL JMS-HX-110 instrument. NMR spectra were obtained on JEOL Eclipse 500, Varion Inova 400, and Varion Unity 400 spectrometers. Chemical shifts are given in δ (ppm), and coupling constants (J) are reported in Hz. HPLC was performed using either Shimadzu LC-8A pumps coupled with a Varian Dynamax preparative silica column (250 × 21.4 mm), or Shimadzu LC-10AT pumps coupled with a Varian Dynamax semi-preparative silica column (250 × 10 mm). Both HPLC systems employed a Shimadzu SPD-M10A diode array detector.
3.2. Plant material
Samples of leaves and fruits of Artabotrys madagascariensis Miq were collected in December 2004. The plant was a well branched shrub with green aromatic fruit growing in a degraded forest, on calcareous rock on the Montagne des Français, Antsiranana province, Madagascar (12.23.27 S / 49.20.01. E, elevation 410 m). The herbarium voucher specimen for the sample is Stephan Rakotonandrasana et al. 884. Duplicate voucher specimens were deposited at herbaria of the Centre National d'Application des Recherches Pharmaceutiques, Madagascar (CNARP), the Parc Botanique et Zoologique de Tsimbazaza, Madagascar (TAN), the Missouri Botanical Garden, St. Louis, Missouri (MO), and the Muséum National d'Histoires Naturelles, Paris, France (P).
3.3. Extraction and isolation
The dried plant sample described above was extracted with EtOH to give 3.69 g of extract designated MG 2898. The A2780 assay was used to guide fractionation. Extract MG 2898 (1.5 g) was suspended in aqueous MeOH (MeOH-H2O, 9:1, 350 mL) and extracted with hexanes (2 × 150 mL). The aqueous MeOH fraction was then adjusted to 60 % aqueous MeOH, and extracted with CHCl3 (2 × 150 mL). The CHCl3 fraction displayed activity (420 mg, IC50 = 2.9 µg/mL), and 100 mg was further purified by chromatography over silica gel using a step gradient of CHCl3-isopropanol to yield three fractions (A–C). Fraction A (54.8 mg, 3.1 µg/mL) was further purified by chromatography using an isocratic flow of 100 % CHCl3 (10.0 mL/min) on a preparative silica gel HPLC column to furnish 6 fractions (D–I). Fraction F was identified as compound 3 (tR 8.7 min, 27 mg). Fraction G was identified as compound 4 (tR 12.0 min, 15 mg). Fraction H was identified as compound 2 (tR 17.6 min, 2.9 mg).
Fraction B (19.5 mg, 6.2 µg/mL) also displayed antiproliferative activity, therefore it was subjected to preparative HPLC over silica gel using the previously described conditions to yield four subfractions (J–M). Fraction K was identified as the novel compound 1 (tR 16.5 min, 0.5 mg). An additional 3.0 mg of 1 was later obtained through similar fractionation techniques. It should be noted that the activity of fraction B was due to the presence of compounds 3 and 4, which proved to be quite abundant throughout the plant. The structures of the known compounds 2–4 were confirmed by interpretation of one and two-dimensional NMR spectra and by comparison with literature data.
3.3.1. Artabotrol A (1S, 2R, 3S, 4R) (1)
Transparent oil, [α]D23 + 89.4° (c 0.10, CHCl3); UV (CH3OH) λmax (log ε) 230 (3.92) nm, 272 (3.42) nm; IR νmax 3454, 2956, 1748, 1722, 1369, 1272, 1241, 1227, 1114, 1044, 1026, 711 cm−1; CD (MeOH) [θ]210 −69, [θ]240 15.7, [θ]260 21.4, [θ]287 10.4; 1H and 13C NMR (CDCl3), see Table 1; HRFABMS m/z 365.1243 [M+1]+ (calcd for C18H21O8, 365.1236).
Table 1.
NMR spectral data of artabotrol A (1) in CDCl3a.
| 1 | ||
|---|---|---|
| position | 13Cb | 1Hb,c (J, Hz) |
| 1 | 76.3 | - |
| 2 | 74.7 | 5.33 d (8.0) |
| 3 | 71.2 | 5.77 m |
| 4 | 73.8 | 4.53 m |
| 5 | 125.4 | 5.62 ddd (10.4, 2.8, 2.8) |
| 6 | 131.6 | 5.83 ddd (10.4, 2.0, 2.0) |
| 7 | 65.1 | 4.72 d (12.8), 4.65 d (12.8) |
| 2-OCOCH3 | 170.3 | - |
| 2-OCOCH3 | 20.6 | 1.87 s |
| 3-OCOCH3 | 170.4 | - |
| 3-OCOCH3 | 20.9 | 2.04 s |
| 1' | 129.3 | - |
| 2', 6' | 129.9 | 8.08 d (8.0) |
| 3', 5' | 128.6 | 7.48 t (8.0) |
| 4' | 133.7 | 7.61 t (7.6) |
| 7' | 168.7 | - |
| 1-OH | - | 3.87 br s |
| 4-OH | - | 2.92 br s |
Assignments based on COSY, HMBC, HSQC.
Chemical shifts (δ) in ppm.
br s: broad singlet; d: doublet; m: multiplet.
J values in bracket.
3.4. Cell growth inhibition bioassays
Antiproliferative effects of compounds 2 and 3 were evaluated in four cultured human cancer cell lines: MDA-MB-435 breast cancer cells, HT-29 colon cancer cells, H522-T1 non-small cell cancer cells, and U937 histiocytic lymphoma cells. The cells were placed into 96-well plates and grown in the absence or continuous presence of 0.3 – 10000 nM compounds for 96 h. Cell growth was assessed using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega) according to manufacturer's recommendations. Luminescence was read on a Victor2 V 1420 MultiLabel HTS Counter (Perkin-Elmer/Wallac). IC50 values were determined as the concentration of a compound which inhibits cell growth by 50% compared to untreated cell populations. Two separate replicate experiments were performed.
The A2780 ovarian cancer cell line assay was performed on compounds 1–4 at Virginia Polytechnic Institute and State University as previously described [26].The A2780 cell line is a drug-sensitive human ovarian cancer cell line [27].
Structures.
Acknowledgments
This work was supported by International Cooperative Biodiversity Grant Number TW 00313 from the Fogarty Center, National Institutes of Health, and this support is gratefully acknowledged. We also thank Mr. Bill Bebout and Ms. Anne Campbell for obtaining MS data, Mr. Kim Harich for obtaining CD data, and Mr. Tom Glass for assistance obtaining NMR spectra. Field work essential for this project was conducted under a collaborative agreement between the Missouri Botanical Garden and the Parc Botanique et Zoologique de Tsimbazaza and a multilateral agreement between the ICBG partners, including the Centre National d’Applications et des Recherches Pharmaceutiques. We gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts
Footnotes
See Ref. [1]
References
- 1.Biodiversity Conservation and Drug Discovery in Madagascar, Part 30. For Part 29, see: Karkare S, Adou E, Cao S, Brodie P, Miller JS, Andrianjafy NM, Razafitsalama J, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. doi: 10.1021/np070336n. in press.
- 2.Mabberley DJ. The Plant Book. 2nd ed. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- 3.Sagen A-L, Sahpaz S, Mavi S, Hostettmann K. Biochem. Sys. Ecol. 2003;31:1447. [Google Scholar]
- 4.Wu YC, Chen CH, Yang TH, Lu ST, McPhail DR, McPhail AT, Lee KH. Phytochemistry. 1989;28:2191. [Google Scholar]
- 5.Wong H-F, Brown GD. Phytochemistry. 2002;59:99. doi: 10.1016/s0031-9422(01)00433-2. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer TC, Waigh RD, Waterman PG. J. Nat. Prod. 1997;60:1054. [Google Scholar]
- 7.Singh AP, Sahai M. Planta Med. 1996;62:192. doi: 10.1055/s-2006-957860. [DOI] [PubMed] [Google Scholar]
- 8.Liang X-T, Yu D-Q, Wu W-L, Deng H-C. Huaxue Xuebao. 1979;37:215. [Google Scholar]
- 9.Liang X-T, Yu D-Q, Pan W-D. Huaxue Xuebao. 1979;37:231. [Google Scholar]
- 10.Zhang L, Zhou W-S, Xu X-X. J. Chem. Soc. Chem. Commun. 1988;8:523. [Google Scholar]
- 11.Kupchan SM, Hemingway RJ, Smith RM. J. Org. Chem. 1969;34:3898. doi: 10.1021/jo01264a033. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Yang S-P, Liao S-G, Wu Y, Yue J-M. Helv. Chim. Acta. 2006;89:1408. [Google Scholar]
- 13.Jung JH, Pummangura S, Patarapanich C, Chaichantipyuth C, Fanwick PE, Chang C-J, McLaughlin JL. Tetrahedron. 1990;46:5043. [Google Scholar]
- 14.Tuchinda P, Udchachon J, Reutrakul V, Santisuk T, Taylor WC, Farnsworth NR, Pezzuto JM, Kinghorn AD. Phytochemistry. 1991;30:2685. [Google Scholar]
- 15.Jung JH, Chang CJ, Smith DL, McLaughlin JL, Pummangura S, Chaichantipyuth C, Patarapanich C. J. Nat. Prod. 1991;54:500. doi: 10.1021/np50074a023. [DOI] [PubMed] [Google Scholar]
- 16.Shen C, Chou S, Chou C. Tetrahedron: Asymmetry. 1996;7:3141. [Google Scholar]
- 17.Lu X, Chen G, Xia L, Guo G. Tetrahedron: Asymmetry. 1997;8:3067. [Google Scholar]
- 18.Pohmakotr M, Tuchinda P, Premkaisorn P, Limpongpan A, Reutrakul V. Heterocycles. 1999;51:795. [Google Scholar]
- 19.Ahmed MM, Akmedhov NG, Novruz G, Cui H, Friedrich D, O’Doherty GA. Heterocycles. 2006;70:223. [Google Scholar]
- 20.Boukouvalis J, Beltran PP, Lachance N, Cote S, Maltais F, Pouliot M. Synlett. 2007;2:219. [Google Scholar]
- 21.Hamonniere M, Leboeuf M, Cave A. Phytochemistry. 1977;16:1029. [Google Scholar]
- 22.Jayasuriya H, Herath KB, Ondeyka JG, Guan Z, Borris RP, Tiwari S, Jong W, Chavez F, Moss J, Stevenson DW, Beck HT, Slattery M, Zamora N, Schulman M, Ali A, Sharma N, MacNaul K, Hayes N, Menke JG, Singh SB. J. Nat. Prod. 2005;68:1247. doi: 10.1021/np050182g. [DOI] [PubMed] [Google Scholar]
- 23.Matos MFC, Leite LISP, Brustolim D, de Siqueira JM, Carollo CA, Hellmann AR, Pereira NFG, da Silva DB. Phytoterepia. 2006;77:227. doi: 10.1016/j.fitote.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Nyasse B, Ngantchou I, Nono JJ, Schneider B. Nat. Prod. Res. 2006;20:391. doi: 10.1080/14786410600661377. [DOI] [PubMed] [Google Scholar]
- 25.Jung JH, Pummangura S, Chaichantipyuth C, Patarapanich C, McLaughlin JL. Phytochemistry. 1990;29:1667. [Google Scholar]
- 26.Cao S, Brodie PJ, Miller JS, Randrianaivo R, Ratovoson F, Birkinshaw C, Andriantsiferana R, Rasamison VE, Kingston DGI. J. Nat. Prod. 2007;70:686. doi: 10.1021/np060627g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Cancer Res. 1985;45:2110. [PubMed] [Google Scholar]