Abstract
Congenital central hypoventilation syndrome (CCHS) patients show hypoventilation during sleep and severe autonomic impairments, including aberrant cardiovascular regulation. Abnormal sympathetic patterns, together with increased and variable CO2 levels, lead to the potential for sustained cerebral vasculature changes. We performed high-resolution T1-weighted imaging in 13 CCHS and 31 control subjects using a 3.0-Tesla magnetic resonance imaging scanner, and evaluated resting basilar and bilateral middle cerebral artery cross-sections. Two T1-weighted image series were acquired; images were averaged and reoriented to common space, and regions containing basilar and both middle cerebral arteries were oversampled. Cross-sections of the basilar and middle cerebral arteries were manually outlined to calculate cross-sectional areas, and differences between and within groups were evaluated. Basilar arteries in CCHS were significantly dilated over control subjects, but both middle cerebral artery cross-sections were similar between groups. No significant differences appeared between left and right middle cerebral arteries within either group. Basilar artery dilation may result from differential sensitivity to high CO2 over other vascular beds, damage to serotonergic or other chemosensitive cells accompanying the artery, or enhanced microvascular resistance, and that dilation may impair tissue perfusion, leading to further neural injury in CCHS.
Keywords: Magnetic Resonance Imaging, Respiration, Middle cerebral artery, Chemosensitivity, Perfusion
Introduction
Congenital central hypoventilation syndrome (CCHS) is a genetic condition characterized by mutations in a transcription protein for cellular nervous system development, PHOX2B [2, 34], which in the mouse, targets the expression of neurons in autonomic and certain brainstem respiratory ganglia [7, 8, 25, 30]. Affected patients show multiple autonomic and physiological deficits, including impaired CO2 and O2 sensitivity, and reduced breathing drive during sleep [1, 9, 27]. Autonomic impairments in CCHS include increased and poorly-regulated sympathetic tone, with associated impaired blood pressure control, and a range of parasympathetic alterations [1, 16, 31, 35]. Impaired sympathetic regulation, as well as increased and variable CO2 levels from hypoventilation during sleep, and occasionally during waking, lead to the potential for cerebral vasculature constriction or dilation.
Changes in cerebral vessel diameter exert substantial blood flow and arterial pressure effects [11]. Increased CO2 levels in blood or surrounding tissue dilate cerebral vessels [4], with hypotension accompanying the dilatation in vasculature [36]. Since CCHS subjects show increased and inconsistent CO2 levels, as well as day-time hypotension [31], cerebral blood vessels may show long-lasting dilation after sustained exposure to hypercapnia during development.
Our aim was to assess dilation in major cerebral vessels in CCHS patients, relative to control subjects. We evaluated middle cerebral and basilar arteries, since these two arterial systems supply many of the structures affected in the syndrome [13]. We hypothesized that CCHS subjects would show dilated major arteries relative to control subjects.
Materials and Methods
We studied 13 CCHS patients (mean age ± SD: 15.1 ± 2.4 years; range: 12-18 years; mean body-mass-index ± SD: 20.8 ± 3.9 kg/m2; 8 male) and 31 control subjects (15.0 ± 2.3 years; 10-19 years; 21.2 ± 4.3 kg/m2; 18 male) during unsedated awake conditions. CCHS subjects were recruited from the CCHS family network (http://www.cchsnetwork.org), and were diagnosed based on standard criteria [1]. Of the 13 CCHS subjects, five showed PHOX2B mutations, tests on two were inconclusive, and the remaining six were untested. We included only those CCHS subjects who required ventilatory support during sleep, but not during waking. CCHS subjects with other conditions affecting the cerebral vasculature, e.g., cardiac disease, were also excluded. Control subjects were in good health, without neurological or cardiovascular disorders, and were recruited through advertisements at the university campus. The study was approved by the Institutional Review Board of the University of California at Los Angeles, and all subjects and their parents/guardians gave informed written consent/assent before the study.
We performed brain studies with a 3.0-Tesla magnetic resonance imaging (MRI) scanner (Magnetom Trio; Siemens, Erlangen, Germany). During data collection, subjects lay supine; foam pads on both sides of the head reduced head-motion. The present vascular study was performed as part of other neurological evaluations; thus, angiographic procedures were not performed. Two high-resolution T1-weighted image series were collected in the axial plane (minimal oblique), using a magnetization-prepared-rapid-acquisition gradient-echo pulse sequence (repetition-time = 2200 ms; echo-time = 3.05 ms; inversion-time = 1100 ms; flip-angle = 10°; matrix size = 256 × 256; field-of-view = 220 × 220 mm; slice-thickness = 1.0 mm; phase-encoding = R≫L; magnetization-preparation = slice-selective inversion-recovery). Proton-density (PD) and T2-weighted images were also collected in the axial plane, using a dual-echo turbo spin-echo pulse sequence (repetition-time = 8000 ms; echo-time 1, echo-time 2 = 17, 133 ms; flip-angle = 150°; matrix size = 256 × 256; field-of-view = 240 × 240 mm; slice-thickness = 5.0 mm), for visual examination of clinical pathology.
Both T1-weighted image series were examined for head motion-related or other image artifacts. T1-, T2-, and PD-weighted images were assessed to verify absence of major brain pathology.
The statistical parametric mapping package SPM5 (http://www.fil.ion.ucl.ac.uk/spm/), MRIcron [28], and MATLAB-based (The MathWorks Inc, Natick, MA) custom software were used to process the high-resolution T1-weighted images, outline cerebral artery cross-sections, and calculate cross-sectional areas.
We realigned both high-resolution T1-weighted image series, and averaged the images to increase signal-to-noise ratio. The averaged images were bias-corrected for any signal inhomogeneity, reoriented into a common space, using a 6-parameter rigid-body (non-distorting) affine transformation, and sampled to a resolution of 0.9×0.9×0.9 mm.
Brain regions containing basilar and both middle cerebral arteries were oversampled to a resolution of 0.2×0.2×0.2 mm. If required, images containing the basilar artery were manually rotated such that the artery was perpendicular in sagittal and coronal views. Image contrast was visually standardized for all brain images containing basilar and both middle cerebral arteries. An investigator, blinded to subject group, outlined cross-sections of both sets of arteries. For each subject, the basilar artery was identified in sagittal views, and the cross-section was manually outlined in an axial view at the level of the mid basal-pons. Both left and right middle cerebral arteries were identified in axial views, and were traced in sagittal views at a level immediately after exit from the Circle of Willis. Pixels in each artery cross-section were counted, and cross-sectional areas were calculated.
We used the SPSS (v 15.0) statistical software for statistical assessment. Demographic data were compared with independent-samples t-tests and the Chi-square test. Cross-sectional areas of the basilar and left and right middle cerebral arteries between the CCHS and control groups and left and right middle cerebral arteries within CCHS and control groups were assessed using independent-samples t-tests. Intra- and inter-tracer reliabilities were measured with intraclass correlation coefficient (ICC) procedures.
Intra- and inter-tracer reliabilities for outlining basilar and middle cerebral artery cross-sections were established. An investigator, who outlined all cross-sections, re-outlined cross-sections of the arteries in 8 randomly-selected subjects (5 controls, 3 CCHS). A second investigator also outlined cross-sections in the same subjects. We calculated intra-tracer and inter-tracer reliabilities, which were high (intra-tracer: basilar artery, ICC = 0.99; middle cerebral artery, ICC = 0.87; inter-tracer: basilar artery, ICC = 0.98; middle cerebral artery, ICC = 0.86).
Results
No significant differences in age, gender, or body-mass-index appeared between CCHS and control groups.
Both middle cerebral arteries were visualized in all 13 CCHS and 31 control subjects, and the basilar artery was visualized in 12 CCHS and 29 control subjects; thus, one CCHS and two control subjects were excluded for the basilar artery evaluation. Substantially dilated basilar arteries appeared in CCHS over control subjects, but the middle cerebral arteries showed similar cross-sections in both groups. The significant expansion of the basilar artery and the minimal changes in both middle cerebral arteries are visually apparent in sample images from each group (Fig. 1, 2).
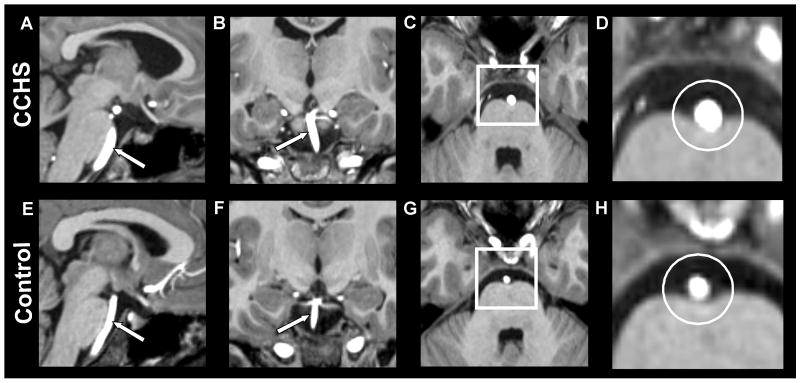
Fig. 1.
High-resolution T1-weighted images show the basilar artery in a CCHS and control subject. The upper panel shows longitudinal (A, sagittal section; B, coronal section) and cross-sectional views (C, axial section, white square; D, magnified area of the square in image C, white circle) of the basilar artery in a CCHS subject (age 14.3 years, male). The lower panel shows longitudinal (E, sagittal section; F, coronal section) and cross-sectional views (G, axial section, white square; H, magnified area of the square in image G, white circle) of the basilar artery in a control subject (14.4 years, male). The basilar artery cross-section in CCHS is larger than the control subject (D vs. H).
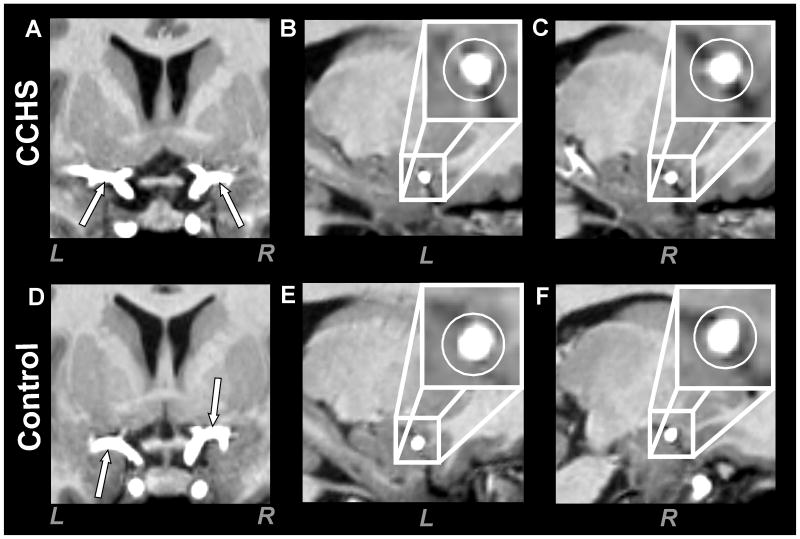
Fig. 2.
High-resolution T1-weighted images show both left (L) and right (R) middle cerebral arteries in a CCHS and control subject. The upper panel is a longitudinal view (A, coronal section) and cross-sectional views (B, sagittal section; C, sagittal section) of middle cerebral arteries in a CCHS subject (age 13.5 years, female). The lower panel shows longitudinal (D, coronal section) and cross-sectional views (E, sagittal section; F, sagittal section) of middle cerebral arteries in a control subject (14.0 years, female). Both left and right middle cerebral arteries in CCHS show comparable cross-sections as found in the control subject.
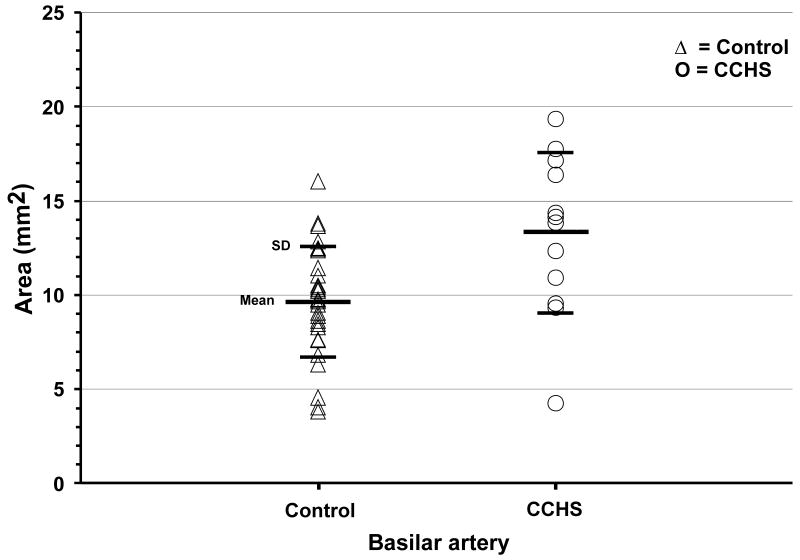
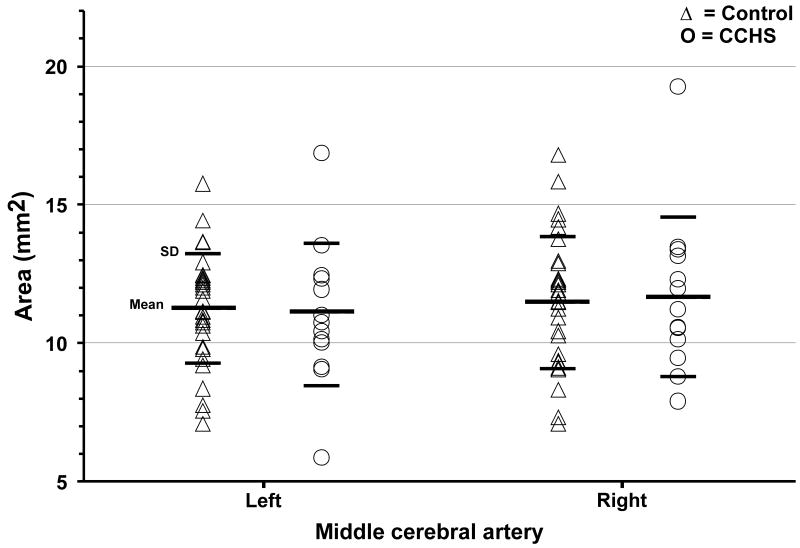
Basilar and both middle cerebral artery mean cross-sectional areas of individual CCHS and control subjects are shown on scatter plots (Fig. 3, 4). Basilar artery cross-sections were significantly enlarged in CCHS over control subjects [CCHS (n = 12) vs. controls (n = 29); 13.31 ± 4.27 vs. 9.67 ± 2.92 mm2, p = 0.003], confirming the visual impression from individual subjects in Figure 1. Left and right middle cerebral artery cross-sectional areas did not differ significantly between CCHS and control groups [CCHS (n = 13) vs. controls (n = 31); left, 11.06 ± 2.60 vs. 11.22 ± 1.98 mm2, p = 0.83; right, 11.72 ± 2.87 vs. 11.46 ± 2.38 mm2, p = 0.76]. No significant differences appeared between left and right middle cerebral artery cross-sections either in CCHS (p = 0.54) or control groups (p = 0.66).
Fig. 3.
Basilar artery cross-sectional areas from individual control (△) and CCHS (○) subjects. Basilar cross-sectional areas were significantly enlarged in CCHS over control subjects.
Fig. 4.
Left and right middle cerebral arteries' cross-sectional areas from individual control (△) and CCHS (○) subjects. Neither left nor right cross-sections showed any significant differences between groups.
Discussion
Basilar arteries were dilated in CCHS, relative to control subjects. The left and right middle cerebral arteries, however, were unaffected. Both the forebrain and more-caudal sites, including the midbrain, pons, medulla, and cerebellum, show neural injury in CCHS [13-15]; functional MRI studies also revealed abnormal functional responses to cardiovascular and respiratory challenges in these areas [10, 20, 21]. Dilation of the principal artery supplying portions of the cerebellum, medulla, pons, and midbrain may result in reduced tissue perfusion by lowering blood pressure, and thus, blood flow to the microvasculature in these areas, and may lead to further damage found in these brain sites [13].
The basilar artery is formed from the vertebral arteries that ascend with the spinal cord and combine at the caudal pons, and bifurcates at the level of the midbrain into the posterior cerebral arteries. Before bifurcating, the basilar artery gives rise to the posterior inferior cerebellar artery, which supplies areas within the cerebellum and medulla [29], and the superior cerebellar artery, which supplies the cerebellum and pons. Several pontine branches from the basilar artery perfuse the pons [29]. Thus, major regions of the cerebellum, medulla, pons, and midbrain are predominantly supplied by the basilar artery and its branches, and many of these areas are damaged in CCHS.
Several possibilities emerge that may lead to dilation of blood vessels, including increased CO2 levels in the blood and nearby tissue, enhanced nitric oxide production in endothelial cells, chronic hypotension, increased microvascular resistance, or damage to chemosensitive or other cells accompanying the major ventral medullary arteries. Congenital central hypoventilation syndrome patients show increased and variable levels of blood CO2, principally resulting from hypoventilation during sleep. Enhanced CO2 levels induce vasodilation, mediated by H+ actions on vascular smooth muscle accompanying alterations in pH [12]. Hydrogen ions activate membrane phospholipase, and release vasodilator prostaglandins [33], which reduce vascular wall resistance, and dilate the blood vessels.
Enhanced nitric oxide production in endothelial cells can also contribute to vasodilation, although such alterations in CCHS patients have not been demonstrated. Nitric oxide, together with hemoglobin, plays a significant role in gas exchange, and helps control blood pressure [3, 6].
Any consideration of potential mechanisms underlying the dilation, however, must account for the specificity of the effect; the basilar, but not the middle cerebral arteries were affected. The lack of dilation in the middle cerebral arteries would appear to preclude a generalized effect, e.g., high CO2, operating overall on cerebral vasculature. However, responses to CO2 or other vasoactive stimuli are not uniform across all vascular beds, with differing reactions to CO2 between the middle cerebral and basilar arteries in canines [32], and to endothelium-dependent vasopressin-mediated contractions in humans [26]. Therefore, the specificity of the dilation to the basilar arteries does not exclude high CO2 levels in CCHS as a source of the effect.
Chronic hypotension, regardless of the source, typically is accompanied by cerebral vasodilation; CCHS patients are hypotensive during wakefulness, but hypertensive at night, with an absence of nocturnal “dipping” in blood pressure [31]. The impaired autonomic nervous system activity in CCHS likely underlies the abnormal cerebral autoregulation in the condition, an outcome apparent through grossly distorted global blood-oxygen-level-dependent (BOLD) signals found in functional MRI studies [19]. The global BOLD signals in CCHS, resulting from cerebral blood flow and oxygenation actions, differ from controls in response to CO2 and O2 challenges [19], and could reflect impaired vasculature control here.
The vessel diameters represent resting values. It may be the case that vasculature diameters will react inappropriately to transient changes in ventilatory, sympathetic, or other challenges in CCHS, as suggested by the global BOLD findings. Evaluations to dynamic challenges have yet to be performed, but may help reveal the pathology underlying the baseline dilatation.
Congenital central hypoventilation syndrome patients show PHOX2B mutations, the principal mechanism underlying the condition [2]. Mutations in Phox2b in the mouse target autonomic ganglia in the viscera and brainstem [7, 30], as well as neurons in the vicinity of the retrofacial nucleus [8], and selected more-rostral areas (http://www.brain-map.org/), and affect cellular development in these sites. PHOX2B mutations may introduce injury in CCHS patients to comparable brainstem autonomic and respiratory regulatory areas as in murine models, with significant effects on vasculature regulation. Damage to vascular regulatory areas occurs in the human condition; the injury appears, among other sites, in the midline raphé, origin of serotonergic fibers which contribute significantly to regulation of vascular tone [22]. Moreover, damage appears in the lateral medulla and cerebellar cortex and deep nuclei, all sites having significant autonomic control roles [17, 18]. The damage has the potential to modify autonomic influences altering microvasculature diameter, thereby increasing microvascular resistance and contributing to basilar artery dilation.
Primary chemoreceptor surface cells, which monitor perivascular pH levels, are sited below the major surface vessels inside the glial layer of the medullary surface, as well as surrounding areas of small branches from large surface vessels [23, 24]. Serotonergic neurons in the medulla follow the basilar artery and its branches in close proximity [5]. These cells respond to CO2, and may serve significant roles in CO2–related actions on the vasculature. Although the midline raphé, containing serotonergic neurons, is injured in CCHS [13], it is unclear whether 5-HT neurons, or other chemosensitive surface cells accompanying the major medullary vessels are similarly damaged. Serotonergic neurons do not express Phox2b in mice, and are relatively unaffected by Phox2b mutations [8], suggesting that a secondary process elicits the raphé injury, possibly from ischemia resulting from loss of differentiation of autonomic neurons in the condition. Phox2b is found in glutamatergic neurons, however [8], and such neurons also accompany large vessels in the medulla [23, 24], possibly contributing to the dilation here.
Congenital central hypoventilation syndrome subjects show structural injury in both rostral and caudal brain areas [13-15]; in caudal areas supplied by the basilar artery, damage is found from the midbrain through the periaqueductal gray, raphé, superior cerebellar decussation, basal-pons, superior and inferior cerebellar peduncles, cerebellar cortex and deep nuclei, and the lateral medulla [13]. While initial injury in CCHS may result from developmental consequences in several of these sites, dilated basilar arteries might lead to localized hypotension which would fail to perfuse the regional microvasculature, resulting in additional injury in brainstem and cerebellar areas.
In conclusion, the basilar, but not the middle cerebral arteries, are dilated in CCHS patients. Changes in vascular tension accompanying vessel dilation have the potential to impair tissue perfusion, and possibly contribute to further injury in the syndrome. The specificity of the dilation to the basilar arteries suggests that differences in reactivity of separate vascular beds to vascular stimuli, or microvascular resistance underlie the enlargement. Since serotonergic and other chemosensitive surface cells accompany the basilar arteries on the ventral medulla, injury in those cells, paralleling damage already found in raphé sites in the syndrome, may also contribute to the specific dilation.
Acknowledgments
We thank Ms. Rebecca Harper for assistance with data collection, and Dr. Jennifer Ogren for editorial assistance. This research was supported by HD-22695.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Thoracic Society. Idiopathic congenital central hypoventilation syndrome: diagnosis and management. Am J Respir Crit Care Med. 1999;160:368–373. doi: 10.1164/ajrccm.160.1.16010. [DOI] [PubMed] [Google Scholar]
- 2.Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- 3.Anggard E. Nitric oxide: mediator, murderer, and medicine. Lancet. 1994;343:1199–1206. doi: 10.1016/s0140-6736(94)92405-8. [DOI] [PubMed] [Google Scholar]
- 4.Bradac GB, Simon RS, Heidsieck CH. Angiographically verified transient alteration of the intracranial arteries and veins in dependence of different CO2 tensions. Neuroradiology. 1976;10:257–262. doi: 10.1007/BF00327574. [DOI] [PubMed] [Google Scholar]
- 5.Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- 6.Calver A, Collier J, Vallance P. Nitric oxide and cardiovascular control. Exp Physiol. 1993;78:303–326. doi: 10.1113/expphysiol.1993.sp003687. [DOI] [PubMed] [Google Scholar]
- 7.Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet JF. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- 8.Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haddad GG, Mazza NM, Defendini R, Blanc WA, Driscoll JM, Epstein MA, Epstein RA, Mellins RB. Congenital failure of automatic control of ventilation, gastrointestinal motility and heart rate. Medicine (Baltimore) 1978;57:517–526. doi: 10.1097/00005792-197811000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Harper RM, Macey PM, Woo MA, Macey KE, Keens TG, Gozal D, Alger JR. Hypercapnic exposure in congenital central hypoventilation syndrome reveals CNS respiratory control mechanisms. J Neurophysiol. 2005;93:1647–1658. doi: 10.1152/jn.00863.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontos HA, Raper AJ, Patterson JL. Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke. 1977;8:358–360. doi: 10.1161/01.str.8.3.358. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatr Res. 2008;64:275–280. doi: 10.1203/PDR.0b013e31817da10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Elevated mean diffusivity in widespread brain regions in congenital central hypoventilation syndrome. J Magn Reson Imaging. 2006;24:1252–1258. doi: 10.1002/jmri.20759. [DOI] [PubMed] [Google Scholar]
- 15.Kumar R, Macey PM, Woo MA, Alger JR, Keens TG, Harper RM. Neuroanatomic deficits in congenital central hypoventilation syndrome. J Comp Neurol. 2005;487:361–371. doi: 10.1002/cne.20565. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z, Chen ML, Keens TG, Ward SL, Khoo MC. Noninvasive assessment of cardiovascular autonomic control in congenital central hypoventilation syndrome. Conf Proc IEEE Eng Med Biol Soc. 2004;5:3870–3873. doi: 10.1109/IEMBS.2004.1404083. [DOI] [PubMed] [Google Scholar]
- 17.Lutherer LO, Lutherer BC, Dormer KJ, Janssen HF, Barnes CD. Bilateral lesions of the fastigial nucleus prevent the recovery of blood pressure following hypotension induced by hemorrhage or administration of endotoxin. Brain Res. 1983;269:251–257. doi: 10.1016/0006-8993(83)90134-8. [DOI] [PubMed] [Google Scholar]
- 18.Macefield VG, Henderson LA. Real-time imaging of the medullary circuitry involved in the generation of spontaneous muscle sympathetic nerve activity in awake subjects. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macey PM, Alger JR, Kumar R, Macey KE, Woo MA, Harper RM. Global BOLD MRI changes to ventilatory challenges in congenital central hypoventilation syndrome. Respir Physiol Neurobiol. 2003;139:41–50. doi: 10.1016/j.resp.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Macey PM, Macey KE, Woo MA, Keens TG, Harper RM. Aberrant neural responses to cold pressor challenges in congenital central hypoventilation syndrome. Pediatr Res. 2005;57:500–509. doi: 10.1203/01.PDR.0000155757.98389.53. [DOI] [PubMed] [Google Scholar]
- 21.Macey PM, Woo MA, Macey KE, Keens TG, Saeed MM, Alger JR, Harper RM. Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic deficits in congenital central hypoventilation syndrome. J Appl Physiol. 2005;98:958–969. doi: 10.1152/japplphysiol.00969.2004. [DOI] [PubMed] [Google Scholar]
- 22.Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada Y, Chen Z, Jiang W, Kuwana S, Eldridge FL. Anatomical arrangement of hypercapnia-activated cells in the superficial ventral medulla of rats. J Appl Physiol. 2002;93:427–439. doi: 10.1152/japplphysiol.00620.2000. [DOI] [PubMed] [Google Scholar]
- 24.Okada Y, Kuwana S, Oyamada Y, Chen Z. The cell-vessel architecture model for the central respiratory chemoreceptor. Adv Exp Med Biol. 2006;580:233–238. doi: 10.1007/0-387-31311-7_36. [DOI] [PubMed] [Google Scholar]
- 25.Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onoue H, Kaito N, Tomii M, Tokudome S, Nakajima M, Abe T. Human basilar and middle cerebral arteries exhibit endothelium-dependent responses to peptides. Am J Physiol. 1994;267:H880–886. doi: 10.1152/ajpheart.1994.267.3.H880. [DOI] [PubMed] [Google Scholar]
- 27.Paton JY, Swaminathan S, Sargent CW, Keens TG. Hypoxic and hypercapnic ventilatory responses in awake children with congenital central hypoventilation syndrome. Am Rev Respir Dis. 1989;140:368–372. doi: 10.1164/ajrccm/140.2.368. [DOI] [PubMed] [Google Scholar]
- 28.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 29.Ryan S, McNicholas M, Eustace S. Anatomy for Diagnostic Imaging. second edition. Saunders; London: 2004. The central nervous system; pp. 49–84. [Google Scholar]
- 30.Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trang H, Boureghda S, Denjoy I, Alia M, Kabaker M. 24-hour BP in children with congenital central hypoventilation syndrome. Chest. 2003;124:1393–1399. doi: 10.1378/chest.124.4.1393. [DOI] [PubMed] [Google Scholar]
- 32.Tsukui A, Fukuda S, Shimoji K. Heterogeneous responses of canine basilar and middle cerebral arteries to serotonin at normal and high CO2 tension. Experientia. 1992;48:1118–1121. doi: 10.1007/BF01948002. [DOI] [PubMed] [Google Scholar]
- 33.Wagerle LC, Mishra OP. Mechanism of CO2 response in cerebral arteries of the newborn pig: role of phospholipase, cyclooxygenase, and lipoxygenase pathways. Circ Res. 1988;62:1019–1026. doi: 10.1161/01.res.62.5.1019. [DOI] [PubMed] [Google Scholar]
- 34.Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Rand CM. Congenital central hypoventilation syndrome (CCHS) and sudden infant death syndrome (SIDS): Kindred disorders of autonomic regulation. Respir Physiol Neurobiol. 2008;164:38–48. doi: 10.1016/j.resp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Woo MS, Woo MA, Gozal D, Jansen MT, Keens TG, Harper RM. Heart rate variability in congenital central hypoventilation syndrome. Pediatr Res. 1992;31:291–296. doi: 10.1203/00006450-199203000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Yoshino H, Sakurai T, Oizumi XS, Akisaki T, Wang X, Yokono K, Kondoh T, Kohmura E, Umentani K. Dilation of perforating arteries in rat brain in response to systemic hypotension is more sensitive and pronounced than that of pial arterioles: simultaneous visualization of perforating and cortical vessels by in-vivo microangiography. Microvasc Res. 2009;77:230–233. doi: 10.1016/j.mvr.2008.09.011. [DOI] [PubMed] [Google Scholar]