Abstract
Leukocyte-specific CD18 integrins are critical in mediating cell recruitment and activation during host defense responses to bacterial infection. The signaling pathways downstream of CD18 integrins are dependent on the spleen tyrosine kinase, Syk. To investigate the role integrin signaling plays in host defense, we examined the responses of Syk-deficient neutrophils to bacterial challenge with serum-opsonized Staphylococcus aureus and Escherichia coli. Syk-conditional knockout mice lacking this kinase specifically in myeloid cells or just neutrophils were also used to investigate host responses in vivo. Syk-deficient neutrophils manifested impaired exocytosis of secondary and tertiary granules, reduced cytokine release, and very poor activation of the NADPH oxidase in response to serum-opsonized S aureus and E coli. These functional defects correlated with impaired activation of c-Cbl, Pyk2, Erk1/2, and p38 kinases. Bacterial phagocytosis, neutrophil extracellular trap formation, and killing were also reduced in Syk-deficient cells, with a more profound effect after S aureus challenge. In vivo, loss of Syk in myeloid cells or specifically in neutrophils resulted in reduced clearance of S aureus after subcutaneous or intraperitoneal infection, despite normal recruitment of inflammatory cells. These results indicate that loss of Syk kinase-mediated integrin signaling impairs leukocyte activation, leading to reduced host defense responses.
Introduction
Neutrophils are critical innate immune effectors during responses to infection and inflammation. These cells are activated by a diverse repertoire of receptors; chief among them are the leukocyte-specific CD18 integrins, which mediate neutrophil adhesion during migration and function as phagocytic receptors for bacterial uptake and killing. CD18 deficiency leads to leukocyte adhesion deficiency type I syndrome.1 These patients present early in life with repeated bacterial infections primarily from impaired phagocyte function, a phenotype that is recapitulated in CD18-deficient mice.2–4
The engagement of neutrophil integrins activates a variety of effector functions critical for host defense, including secretion of granule components, release of cytokines, and generation of reactive oxygen intermediates (ROIs).5 The production of distinct cytokines by neutrophils has been demonstrated to influence both the differentiation of macrophages and responses to antibiotic resistant strains of Staphylococcus aureus.6 In neutrophils, after integrin engagement, signaling events are mediated by activation of Src-family and Syk tyrosine kinases, via coupling to immunoreceptor tyrosine-based activation motif (ITAM)-bearing molecules.7 The downstream substrates of Src and Syk kinases that have been implicated in the effector functions elicited by integrin ligation include Pyk2, Cbl, SLP-76, Vav1/3, PLCγ2, and Erk.8,9 Signaling events triggered by integrins are thought to be important for cellular migration; however, syk−/− neutrophils undergo normal migration in CD18-dependent transwell assays, sterile peritonitis,10 and thrombohemorrhagic vasculitis.11 Whereas these observations may suggest that CD18-dependent migration is independent of signaling through Syk, more recent studies indicate that the integrin-ITAM pathway regulates the velocity of neutrophil migration in tissues, rather than overall directionality.12 In contrast, it is clear that activation of adhesion-dependent neutrophil effector functions, such as release of specific granules or production of ROIs after adhesion to integrin ligands, is dependent on Syk signaling.10 Loss of this signaling pathway blocks tissue injury in the thrombohemorrhagic vasculitis model, indicating the importance of integrin signaling pathways in inflammatory disease. However, the physiologic effect of alterations in integrin signaling in host defense to pathogen infection in vivo remains unknown.
Systemic loss of Syk leads to vascular abnormalities, perinatal lethality, and loss of immune receptor signaling,13,14 which is phenocopied in radiation chimeras15 and complicates their use in models of infection. This complication is alleviated by the use of a genetically modified syk allele that allows for conditional deletion after expression of Cre recombinase.16 To study the role of Syk signaling in host defense mediated by myeloid cells, mice containing the loxP flanked-allele of syk (sykf/f) were crossed to mice containing Cre under the control of the Lysozyme M17 or human MRP818 promoter. These mice provide a system to assess the importance of Syk in neutrophils during acute bacterial infections in a skin air pouch model. Syk-deficient neutrophils fail to secrete granule components, release induced cytokines, and generate ROIs while retaining elastase activity in response to both S aureus and Escherichia coli. After bacterial infection, Syk-deficient neutrophils migrate normally; but because of the defects in neutrophil function, killing of S aureus, and to a lesser extent E coli, is impaired. These data support a critical role for Syk signaling in the induction of neutrophil effector functions during innate immune responses.
Methods
Mice
Sykf/f mice,16 backcrossed to C57BL/6 for 8 generations, were crossed to LysMcreTg/Tg17 or Mrp8creTg18 mice. Sykf/fVav1creTg mice were generated for hematopoietic system deletion.19 Syk−/− mice have been described,13,14; syk−/− and wild-type (WT) cells were obtained from radiation chimeras, as described.10 CD18−/− and CD11b−/− mice were purchased from The Jackson Laboratory. Animals were kept in a specific pathogen–free facility at University of California, San Francisco and used according to protocols approved by the University of California, San Francisco Committee on Animal Research. All experiments were conducted with animals 8 to 12 weeks of age.
Antibodies and flow cytometry
Neutrophils were delineated with antibodies to Gr-1 (RB6; eBioscience), Ly6G (1A8; BD Biosciences PharMingen), CD11b (M1/70; BD Biosciences PharMingen), 7/4 (Abcam), and CD62L (MEL-14; eBioscience). Cells were surfaced stained, then fixed and permeabilized (eBioscience), and intracellular stained with a monoclonal antibody against Syk.20 For pErk and pp38 staining, cells were fixed with 1% para-formaldehyde, permeabilized in 90% methanol, and stained for neutrophils, and pErk1/2 PE or pp38 AlexaFluor 488 (BD Biosciences PharMingen). Data were collected on a modified FACScan (BD Biosciences; Cytek Development) and analyzed using FlowJo software (TreeStar).
Neutrophil functional assays
Bone marrow neutrophils from WT, syk−/−, CD11b−/−, and CD18−/− mice were isolated as described.21 Unless otherwise noted, all bacterial stimulations were conducted by opsonizing S aureus or E coli with 10% rag1−/− serum for 15 minutes at 37°C with end-over-end rotation. Induction of neutrophil oxidative burst was performed as described,22 except that ImmulonIV plates were blocked with 0.1% milk and stimulations were with S aureus (multiplicity of infection [MOI] = 5) or E coli (MOI = 10). Superoxide production was monitored by flow cytometry of 106 neutrophils loaded with 5μM 3′-(p-aminophenyl) fluorescein (APF; Invitrogen). Secretory vesicle degranulation was performed similarly and analyzed by flow cytometry after surface staining for CD11b and CD62L.
Gelatinase zymography was performed as described.7 Elastase activation was detected by flow cytometry of 106 neutrophils with 10μM ElastoLux (OncoImmunin), stimulated with S aureus or E coli (MOI = 5). Degranulation of lactoferrin was determined by enzyme-linked immunosorbent assay (ELISA; antihuman Lactoferrin antibody, Immunology Consultants Laboratory) as described.23
Inhibitors of MAPKs (PD98059, SB203580) were from EMD Biosciences. Bone marrow or purified neutrophils were incubated for 30 minutes with 20μM PD98059, 3μM SB203580, or dimethyl sulfoxide before stimulation.
Cytokine analysis
Neutrophil supernatants after 16-hour culture in Dulbecco modified Eagle medium, 10% fetal calf serum, penicillin, streptomycin, plus E coli or S aureus (MOI = 2) were analyzed in triplicate for tumor necrosis factor-α (TNF-α) by ELISA (BioSource), or for interleukin-1β (IL-1β), IL-6, TNF-α, macrophage inflammatory protein 1α (MIP1α), macrophage inflammatory protein 2 (MIP2), chemokine (C-X-C motif) ligand 1 (KC), interferon-γ-induced protein (IP-10), and monocyte chemotactic protein 1 (MCP1) by multiplex bead array assay (Milliplex) and acquired on a Bio-Rad Bio-Plex instrument.
Western blotting
Neutrophils were stimulated with TNF-α (50 ng/mL), and S aureus or E coli (MOI = 5) for 10 or 30 minutes at 37°C, or with 10nM phorbol myristate acetate. Cell lysates were prepared for Western blotting as described.10 The following antibodies were used: phospho-p40phox Thr154 (Cell Signaling Technology, 4311), Phospho-c-Cbl Tyr774 (Cell Signaling Technology, 3555), c-Cbl (Santa Cruz Biotechnology, 170), phospho-Pyk2 Tyr579 (Biosource 44632), and Pyk2 (Santa Cruz Biotechnology, 1514).
Bactericidal assay and phagocytosis
Neutrophils were allowed to bind opsonized E coli or S aureus (MOI = 10) for 10 minutes before the addition of 50 μg/mL gentamicin. At the indicated time points, neutrophils were washed, lysed in Luria broth (LB) + 0.1% Triton X-100, and dilutions were plated to count viable bacteria.
Phagocytosis was assessed by flow cytometry as described.24 Heat shock killed S aureus or E coli were labeled with fluorescein isothiocyanate (FITC; Pierce Chemical), opsonized, and incubated with neutrophils (MOI = 10). Before acquisition, extracellular FITC-labeled bacteria were quenched with 0.4% trypan blue.
Microscopy
Neutrophils were incubated for 2 hours in the presence of 50 ng/mL TNF-α with S aureus (MOI = 5) or E coli (MOI = 10). Cytospins were prepared from 5 × 104 neutrophils and were fixed in 4% para-formaldehyde. Slides were blocked with 4% goat serum and 1% bovine serum albumin, followed by staining with antihistone (Chemicon, 3422), phalloidin-Alexa 546 (Invitrogen), and 2 μg/mL 4,6 diamidino-2-phenylindole. Images were acquired with an inverted Axiovert 200 M microscope and a 63×/1.4 lens (Carl Zeiss) equipped with a 175-W xenon high-speed DG4 wavelength selector and a single emission filter wheel (Sutter Instruments), a PI piezoelectric z-drive (Physik Instrument), and a cooled CCD Coolsnap camera (Roper Instruments). Data were acquired with MetaMorph software (Molecular Devices), and images were colored and overlaid using the MetaMorph “Color Combine” command.
Nonlethal skin abscess infection model
A total of 2 × 107 early exponential phase S aureus (ATCC strain SA113) or 6 × 106 E coli serotype K1, isolated from the blood of a patient with biliary sepsis,25 were injected into a 7-day air pouch created by subcutaneous injection of 5 mL of sterile air on day 1 and reinflation with 2.5 mL on day 4. After 24 hours, the air pouch was lavaged with 3 mL of 0.315% sodium citrate in Hanks balanced salt solution. Lavage cells were enumerated, and an aliquot was lysed in LB/0.1% Triton X-100. Dilutions were plated on LB-agar to determine the viable bacterial counts.
Results
Neutrophil degranulation
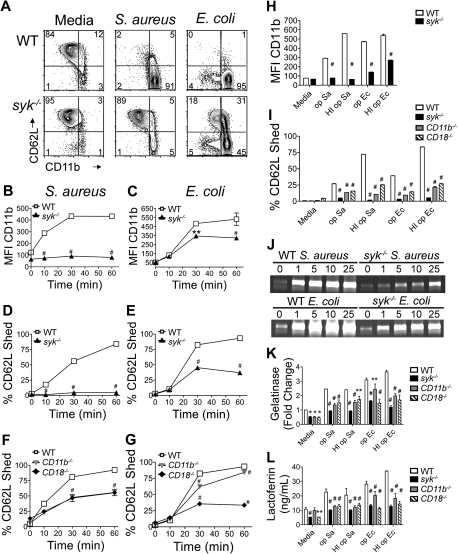
After neutrophil activation, secretory vesicles fuse with the plasma membrane. CD11b is stored in these vesicles26 and is redistributed on the plasma membrane after degranulation,27 coincident with loss of CD62L (L-selectin) from the surface.28 The requirement for Syk in bacterial-induced secretory vesicle degranulation was determined by the cell surface levels of CD11b and CD62L (Figure 1A). Bacteria were opsonized with serum isolated from rag1−/− mice to ensure that integrins were the primary leukocyte receptors engaged while avoiding antibody opsonization that could trigger Fc receptors. Exposure of WT neutrophils to opsonized S aureus or E coli resulted in a significant increase in CD11b on the cell surface. The median fluorescence intensity (MFI) of CD11b increased 4-fold at 30 to 60 minutes after stimulation with either bacteria (Figure 1B-C). Concomitant with increased CD11b expression was loss of CD62L from the surface of approximately 80% of WT neutrophils by 60 minutes (Figure 1D-E). Syk−/− neutrophils failed to up-regulate CD11b and shed CD62L after bacterial stimulation (Figure 1B-E), with a greater effect after S aureus incubation. Albumin secretion, another marker of vesicle degranulation, was also decreased in the absence of Syk (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). The ability to shed CD62L in response to S aureus was partially dependent on CD18 integrins, as neutrophils from CD11b−/− or CD18−/− mice showed an intermediate ability to shed CD62L (Figure 1F). CD62L shedding in response to E coli depended more highly on CD18 expression than CD11b expression (Figure 1G) and probably results from the contribution of CD11c (Complement Receptor 4). Secretory vesicle degranulation was also monitored in response to bacteria opsonized with heat-inactivated (HI) rag1−/− serum, to eliminate the presence of the complement ligand. However, up-regulation of CD11b and shedding of CD62L were still compromised in the absence of Syk, and CD62L shedding was compromised in the absence of CD11b and CD18 (Figure 1H-I). This suggests that there are other integrin ligands on the surface of S aureus and E coli, in addition to iC3b. It has been demonstrated that lipopolysaccharide and intact E coli can bind directly to CD18 integrins.29 In addition, S aureus expresses gene products that bind to fibronectin (Gene ID 3920926) or fibrinogen (Gene ID 3920717), allowing indirect associations with CD18 integrins, as well as β1 integrins.30
Figure 1.
Impaired degranulation in syk−/− neutrophils in response to bacteria. (A) Flow cytometric analysis of CD11b and CD62L levels on WT or syk−/− neutrophils stimulated for 60 minutes with rag1−/− serum-opsonized (op) S aureus (MOI = 5) or E coli (MOI = 10). Neutrophils of the indicated genotype were quantified for CD11b (MFI) after stimulation with (B) S aureus or (C) E coli; percentage CD62L− (shed) after stimulation with (D,F) S aureus, (E,G) E coli, or (H-I) bacteria opsonized with heat-inactivated (HI op) serum. (J) Release of gelatinase granules by WT or syk−/− neutrophils stimulated in suspension with S aureus or E coli for 60 minutes and analyzed by gelatinase zymogram. (K) Quantitation of gelatinase zymogram (MOI = 1). (L) Lactoferrin release (MOI = 1). Error bars represent SD. *P < .05, **P < .01, #P < .001 compared with WT by 2-way analysis of variance (ANOVA) with Bonferroni posttests. All data are representative of 3 or more independent experiments.
Release of the 3 major granule types was analyzed to determine the requirement for Syk in their exocytosis. Release of gelatinase granules (Figure 1J-K) and the secondary granule marker lactoferrin (Figure 1L) was impaired in syk−/−, CD11b−/−, and CD18−/− neutrophils in response to bacteria opsonized with rag1−/− and HI rag1−/− serum. There was no appreciable defect in the release of the azurophilic granule markers myeloperoxidase and β-glucoronidase (supplemental Figure 1).
Neutrophil cytokine secretion
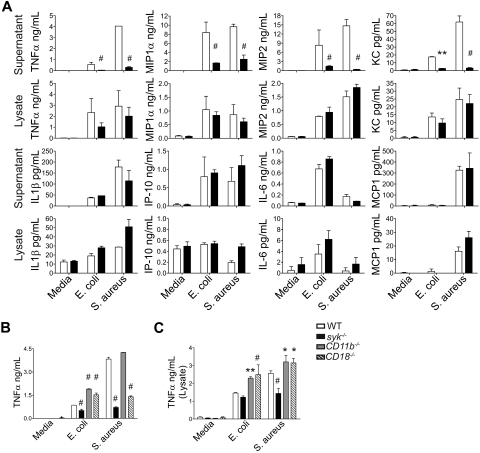
Neutrophils synthesize and secrete a variety of cytokines on encountering inflammatory stimuli.31 To determine whether Syk is required for neutrophil cytokine synthesis or secretion, a multiplex bead array was used to assess cytokine levels in supernatants and cell lysates of neutrophils stimulated with opsonized S aureus or E coli (Figure 2). Syk deficiency resulted in the decreased secretion of TNF-α, MIP1α, MIP2, and KC in response to bacterial stimuli, but relatively normal secretion of IL-1β, IL-6, 10 kDa IP-10, and MCP1. Analysis of the cytokine profiles in the cell pellets indicates that those dependent on Syk for secretion (TNF-α, MIP1α, MIP2, and KC) were induced and stored on stimulation. This is in contrast to cytokines that were not dependent on Syk for secretion (IL-1β, IL-6, IP-10, and MCP1), which were preformed and stored in granules before stimulation and release (IL-1β, IP-10) or directly secreted from the neutrophil without significant storage (IL-6, MCP-1). The ability of neutrophils to secrete TNF-α in response to S aureus was dependent on CD18 integrins, as demonstrated by reduced TNF-α secretion in supernatants from stimulated CD18−/− neutrophils. However, this response was not mediated through CD11b (Figure 2B). The decrease in TNF-α secretion is not the result of a reduction in mRNA transcription, as syk−/− neutrophils induced TNF-α mRNA equivalently to WT (supplemental Figure 2). These data indicate that Syk signaling through CD18 is required for release of a subset of inflammatory neutrophil cytokines.
Figure 2.
Syk-deficient neutrophils have altered cytokine profiles after stimulation with bacteria. (A) WT (white bars) or syk−/− (black bars) neutrophils were stimulated with rag1−/− serum-opsonized S aureus or E coli (MOI = 5) for 16 hours. Culture supernatants or cell pellets lysed in low detergent lysis buffer were analyzed for TNF-α, MIP1α, MIP2, KC, IL-1β, IP-10, or MCP1 by multiplex bead array (Milliplex). Data are the mean of 2 independent experiments, each with an N = 2 or N = 3. (B) WT, syk−/−, CD11b−/−, or CD18−/− neutrophils were stimulated with rag1−/− serum-opsonized S aureus or E coli (MOI = 2) for 16 hours and assessed for (B) TNF-α secretion into the supernatant or (C) TNF-α synthesis and storage in cell lysates by plate-based ELISA, and represent at least 3 independent experiments. Data are presented as mean ± SD. *P < .05, **P < .01, # P < .001 compared with WT by 2-way ANOVA with Bonferroni posttests.
Respiratory burst and elastase activation
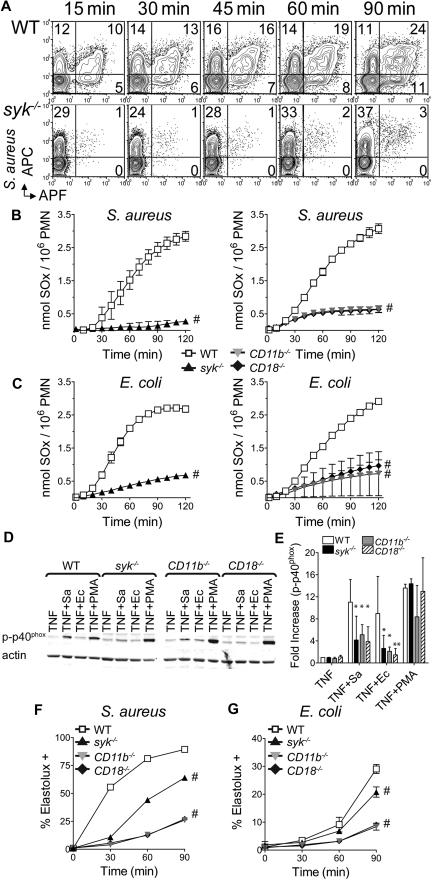
The requirement for neutrophil superoxide production in host defense against S aureus has been demonstrated in both human patients with chronic granulomatous disease32 and mouse mutants lacking the gp91phox or p40phox subunits of the NADPH-oxidase.33,34 The ability of WT or syk−/− neutrophils to generate superoxide in response to S aureus was assessed using a nonadherent flow cytometric assay. WT and syk−/− neutrophils bound similar levels of allophycocyanin (APC)–labeled S aureus, but a reduced percentage of syk−/− neutrophils produced superoxide, as detected by the fluorescent conversion of APF (Figure 3A). To independently measure the amount of superoxide produced, a plate-based cytochrome c reduction assay was used. After stimulation with S aureus or E coli, syk−/−, CD11b−/−, and CD18−/− produced less superoxide than WT neutrophils (Figure 3C-D). To determine whether the failure to produce superoxide was the result of a defect in activation of the NADPH-oxidase complex, the phosphorylation of p40phox, known to occur during NADPH-oxidase activation,35 was assessed. Compared with WT, phosphorylation of p40phox was reduced 2- to 4-fold in syk−/− neutrophils stimulated with S aureus or E coli (Figure 3D-E). A similar level of impairment of p40phox phosphorylation was observed with CD11b−/− and CD18−/− cells, again confirming that the majority of this signaling response was proceeding through the CD18/Syk pathway.
Figure 3.
Syk-deficient neutrophils display reduced integrin-mediated superoxide production in response to bacteria. (A) Flow cytometric detection of superoxide by fluorescence conversion of APF in WT or syk−/− neutrophils after stimulation with APC-labeled S aureus (MOI = 5). (B) WT, syk−/−, CD11b−/−, or CD18−/− neutrophils were plated in microtiter wells containing S aureus (MOI = 5) or (C) E coli (MOI = 10), in cytochrome c media. Production of superoxides (SOx) by respiratory burst was measured as reduction of cytochrome c. Data are representative of at least 3 independent experiments and are mean ± SD. (D) Western blot analysis of p40phox phosphorylation (Thr 154) after priming of neutrophils for 30 minutes with 50 ng/mL TNF-α and, where indicated, 30 minutes with S aureus (Sa) MOI = 20, E coli (Ec) MOI = 40, or 10nM PMA. (E) Quantitation of phospho-p40phox normalized to actin. (F-G) Intracellular activation of elastase was detected by flow cytometry after cleavage of ElastoLux. Neutrophils of the indicated genotype were stimulated with (F) S aureus or (G) E coli (MOI = 5) for the indicated time points. Data are representative of 3 independent experiments and are mean ± SD. *P < .05, **P < .01, #P < .001 compared with WT by ANOVA.
The requirement for neutrophil elastase has been demonstrated to have a specific role in host defense against Gram-negative bacteria, including E coli.36 To determine whether the CD18/Syk pathway is involved in elastase activation after stimulation with bacteria, WT, syk−/−, CD11b−/−, or CD18−/− neutrophils were stimulated with S aureus or E coli, and assessed by flow cytometry for intracellular elastase activity (Figure 3F-G). Both bacteria activated elastase in WT neutrophils, and this was decreased slightly in the absence of Syk. However, elastase activation in response to both bacteria was dependent on CD11b and CD18, indicating that bacterial killing by elastase activation remained intact in syk−/− neutrophils.
Signaling events
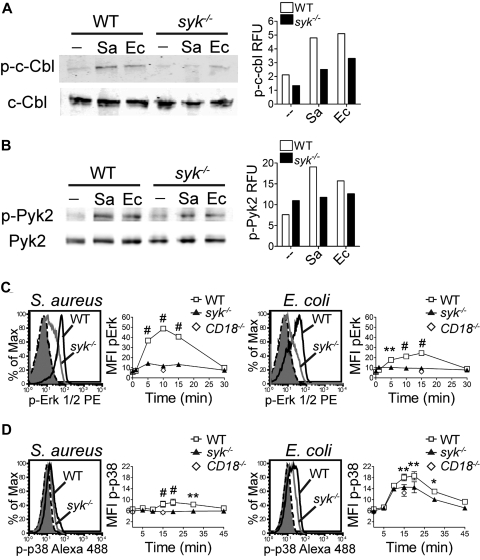
An assessment of the signaling events after stimulation with opsonized bacteria indicated that the phosphorylation of c-Cbl (Figure 4A) and Pyk2 (Figure 4B) was reduced in syk−/− neutrophils compared with WT. Given the reduced phosphorylation of the upstream signaling molecules c-Cbl and Pyk2, it was of interest to determine the phosphorylation status of Erk1/2 and p38. The magnitude of Erk1/2 phosphorylation was decreased in syk−/− neutrophils stimulated with S aureus or E coli (Figure 4C), with a maximal pErk1/2 signal in WT neutrophils at 10 minutes in response to S aureus, and at 15 minutes in response to E coli. The phosphorylation of p38 was marginally decreased in syk−/− neutrophils (Figure 4D). E coli stimulation induced a more robust level of p38 phosphorylation than S aureus, peaking at 20 minutes. CD18−/− neutrophils showed poor phosphorylation of Erk1/2, at levels equivalent to syk−/− cells, indicating that these pathways are activated, in part, through engagement of integrins. It is probable that much of the p38 phosphorylation caused by E coli reflects signaling through neutrophil TLR4 and hence is independent of the CD18/Syk pathway.
Figure 4.
Syk is required for normal phosphorylation events after bacterial stimulation. Western blot analysis of (A) c-Cbl or (B) Pyk2 phosphorylation after stimulation of WT or syk−/− neutrophils with S aureus (Sa) or E coli (Ec) for 10 minutes. For quantitation, c-Cbl and p-c-Cbl were detected on separate gels and were each normalized to actin; p-Pyk2 was normalized to total Pyk2 detected on the same gel. (C-D) Flow cytometric histograms of intracellular (C) p-Erk1/2 at 10 minutes in response to S aureus or at 15 minutes in response to E coli or (D) p-p38 at 20 minutes in response to S aureus or E coli. Histograms of unstimulated WT (black dash) or syk−/− (filled gray) and stimulated WT (black) or syk−/− (gray) neutrophils are shown. Time course quantitation is of the MFI. Error bars represent ± SD. *P < .05, **P < .01, #P < .001 by 2-way ANOVA with Bonferroni posttests. All data are representative of 3 or more independent experiments.
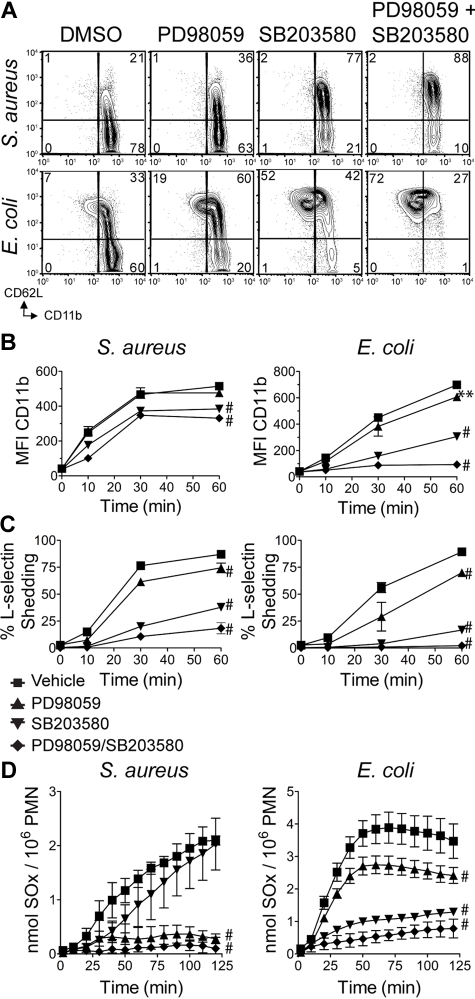
Given the strong phosphorylation of Erk1/2 and p38 in response to bacterial stimulation, it was important to determine whether decreased signaling through MEK/Erk and p38 affected neutrophil degranulation responses to rag1−/− serum-opsonized bacteria. WT neutrophils were treated with either the MEK-1 inhibitor PD98059 or the p38 inhibitor SB203580 before bacterial challenge. Treatment with SB203580 impaired secretory vesicle degranulation, as assessed by CD11b up-regulation and CD62L shedding, whereas treatment with PD98059 only weakly impaired CD62L shedding (Figure 5A-C). Interestingly, inhibition of p38 had a greater effect on both S aureus– and E coli–treated neutrophils, even though S aureus induced more robust Erk1/2 responses and less p38 activation. Treatment of WT neutrophils with both PD98059 and SB203580 synergized to inhibit CD11b up-regulation and CD62L shedding.
Figure 5.
Signaling through Erk and p38 is required for neutrophil degranulation events. (A) WT neutrophils pretreated with dimethyl sulfoxide vehicle, 20μM PD98059, 3μM SB203580, or both compounds were stimulated for 60 minutes with S aureus (MOI = 5) or E coli (MOI = 10), then analyzed by flow cytometry for CD11b and CD62L. Quantitation of (B) CD11b MFI ± SD, and (C) percentage of CD62L− mean ± SD. Data are ± SD and are representative of 3 or more independent experiments. (D) WT neutrophils pretreated as in panel A were plated in microtiter wells containing S aureus (MOI = 5) or E coli (MOI = 10), in cytochrome c media, and assessed for superoxide (SOx) production as in Figure 3. Data are representative of at least 3 independent experiments. Error bars represent ± SD. **P < .01, #P < .001, by 2-way ANOVA with Bonferroni posttests.
The relative requirement for signaling through the MEK/Erk versus p38 pathways for neutrophil oxidative burst in response to opsonized bacteria was compared. Treatment of WT neutrophils with PD98059 inhibited oxidative burst in response to S aureus, whereas treatment with SB203580 had a minimal effect (Figure 5D). In contrast, treatment of WT neutrophils with SB203580 significantly decreased the oxidative burst in response to E coli, whereas treatment with PD98059 resulted in a modest decrease (Figure 5D). Treatment of WT neutrophils with both PD98059 and SB203580 had a cumulative effect, causing significant inhibition of superoxide production to both organisms. These data support the predominant requirement for Erk signaling downstream of the CD18/Syk pathway in response to S aureus, whereas p38 signaling plays the major role in E coli–induced oxidative burst.
Phagocytosis and killing
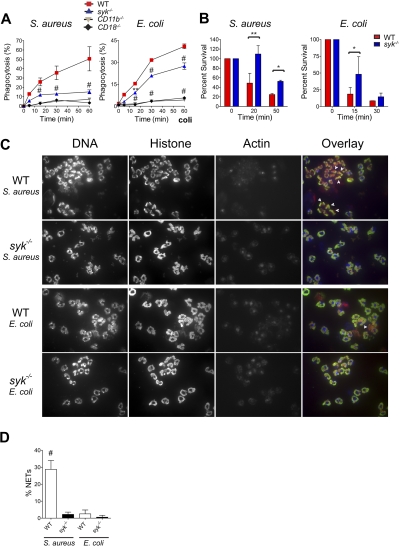
To determine whether Syk is required for the phagocytosis of opsonized bacteria, a flow cytometric assay was used. Syk−/− neutrophils displayed reduced S aureus phagocytosis but a more modest defect in E coli phagocytosis (Figure 6A). Phagocytosis of both of these bacteria is dependent on CD11b and CD18, as neutrophils deficient in either of these integrin components fail to take up bacteria. After stimulation with either bacteria, there is a rapid burst in actin polymerization, which is maintained in the absence of Syk (supplemental Figure 3), and is thus not the defect leading to reduced phagocytosis. To assess whether syk−/− neutrophils kill internalized bacteria, an in vitro phagocytosis and killing assay was used. In this assay, syk−/− neutrophils exhibited a reduced ability to kill internalized S aureus and, to a lesser extent, a reduced ability to kill internalized E coli (Figure 6B). Concomitant with uptake of S aureus or E coli, WT neutrophils formed large cellular aggregates, containing cells with nuclear disruption, loss of actin, and extracellular chromatin with trapped bacteria, suggestive of neutrophil extracellular trap (NET) formation (Figure 6C). The percentage of neutrophils that had formed NETs was quantified as those that had lost both the ring-shaped nuclear structure and excluded the actin cytoskeleton (Figure 6D). The aggregation of WT neutrophils and the presence of NETs were more pronounced in response to S aureus than E coli. In contrast, syk−/− neutrophils did not aggregate or form NETs after challenge with bacteria (Figure 6C-D).
Figure 6.
Syk is required for optimal phagocytosis and killing of rag1−/− serum-opsonized bacteria. (A) Phagocytosis was determined by flow cytometric analysis of FITC-labeled S aureus or E coli by WT, syk−/−, CD11b−/−, or CD18−/− neutrophils. (B) Intracellular bacterial killing capacity assessed by viable S aureus or E coli colony-forming units (CFUs). Data are the mean (n = 3 replicates) of the percentage remaining CFU relative to the addition of gentamicin (set as time = 0 minutes). (C) Cytospins of TNF-α primed neutrophils stimulated for 2 hours with S aureus or E coli and stained with 4,6-diamidino-2-phenylindole (DNA/red), antihistone antibody (green), and phalloidin (actin/blue). White arrowheads indicate cells classified as NETs. (D) NETs quantified by both loss of nuclear structure and exclusion of the actin cytoskeleton, expressed as a percentage of total neutrophils scored. Error bars represent ± SD. Data are representative of 3 independent experiments. *P < .05, **P < .01, #P < .001 by ANOVA.
Lineage-specific deletion of syk
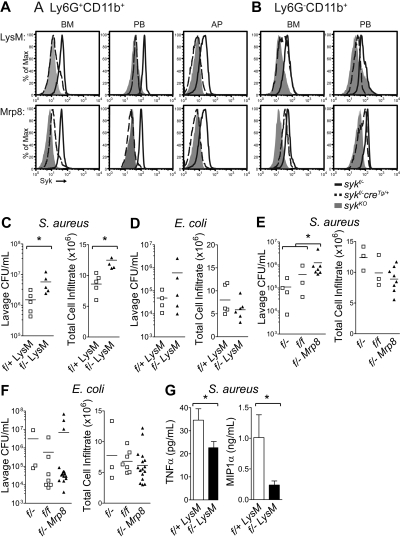
To determine the role of Syk in neutrophils in vivo, mice with loxP sites flanking exon 1 of syk16 were crossed to mice expressing the Cre recombinase under the control of the Lysozyme M promoter (LysMcreTg/Tg), restricting Cre activity to myeloid cells,17 or under the control of the human Mrp8 promoter (Mrp8creTg), restricting Cre activity to neutrophils.18 Sykfl/−LysMcreTg/+ and sykf/−Mrp8creTg mice were born at the expected Mendelian ratio and had normal differential blood leukocyte counts (Table 1), and normal vasculature (data not shown). Intracellular staining for Syk was used to investigate the loss of Syk protein (Figure 7A-B; Table 2). Analysis of bone marrow from sykfl/−LysMcreTg/+ revealed that approximately 40% of neutrophils (Ly6G+CD11b+) and approximately 60% of monocytes (Ly6G−CD11b+) were positive for Syk protein, whereas approximately 11% of neutrophils and approximately 60% of monocytes were positive for Syk in sykfl/−Mrp8creTg mice. Analysis of peripheral blood from sykfl/−LysMcreTg/+ mice indicates that approximately 20% of neutrophils and 40% to 50% of monocytes retained Syk protein, at a level lower than that of WT expression. In contrast, peripheral blood from sykfl/−Mrp8creTg mice indicates that approximately 12% of neutrophils and approximately 75% of monocytes retained Syk protein. Analysis of Syk expression in cells migrating into an inflammatory air pouch indicated that approximately 10% of neutrophils were positive for Syk protein in mice from either Cre strain. In summary, loss of Syk protein was incomplete in the bone marrow where neutrophils develop; however, as mature neutrophils enter the peripheral blood and sites of inflammation, protein loss increased up to 90%. Furthermore, LysMcre-mediated syk deletion occurred in neutrophils and monocytes, whereas Mrp8cre-mediated deletion occurred predominantly in neutrophils.
Table 1.
Complete blood leukocyte counts in conditionally deleted mice
| Neutrophils |
Monocytes |
Lymphocytes |
n | ||||
|---|---|---|---|---|---|---|---|
| Percentage | No. (×103 cells/uL) | Percentage | No. (×103 cells/uL) | Percentage | No. (×103 cells/uL) | ||
| sykf/−LysMcre | 20.8 ± 4.0 | 1.9 ± 0.4 | 3.0 ± 0.6 | 0.3 ± 0.1 | 76.2 ± 3.7 | 6.8 ± 0.4 | 5 |
| sykf/−Mrp8cre | 20.2 ± 4.7 | 1.9 ± 0.4 | 4.0 ± 2.8 | 0.3 ± 0.2 | 75.9 ± 7.0 | 7.8 ± 2.8 | 5 |
| sykf/f Vavcre | 51.3 ± 5.2* | 3.5 ± 1.3 | 3.1 ± 0.3 | 0.2 ± 0.04 | 44.5 ± 5.7* | 2.9 ± 0.5 | 3 |
| syk−/−chimera | 46.0 ± 0.9* | 1.6 ± 0.1 | 5.4 ± 1.0 | 0.2 ± 0.1 | 46.0 ± 1.8* | 1.6 ± 0.1* | 2 |
| sykf/f | 23.1 ± 9.0 | 1.6 ± 0.7 | 3.4 ± 1.1 | 0.3 ± 0.1 | 73.1 ± 9.0 | 5.8 ± 3.5 | 3 |
| sykf/− | 22.6 ± 3.9 | 2.2 ± 0.7 | 3.1 ± 0.7 | 0.3 ± 0.1 | 73.6 ± 3.1 | 7.2 ± 1.8 | 5 |
| sykf/+LysMcre | 22.0 ± 2.7 | 2.0 ± 0.2 | 2.6 ± 0.3 | 0.2 ± 0.1 | 75.1 ± 3.5 | 6.8 ± 1.3 | 3 |
The data represent the mean ± SD of the indicated measurement of complete blood count with cellular differential, assessed by a Hemavet 950FS (Drew Scientific) multispecies hematology instrument.
P < .05 by one-way analysis of variance with Bonferroni posttests.
Figure 7.
Neutrophil-specific deletion of Syk results in a defective host response to S aureus. Analysis of syk deletion by flow cytometry of Syk protein in (A) neutrophils (Ly6G+CD11b+) and (B) monocytes (Ly6G−CD11b+) from the bone marrow (BM), peripheral blood (PB), and air pouch lavage (AP). SykKO cells from syk−/− chimeras or syk f/fVav1creTg mice are negative controls (gray histograms) relative to sykf/−LysMcreTg/+, sykf/−Mrp8creTg (dashed black lines), or sykf/− (solid black line) mice. Histograms are representative of at least 4 animals. (C) CFU of S aureus and neutrophil infiltrate and (D) CFU of E coli and neutrophil infiltrate in the air pouch lavage of sykf/+LysMcreTg/+ and sykf/−LysMcreTg/+ mice 24 hours after the initiation of infection. (E) CFU of S aureus and neutrophil infiltrate and (F) CFU of E coli and neutrophil infiltrate in the air pouch lavage of sykf/f, sykf/−, and sykf/−Mrp8creTg mice 24 hours after the initiation of infection. Data represent at least 3 independent experiments, with at least 3 animals per group. The difference in S aureus counts in the air pouch is statistically significant; *P < .05 by Mann-Whitney U test. (G) Analysis of TNF-α and MIP-1α levels in S aureus–infected air pouches from sykf/+LysMcreTg/+ and sykf/−LysMcreTg/+ mice. *P < .05, unpaired t test.
Table 2.
Syk protein level in conditionally deleted mice
| Bone marrow |
Peripheral blood |
Air pouch |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Ly6G+ CD11b+ | Ly6G− CD11b+ | n | Ly6G+ CD11b+ | Ly6G− CD11b+ | n | Ly6G+ CD11b+ | Ly6G− CD11b+ | n | |
| sykf/−LysMcre | 39 ± 10 | 57 ± 7 | 6 | 20 ± 11 | 45 ± 12 | 29 | 9 ± 8 | 29 ± 19 | 11 |
| sykf/−Mrp8cre | 11 ± 2 | 58 ± 10 | 9 | 12 ± 4 | 75 ± 5 | 17 | 9 ± 6 | 46 ± 22 | 9 |
| sykf/f Vavcre | ND | ND | 2 ± 2 | 2 ± 2 | 10 | 1 ± 0 | 5 ± 3 | 7 | |
| syk−/−chimera | 1 ± 0 | 8 ± 2 | 3 | 1 ± 0 | 11 ± 10 | 9 | ND | ND | |
| sykf/f | 95 ± 5 | 93 ± 0 | 5 | 85 ± 3 | 99 ± 1 | 16 | 84 ± 19 | 68 ± 8 | 7 |
| sykf/− | 90 ± 2 | 74 ± 14 | 4 | 92 ± 6 | 62 ± 12 | 8 | 91 ± 3 | 71 ± 11 | 4 |
| sykf/+LysMcre | 98 ± 1 | 78 ± 7 | 5 | 96 ± 3 | 89 ± 3 | 18 | 68 ± 9 | 76 ± 4 | 5 |
The data represent the mean ± SD of the percentage Syk+ cells as assessed by intracellular flow cytometry. For each analysis, the respective cell type from sykf/fVavcreTg or syk−/− mice was used as a negative control for Syk protein level and used to set the lower limit gate.
ND indicates not done.
Bacterial clearance in a skin abscess model
A skin abscess model was used to determine whether the observed defects in neutrophil activation in the absence of Syk resulted in an increased bacterial susceptibility. At 24 hours after bacterial infection, 80% to 90% of the cells recruited into the skin abscess were neutrophils, as defined by cell surface expression of Ly6G and CD11b (supplemental Figure 4). Mice with Syk deficiency in the myeloid lineage (sykf/−LysMcreTg/+) had a 3- to 4-fold increase in the number of viable S aureus recovered from a subcutaneous abscess, despite enhanced migration of neutrophils into the site of infection (Figure 7C). Although there was a tendency toward higher E coli bacterial counts in sykf/−LysMcreTg/+ mice, this result was variable and not statistically significant (Figure 7D). Using the Mrp8cre model for neutrophil-restricted deletion, a similar pattern was observed. Sykf/−Mrp8creTg mice had a 4-fold increase in the number of viable S aureus recovered from a subcutaneous abscess, despite equivalent migration of neutrophils (Figure 7E). Similarly, sykf/−Mrp8creTg mice, although having a tendency toward higher bacterial burden, exhibited a near-normal ability to clear E coli and displayed normal neutrophil migration (Figure 7F). Control mice sykf/f and sykf/− showed no differences, ruling out effects from heterozygosity of the syk gene.
Cytokine analysis after S aureus challenge indicated that the levels of TNF-α and MIP-1α were decreased in the air pouch lavage in sykf/−LysMcreTg/+ animals relative to sykf/+LysMcreTg/+ littermate controls (Figure 7G), supporting the impaired cytokine release by syk−/− neutrophils (Figure 2). The residual level of TNF-α and MIP-1α may be sufficient to induce neutrophil recruitment to the infected site of sykf/−LysMcreTg/+ mice. Alternatively, other cytokines secreted by neutrophils or other cell types may act to recruit neutrophils after bacterial infection.
In a CD11b-dependent S aureus challenge of the peritoneum, both sykf/−LysMcreTg/+ mice and CD11b−/− mice had an increased bacterial burden relative to their WT controls (supplemental Figure 5). This supports the requirement for Syk signaling downstream of CD18 integrins for S aureus clearance at an additional tissue site.
Discussion
In this study, we report that Syk-deficient neutrophils fail to secrete granule components, release induced cytokines, and generate ROIs, while retaining partial elastase activity in response to S aureus and E coli. This results in a reduced ability of syk−/− neutrophils to kill bacteria in vitro. We also measured the consequences of Syk deficiency in vivo; and although neutrophil migration is normal in the absence of Syk, the defects in neutrophil function result in impaired killing of S aureus and, to a lesser extent, E coli. These data indicate a requirement for Syk signaling during innate immune responses to bacteria.
Syk was previously shown to be required for CD18 integrin-dependent adhesive responses.10 Our results implicate Syk in host responses to bacterial infection. In particular, the exocytosis of granules containing cytokines that are induced and stored before secretion are impaired in Syk-deficient neutrophils. The mechanisms and location of neutrophil cytokine production, storage, and exocytosis are relatively poorly understood. It has been demonstrated that human IL-8 is produced and released from activated neutrophils in vitro and is stored in an organelle distinct from classically defined granules and secretory vesicles.37 The production of IL-8 by neutrophils is initiated by accumulation of mRNA transcripts, followed by posttranscriptional and posttranslational regulation, varying in response to the stimulus.31 The normal accumulation of TNF-α mRNA and protein in syk−/− neutrophils suggests that the kinase is not involved in these initial steps but has a primary function in granule release. Why syk−/− neutrophils fail to overaccumulate cytokines resulting from impaired release is unclear. Because neutrophils themselves are responsive to TNF-α, MIP1α, MIP2, and KC, the reduced secretion of these cytokines by Syk-deficiency could result in a reduction in feedback responses, leading ultimately to reduced storage or increased cellular turnover of these cytokines.
Previous studies demonstrated that macrophages38 and myeloid dendritic cells39 require Syk for negative regulation of cytokine secretion in response to bacterial cell wall products. Our data demonstrate that neutrophils require Syk for maximal secretion of inflammatory cytokines in response to serum-opsonized bacteria. This suggests that the use of Syk-dependent signaling events differs between neutrophils, macrophages, and myeloid dendritic cells after stimulation with bacteria. Analysis of signaling events triggered during serum-opsonized bacterial stimulation support this finding, in that syk−/− neutrophils have decreased phosphorylation of Erk1/2 in contrast to hyperphosphorylation of Erk1/2 in macrophages in response to lipopolysaccharide.38 Other studies ruled out a role for Syk in complement-mediated phagocytosis of E coli40 or zymosan15 in macrophages, which contrasts with our results of reduced phagocytosis of S aureus and, to a lesser extent, E coli, in syk−/− neutrophils. This again highlights the diverse roles that Syk plays in different cell types.
Here we show that Syk is required for CD18-dependent responses to bacteria in neutrophils, illustrating the importance of integrin signaling during innate immune responses. Our results support and extend a previous report demonstrating the requirement of CD18 for efficient phagocytosis and ROI production in response to serum-opsonized bacteria.41 One important difference between the receptor, CD18, and the signaling molecules, namely, Syk, is that, whereas CD18 is required for normal intracellular elastase activation in response to both S aureus and E coli, Syk is only partially required for this response. The failure of CD18−/− neutrophils to phagocytose complement-opsonized bacteria could explain this phenotypic divergence. The requirement of Syk signaling for ROI production, but not elastase activation, has important implications for bacterial clearance. Mice deficient in components of the NADPH-oxidase complex are more susceptible to S aureus challenge than E coli, indicating that ROI production has less of a protective role in Gram-negative infections.33,34 Conversely, mice deficient in neutrophil elastase are more susceptible to E coli but relatively resistant to S aureus.36 Thus, the increased susceptibility of mice lacking Syk in neutrophils to S aureus probably results from their inability to produce ROIs, whereas the less pronounced susceptibility to E coli could be the result of retention of elastase activity.
CD11b and CD18 are required for a robust clearance of S aureus after air pouch challenge42 (and J.A.V.Z. and C.A.L., unpublished data, September 2009). The susceptibility of mice lacking Syk in myeloid cells, and specifically in neutrophils, to S aureus after air pouch challenge supports an in vivo requirement for Syk-signaling downstream of CD18 integrins. Given that Syk has a dominant role in neutrophil-dependent host defense responses relative to macrophages, the impaired clearance of bacteria in the sykf/−LysMcreTg/+ mice probably represents neutrophil dysfunction. This is further supported by the impaired clearance of bacteria in the sykfl/−Mrp8creTg mice, where syk deletion is restricted to neutrophils. However, in both our CreTg lines, neutrophil deletion of syk was not complete, as determined by flow cytometry. Hence, the reduced ability of sykf/−LysMcreTg/+ and sykfl/−Mrp8creTg mice to clear bacterial infections probably represents an underestimate of the role of Syk in host defense. More complete deletion would probably result in more impaired bacterial clearance in vivo. However, the syk−/− radiation chimeras are not a suitable choice to resolve this potential issue because of the complication of the vascular phenotype.15 The concept that impaired signaling through neutrophil CD18 integrins can lead to impaired host defense is also evidenced by reduced clearance of S aureus in Vav1/3-deficient mice during a lung infection.43 Vav proteins are known to be required downstream of Syk in the leukocyte integrin signaling pathway.44
E coli and S aureus can trigger a different variety of activating receptors on the neutrophil surface besides CD11b, probably contributing to the differences in neutrophil effector phenotypes between syk−/− and CD18−/− cells. These receptors include TLRs (TLR4 vs TLR2), C-type lectins, and Ig-superfamily members, such as TREM-145 and TREM-2.46 In addition, CD11c/CD18 dimers may recognize bacterial ligands, for example, on S aureus, which would explain normal TNF-α release from CD11b−/− compared with CD18−/− neutrophils. It is probable that several receptors, not just CD18, are engaged during neutrophil responses to bacteria, particularly in vivo where the bacteria are not directly incubated with serum opsonins. In addition, Syk is required for signal transduction from receptors other than CD18, such as TREM-1 and TREM-2, which are known to signal via ITAM-bearing molecules, and some C-type lectins, including Dectin-1, which signal via an ITAM-like motif.47 Hence, it is possible that some of the host defense deficits in Syk-deficient neutrophils may represent defects in signaling pathways other than CD18 integrins. This may explain why some of the functional defects of syk−/− cells, such as TNF-α secretion, appear to be CD18 independent. For example, Syk is involved in coupling TLR signaling to downstream pathways through CARD9,48 which could lead to impaired TNF-α release after E coli challenge.
Overall, these results demonstrate that Syk is a critical signaling molecule for neutrophil effector responses to bacteria. Although inhibitors of Syk are being actively pursued in clinical trials for the treatment of neutrophil-dependent inflammatory diseases, the data presented here raise the possibility that the use of such inhibitors may reduce the ability of patients to clear certain types of bacterial infections, in particular those arising from Gram-positive bacteria. Recent demonstrations of the involvement of Syk in antifungal host defenses, through signaling involving inflammasome activation or Dectin-1, also raise concern that anti-Syk therapeutics may put patients at risk for these types of infections.49,50 A deeper understanding of Syk signaling in neutrophils during infection and inflammation may provide a rationale for differential dosing of Syk inhibitors, allowing attenuation of tissue-destructive inflammation while maintaining Syk-dependent microbicidal activity.
Supplementary Material
Acknowledgments
The authors thank P. Scapini, C. Abram, A. Miller, and L. Kamen for thoughtful discussion and assistance throughout this project; Dr A. Weiss for the Syk antibody; Dr A. Tarakhovsky for the syk conditional knockout mice; and Dr E. Passegué for the Mrp8-cre transgenic mice.
This work was supported by the National Institutes of Health (grants RO1 AI065495 and RO1 AI068150) (C.A.L.) and a Canadian Institutes of Health Research doctoral research award (J.A.V.Z.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.A.V.Z. performed experiments; and J.A.V.Z. and C.A.L. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clifford A. Lowell, Department of Laboratory Medicine, University of California, San Francisco, 513 Parnassus Ave, San Francisco, CA 94143-0451; e-mail: clifford.lowell@ucsf.edu.
References
- 1.Etzioni A, Doerschuk CM, Harlan JM. Of man and mouse: leukocyte and endothelial adhesion molecule deficiencies. Blood. 1999;94(10):3281–3288. [PubMed] [Google Scholar]
- 2.Bunting M, Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. Leukocyte adhesion deficiency syndromes: adhesion and tethering defects involving beta 2 integrins and selectin ligands. Curr Opin Hematol. 2002;9(1):30–35. doi: 10.1097/00062752-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Rosenzweig SD, Holland SM. Phagocyte immunodeficiencies and their infections. J Allergy Clin Immunol. 2004;113(4):620–626. doi: 10.1016/j.jaci.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Scharffetter-Kochanek K, Lu H, Norman K, et al. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188(1):119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berton G, Lowell CA. Integrin signalling in neutrophils and macrophages. Cell Signal. 1999;11(9):621–635. doi: 10.1016/s0898-6568(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 6.Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21(2):215–226. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7(12):1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26(4):208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Abram CL, Lowell CA. Convergence of immunoreceptor and integrin signaling. Immunol Rev. 2007;218:29–44. doi: 10.1111/j.1600-065X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 10.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16(4):547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 11.Hirahashi J, Mekala D, Van Ziffle J, et al. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 2006;25(2):271–283. doi: 10.1016/j.immuni.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Graham DB, Zinselmeyer BH, Mascarenhas F, Delgado R, Miller MJ, Swat W. ITAM signaling by Vav family Rho guanine nucleotide exchange factors regulates interstitial transit rates of neutrophils in vivo. PLoS ONE. 2009;4(2):e4652. doi: 10.1371/journal.pone.0004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner M, Mee PJ, Costello PS, et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378(6554):298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 14.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378(6554):303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 15.Kiefer F, Brumell J, Al-Alawi N, et al. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol Cell Biol. 1998;18(7):4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saijo K, Schmedt C, Su IH, et al. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4(3):274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 17.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 18.Passegue E, Wagner EF, Weissman IL. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell. 2004;119(3):431–443. doi: 10.1016/j.cell.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 19.de Boer J, Williams A, Skavdis G, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33(2):314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 20.Palacios EH, Weiss A. Distinct roles for Syk and ZAP-70 during early thymocyte development. J Exp Med. 2007;204(7):1703–1715. doi: 10.1084/jem.20070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereira S, Lowell C. The Lyn tyrosine kinase negatively regulates neutrophil integrin signaling. J Immunol. 2003;171(3):1319–1327. doi: 10.4049/jimmunol.171.3.1319. [DOI] [PubMed] [Google Scholar]
- 22.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133(4):895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mocsai A, Ligeti E, Lowell CA, Berton G. Adhesion-dependent degranulation of neutrophils requires the Src family kinases Fgr and Hck. J Immunol. 1999;162(2):1120–1126. [PubMed] [Google Scholar]
- 24.Bjerknes R, Bassoe CF. Phagocyte C3-mediated attachment and internalization: flow cytometric studies using a fluorescence quenching technique. Blut. 1984;49(4):315–323. doi: 10.1007/BF00320205. [DOI] [PubMed] [Google Scholar]
- 25.Matute-Bello G, Frevert CW, Kajikawa O, et al. Septic shock and acute lung injury in rabbits with peritonitis: failure of the neutrophil response to localized infection. Am J Respir Crit Care Med. 2001;163(1):234–243. doi: 10.1164/ajrccm.163.1.9909034. [DOI] [PubMed] [Google Scholar]
- 26.Sengelov H, Kjeldsen L, Diamond MS, Springer TA, Borregaard N. Subcellular localization and dynamics of Mac-1 (alpha m beta 2) in human neutrophils. J Clin Invest. 1993;92(3):1467–1476. doi: 10.1172/JCI116724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borregaard N, Kjeldsen L, Sengelov H, et al. Changes in subcellular localization and surface expression of L-selectin, alkaline phosphatase, and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J Leukoc Biol. 1994;56(1):80–87. doi: 10.1002/jlb.56.1.80. [DOI] [PubMed] [Google Scholar]
- 28.Tellier E, Canault M, Rebsomen L, et al. The shedding activity of ADAM17 is sequestered in lipid rafts. Exp Cell Res. 2006;312(20):3969–3980. doi: 10.1016/j.yexcr.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Wright SD, Jong MT. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164(6):1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz-Linek U, Hook M, Potts JR. The molecular basis of fibronectin-mediated bacterial adherence to host cells. Mol Microbiol. 2004;52(3):631–641. doi: 10.1111/j.1365-2958.2004.04027.x. [DOI] [PubMed] [Google Scholar]
- 31.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 32.Liese J, Kloos S, Jendrossek V, et al. Long-term follow-up and outcome of 39 patients with chronic granulomatous disease. J Pediatr. 2000;137(5):687–693. doi: 10.1067/mpd.2000.109112. [DOI] [PubMed] [Google Scholar]
- 33.Pollock JD, Williams DA, Gifford MA, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9(2):202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 34.Ellson CD, Davidson K, Ferguson GJ, O'Connor R, Stephens LR, Hawkins PT. Neutrophils from p40phox−/− mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J Exp Med. 2006;203(8):1927–1937. doi: 10.1084/jem.20052069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouin AP, Grandvaux N, Vignais PV, Fuchs A. p40(phox) is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase: implication of a protein kinase c-type kinase in the phosphorylation process. J Biol Chem. 1998;273(46):30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- 36.Belaaouaj A, McCarthy R, Baumann M, et al. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4(5):615–618. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- 37.Pellme S, Morgelin M, Tapper H, Mellqvist UH, Dahlgren C, Karlsson A. Localization of human neutrophil interleukin-8 (CXCL-8) to organelle(s) distinct from the classical granules and secretory vesicles. J Leukoc Biol. 2006;79(3):564–573. doi: 10.1189/jlb.0505248. [DOI] [PubMed] [Google Scholar]
- 38.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6(6):579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu CL, Yu YL, Shen KY, Lowell CA, Lanier LL, Hamerman JA. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRgamma. Eur J Immunol. 2008;38(1):166–173. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley MT, Costello PS, Fitzer-Attas CJ, et al. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186(7):1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson KE, Boyle K, Davidson K, et al. CD18-dependent activation of the neutrophil NADPH oxidase during phagocytosis of Escherichia coli or Staphylococcus aureus is regulated by class III but not class I or II PI3Ks. Blood. 2008;112(13):5202–5211. doi: 10.1182/blood-2008-04-149450. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Mocsai A, Zhang H, et al. Role for plastin in host defense distinguishes integrin signaling from cell adhesion and spreading. Immunity. 2003;19(1):95–104. doi: 10.1016/s1074-7613(03)00172-9. [DOI] [PubMed] [Google Scholar]
- 43.Graham DB, Robertson CM, Bautista J, et al. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCgamma2 signaling axis in mice. J Clin Invest. 2007;117(11):3445–3452. doi: 10.1172/JCI32729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gakidis MA, Cullere X, Olson T, et al. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166(2):273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164(10):4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 46.Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171(2):594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- 47.Reid DM, Gow NA, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21(1):30–37. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruland J. CARD9 signaling in the innate immune response. Ann N Y Acad Sci. 2008;1143:35–44. doi: 10.1196/annals.1443.024. [DOI] [PubMed] [Google Scholar]
- 49.Gross O, Poeck H, Bscheider M, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459(7245):433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 50.Gringhuis SI, den Dunnen J, Litjens M, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009;10(2):203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.