Abstract
Carotenoids have been advocated as potential therapeutic agents in treating age-related macular degeneration (AMD). In ocular tissues carotenoids may undergo oxidation and form carotenoid-derived aldehydes (CDA), which would be toxic to tissues. We have investigated the cytotoxic effects of CDA from β–carotene, Lutein and Zeaxanthin on human retinal pigment epithelial cells (ARPE-19). The serum-starved ARPE-19 cells were treated with CDA without or with antioxidant, N-Acetylcysteine (NAC) and cell viability, apoptosis, reactive oxygen species (ROS) levels, nuclear chromatin condensation as well as fragmentation, change in mitochondrial membrane potential (MMP) and activation of transcription factors NF- κB and AP-1 were determined. We observed a dose and time-dependent decline in cell viability upon incubation of ARPE-19 cells with CDA. The CDA treatment also leads to elevation in ROS levels in a dose-dependent manner. Upon CDA treatment a significant number of apoptotic cells were observed. Also early apoptotic changes in ARPE-19 cells induced by CDA were associated with change in MMP. Increased nuclear chromatin condensation and fragmentation were also observed in cells treated with CDA. The cytotoxicity of CDA in ARPE-19 cells was significantly ameliorated by antioxidant, NAC. Furthermore, CDA induced the activation of NF-κB and AP-1 which was significantly inhibited by NAC. Thus our results demonstrate that CDA could increase the oxidative stress in ARPE-19 cells by elevating ROS levels that would cause imbalance in cellular redox status which could lead to cell death. This would suggest that high carotenoid supplementation for treatment of AMD should be used cautiously.
Keywords: Carotenoid, Oxidative stress, Aldehydes, Antioxidants, Age-related Macular Degeneration
1. Introduction
Age-related macular degeneration (AMD) is a degenerative disease that affects the small central region of the retina called macula which is responsible for central vision. Epidemiologic studies have shown that approximately 10% individuals older than 65 years and 28% of those aged 75 to 85 years develop AMD (Leibowitz et al., 1980). Although AMD is considered to have a multifactorial pathogenesis, several lines of evidence suggest that dysfunction of retinal pigment epithelium (RPE) is crucial in triggering molecular pathways contributing to clinically relevant macular degenerative changes (Holz et al., 2004). The RPE is essential for maintenance of retinal health because it is necessary for the phagocytic uptake and degradation of the constantly shed photoreceptor outer segments (POS) (Bok, 1993). Since RPE lysosomes are the major source for degradation of POS everyday, impaired lysosomal function due to aging is the major cause of accumulation of POS, a precursor of lipofucsin granules and extracellular drusen. The increased lipofucsin granules and drusen in retina are the hallmarks of an early-stage of AMD. To explain the complex etiology of AMD, other pathogenic mechanisms such as RPE cell death (Dunaief et al., 2002), oxidative damage of cellular components (Winkler et al., 1999), mitochondrial dysfunction (Liang and Godley, 2003), inflammation & activation of the innate immune system (Johnson et al., 2002) and the accumulation of toxic compounds such as advanced glycation end products (Howes et al., 2004; Kopitz et al., 2004) have been proposed. However, the molecular events that mediate these pathogenic mechanisms are not clearly defined. To date no good therapy to prevent or cure AMD is available, although increased dietary intake of specific antioxidants has been advocated. The most widely used are carotenoids (Delcourt et al., 2006; Leung et al., 2005; Muriach et al., 2006; Chichili et al., 2006; Trevithick-Sutton et al., 2006). The carotenoids are believed to prevent or delay AMD by filtering out photo-toxic short-wavelength visible light and also by acting as an antioxidant which could protect the retina from oxidative stress induced by light and aging.
Carotenoids, besides acting as antioxidants, are also reported to act as prooxidants under high oxygen tension, high carotenoid concentration and imbalanced intracellular redox status (Palozza et al., 2003a). Under oxidative stress, while scavenging reactive oxygen species (ROS), carotenoids undergo oxidation and generate a variety of oxidized products in vitro as well as in vivo (Handelman et al., 1991; Hurst et al., 2004; 2005; Prasain et al., 2005). The presence of carotenoid cleavage products has been demonstrated in human and monkey ocular tissues including retina (Prasain et al., 2005; Khachik et al., 1997; Bernstein et al., 2001; Bhosale and Bernstein, 2005). Moreover, recent studies have shown that carotenoid supplementation in humans and monkeys significantly increases levels of their metabolites in serum and ocular tissues (Khachik et al., 2006a; 2006b). In our previous study we identified various products resulted from the in vitro autoxidation of β-carotene such as β-apo-14′-carotenal, β-apo-12′-carotenal, β-apo-10′-carotenal, β-apo-15-carotenal, β-apo-13-carotenone, β-carotene-5,6-epoxide and various unknown carbonyls (Handelman et al. 1991). Many of the carotenoid cleavage products, characterized either in vivo or in vitro are identified as aldehydes such as retinaldehyde, apocarotenaldehyde and various other long and short chained products which are addressed as carotenoid-derived aldehydes (CDA) (Hurst et al., 2005). The aldehydes particularly from polyunsaturated fatty acids, such as 4-hydroxy-2-nonenal (HNE), are known to be most abundant and toxic. They form conjugates with sulfhydryl, lysyl and histidine residues even at low cellular levels and incur toxicity (Kopitz et al., 2004; Choudhary et al., 2005; Tanito et al., 2005; Kapphahn et al., 2006; Zhou et al., 2005; Uchida, 2003). Since carotenoids also form highly reactive aldehydes, it is likely that excessive use of carotenoids could result in increased carotenoid aldehydes which could cause oxidative stress similar to that caused by lipid peroxidation products. Therefore, carotenoids, especially Lutein and Zeaxanthin, commonly prescribed for the prevention of AMD could generate cytotoxic aldehydes which could potentiate AMD rather than preventing it. Hence, in this study we have investigated the cytotoxic effects of CDA obtained from oxidizing β-Carotene, Lutein and Zeaxanthin on human retinal pigment epithelial cells (ARPE-19) apoptosis as well as activation of redox sensitive transcription factors such as NF-κB and AP-1. We observed that CDA induce apoptosis in ARPE-19 cells by elevating ROS levels and activating NF-κB and AP-1. The oxidative stress-induced changes were ameliorated by antioxidant, N-Acetylcysteine (NAC).
2. Materials and Methods
2.1 Materials
Human retinal pigment epithelial cells (ARPE-19) were purchased from American Type Culture Collection (ATCC). β–carotene was purchased from Fluka, USA. Lutein was purchased from Sigma, USA. Zeaxanthin was a gift from Hoffman La-Roche, Nutley, NJ. JC-1, TACS™ Annexin V- FITC Apoptosis detection kit and Vybrant Apoptosis Assay Kit #5 (containing Hoechst 33342) were purchased from Molecular Probes, USA. The Cell Death ELISA kit was purchased from Roche Inc. USA. CellTiter 96® AQueous one solution cell proliferation assay kit was purchased from Promega. USA. TNF-α was obtained from Research Diagnostics Inc. USA. Protein assay reagent was obtained from Bio-Rad, USA. Fetal Bovine Serum, Trypsin/EDTA, Antibiotics, N-Acetylcysteine (NAC), 2′, 7′-Dichlorofluorescin diacetate (DCF-DA) and all other chemicals were obtained from Sigma, USA.
2.2 Cell Culture and Treatment
The ARPE-19 cells were grown to confluency in DMEM/F-12 medium supplemented with 10% Fetal Bovine Serum (FBS) and 1% penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2. Sub-confluent cells were growth-arrested in 0.1% FBS medium. Sub culturing was done using Trypsin/EDTA solution and the cells were sub cultivated with a split ratio of 1:4. The CDA treatment was done in serum-free medium to avoid binding of CDA with serum proteins. Whenever NAC was used, cells were preincubated with it for 1 h. Control cells were incubated with vehicle, PBS. In all the experiments, concentration of PBS was not greater than 0.2%.
2.3 Preparation of CDA
The CDA were prepared as described previously (Hurst et al., 2005). The use of NaOCl to oxidize carotenoids is based on the fact that hypochlorite is a product of myeloperoxidase, hydrogen peroxide and Cl− in activated phagocytic cells (Harrison and Schultz., 1976; Whiteman et al., 2005; Vissers et al., 2001; Krasowska and Konat, 2004; Ottonello et al., 1994). Since retinal pigment epithelial cells are phagocytic cells, we used NaOCl as a model system for possible harmful effects of carotenoid oxidation products. Briefly, 5 mg of β-carotene or Lutein or Zeaxanthin dissolved in 5 ml dichloromethane and 5 ml methanol were oxidized with 80 mM NaOCl in 1.25 ml water at room temperature for 15 min. The concentration of NaOCl used was similar to that reported by Lee et al. (2002) in activated macrophages. The CDA were extracted by a modification of the Bligh and Dyer (1959) method as used by van Kuijk et al., (1985). To each ml of oxidized carotenoid solution, 5 ml of dichloromethane was added, followed by vortexing for 1 min; 3.75 ml water was added and continued vortexing for another min. The samples were centrifuged for 2 min at 1000 × g and the lower organic phase from each sample was collected. The extraction was repeated and the organic phase from both extractions was pooled. The solvent was evaporated under argon to a small volume of about 0.1 ml which was diluted with sterile Phosphate-Buffered Saline (PBS) without calcium and magnesium (pH 7.4; Cellgro) to make CDA stock solution. The excess organic phase was evaporated again. The CDA solution was ultra filtered by centrifugation using Centricon Tube (Millipore Corp. Bedford, MA, USA) for 1 h at 10,000 × g at 4° C. The concentration of CDA in the ultra filtrate was determined by measuring the optical density at 220 nm on UV-2101 PC recording spectrophotometer (Shimadzu, Columbia, MD). The characterization of NaOCl oxidized β-Carotene products revealed the presence of β-apo-14′-carotenal (C22H30O), β-apo-15-carotenal (C20H28O), β-apo-13-carotenone (C18H26O) and various unidentified carbonyls (Handelman et al., 1991). The CDA stock solution was stored at -20° C in dark. The oxidized products of β-Carotene, Lutein and Zeaxanthin are designated as B-CDA, L-CDA and Z-CDA respectively. Vehicle was also prepared by the same method without carotenoid.
2.4 Cell Viability Assay
The ARPE-19 cells were plated (5000 cells/well) in a 96-well plate. After 24 h, cells were serum starved in 0.1% FBS medium for 24 h and CDA or vehicle was added to the media after pretreating the cells with NAC for 1 h. Cells incubated without CDA and NAC served as control. Cell viability was determined by CellTiter 96® AQueous one solution cell proliferation assay kit. CellTiter reagent was added to culture well, incubated for 3 h and absorbance recorded at 490 nm using a 96-well plate spectra count (Packard, USA). This kit contains a novel MTT tetrazolium compound which is enzymatically reduced in the live cells into a colored formazan product.
2.5 ROS Determination
The intracellular ROS levels were measured using a fluorescent dye, DCF-DA. The cells were plated (5000 cells/well) in a 96-well plate. After 24 h, cells were serum starved for 24 h, washed with PBS and incubated with 10μM DCF-DA at 37°C for 30 min in media without phenol red. Cells were washed again to remove excess DCF-DA and treated with CDA in media without FBS for 30 min. At the end of the treatment, cells were washed twice with PBS. Media without phenol red was added and absorbance determined after 1 h at 485 nm excitation and 538 nm emission wavelengths. Relative ROS production was expressed as a change in fluorescence compared to fluorescence of the appropriate control.
2.6 Determination of Apoptosis
Apoptosis was determined by using “TACS™ Annexin V- FITC Apoptosis kit” (Molecular Probes) that detects cell surface changes that occur early in the apoptotic process. The assay was performed according to manufacturer’s instructions and cell death was detected by flow cytometry. Quantification was performed by using “Cell Death Detection ELISA Kit” (Roche Inc.) that measures cytoplasmic DNA-histone complexes formed during apoptotic DNA fragmentation. The assay was performed according to manufacturer’s instructions and cell death was detected spectrophotometrically at 405 nm. Apoptotic cells were also visualized by using Hoechst 33342 fluorescent dye. Briefly, ARPE-19 cells were cultured in multichamber slides and treated for 12 h with CDA and H2O2 without or with NAC for 1 h. Subsequently the cells were washed two times with 1 ml PBS. The cells were incubated with Hoechst 33342 (1μl/100μl PBS) at 37°C for 30 min in dark, washed with PBS and mounted with fluorosave (Molecular Probe), covered with the coverslip and the coverslip borders were sealed with nail polish. The cells were visualized under a Nikon Eclipse 800 epifluorescence microscope equipped with a xenon arc lamp using a DAPI filter set (excitation 340–380 nm, dichroic mirror 400 nm and emission 435–485 nm) for blue fluorescence. Photographs were taken using a ROPER Scientific CoolSNAP Fx monochrome cooled CCD 12 bit digital camera. The cells with fragmented and/or condensed nuclei were classified as apoptotic cells.
2.7 Measurement of Mitochondrial Membrane Potential (MMP)
Flow cytometry was performed using the cationic dye JC-1 that exhibits it’s membrane potential-dependent accumulation in the mitochondria as determined by a fluorescence emission shift from green (~525 nm) to red (~590 nm). After growth and starvation the cells were washed with PBS and incubated with CDA or H2O2 for 4 h without or with NAC. Pellets of approximately 1 × 106 cells were suspended in 130 μl of warm 1:1 mixture of DMEM/F-12 medium containing 1% FBS to which 250 μl of JC-1 solution (20 μg/ml) was added. After incubation at 37° C for 30 min, cells were washed with PBS, resuspended in 500 μl PBS, and subjected to flow cytometry.
2.8 Electrophoretic Mobility Gel Shift Assay (EMSA)
The ARPE-19 cells were treated for 1 h at 37°C without or with pretreatment with NAC. The EMSA was performed as described earlier (Ramana et al., 2002). Briefly, consensus oligonucleotides for NF-κB and AP-1 transcription factors were 5′-end labeled using T4 polynucleotide kinase. Nuclear extracts prepared from various control and treated cells were incubated with the labeled oligonucleotide for NF-κB or AP-1 for 30 min at 37 °C, and the DNA-protein complex formed was resolved on 7.1% native polyacrylamide gels. The specificity of binding was examined by competition with excess of unlabeled oligonucleotide.
2.9 Statistical Analysis
Results are expressed as mean ± SD and the P values were determined by Student’s t-test using Microsoft Excel software.
3. Results
3.1 CDA cause decreased cell viability
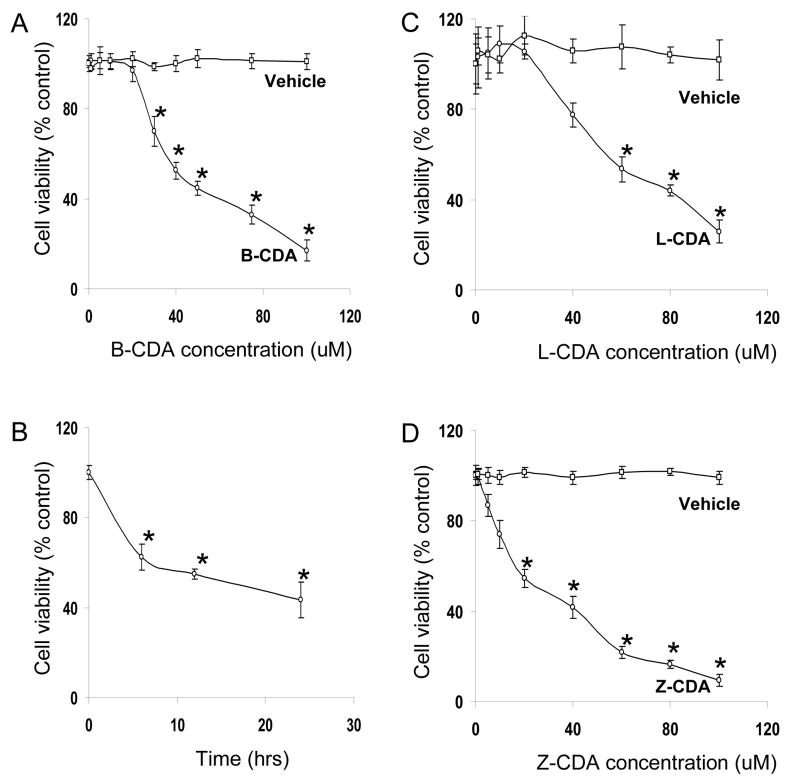
In order to investigate the effect of CDA on cell viability, ARPE-19 cells were incubated with CDA for 24 h. A concentration-dependent (0–100 μM CDA) decrease in cell viability was observed (Figure 1A, C & D). The LC50 for B-CDA, L-CDA and Z-CDA were approximately 40 μM (Figure 1A), 60 μM (Figure 1C) and 25 μM (Figure 1D) respectively, whereas the corresponding amounts of vehicle had no cytotoxic effect. These results indicate that CDA induce cytotoxicity in ARPE-19 cells. To elucidate the time-dependent effect of CDA, ARPE-19 cells were incubated with 40 μM B-CDA for various time intervals. A 40% decline in cell viability occurred in the first 6 h and 44% and 50% by 12 h and 24 h, respectively (Figure 1B). Since we observed, almost similar percent of cell viability in 12 h and 24 h, we incubated the cells for only 12 h in rest of our experiments.
Fig. 1. Effect of CDA on ARPE-19 cell viability.
(A) B-CDA caused concentration-dependent decrease in ARPE-19 cell viability. Cells were exposed to various concentrations of B-CDA (0–100 μM) as well as corresponding amount of vehicle and after 24 h cell viability was determined. (B) Time-dependent effect of B-CDA on ARPE-19 cell viability. Cells were exposed to 40 μM B-CDA for 6 h, 12 h and 24 h and cell viability was determined. (C) L-CDA caused concentration-dependent decrease in ARPE-19 cell viability. Cells were exposed to various concentrations of L-CDA (0–100 μM) as well as corresponding amount of vehicle and after 24 h cell viability was determined. (D) Z-CDA caused concentration-dependent decrease in ARPE-19 cell viability. Cells were exposed to various concentrations of Z-CDA (0–100 μM) as well as corresponding amount of vehicle and after 24 h cell viability was determined. There was no effect on cell viability when cells were incubated with vehicle. Data represents the mean ± SD of three experiments (*p < 0.001).
3.2 Increase in ROS levels by CDA & Protection by NAC
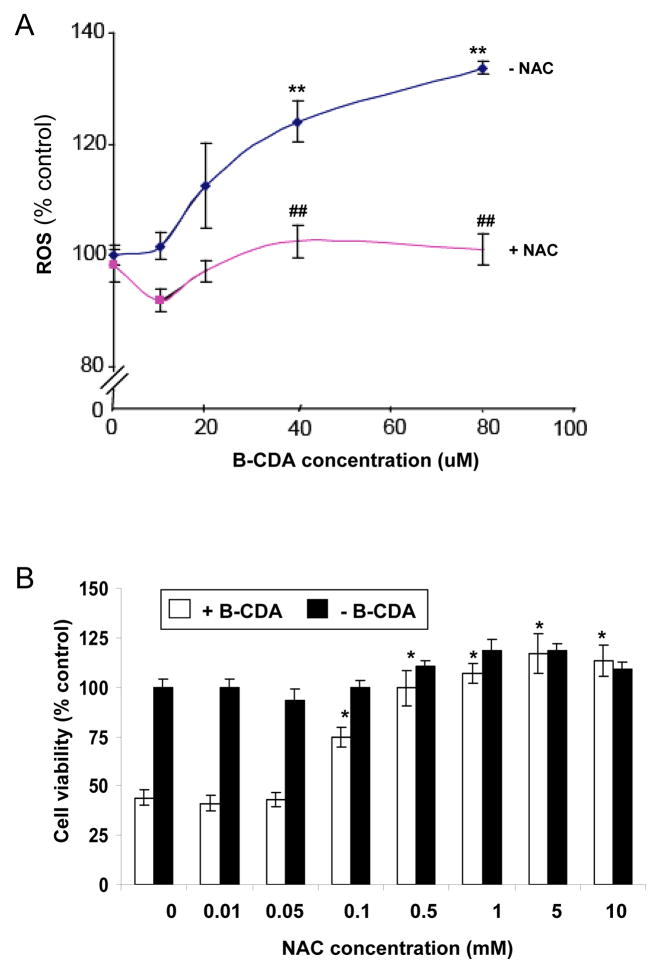
Incubation of ARPE-19 cells with B-CDA resulted in a dose-dependent (0–80 μM) increase in ROS levels (Figure 2A). The B-CDA at lower concentrations (10 μM) did not have a marked effect on ROS generation. However, there was a gradual increase in ROS levels with ≥ 20 μM B-CDA. The increase in ROS was statistically significant at higher concentrations (p<0.001). This observation indicates that CDA, like other antioxidants, at higher concentration could act as pro-oxidant. Therefore, we used ROS scavenger, NAC, to ameliorate the oxidative stress. Pretreatment of cells with NAC significantly (p<0.001) attenuated ROS generation by B-CDA in ARPE-19 cells (Figure 2A). Similarly NAC also provided protection against B-CDA-induced cytotoxicity (Figure 2B). Lower concentrations of NAC (0.01 & 0.05 mM) did not protect against the B-CDA-induced cytotoxicity. However, with increasing concentrations of NAC the protection was evident. For complete protection the lowest concentration of NAC was 1 mM (Figure 2B).
Fig. 2. Effect of CDA on ROS levels and protective effect of NAC in ARPE-19 cells.
(A) B-CDA generates concentration-dependent elevation in ROS levels in ARPE-19 cells. The cells were incubated with 10μM DCF-DA dye for 30 mints. After washing with PBS, cells were treated with B-CDA (0–80 μM) for 30 min without or with NAC. (**p < 0.001 vs control; ## p < 0.001 vs B-CDA treated cells). (B) Concentration-dependent protective effect of NAC on ARPE-19 cell viability. Cells were preincubated with various concentrations of NAC (0–10 mM) for 1 h and exposed to B-CDA (40 μM) for 12 h and cell viability was determined by MTT assay. Data represents the mean ± SD of three experiments (*p < 0.001)
3.3 CDA induced apoptosis in RPE cells
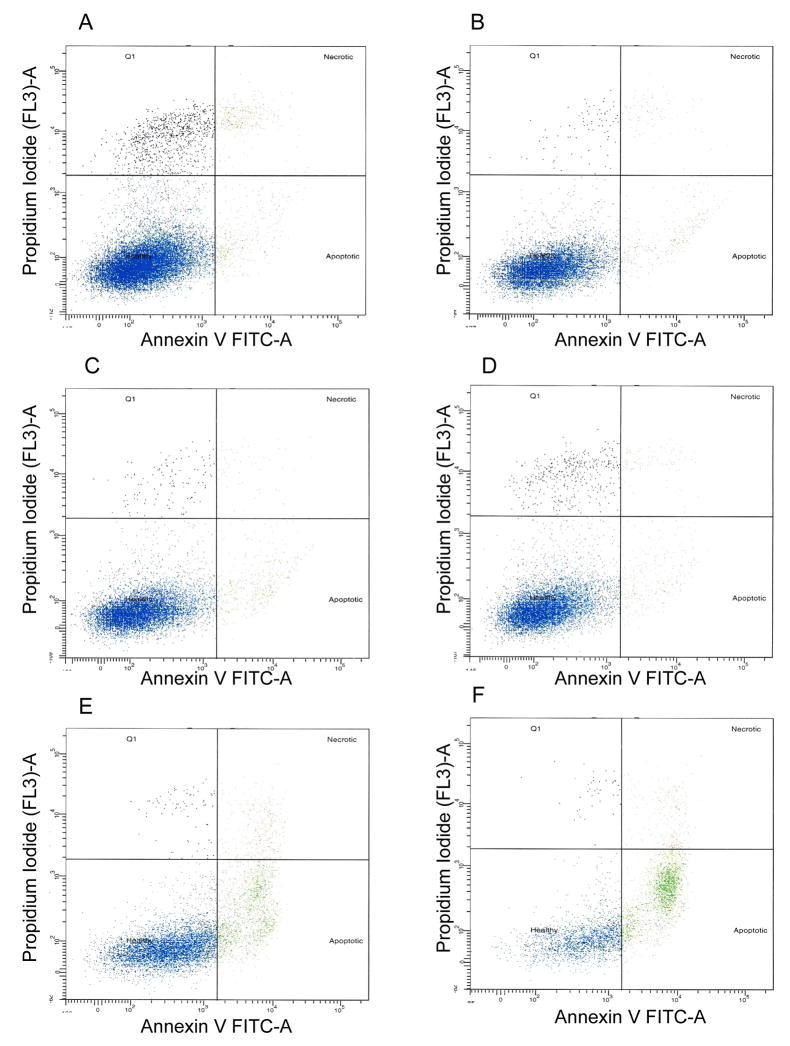
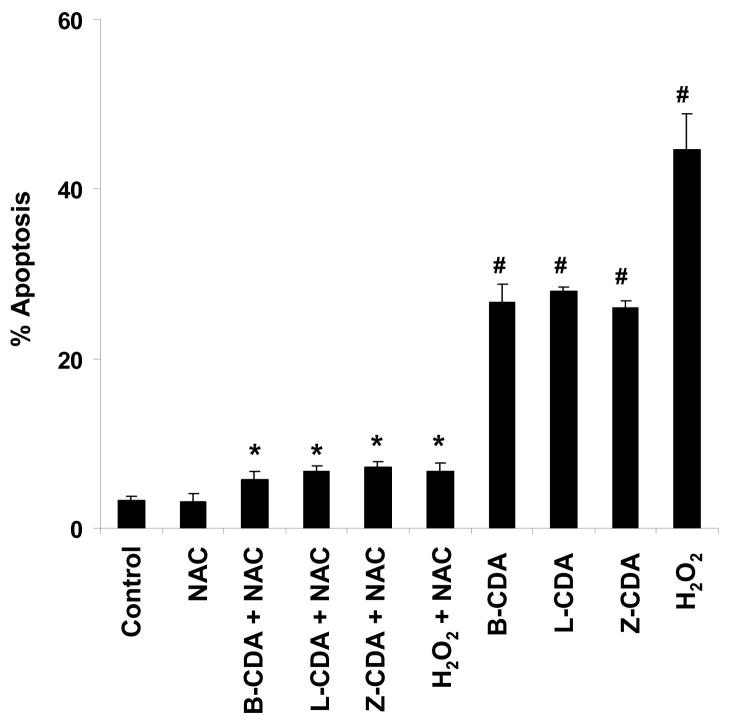
Increased oxidative stress in RPE cells has been known to cause cell death, a pathogenic event in AMD. Therefore, ARPE-19 cells were incubated with B-CDA (40 μM) for 12 h. An increase in apoptosis was observed when cells were treated with B-CDA or H2O2 (200 μM), a positive control (Figures 3E & F). The B-CDA- as well as H2O2- induced apoptosis was 26% and 49%, respectively and NAC significantly (p<0.001) prevented it (Figures 3C & D and Figure 4). Similarly when cells were treated with 60 μM L-CDA and 25 μM Z-CDA, we observed 28% and 25% apoptosis, respectively which was also significantly prevented by NAC (Figure 4). Control and NAC-treated groups showed only approximately 5% apoptosis (Figures 3A & B and Figure 4).
Fig. 3. Effect of CDA on ARPE-19 cell apoptosis and protection by NAC.
Cells (1 × 106) were incubated with B-CDA (40 μM) or H2O2 (200 μM) and harvested after 12 h. Apoptosis was detected by Annexin V- FITC as described in the text. Panels: (A) Control, (B) 1 mM NAC, (C) 40 μM B-CDA + 1 mM NAC, (D) 200 μM H2O2 + 1 mM NAC, (E) 40 μM B-CDA and (F) 200 μM H2O2. Picture is a representative of three different sets of experiments.
Fig. 4. Quantification of protective effect of NAC on CDA- induced apoptosis in ARPE-19 cells.
Cells (1 × 106) were preincubated with 1 mM NAC for 1 h prior to the addition of 40 μM B-CDA or 60 μM L-CDA or 25 μM Z-CDA or 200 μM H2O2. The cells were harvested after 12 h and apoptosis was measured by ELISA as described in the text. Data represents the mean ± SD of three experiments (# p < 0.001 vs Control; * p < 0.001 vs CDA and H2O2 treated cells).
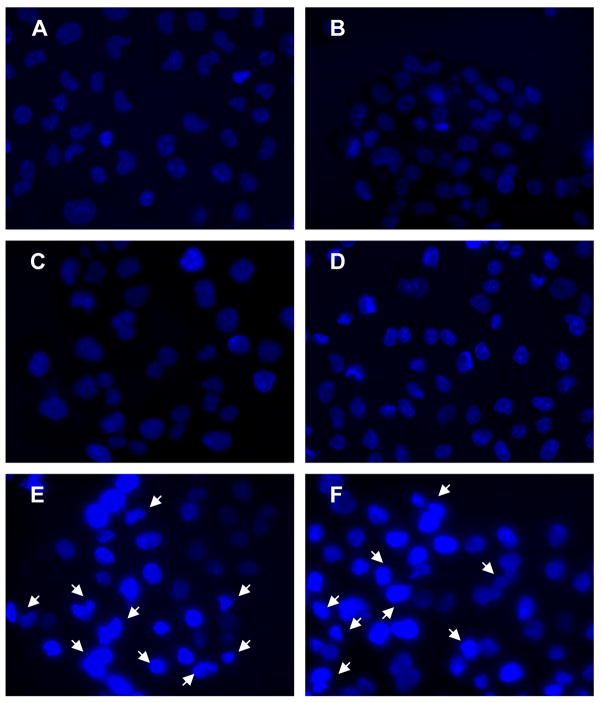
The fluorescence microscopic study was also carried out to visualize B-CDA- and H2O2-induced apoptosis as well as to investigate the protective effect of NAC (Figure 5). B-CDA- or H2O2-treated cells showed condensed and fragmented nuclei (Figures 5E & F) which were prevented by pretreating the cells with NAC (Figures 5C & D). Cells in control and NAC-treated groups showed normal nuclei (Figures 5A & B).
Fig. 5. Protective effect of NAC on CDA-induced ARPE-19 cell apoptosis.
After treating the ARPE-19 cells with 40 μM B-CDA or 200 μM H2O2 for 12 h without or with pretreatment with 1 mM NAC for 1 h, the cells were washed with PBS and stained with Hoechst 33342 as described in the text. The arrows indicate condensed and fragmented nuclei associated with apoptosis. Panel represents (A) Control, (B) 1 mM NAC, (C) 40 μM B-CDA + 1 mM NAC, (D) 200 μM H2O2 + 1 mM NAC, (E) 40 μM B-CDA and (F) 200 μM H2O2. Picture is a representative of three different sets of experiments.
3.4 Regulation of MMP by CDA
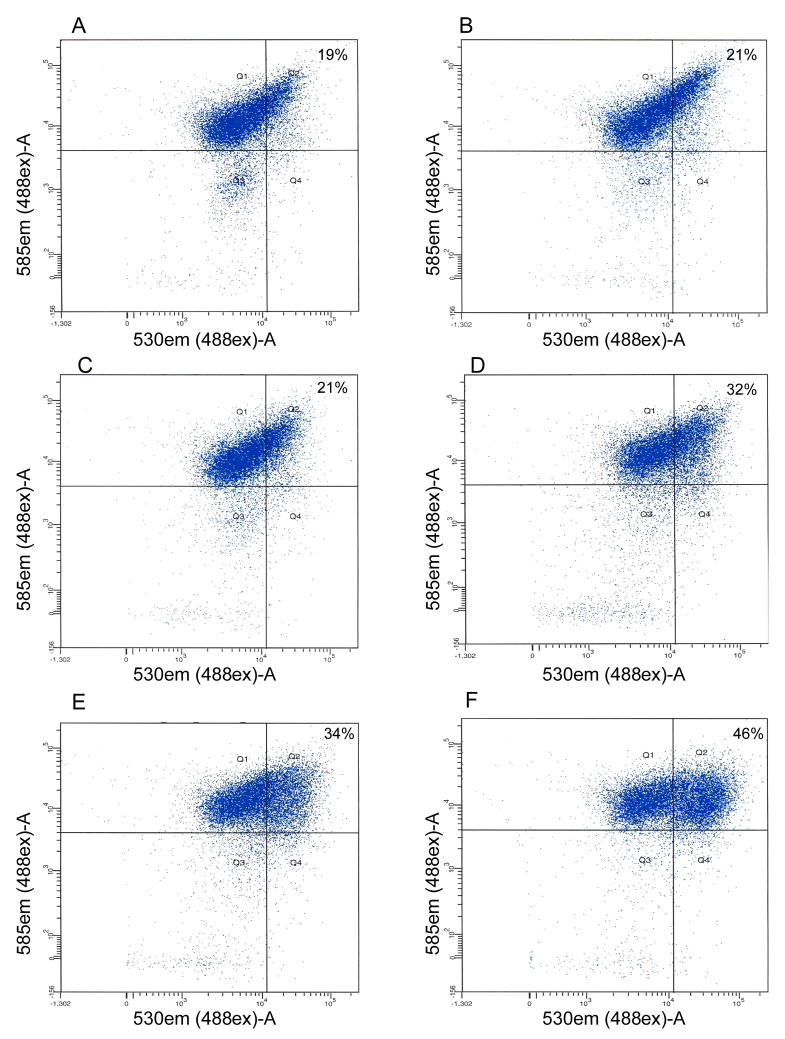
The change in mitochondrial membrane potential has been shown to play an important role in early stages of RPE cell apoptosis. Therefore the effect of B-CDA as well as H2O2 on the early stages of apoptosis was also assessed by measuring MMP. ARPE-19 cells were exposed to 40 μM B-CDA or 200 μM H2O2 for 4 h without or with pretreatment with NAC, followed by incubation with JC-1 dye. Flow cytometric analysis revealed that B-CDA as well as H2O2 treatment caused approximately 40% of the cells to undergo early apoptotic changes (Figures 6E & F) and pretreatment with NAC attenuated B-CDA-induced change in MMP (Figures 6C & D). Figures 6A & B represent control and NAC- treated groups, respectively.
Fig. 6. CDA- or H2O2- induced changes in ARPE-19 cell MMP represent an early apoptotic change.
Cells (0.5 × 106) were exposed to 40 μM B-CDA or 200 μM H2O2 for 4 h without or with pretreatment with 1 mM NAC for 1 h. At the end of experiment, the cells were harvested, washed with PBS, incubated with JC-1 dye for 30 min, followed by 3X wash with PBS, suspended in PBS and subjected to flow cytometry. The percentage in upper right corner of quadrant Q2 represents the percent of cells with altered MMP. Panels: (A) Control, (B) 1 mM NAC, (C) 40 μM B-CDA + 1 mM NAC, (D) 200 μM H2O2 + 1 mM NAC, (E) 40 μM B-CDA and (F) 200 μM H2O2. Representative pictures from three different sets of experiments.
3.5 CDA induce activation of NF-κB and AP-1 in RPE cells
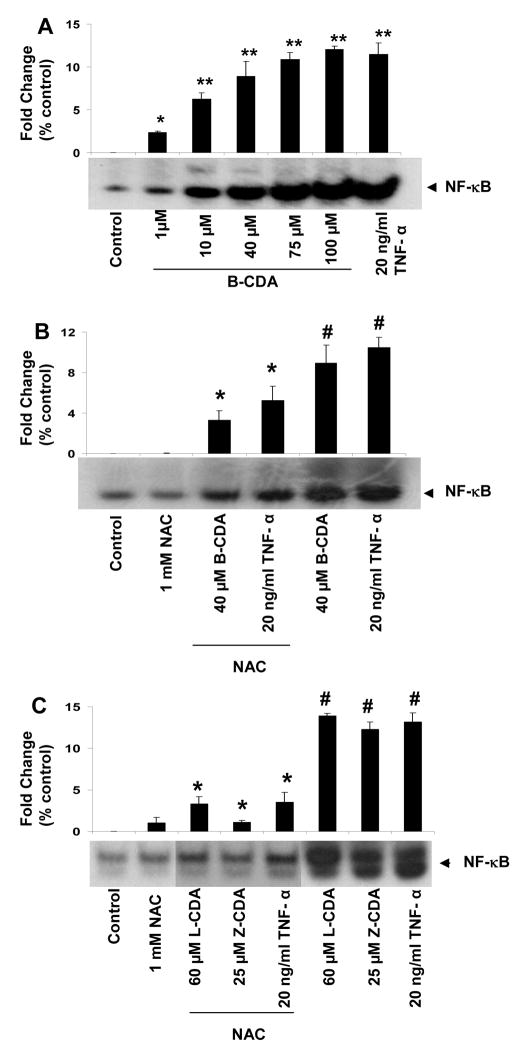
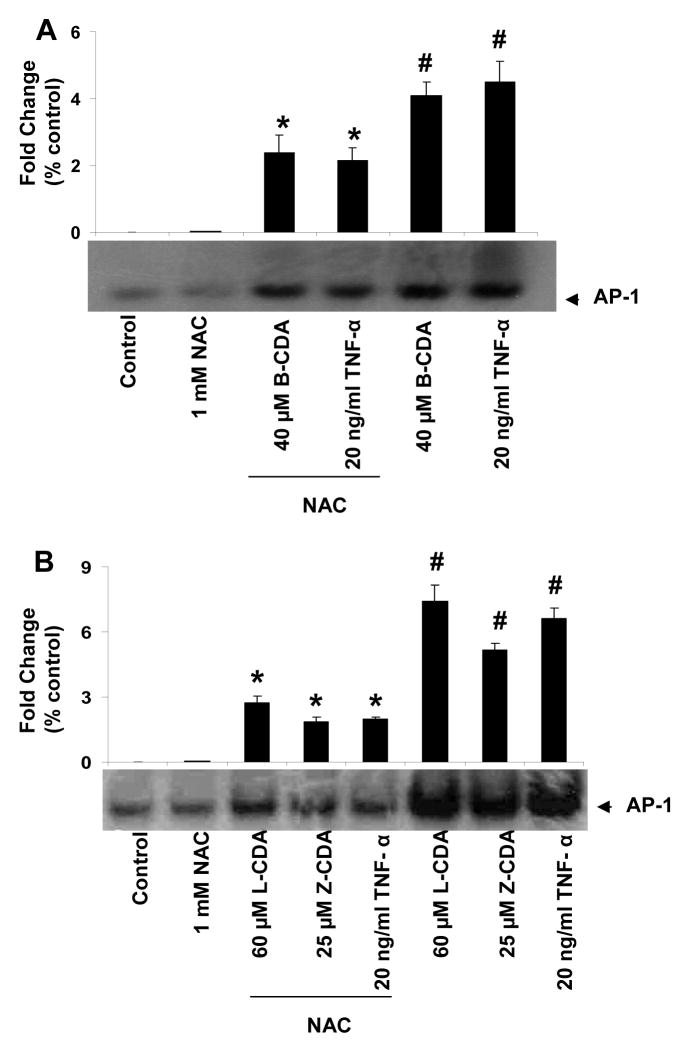
Since it is well known that oxidative stress causes activation of NF-κB and AP1 and our results show that CDA cause oxidative stress, we have examined the effect of CDA on redox sensitive transcription factors, NF-κB and AP-1 in RPE cells. To study activation of NF-κB and AP1, TNF-α was used as positive control (Ramana et al., 2004). We first examined the concentration-dependent (1 – 100 μM) effect of B-CDA on NF-κB activation (Figure 7A). Moreover, B-CDA, L-CDA and Z-CDA-induced activation of both NF-κB and AP1 in ARPE-19 cells was significantly prevented by NAC (Figure. 7B & C and Figure. 8A & B), suggesting that CDA could cause cytotoxicity by activating oxidative stress signals leading to activation of redox-sensitive transcription factors.
Fig. 7. Effect of CDA on activation of NF-κB.
(A) B-CDA-induced concentration-dependent activation of NF-κB. EMSA autoradiographs of nuclear extracts prepared from serum-starved ARPE-19 cells treated with 1, 10, 40, 75 & 100 μM B-CDA for 1h. TNF-α (20 ng/ml) was used as positive control (* p < 0.01; ** p < 0.001). (B) Inhibition of B-CDA and TNF-α induced NF-κB activation by NAC. (C) B-CDA and TNF-α induced AP-1 activation and significant inhibition by 1 mM NAC. (C) Inhibition of L-CDA, Z-CDA and TNF-α induced NF-κB activation by NAC. (E) L-CDA, Z-CDA and TNF-α induced AP-1 activation and significant inhibition by 1 mM NAC. Data represent the mean ± SD of three experiments (# p < 0.001 vs Control; *p < 0.001 vs CDA or TNF- α treated cells).
Fig. 8. Effect of CDA on activation of AP-1.
EMSA autoradiographs of nuclear extracts prepared from ARPE-19 cells. (A) B-CDA and TNF-α induced AP-1 activation and significant inhibition by 1 mM NAC. (B) L-CDA, Z-CDA and TNF-α induced AP-1 activation and significant inhibition by 1 mM NAC. Data represent the mean ± SD of three experiments (# p < 0.001 vs Control; *p < 0.001 vs CDA or TNF- α treated cells).
4. Discussion
Carotenoids are widely used to treat oxidative stress-induced ocular diseases such as AMD and cataract (Delcourt et al., 2006; Leung et al., 2005; Muriach et al., 2006; Chichili et al., 2006; Trevithick-Sutton et al., 2006; Vu et al., 2006a). However, carotenoid supplementation is known to generate various short and long chain carotenoid cleavage products (Nagao, 2004; Sommerburg et al., 2003). Many carotenoid cleavage products including apocarotenal (CDA) have been identified in human and primate tissues (Prasain et al., 2005; Khachik et al., 1997; Bernstein et al., 2001; Bhosale et al., 2005; Ho et al., 2007). It has been recently reported that upon giving β-carotene to a healthy person, β-apo-8′-carotenal increases in plasma (Ho et al., 2007). The concentration of Lutein, Zeaxanthin and other carotenoid products in the donor ocular tissues has been reported to be unusually high relative to serum and unmetabolized carotenoid concentration in the ocular tissues (Bernstein et al., 2001). Moreover, carotenoid supplementation correlates with rise in carotenoid metabolites in serum, plasma and retina of human and monkey (Khachik et al., 2006a; 2006b). However, increase in CDA levels with carotenoid supplementation remains to be determined.
The physiological role of various carotenoid cleavage products is not yet known. However, various cellular as well as animal studies have shown cytotoxic effects of carotenoid cleavage products (Siems et al., 2002; Alija et al., 2006; Liu et al., 2004; Nara et al., 2001; Salgo et al., 1999; Hurst et al., 2005). Like lipid aldehydes, the aldehyde product of β-carotene, i.e. β-apo-8′-carotenal, has been known to induce DNA damage in A549 cells (Yeh and Wu, 2006). Earlier we have shown that increased levels of CDA from β-carotene are toxic in human K562 erythroleukaemic and 28 SV40 retinal pigment epithelial cells (Hurst et al., 2005). We now demonstrate that CDA derived from β-carotene, Lutein and Zeaxanthin could induce apoptosis in ARPE-19 cells. These results are consistent with previous studies which have shown that micro-molar concentrations of lipid aldehyde (HNE) induce apoptosis in ARPE-19 (Chaudhary et al., 2005) and leukemic (Zhang et al., 2001) cells. The mechanism for CDA-induced apoptosis is not known so far. However, it is likely that CDA forms adducts with thiol groups of the mitochondrial proteins, such as the permeability transition pore, which has been proposed to trigger apoptosis (Liang and Godley, 2003; Takeyama et al., 2002; Zamzami et al., 1995). This is supported by our observations that NAC prevents the CDA-induced cytotoxicity in ARPE-19 cells. Such findings suggest that CDA could have similar effects as observed with lipid-derived aldehydes since they are well known for their cytotoxicity including apoptosis and DNA damage (Uchida 2003). Moreover, similar to other antioxidants, carotenoids could also act as pro-oxidants at higher concentrations by forming CDA. Indeed our results demonstrate that CDA increases ROS levels in a concentration-dependent manner in ARPE-19 cells (Figure 2A). Consistent with our results, Siems et al (2002) and Alija et al (2006) have also shown the prooxidant properties of CDA in primary rat hepatocytes.
Increased ROS in cells, irrespective of the cause such as cytokines, chemokines, growth factors, bacterial endotoxins and toxic aldehydes result in cellular toxicity by activating NF-κB and AP-1 (Srivastava et al., 2005). Our demonstration of significant activation of transcription factors, NF-κB and AP1, in ARPE-19 cells by CDA suggests the involvement of ROS. This is strengthened by our observation that CDA-induced activation of NF-κB and AP1 are inhibited by NAC. Further, our results indicate that CDA could be a potential apoptotic agent, since NF-κB and AP-1 activation is associated with apoptotic cell death (Ramana et al. 2002). These results are in agreement with the previous reports showing that carotenoid oxidation products as well as unmetabolised β-carotene cause cytotoxicity in human promyelocytic leukemia cells (HL-60) as well as in colon adenocarcinoma cells (Nara et al., 2001; Palozza et al., 2002; 2003a; 2003b). Although a large number of studies have shown the cytotoxic effects of β-carotene products, this is the first in vitro study that demonstrates cytotoxicity in ARPE-19 cells induced by NaOCl oxidized products of Lutein and Zeaxanthin. Hypochlorous acid (HOCl) generated by activated macrophages, which are known to infiltrate in neural tissues during inflammation, cause apoptosis and other toxic effects (Whiteman et al., 2005; Vissers et al., 2001; Krasowska and Konat, 2004; Ottonello et al., 1994). Since inflammation has been shown to be associated with AMD, it is likely that the increased oxidative stress by HOCl and H2O2 generated by activated macrophages could be the major player of macular degeneration. It is likely that in patients given large doses of carotenoids, the oxidation products of carotenoids such as Lutein and Zeaxanthin could increase the toxicity rather than prevent it. Some of the products of carotenoid oxidation such as apocarotenal, ionone, etc. have indeed been characterized in the retinal tissues (Prasain et al., 2005; Khachik et al., 1997; Bernstein et al., 2001; Bhosale and Bernstein, 2005). However, NaOCl may not be the only or main oxidizing agent in vivo. Further, oxidative environment in vivo could be different since the carotenoids will be in the lipid and/or protein environment. Thus the NaOCl is a model system to simulate the possible deleterious effects of excessive use of catotenoids commonly used in the therapy of progressive AMD. Our results are however potentially important in explaining the Melbourne Visual Impairment Project (MVIP) (Vu et al., 2006b) report which found harmful effects of higher Lutein and Zeaxanthin intake on AMD in subjects concurrently consuming high levels of linoleic acid. Although recently there have been several editorial letters that question the validity of this retrospective study, a prospective study similar to AREDS-2 will greatly help in understanding the beneficial vs harmful effects of carotenoids, particularly Lutein and Zeaxanthin already commonly used in the prevention and treatment of AMD.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants GM71036 (to K.V.R.), DK36118 (to S.K.S.) and Wilkins AMD Fund (to FJGMvK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alija AJ, Bresgen N, Sommerburg O, Langhans CD, Siems W, Eckl PM. Beta-carotene breakdown products enhance genotoxic effects of oxidative stress in primary rat hepatocytes. Carcinogenesis. 2006;27:1128–1133. doi: 10.1093/carcin/bgi342. [DOI] [PubMed] [Google Scholar]
- Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Bernstein PS. Quantitative measurement of 3′-oxolutein from human retina by normal-phase high-performance liquid chromatography coupled to atmospheric pressure chemical ionization mass spectrometry. Anal Biochem. 2005;345:296–301. doi: 10.1016/j.ab.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;7:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- Chichili GR, Nohr D, Frank J, Flaccus A, Fraser PD, Enfissi EMA, Biesalski HK. Protective effects of tomato extract with elevated β-carotene levels on oxidative stress in ARPE-19 cells. Br J Nutr. 2006;96:643–649. [PubMed] [Google Scholar]
- Choudhary S, Xiao T, Srivastava S, Zhang W, Chan LL, Vergara LA, Van Kuijk FJ, Ansari NH. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol Appl Pharmacol. 2005;204:122–134. doi: 10.1016/j.taap.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Delcourt C, Carrière I, Delage M, Barberger-Gateau P, Schalch W The POLA Study Group. Plasma Lutein and Zeaxanthin and Other Carotenoids as Modifiable Risk Factors for Age-Related Maculopathy and Cataract: The POLA Study. Invest Ophthalmol Vis Sci. 2006;47:2329–2335. doi: 10.1167/iovs.05-1235. [DOI] [PubMed] [Google Scholar]
- Dunaief JL, Dentchev T, Ying G, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- Handelman GJ, van Kuijk FJGM, Chatterjee A, Krinsky NI. Characterization of products formed during the autoxidation of β-carotene. Free Radic Biol Med. 1991;10:427–437. doi: 10.1016/0891-5849(91)90051-4. [DOI] [PubMed] [Google Scholar]
- Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- Ho CC, de Moura FF, Kim S, Clifford AJ. Excentral cleavage of β-carotene in vivo in a healthy man. Am J Clin Nutr. 2007;85:770–777. doi: 10.1093/ajcn/85.3.770. [DOI] [PubMed] [Google Scholar]
- Holz FG, Pauleikhoff D, Klein R, Bird AC. Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol. 2004;137:504–510. doi: 10.1016/j.ajo.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Howes KA, Liu Y, Dunaief JL, Milam A, Frederick JM, Marks A, Baehr W. Receptor for advanced glycation end products and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:3713–3720. doi: 10.1167/iovs.04-0404. [DOI] [PubMed] [Google Scholar]
- Hurst JS, Contreras JE, Siems WG, van Kuijk FJGM. Oxidation of carotenoids by heat and tobacco smoke. Biofactors. 2004;20:23–35. doi: 10.1002/biof.5520200103. [DOI] [PubMed] [Google Scholar]
- Hurst JS, Saini MK, Jin G, Awasthi YC, van Kuijk FGJM. Toxicity of oxidized β-carotene to cultured human cells. Exp Eye Res. 2005;81:239–243. doi: 10.1016/j.exer.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer’s Aβ-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapphahn RJ, Giwa BM, Berg KM, Roehrich H, Feng X, Olsen TW, Ferrington DA. Retinal proteins modified by 4-hydroxynonenal: identification of molecular targets. Exp Eye Res. 2006;83:165–175. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–1811. [PubMed] [Google Scholar]
- Khachik F, de Moura FF, Chew EY, Douglass LW, Ferris FL, 3rd, Kim J, Thompson DJ. The Effect of Lutein and Zeaxanthin Supplementation on Metabolites of These Carotenoids in the Serum of Persons Aged 60 or Older. Invest Ophthalmol Vis Sci. 2006a;47:5234–5242. doi: 10.1167/iovs.06-0504. [DOI] [PubMed] [Google Scholar]
- Khachik F, London E, de Moura FF, Johnson M, Steidl S, Detolla L, Shipley S, Sanchez R, Chen XQ, Flaws J, Lutty G, McLeod S, Fowler B. Chronic Ingestion of (3R, 3′R, 6′R)-Lutein and (3R, 3′R)-Zeaxanthin in the Female Rhesus Macaque. Invest Ophthalmol Vis Sci. 2006b;47:5476–5486. doi: 10.1167/iovs.06-0194. [DOI] [PubMed] [Google Scholar]
- Kopitz J, Holz FG, Kaemmerer E, Schutt F. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie (Paris) 2004;86:825–831. doi: 10.1016/j.biochi.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Krasowska A, Konat GW. Vulnerability of brain tissue to inflammatory oxidant, hypochlorous acid. Brain Res. 2004;997:176–184. doi: 10.1016/j.brainres.2003.09.080. [DOI] [PubMed] [Google Scholar]
- Lee CS, Jang YY, Song JS, Han ES. Ambroxol inhibits peroxynitrite-induced damage of α1-antiproteinase and free radical production in activated phagocytic cells. Pharma & Toxico. 2002;91:140–149. doi: 10.1034/j.1600-0773.2002.910309.x. [DOI] [PubMed] [Google Scholar]
- Leibowitz HM, Krueger DE, Maunder LR. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24:335–610. [PubMed] [Google Scholar]
- Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Max Snodderly D. Nutritional manipulation of primate retinas. IV Effects of n--3 fatty acids, lutein, and zeaxanthin on S-cones and rods in the foveal region. Exp Eye Res. 2005;81:513–529. doi: 10.1016/j.exer.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Liu C, Russell RM, Wang XD. α-tocopherol and ascorbic acid decrease the production of β-apo-carotenals and increases the formation of retinoids from β-carotene in the lung tissues of cigarette-smoke-exposed ferrets in vitro. J Nutr. 2004;134:426–430. doi: 10.1093/jn/134.2.426. [DOI] [PubMed] [Google Scholar]
- Muriach M, Bosch-Morell F, Alexander G, Blomhoff R, Barcia J, Arnal E, Almansa I, Romero FJ, Miranda M. Lutein effect on retina and hippocampus of diabetic mice. Free Radic Biol Med. 2006;41:979–984. doi: 10.1016/j.freeradbiomed.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Nagao A. Oxidative conversion of carotenoids to retinoids and other products. J Nutr. 2004;134:237S–240S. doi: 10.1093/jn/134.1.237S. [DOI] [PubMed] [Google Scholar]
- Nara E, Hayashi H, Kotake M, Miyashita K, Nagao A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr Cancer. 2001;39:273–283. doi: 10.1207/S15327914nc392_18. [DOI] [PubMed] [Google Scholar]
- Ottonello L, Dapino P, Scirocco M, Dallegri F, Sacchetti C. Proteolytic inactivation of alpha-1-antitrypsin by human neutrophils: involvement of multiple and interlinked cell responses to phagocytosable targets. Eur J Clin Invest. 1994;24:42–49. doi: 10.1111/j.1365-2362.1994.tb02058.x. [DOI] [PubMed] [Google Scholar]
- Palozza P, Serini S, Torsello A, Boninsegna A, Covacci V, Maggiano N, Ranelletti FO, Wolf FI, Calviello G. Regulation of cell cycle progression and apoptosis by beta-carotene in undifferentiated and differentiated HL-60 leukemia cells: possible involvement of a redox mechanism. Int J Cancer. 2002;97:593–600. doi: 10.1002/ijc.10094. [DOI] [PubMed] [Google Scholar]
- Palozza P, Serini S, Di Nicuolo F, Piccioni E, Calviello G. Prooxidant effects of β-carotene in cultured cells. Mol Aspects Med. 2003a;24:353–362. doi: 10.1016/s0098-2997(03)00031-1. [DOI] [PubMed] [Google Scholar]
- Palozza P, Serini S, Torsello A, Di Nicuolo F, Piccioni E, Ubaldi V, Pioli C, Wolf FI, Calviello G. Beta-carotene regulates NF-kappaB DNA-binding activity by a redox mechanism in human leukemia and colon adenocarcinoma cells. J Nutr. 2003b;133:381–388. doi: 10.1093/jn/133.2.381. [DOI] [PubMed] [Google Scholar]
- Prasain JK, Moore R, Hurst JS, Barnes S, van Kuijk FJGM. Electrospray tandem mass spectrometric analysis of zeaxanthin and its oxidation products. J Mass Spectrom. 2005;40:916–923. doi: 10.1002/jms.868. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- Salgo M, Cueto R, Winston G, Pryor W. β-carotene and its oxidation products have different effects on microsome mediated binding of benzo[a]pyrene to DNA. Free Radic Biol Med. 1999;26:162–173. doi: 10.1016/s0891-5849(98)00172-5. [DOI] [PubMed] [Google Scholar]
- Siems W, Sommerburg O, Schild L, Augustin W, Langhans CD, Wiswedel I. β-Carotene cleavage products induce oxidative stress in vitro by impairing mitochondrial respiration. FASEB J. 2002;16:1289–1291. doi: 10.1096/fj.01-0765fje. [DOI] [PubMed] [Google Scholar]
- Sommerburg O, Langhans CD, Arnhold J, Leichsenring M, Salerno C, Crifo C, Hoffmann GF, Debatin KM, Siems WG. Beta-carotene cleavage products after oxidation mediated by hypochlorous acid—a model for neutrophil-derived degradation. Free Rad Biol Med. 2003;35:1480–1490. doi: 10.1016/j.freeradbiomed.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- Takeyama N, Miki S, Hirakawa A, Tanaka T. Role of the mitochondrial permeability transition and cytochrome C release in hydrogen peroxide-induced apoptosis. Exp Cell Res. 2002;274:16–24. doi: 10.1006/excr.2001.5447. [DOI] [PubMed] [Google Scholar]
- Tanito M, Elliott MH, Kotake Y, Anderson RE. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest Ophthalmol Vis Sci. 2005;46:3859–3868. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- Trevithick-Sutton CC, Foote CS, Collins M, Trevithick JR. The retinal carotenoids, zeaxanthin and lutein scavenge superoxide and hydroxyl radicals: a chemiluminescence and ESR study. Mol Vis. 2006;12:1127–1135. [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- van Kuijk FJ, Thomas DW, Stephens RJ, Dratz EA. Gas chromatography-mass spectrometry method for determination of phospholipid peroxides; I. Transesterification to form methyl esters. J Free Radic Biol Med. 1985;1:215–225. doi: 10.1016/0748-5514(85)90121-7. [DOI] [PubMed] [Google Scholar]
- Vissers MC, Lee WG, Hampton MB. Regulation of apoptosis by vitamin C. Specific protection of the apoptotic machinery against exposure to chlorinated oxidants. J Biol Chem. 2001;276:46835–46840. doi: 10.1074/jbc.M107664200. [DOI] [PubMed] [Google Scholar]
- Vu HT, Robman L, Hodge A, McCarty CA, Taylor HR. Lutein and zeaxanthin and the risk of cataract: the Melbourne visual impairment project. Invest Ophthalmol Vis Sci. 2006a;47:3783–3786. doi: 10.1167/iovs.05-0587. [DOI] [PubMed] [Google Scholar]
- Vu HT, Robman L, McCarty CA, Taylor HR, Hodge A. Does dietary lutein and zeaxanthin increase the risk of age related macular degeneration? The Melbourne Visual Impairment Project. Br J Ophthalmol. 2006b;90:389–393. doi: 10.1136/bjo.2005.078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Bassiri M, Najafi R, Najafi K, Yang J, Khosrovi B, Hwong W, Barati E, Belisle B, Celeri C, Robson MC. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007;11:65–79. [PMC free article] [PubMed] [Google Scholar]
- Whiteman M, Rose P, Siau JL, Cheung NS, Tan GS, Halliwell B, Armstrong JS. Hypochlorous acid-mediated mitochondrial dysfunction and apoptosis in human hepatoma HepG2 and human fetal liver cells: role of mitochondrial permeability transition. Free Radic Biol Med. 2005;38:1571–1584. doi: 10.1016/j.freeradbiomed.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- Yeh Shu-Lan, Wu Shu-Hsuan. Effects of quercetin on β-apo-8′-carotenal-induced DNA damage and cytochrome P1A2 expression in A549 cells. Chemico-Biol Inter. 2006;163:199–206. doi: 10.1016/j.cbi.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, He Q, Chan LL, Zhou F, El Naghy M, Thompson EB, Ansari NH. Involvement of caspases in 4-hydroxy-alkenal-induced apoptosis in human leukemic cells. Free Radic Biol Med. 2001;30:699–706. doi: 10.1016/s0891-5849(01)00465-8. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cai B, Jang YP, Pachydaki S, Schmidt AM, Sparrow JR. Mechanisms for the induction of HNE- MDA- and AGE-adducts, RAGE and VEGF in retinal pigment epithelial cells. Exp Eye Res. 2005;80:567–580. doi: 10.1016/j.exer.2004.11.009. [DOI] [PubMed] [Google Scholar]