Abstract
Mother rabbits nurse their young once a day with circadian periodicity. Nursing bouts are brief (ca. 3 min) and occur inside the maternal burrow. Despite this limited contact mother rabbits and their pups are tuned to each other to ensure that the capacities of each party are used efficiently to ensure the weaning of a healthy litter. In this review we present behavioral, hormonal, metabolic and hormonal correlates of this phenomenon in mother rabbits and their pups. Research is revealing that the circadian rhythm of locomotion shifts in parallel to the timing of nursing in both parties. In pups corticosterone has a circadian rhythm with highest levels at the time of nursing. Other metabolic and hormonal parameters follow an exogenous or endogenous rhythm which is affected by the time of nursing. In the brain clock genes (e.g., Per1) are differentially expressed in specific brain regions (e.g., suprachiasmatic nucleus, paraventricular nucleus) in relation to providing or ingesting milk in mothers and young, respectively. These findings suggest that circadian activities are modulated, in the mothers, by suckling stimulation and, in the young, by the ingestion of milk and/or the perception of the mammary pheromone. In conclusion the rabbit pup is an extraordinary model for studying the entraining by a single daily food pulse with minimal manipulations. The mother offers the possibility of studying nursing as a non-photic synchronizer, also with minimal manipulation, as suckling stimulation from the litter occurs only once daily
Keywords: corticosterone, clock genes, lactation, circadian rhythms, developmental biology
Introduction
The rabbit has a unique pattern of nursing: it occurs only once a day, with circadian periodicity, throughout lactation. Moreover, studies in the wild (Lloyd & McCowan, 1968; Broekhuizen & Mulder, 1983) and in the laboratory (Zarrow et al., 1965; Lincoln, 1974; Drewett et al., 1982; González-Mariscal et al., 1994) have consistently found that the daily nursing bout is very brief (ca. 3 min). Nursing takes place inside the maternal nest, which the mother constructs (with straw and body hair) across late pregnancy (González-Mariscal et al., 1994). When allowed free access to their litters doe rabbits kept under a 12:12 h L:D cycle nurse the pups during the dark phase (Jilge, 1993). When kept under continuous light (in a noise-isolated facility) the time when the single daily nursing bout occurs is slightly advanced across the first week, by an average of 42 min ±16 min/day. By around day 10 the nursing rhythm free-runs in parallel with other rhythms (i.e., locomotion and hard faeces excretion; Jilge, 1995).
Pups show a clear locomotor anticipatory behavior to the arrival of the nursing mother (Jilge 1993, 1995; Caba et al., 2008) and the locomotor behavior of the lactating mother also shifts in parallel to the timing of nursing (Meza et al., 2008). Thus, the rabbit provides a unique model for studying non-photic synchronization from a dual perspective, i.e., the mother’s and the litter’s, which are the provider and the recipient of nourishment, respectively. The synchronization of the activity rhythms of both parties is essential for the progress of lactation, the process culminating the reproductive cycle in mammals.
The suprachiasmatic nucleus (SCN) is the master biological clock responsible for the generation of physiological and behavioral circadian rhythms in mammals (Klein et al., 1991). Light is the main synchronizer of the SCN (Takahashi et al., 1984) but there are other stimuli capable of achieving the same effect, known collectively as non-photic zeitgebers. These stimuli include: novelty, and food availability, as well as internal cues like melatonin, GABA and serotonin (Hastings et al., 1998; Challet & Pevet, 2003).The impact of restricting food availability to certain times of the day on the rhythms of specific behavioral and physiological processes has been studied mainly in male rodents. Rats fed one meal per day display an increase in wheel running activity hours before mealtime, an effect known as food anticipatory activity (FAA; Mistlberger, 1994). The experimental protocol to induce FAA in rodents consists of initially providing subjects with food ad libitum, then restricting it to specific hours, usually during daytime, switching to ad libitum again, and finally to fasting (Mistlberger, 1994; Stephan, 2002). In addition to locomotor activity other physiological and neural parameters are entrained by the periodic exposure to food, a finding suggesting the existence of a food-entrainable oscillator (FEO), separable from the SCN (reviewed in: Mistlberger, 1994; Stephan, 2002). Because rabbit pups are normally fed by nursing only once a day and this event entrains specific physiological and behavioral parameters in them (see below), as occurs in adult rodents under FAA, we will call this phenomenon “nursing anticipatory activity” (NAA). Because in rabbit pups a variety of behavioral and physiological parameters are entrained by the nursing pulse it was proposed (Jilge 1993, 1995; Jilge et al., 2000) that such anticipatory activity could be controlled by a FEO similar to the one proposed for adult animals. Thus, the rabbit offers the conditions that make it an ideal model for studying the phenomenon of entraining by a single daily food pulse with minimal manipulations.
Circadian rhythms in the mother rabbit
Little is known about the circadian periodicity of behavioral or physiological rhythms in pregnant does. If kept under constant light and sound isolation their body temperature shows a circadian pattern characteristic of nocturnal animals. Across the last two thirds of pregnancy a gradual but steady decline in average body temperature is apparent. During delivery it rises and, across lactation, it remains stable at the levels seen in early gestation (Jilge et al., 2001). Parturition itself is a rapid event (lasting around 10 min; Hudson et al., 1999), tightly controlled by the decline in progesterone seen in late pregnancy (González-Mariscal et al., 1994) and the massive release of oxytocin which triggers uterine contractions (Fuchs & Dawood, 1980). Under constant light and sound isolation conditions parturitions occur at any time of day, but usually coinciding with the resting period of the does (Jilge, 1995). When kept under a 12:12 h L:D cycle no clear pattern of the timing of delivery was evident but half of the does gave birth within 2 h of lights-on (Jilge, 1993). Whether the timing of parturition is associated with a preferential release of oxytocin at specific hours of the day remains to be determined, but it has been reported that the injection of this peptide during the light period to late pregnant rabbits provokes aberrant deliveries (Hudson et al., 1995).
Jilge and colleagues (2001) were the first to recognize that daily suckling by the pups is also a strong signal for the does. During pregnancy the doe has a circadian rhythm of temperature with a peak that free runs with a period length greater than 24 h (Jilge et al., 2001). Interestingly, after parturition, the temperature peak coincides with the phase of the daily nursing bout (Jilge et al., 2001). Moreover, before nursing there is a clear elevation of body temperature that peaks at the time of nursing (see Fig. 6 in Jilge et al., 2001). Thus, mother rabbits show an anticipation of a specific physiological event (elevation of peak body temperature) in relation to the daily suckling episode, as occurs with the pups (see below).
Fig 6.

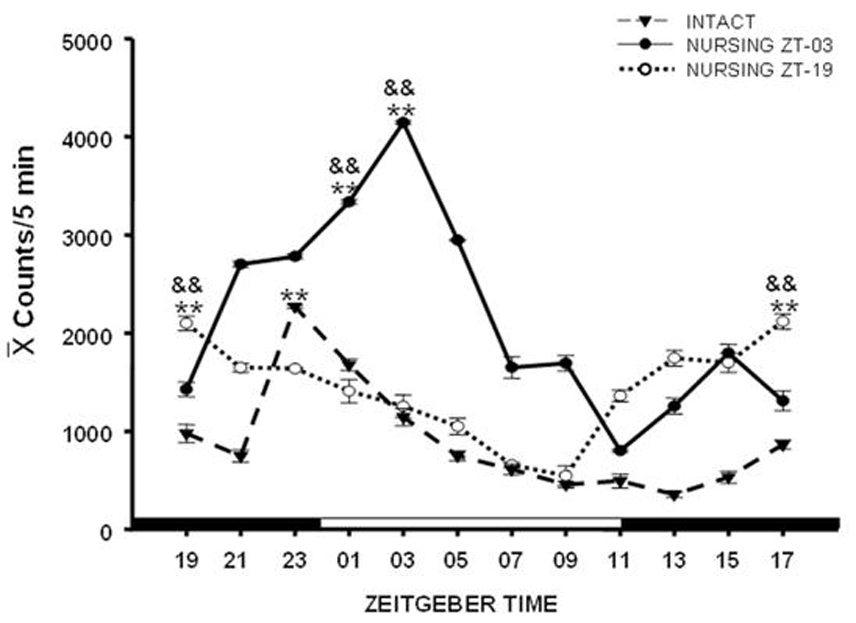
Additionally, the locomotor behavior of the doe shifts in parallel to the timing of suckling (Fig. 1; Meza et al., 2008). Moreover, the PERIOD1 (PER1) protein, product of the Per1 clock gene has a rhythmic expression in the supraoptic (SON) and paraventricular (PVN) nuclei of the hypothalamus in intact, non-pregnant, non-lactating rabbits. Yet, during lactation the rhythm of PER1 in both nuclei shifts in parallel to the timing of suckling (Meza et al., 2008). As this effect occurs mainly in oxytocinergic cells we proposed (Meza et al., 2008) that suckling stimulation could regulate neuronal activities in these nuclei, among them the synthesis and/or release of oxytocin. In summary, the above evidence indicates that the pups, probably through their suckling stimulation, are a powerful entraining signal for the mother doe. On the other hand, the observation that specific clock genes in the hypothalamus of the lactating doe oscillate with a different phase from that seen in the SCN supports the proposal that entraining of clock genes can vary in different areas of the brain to support a particular physiological condition in the subject (Kriegsfeld & Silver, 2006; Guilding & Piggins, 2007).
Fig 1.
As mentioned earlier, independent groups of investigators have confirmed in several breeds that does nurse their litter for around 3 min (Lincoln, 1974; Drewett et al., 1982; González-Mariscal et al., 1994). Yet, the apparent invariability in the duration of the nursing bout is challenged when the number of suckling pups is deliberately varied. Providing three or more young results in a normal 3 min nursing bout but when only 1 or 2 young are given mothers stay a much longer time inside the nest box. Various experimental manipulations that alter the quantity or quality of suckling stimulation (e.g., sealing the pups’ mouths, covering the mother’s nipples) provoke the same effect (González-Mariscal, 2007). From these results we can conclude that a threshold of suckling stimulation is required to trigger the operation of a neural mechanism that determines a fixed duration of the daily nursing bout. Moreover, in preliminary studies we have found that the number of pups may also modulate the circadian rhythms of nursing as mother rabbits given only 1–2 pups enter the nest box several times a day (González-Mariscal et al., in preparation).
Behavioural and physiological anticipation of nursing in rabbit pups
Rabbit pups are born altricial, i.e. furless, with their eyelids and ears sealed, unable to regulate their body temperature and showing a poor motor coordination. Most of the time pups remain huddled together, well covered by the material of the nest, a strategy allowing them to maintain a stable body temperature. However, around 2 hours before nursing, they become more active and rid themselves of the nest material. Thus, when the mother enters the nest she simply crouches over the pups, which immediately start searching for her nipples, guided by the olfactory cue of the so-called “mammary pheromone” (MP; Hudson & Distel, 1983), recently identified as 2-methyl-but-2-enal (Schaal et al., 2003). Following the brief nursing bout (see above) they again cover themselves with the nest material and repeat this cycle approximately 24 hrs later (Hudson & Distel, 1989).
Locomotor behavior
The nursing anticipatory activity (NAA) of rabbit pups has been recorded by three methods: visual inspection (Hudson & Distel, 1989), vibration on a scale (where the litter and the nest are placed; Pongrácz & Altbäcker, 2003) and automatic generation of actograms (Jilge, 1993, 1995). These three methods coincide in showing that the daily visit of the mother is preceded by an increase in the locomotor activity of the pups, which occurs between 1–3 h before the usual time of nursing. As occurs with the mother (see above) the pup’s locomotor activity gradually shifts across the first week of lactation to the middle or the first half of the dark phase with τ<24 h (Jilge, 1993, 1995).
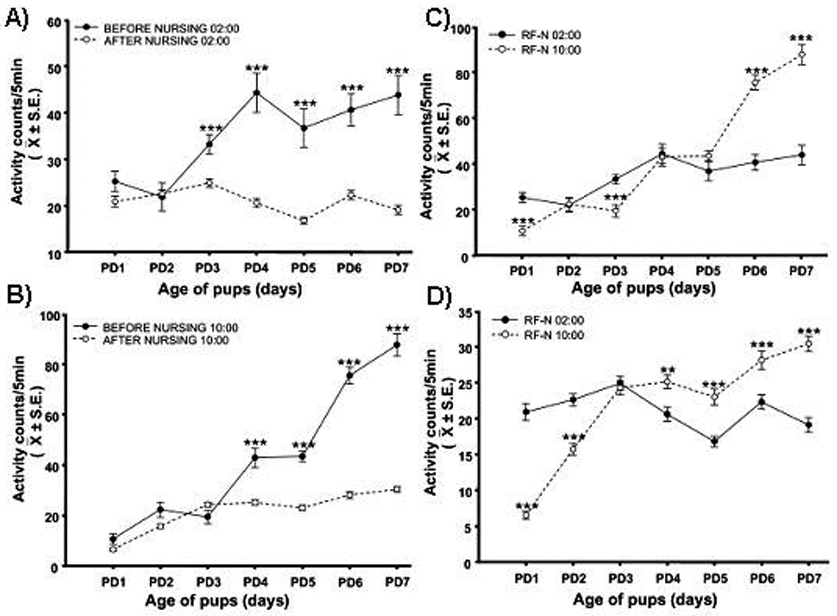
We have studied the nursing visit of the mother and the locomotor activity of pups at different times under constant darkness (D/D) conditions, in circadian activity-recording cages equipped with two compartments, one for the mother and one for the pups, equipped with thermal sensors (Caba et al., 2008). Fig. 2 is a representative actogram from our laboratory showing the free-running rhythm of nursing in a doe with free access to her pups. Note the advancement of nursing every day in agreement with the anticipatory locomotor behavior of the pups. In order to explore in more detail the pup’s NAA we scheduled nursing either during daytime (10:00 h) or nighttime (02:00 h). In both cases actograms and periodograms of activity show a circadian component of 24 h (Fig 3; Caba et al., 2008). Furthermore, as shown in Fig. 3, even if the doe fails to nurse the pups at the scheduled time, the locomotor behavior of the pups does not drop after nursing but is maintained for several hours before declining. However, 48 h after the last nursing NAA is present again at the time of the expected next nursing bout, an observation that confirms that the nursing visit of the mother is a powerful zeitgeber for the pup’s NAA (Jilge, 1993, 1995; Caba et al., 2008; Morgado et al., 2008). We found significant differences in NAA depending on the timing of nursing. Locomotor activity was higher both before and after nursing in the daytime nursing group than in nighttime fed pups (Fig. 4). This finding is important as most studies in rabbit pups involve daytime nursing and, in all mammals studied, providing food during either the day or the night profoundly affects several neuroendocrine parameters (Belda et al., 2005; Goel et al., 2009).
Fig 2.
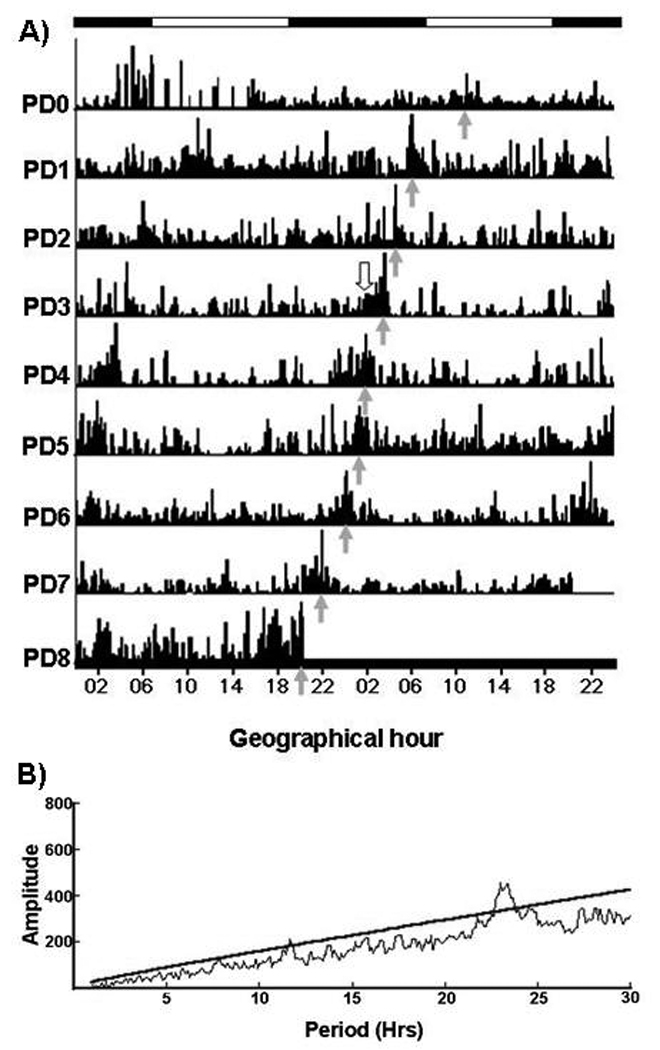
Fig 3.
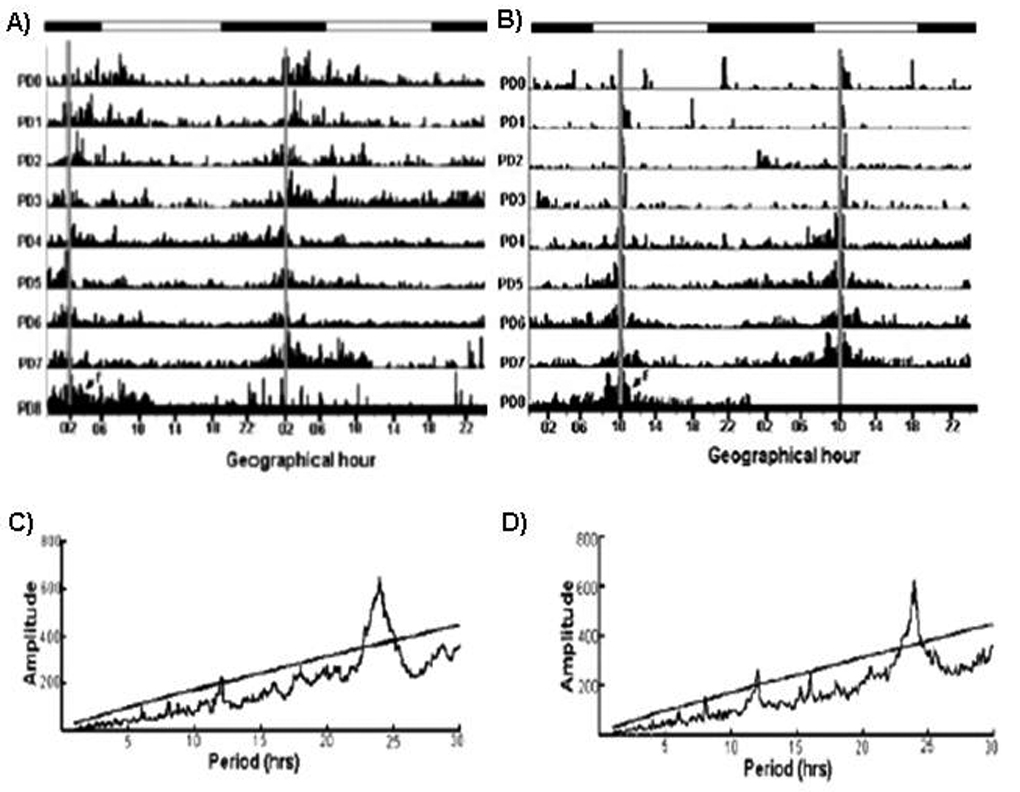
Fig 4.
Core body temperature
The zeitgeber properties of nursing have also been explored in relation to the entrainment of core body temperature. Pups scheduled to nurse during subjective morning and implanted i.p. with a transmitter on PD3 showed, on the following day, a significant anticipatory rise in body temperature of 0.4 to 0.6 °C during NAA, in addition to the 24-h rhythm, which starts 2.5–3.5 h prior to nursing (Jilge et al., 2000). By PD7 the rhythm consolidated into two components: a) the anticipatory one, which was followed by an additional elevation of temperature of 0.3 to 0.6 °C starting 4 to 8 min after the onset of milk ingestion; b) a post-suckling component, appearing across the 3 hours following nursing, during which body temperature dropped 1.2 to 1.5 °C and returned to the daily average level about 3 to 5 h later (Jilge et al., 2000). As expected, a shift in the timing of nursing resulted in a parallel shift of the body temperature rhythm (Fig. 5; Jilge et al., 2000). When does were not permitted to nurse their pups the circadian anticipatory peak of temperature persisted, but the further elevation of temperature did not occur (Jilge et al., 2000). Together, these experiments confirm that the phase of the rhythm is controlled by an endogenous oscillator that is entrained by the daily nursing bout, which acts as a strong zeitgeber.
Fig 5.
Corticosterone
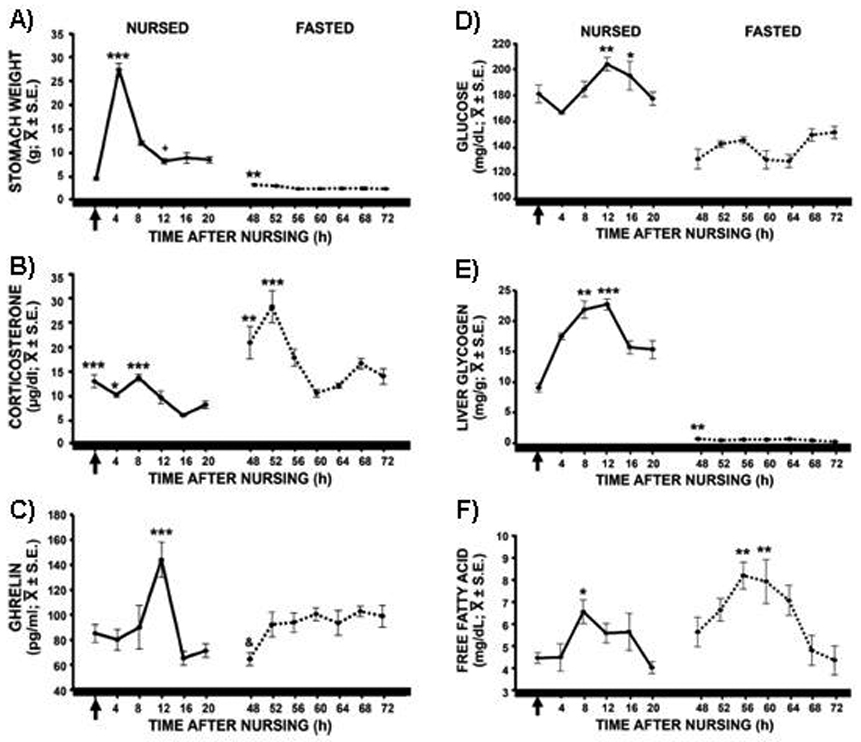
One of the most salient features of animals under a food restriction paradigm is that the peak of plasma corticosterone (CORT) shifts from the onset of activity (night in nocturnal rodents, day in diurnal animals) to the onset of food intake at the time of FAA (Krieger, 1974). Moreover, a bimodal CORT peak has been reported, with both an anticipatory and a nocturnal component (Moberg et al., 1975; Honma et al., 1992). As such studies were performed in adult subjects and, although locomotor behavior of rabbit pups seemed to be entrained by the timing of daily food intake, rabbit pups were considered a very different model from adult rodents under food restriction. The reason was that young rodents show, during PD4–PD14, a stress hyporesponsive period (SHRP) during which no rhythmic secretion of CORT is detected (Levine, 1994; Schmidt et al., 2003). In fact an SHRP was proposed for the PD7rabbit pup (Pradier & Dalle, 1996). To our surprise, we found a 24-h rhythmic secretion of CORT in PD7–9 in rabbit pups fed at ZT03 in L/D condition (Rovirosa et al., 2005). Moreover the rhythmic pattern appeared to be entrained by the daily nursing visit of the mother as the peak CORT secretion coincided with the time of feeding and a clear elevation was observed before the scheduled nursing, i.e., during NAA. After suckling values steadily decreased to reach a nadir 12 h later and to increase again in anticipation of the next nursing bout (Rovirosa et al., 2005). This pattern was confirmed with subjects kept under DD (Fig. 5; Morgado et al., 2008). Following a 48 h fast a further elevation of CORT was observed, compared with subjects nursed 24 h earlier (Fig. 5; Rovirosa et al., 2005; Morgado et al., 2008). This high CORT elevation could be due to the lack of food, as has been well documented in rodents (Suemaru et al., 1986; Dallman et al., 1999). Interestingly, this fast-induced increase remains under a circadian control (Morgado et al., 2008), as occurs in rats (Akana et al., 1994; Dallman et al., 1999).
In our previous studies lowest CORT levels were found at 02:00 (Rovirosa et al., 2005; Morgado et al., 2008), so we shifted nursing to the night at the time of nadir. Accordingly, all pups developed NAA and a rhythm of CORT with highest levels at the time of nursing or expected nursing (Caba et al., 2009). We conclude that nursing is a zeitgeber that also entrains the circadian rhythm of CORT in rabbit pups.
Ghrelin
Ghrelin is a peptide hormone produced and released mainly by the endocrine oxyntic cells of the gut (reviewed in: Kojima & Kangawa, 2005). It plays an important role in energy homeostasis; plasma ghrelin levels increase in rats (Lee et al., 2002), sheep (Sugino et al., 2002) and humans (Cummins et al., 2001) before feeding with fixed meal times. In rabbit pups nursed at ZT03 we found a rhythm of circulating ghrelin in which the highest plasma levels occurred around 12 h after nursing, (i.e., at a time when two thirds of the stomach had been evacuated), but not during the NAA (Fig. 5; Morgado et al, 2008). From these results we proposed that the peak of ghrelin seen 12 h after nursing could be a consequence of the emptied of the stomach which then lead to the next period of NAA (Morgado et al., 2008). On the other hand, following an omission of two nursing bouts, ghrelin remained high for most of the sampling period (Fig. 5). To further investigate a role of ghrelin in fed and fasted pups we scheduled nursing during nighttime at ZT19 and found that ghrelin was not elevated before nursing, had a slight peak 12 h after nursing (although not significant, P<0.07), and a large increase during fasting (Caba et al., 2009). From these results we propose that the elevation of ghrelin 12 h after nursing, together with the signals of an “empty” stomach (see below) could trigger the next NAA, in nursed but not in fasted subjects. Support for this possibility comes from the following: a) in rats, the injection of ghrelin, either i.c.v. (Szentirmai et al., 2006) or in discrete areas of the forebrain (Szentirmai et al., 2007) has strong wakefulness-promoting effects; b) i.c.v. ghrelin increases spontaneous locomotor activity and core body temperature and also promotes corticosterone release (Jazberényi et al., 2006). By contrast, during fasting ghrelin values remain high, an observation that, in other species, has been interpreted as indicative of hunger (rev. Kojima & Kangawa, 2005). Moreover, high ghrelin values during fasting support the relationship between this hormone and an empty stomach. Future studies should explore the relationship between circulating levels of ghrelin and the activation of specific brain regions in both nursed and fasted pups.
Metabolites
Rabbit pups ingest up to 35% of their body weight in milk in less than 5 minutes (Hudson & Distel, 1989; González-Mariscal et al., 1994; Caba et al., 2003). In subjects nursed during daytime the large ingestion of milk produces an abrupt filling of the stomach. Two-thirds of the content is evacuated during the first 12 h after nursing while the remaining one-third is eliminated across the next 12 h (Fig. 5; Escobar et al., 2000; Morgado et al., 2008).
Glucose levels in blood fluctuate from around 160–200 mg/dl in 24 h while liver glycogen follows a cycle with lower levels at the time of nursing that increase 8 h later and then decrease in advance of the following nursing bout (Fig. 5; Escobar et al., 2000; Morgado et al., 2008). The effect of nursing on free fatty acids (FFA) is less clear as one study reported a rhythmic fluctuation (Morgado et al., 2008) but in another work this variation was not observed (Escobar et al., 2000, also containing additional metabolic data). During fasting the stomach was almost empty (Fig. 5A) but glucose levels fluctuated between 120–140 mg/dl (Fig. 5D) and liver glycogen was almost undetectable (Fig. 5E). The lack of food depletes liver glycogen stores to maintain stable circulating glucose levels, probably through gluconeogenesis from glycerol obtained from triglycerides. Indeed, triglyceride levels steadily decrease across fasting (Escobar et al., 2000). Interestingly, the rhythm of free FFA persists during fasting with lowest levels at the time of nursing or expected nursing and higher levels around 8 hours later (Fig. 5F; also see Escobar et al., 2000). Furthermore in subjects nursed during nighttime (02:00 h) we also found a rhythm of FFA that also persisted during fasting although with a larger amplitude (Caba et al., 2009). FFA have been associated in the rat with FAA (Escobar et al., 1998; Díaz-Muñoz et al., 2000). The decrease in liver glycogen which leads to a lipid mobilization to induce gluconeogenesis, together with an elevation of CORT, suggests an entrainment of nursing on metabolic parameters and the induction of a catabolic state which has been associated with the appearance of FAA in adult rats (Krieger, 1974; Abe & Rusak, 1992; Escobar et al., 1998; Díaz-Muñoz et al., 2000;). This is supported by the up-regulation of 21 genes related to lipid catabolism and steroid metabolism in the liver during FAA in the adult rat, as the liver changes from a glycogen –to a lipid-based metabolism (Báez-Ruíz et al., 2005). Furthermore, the persistence of a rhythm of FFA during fasting confirms the relevance of lipid metabolism as a phenomenon associated with the FEO in the rat (Escobar et al., 1998).
Neural correlates of NAA
Suprachiasmatic nucleus
To explore the effect of nursing on the SCN newborn pups were maintained unmanipulated, in darkness and were scheduled to nurse, from PD1 onwards, either during daytime (10:00 h = ZT03) or nighttime (02:00 h = ZT19). To determine the phase of the clockwork oscillation we used the expression of the PER1 protein by means of immunohistochemistry. As shown in Fig. 6 the SCN of PD7 rabbits show a robust circadian rhythm of the PER1 protein. In both groups lowest values were observed between 10:00 and 14:00 h but night-fed pups had more PER1-immunoreactive (IR) cells at earlier and later time points than day-fed pups. Cosinor analysis revealed a phase difference of 2.54 h in subjects nursed during the night against those nursed during the day (Caba et al., 2008). A difference of 1.19 h between night- and day-fed groups was also observed in fasted subjects. Similarly, the expression of the c-FOS protein-which has been used as a marker of circadian oscillation in the SCN of rats (Guido et al., 1999) and rabbits (Toledo et al., 2005) showed a robust circadian rhythm. Both, day- and night- fed subjects showed higher numbers of c-FOS-IR cells during daytime but in night-fed subjects such values peaked earlier than in subjects nursed during the day. Cosinor analysis revealed a difference of 0.55 h between both experimental groups (Caba et al., 2008).
Clock genes have also been analyzed by in situ hybridization. Per1, Per2 and Bmal1 show a rhythmic circadian expression in the SCN, with Per1 mRNA peaking in the afternoon at around 18 hrs in PD7 pups nursed at 9:30 a.m. (Caldelas et al., 2007). This pattern is consistent with that of the PER1 protein as highest values of immunoreactive signal were found during the night (Caba et al., 2008). In a different publication, Caldelas and colleagues (2009) reported that delaying nursing time from 09:30 h (PD1–3) to 15:30 h (PD4–7) shifted the peak expression of clock genes from 18:30 h on PD3 to 23:12 h on PD7. However, in the same publication, PD9 subjects nursed at 09:30 h across PD1–9 showed a peak expression of Per1 mRNA at 12:10 h. This finding suggests that a “spontaneous” shift in Per1 mRNA expression is taking place as part of a normal developmental process. Consequently, the shift in clock gene expression observed on PD7 could have ensued from such ontogenetic change rather than from a shift in nursing time. Indeed, ontogenetic changes in the expression of clock genes can be inferred from the finding that Cry1 has a bimodal pattern of expression in the SCN of rabbit pups on PD7 (Caldelas et al., 2007). Moreover, a study from our laboratory indicates that the SCN of rabbit pups at this age is immature (Zavaleta et al., 2007). Thus, the data on PER1 and c-FOS proteins suggest that nursing has modulatory effect on the SCN of rabbit pups. Further studies should compare mRNA and proteins of clock genes in order to distinguish the relative contribution of nursing vs spontaneous developmental changes in the SCN.
Paraventricular thalamic nucleus
In the paraventricular nucleus of the thalamus (PVT) the number of c-FOS-IR cells increases from the low levels seen one hour before nursing to the highest numbers observed two hours after nursing (or expected time of scheduled nursing in un-nursed rabbit pups) on PD7. Thereafter, values decrease to their lowest in both nursed and un-nursed pups (Allingham et al., 1998). The PVT has reciprocal connections with the SCN (Moga et al., 1995; Moga & Moore, 1997; Leak & Moore, 2001) and it can be affected by food restriction schedules (Pereira de Vasconcelos et al., 2006; Angeles-Castellanos et al., 2007; Poulin & Timofeeva, 2008). However, as rats with complete ablation of the PVT show normal FAA (determined by activity at or near a food-bin; Landry et al., 2007), it is unclear what the role of the PVT on the circadian NAA of the rabbit pup is.
Supraoptic and Paraventricular nuclei
The number of c-FOS-IR cells in the paraventricular nucleus of the hypothalamus changes significantly with respect to nursing time. It is low one h before nursing, shows highest levels two hours after nursing (or expected time of scheduled nursing in un-nursed pups) on PD7, and then decreases to its lowest levels 8 h later (Allingham et al., 1998). Although the authors proposed that the c-FOS-IR cells seen at 2 h after nursing were in a parvocellular region, whereas those of un-nursed pups were in a magnocelllular area this possibility is unlikely as the rabbit PVN has a loose organization, with cells of different sizes being intermingled throughout the nucleus (Schimchowitsch et al., 1989). Nursing induces c-FOS expression also in the SON (Allingham et al., 1998; Caba et al., 2003). Most of the c-FOS-IR cells were oxitocinergic and a few were vasopressinergic (Caba et al., 2003). The former effect could be related to: a) the gastric distension provoked by the abrupt ingestion of milk (Renaud et al., 1987), b) a release of oxytocin associated with satiety (Verbalis et al., 1995) or c) a modulation of the vagal digestive motor functions in the brainstem (Flanagan et al., 1992).
Dorsomedial hypothalamic nucleus
In neonatal rabbit pups the dorsomedial hypothalamic nucleus (DMH) exhibits a circadian rhythm of PER1 that shifts in parallel to the timing of nursing (Caba et al., 2008). Regardless of whether pups are fed during day- or nighttime PER1 peaks in the DMH 8 h after nursing, an effect that persists even in fasted pups for 48 h (although the effect is smaller than in nursed subjects). This persistence of the rhythmic expression of PER1 in fasted pups suggests that its expression is not simply due to energy depletion due to a lack of food availability (Caba et al., 2008). In rodents several studies have associated the DMH with FAA as specific changes have been observed during food restriction (Angeles-Castellanos et al., 2004; Gooley et al., 2006; Mieda et al., 2006; Fuller et al., 2008). However, partial or total lesion of the DMH does not affect FAA in rats (Landry et al., 2006; Landry et al., 2007) or mice (Moriya et al., 2009).
What does NAA mean?
Although NAA has been well documented by independent groups (see above) the implications of this state of arousal for the development of rabbit pups has been less investigated. A series of studies has explored the effect of handling pups at different times around nursing on their responsiveness to humans several weeks later (Bilkó & Altbäcker, 2000; Csatádi et al, 2005; Pongrácz & Altbäcker, 1999, 2003). These works have shown that handling the litter briefly during a restricted period around nursing (15 min before and up to 30 min after it) reduces the fear normally shown by rabbits to humans, when tested as adults. This effect is not provoked by nursing per se because when handling is performed around the time of a second (i.e., additional) nursing episode (6 h after the usual one) reduced fearfulness to humans is not observed (Pongrácz & Altbäcker, 2003). Moreover, even wild rabbits handled in early development around nursing show the same response when tested as adults, a finding indicating that the results observed are not a consequence of domestication (Bilkó & Altbäcker, 2000). Rather, such results suggest that NAA is a “window of opportunity”, a critical period during which young rabbits can learn about their environment and retain the acquired information for life. Indeed, while they live in the maternal burrow, rabbits are exposed to specific components of their mother’s diet. By incorporating edible herbs as she builds the maternal nest and depositing fecal pellets after each nursing visit mothers transmit their food preferences to the litter. Such maternal behaviors allow an early form of learning in the young because, early in their development, pups start nibbling on the nest material and the fecal pellets. When tested after weaning, the offspring prefer the herbs they were exposed to in the nest over those that are new to them (Hudson & Altbäcker, 1992).
Little is known about the factors that trigger the state of arousal characteristic of NAA and which, theoretically, could be related with the enhanced capacity for learning that occurs around nursing. One such candidate are glucocorticoids: a clear rise in corticosterone secretion precedes daily nursing and its concentration gradually declines thereafter (Rovirosa et al, 2005; Morgado et al., 2008). As glucocorticoids promote several forms of learning in mammals (Beylin & Shors, 2003; Roozendaal et al., 2006) we propose that the rise in corticosterone normally seen during NAA plays a crucial role in directing the psychobiological development of rabbit pups. Indeed, under opposite conditions, i.e., exposing the young to excessive endogenous concentrations of corticosterone (a consequence of separating them from their mother for 48 hrs), provokes a permanent disorganization of the hypothalamus-pituitary-adrenal gland (HPA) axis. When tested as adults, such individuals show a blunted response to stress, i.e., they release significantly lower concentrations of CORT following stressful stimulation (Boiti et al., in preparation). These findings coincide with the abundant literature, obtained mainly in rodents, confirming the sensitivity of the HPA axis of newborn mammals to maternal stimulation and its vulnerability to corticosteroid action (for recent review see: González-Mariscal & Kingsley, 2009).
Future directions
Although the pups’ capacity to synchronize their activity and physiology to the mother’s nursing visits is well supported (see above) the specific signals that provoke this effect need to be investigated. Because at each suckling episode the young are exposed to both the MP and the milk the relative role of the olfactory signal and the nutrients (plus gut distention, metabolic signals, etc.) received cannot at present be unequivocally established. Moreover, it is possible that the action of these stimuli as zeitgebers varies with age as, for instance, the pup’s response to the pheromone is influenced by age and even prandial state (Montigny et al., 2006). Furthermore, as milk intake is probably rewarding for the pups, one could think that as lactation progresses the young become conditioned to the perception of the MP. Studies in which MP perception is dissociated from milk intake are, therefore, essential to establish which stimulus is the zeitgeber or if they act in combination. Also, it is necessary to establish the ontogeny of clock genes and their protein products in relation to nursing at several ages as the SCN and other brain nuclei may be affected by milk intake only during the first postnatal days. Given that lactation lasts one month and that pups open their eyes at around day 10 future studies should determine when in their postnatal development do young rabbits shift from a nonphotic to a photic synchronizer. The behavioral and physiological parameters entrained by daily nursing in rabbit pups younger than 10 days suggest that they are controlled by a FEO, similar to that described in adult rodents. However, it is necessary to explore the same parameters in older pups, i.e., after they open their eyes and are exposed to both a non-photic (nursing) and a photic (light) synchronizer.
Finally, it is necessary to explore how the mother’s behavior is entrained by the pups. The quality and quantity of suckling stimulation probably plays an important role in this regard: mothers can nurse earlier than usual if the number of pups suckled at the previous nursing bout is reduced (González-Mariscal, 2007). Moreover, ongoing studies in which mothers were given one or two pups are revealing that circadian periodicity of nursing is completely lost and the does enter the nest box many times a day (González-Mariscal et al. unpublished observations). Taken together, these findings suggest that a threshold of suckling stimulation is required for the normal operation of the neural circuit that determines the circadian expression of nursing in mother rabbits. Suckling, per se seems not to impact directly the activity of the SCN, as revealed by immunocytochemistry of the PER1 (Meza et al., 2008) or the c-FOS (González-Mariscal et al., 2009) proteins.
In conclusion the neonatal rabbit pup is an extraordinary model for studying the phenomenon of entraining by a single daily food pulse with minimal manipulations. Furthermore the mother also offers the possibility to study lactation as a phenomenon of non-photic synchronization, again with minimal manipulation, as the suckling stimulus occurs also once a day.
Acknowledgements
This work was supported by a National Institutes of Health/Fogarty Grant R01 TW-006636, and a Grant from CONACYT, Ref. 82764 (M. Caba).
References
- Abe H, Rusak B. Anticipatory activity and entrainment of circadian rhythms in Syrian hamsters exposed to restricted palatable diets. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992;295:R690–R695. doi: 10.1152/ajpregu.1992.263.1.R116. [DOI] [PubMed] [Google Scholar]
- Akana SF, Strack AM, Hanson ES, Dallman MF. Regulation of activity in the hypothalamo-pituitary-adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology. 1994;135:1125–1134. doi: 10.1210/endo.135.3.8070356. [DOI] [PubMed] [Google Scholar]
- Allingham K, von Saldern C, Brennan PA, Distel H, Hudson R. Endogenous expression of c-Fos in hypothalamic nuclei of neonatal rabbits coincides with their circadian pattern of suckling-associated arousal. Brain Res. 1998;783:210–218. doi: 10.1016/s0006-8993(97)01379-6. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Aguilar-Roblero R, Escobar C. C-Fos expression in hypothalamic nuclei of food-entrained rats. Am. J. Physiol. Reg. Integr. Physiol. 2004;286:R159–R165. doi: 10.1152/ajpregu.00216.2003. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and proteín Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144:344–355. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Báez-Ruiz A, Luna-Moreno D, Vázquez-Martínez O, Ramírez-Salcedo J, Díaz-Muñoz M. The food entrainable oscillator studied by DNA microarrays: What is the liver doing during food anticipatory activity? Biol. Rhythm Res. 2005;36:83–98. [Google Scholar]
- Belda X, Ons S, Carrasco J, Armario A. The effects of chronic food restriction on hypothalamic-pituitary-adrenal activity depend on morning versus evening availability of food. Pharmacol. Biochem. Behav. 2005;81:41–46. doi: 10.1016/j.pbb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm. Behav. 2003;43:124–131. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkó A, Altbäcker V. Regular handling early in the nursing period eliminates far responses toward human beings in wild and domestic rabbits. Dev. Psychobiol. 2000;36:78–87. [PubMed] [Google Scholar]
- Broekhuizen S, Mulder JL. Differences and similarities in nursing behaviour of hares and rabbits. Acta Zool. Fenn. 1983;174:61–63. [Google Scholar]
- Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Dev. Brain Res. 2003;143:119–128. doi: 10.1016/s0165-3806(03)00064-6. [DOI] [PubMed] [Google Scholar]
- Caba M, Tovar A, Silver R, Morgado E, Meza E, Zavaleta Y, Juárez C. Nature’s food anticipatory experiment: entrainment of locomotor behavior, suprachiasmatic and dorsomedial hypothalamic nuclei by suckling in rabbit pups. Eur. J. Neurosci. 2008;27:432–443. doi: 10.1111/j.1460-9568.2008.06017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caba M, Morgado E, Juarez C, Meza E. Mealtime entrains behavioral, hormonal, metabolic and neural parameters in the rabbit pup. Proceedings of the XI Congress of the European Biological Rhythms Society; Strasbourg, France. 2009. [Google Scholar]
- Caldelas I, Tejadilla D, González B, Montúfar R, Hudson R. Diurnal pattern of clock gene expression in the hypothalamus of the newborn Rabbit. Neuroscience. 2007;144:395–401. doi: 10.1016/j.neuroscience.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Caldelas I, González B, Montúfar-Chaveznava R, Hudson R. Endogenous clock gene expression in the suprachiasmatic nuclei of previsual newborn rabbits is entrained by nursing. Develop. Neurobiol. 2009;69:47–59. doi: 10.1002/dneu.20687. [DOI] [PubMed] [Google Scholar]
- Challet E, Pevet P. Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals. Front. Biosci. 2003;8:246–257. doi: 10.2741/1039. [DOI] [PubMed] [Google Scholar]
- Csatádi K, Kustos K, Eeiben C, Bilkó A, Altbäcker V. Even minimal human contact linked to nursing reduces fear responses toward humans in rabbits. Appl. Anim. Behav. Sci. 2005;95:123–128. [Google Scholar]
- Cummins DE, Purnell JQ, Frayo RS, Schmidova K, Wiesse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LZ, Strack AM, Viau V. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz M, Vázquez-Martínez O, Aguilar-Roblero R, Escobar C. Anticipatory changes in liver metabolism and entrainment of insulin, glucagon, and corticosterone in food-restricted rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R2048–R2056. doi: 10.1152/ajpregu.2000.279.6.R2048. [DOI] [PubMed] [Google Scholar]
- Drewett RF, Kendrick KM, Sanders DJ, Trew AM. A quantitative analysis of the feeding behavior of suckling rabbits. Dev. Psychobiol. 1982;15:25–32. doi: 10.1002/dev.420150106. [DOI] [PubMed] [Google Scholar]
- Escobar C, Díaz-Muñoz M, Encinas F, Aguilar-Roblero R. Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;295:R690–R695. doi: 10.1152/ajpregu.1998.274.5.R1309. [DOI] [PubMed] [Google Scholar]
- Escobar C, Hudson R, Martínez-Gómez M, Aguilar-Roblero R. Metabolic correlates of the circadian pattern of suckling-associated arousal in young rabbits. J. Comp. Physiol. A. 2000;186:33–38. doi: 10.1007/s003590050004. [DOI] [PubMed] [Google Scholar]
- Flanagan LM, Dohanics J, Verbalis JG, Stricker EM. Gastric motility and food intake in rats after lesions of hypothalamic paraventricular nucleus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992;263:R39–R44. doi: 10.1152/ajpregu.1992.263.1.R39. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Dawood Y. Oxytocin release and uterine activation during parturition in rabbits. Endocrinoogyl. 1980;107:1117–1126. doi: 10.1210/endo-107-4-1117. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Lu J, Saper CB. Differential rescue of light-and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Stunkard AJ, Rogers NL, Van Dongen HPA, Allison KC, O’Reardon JP, Ahima RX, Cummings DE, Heo M, Dinges DF. Circadian rhythm profiles in women with night eating syndrome. J. Biol. Rhythms. 2009;24:85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mariscal G, Díaz-Sánchez V, Melo AI, Beyer C, Rosenblatt JS. Maternal behavior in New Zealand White rabbits: quantification of somatic events, motor patterns and steroid plasma levels. Physiol. Behav. 1994;55:1081–1089. doi: 10.1016/0031-9384(94)90391-3. [DOI] [PubMed] [Google Scholar]
- González-Mariscal G. Mother rabbits and their offspring: timing is everything. Dev. Psychobiol. 2007;49:71–76. doi: 10.1002/dev.20196. [DOI] [PubMed] [Google Scholar]
- González-Mariscal G, Kingsley CH. From indifference to ardor: the onset, maintenance and meaning of the maternal brain. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2009. IN PRESS. [Google Scholar]
- González-Mariscal G, Jiménez A, Chirino R, Beyer C. Motherhood and nursing stimulate c-fos expression in specific regions of the rabbit forebrain. Behav. Neurosci. 2009 doi: 10.1037/a0016487. IN PRESS. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat. Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Guido ME, de Guido LB, Goguen D, Robertson HA, Rusak B. Daily rhythm of spontaneous immediate-early gene expression in the rat suprachiasmatic nucleus. J. Biol. Rhythms. 1999;14:275–280. doi: 10.1177/074873099129000687. [DOI] [PubMed] [Google Scholar]
- Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur. J. Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Duffield GE, Smith EJ, Maywood ES, Ebling FJ. Entrainment of the circadian system of mammals by nonphotic cues. Chrobiol. Int. 1998;15:424–445. doi: 10.3109/07420529808998700. [DOI] [PubMed] [Google Scholar]
- Honma K-I, Noe Y, Honma S, Katsuno Y, Hiroshige T. Roles of paraventricular catecholamines in feeding-associated corticosterone rhythm in rats. Am J. Physiol. Endocrinol. Metab. 1992;25:E948–E955. doi: 10.1152/ajpendo.1992.262.6.E948. [DOI] [PubMed] [Google Scholar]
- Hudson R, Distel H. Nipple location by newborn rabbits: behavioral evidence for pheromonal guidance. Behaviour. 1983;85:260–275. [Google Scholar]
- Hudson R, Distel H. Temporal pattern of suckling in rabbit pups: a model of circadian synchrony between mother and young. In: Reppert SM, editor. Development of circadian rhythmicity and photoperiodism in mammals. Ithaca, NY: Perinatology Press; 1989. pp. 83–102. [Google Scholar]
- Hudson R, Altbäcker V. Development of feeding and food preference in the european rabbit: environmental and maturational determinants. In: Galef SM, Mainardi M, Valsecchi P, editors. Ontogeny and social transmission of food preferences in mammals: basic and applied research. London: Harwood Academic Publishers; 1992. pp. 125–145. [Google Scholar]
- Hudson R, Müller A, Kennedy GA. Parturition in the rabbit is compromised by daytime nursing: the role of oxytocin. Biol. Reprod. 1995;53:519–524. doi: 10.1095/biolreprod53.3.519. [DOI] [PubMed] [Google Scholar]
- Hudson R, Cruz Y, Lucio A, Ninomiya J, Martínez-Gómez M. Temporal and behavioural patterning of parturition in rabbits and rats. Physiol. Behav. 1999;66:599–604. doi: 10.1016/s0031-9384(98)00331-x. [DOI] [PubMed] [Google Scholar]
- Jilge B. The ontogeny of circadian rhythms in the Rabbit. J. Biol. Rhythms. 1993;8:247–260. doi: 10.1177/074873049300800307. [DOI] [PubMed] [Google Scholar]
- Jilge B. Ontogeny of the rabbit’s circadian rhythms without an external zeitgeber. Physiol. Behav. 1995;58:131–140. doi: 10.1016/0031-9384(95)00006-5. [DOI] [PubMed] [Google Scholar]
- Jilge B, Kuhnt B, Landerer W, Rest S. Circadian thermoregulation in suckling rabbit pups. J. Biol. Rhythms. 2000;15:329–335. doi: 10.1177/074873000129001431. [DOI] [PubMed] [Google Scholar]
- Jilge B, Kuihnt B, Landerer W, Rest S. Circadian temperature rhythms in rabbit pups and in their does. Lab. animal. 2001;35:364–373. doi: 10.1258/0023677011911831. [DOI] [PubMed] [Google Scholar]
- Jaszberényi M, Bujdoso E, Bagosi Z, Telegdy G. Mediation of the behavioral, endocrine and thermoregulatory actions of ghrelin. Horm. Behav. 2006;50:266–273. doi: 10.1016/j.yhbeh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind’s clock. New York: Oxford University Press; 1991. p. 467. [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol. Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Krieger DG. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: timing is everything. Hormones and Behavior. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistance of a behavioral food anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:1527–1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Yamakawa GR, Mistlberger RE. Robust food anticipatory circadian rhythms in rats with complete ablation of the thalamic paraventricular nucleus. Brain Res. 2007;1141:108–118. doi: 10.1016/j.brainres.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J. Comp. Neurol. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- Lee HM, Wang G, Englander EW, Kojima M, Greeley GH. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine and dietary manipulations. Endocrinology. 2002;143:185–190. doi: 10.1210/endo.143.1.8602. [DOI] [PubMed] [Google Scholar]
- Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann. N.Y. Acad. Sci. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- Lincoln DW. Suckling: a time constant in the nursing behaviour of the rabbit. Physiol. Behav. 1974;13:711–714. doi: 10.1016/0031-9384(74)90247-9. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, McCowan D. Some observations on the breeding burrows of the wild rabbit, Oryctolagus cuniculus, on the island of Skokholm. J. Zool. (Lond.) 1968;156:540–549. [Google Scholar]
- Meza E, Juárez C, Morgado E, Zavaleta Y, Caba M. Brief daily suckling shifts locomotor behavior and induces PER1 protein in paraventricular and supraoptic nuclei, but not in the suprachiasmatic nucleus, of Rabbit does. Eur. J. Neurosci. 2008;28:1394–1403. doi: 10.1111/j.1460-9568.2008.06408.x. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc. Nat. Acad. Sci. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosc. Biobehav. Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Moberg GP, Bellinger LL, Mendel VE. Effect of meal feeding on daily rhythms of plasma corticosterone and growth hormone in the rat. Neuroendocrinology. 1975;19:160–169. doi: 10.1159/000122436. [DOI] [PubMed] [Google Scholar]
- Moga MM, Weis RP, Moore RY. Efferent projections of the paraventricular thalamic nucleus in the rat. J. Comp. Neurol. 1995;359:221–238. doi: 10.1002/cne.903590204. [DOI] [PubMed] [Google Scholar]
- Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J. Comp. Neurol. 1997;389:508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Montigny D, Coureaud G, Schaal B. Rabbit pup response to the MP: from automatism to prandial control. Physiol. Behav. 2006;89:742–749. doi: 10.1016/j.physbeh.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, Shibata S. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur. J. Neurosci. 2009;29:1447–1460. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- Morgado E, Gordon MK, Miñana-Solís MC, Meza E, Levine S, Escobar C, Caba M. Hormonal and metabolic rhythms associated with the daily scheduled nursing in rabbit pups. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R690–R695. doi: 10.1152/ajpregu.00162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira de Vasconcelos A, Bartol-Munier I, Feiller CA, Gourmelen S, Pevet P, Challet E. Modifications of local cerebral glucose utilization during circadian food-anticipatory activity. Neuroscience. 2006;139:741–748. doi: 10.1016/j.neuroscience.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Pongrácz P, Altbäcker V. The effect of early handling is dependent upon the state of the rabbit (Oryctolagus cuniculus) pups around nursing. Dev. Psychobiol. 1999;35:241–251. doi: 10.1002/(sici)1098-2302(199911)35:3<241::aid-dev8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Pongrácz P, Altbäcker V. Arousal, but not nursing, is necessary to elicit a decreased fear reaction toward humans in rabbit (Oryctolagus cuniculus) pups. Dev. Psychobiol. 2003;43:192–199. doi: 10.1002/dev.10132. [DOI] [PubMed] [Google Scholar]
- Poulin AM, Timofeeva E. The dynamics of neuronal activation during food anticipation and feeding in the brain of food-entrained rats. Brain Res. 2008;1227:128–141. doi: 10.1016/j.brainres.2008.06.039. [DOI] [PubMed] [Google Scholar]
- Pradier P, Dalle M. Effects of corticotrophin-releasing factor and vasopressin on plasma adrenocorticotropin molecular forms, aldosterone and corticosterone in young and adult rats and rabbits. Reprod. Fertil. Dev. 1996;8:111–116. doi: 10.1071/rd9960111. [DOI] [PubMed] [Google Scholar]
- Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1987;253:R661–R665. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, De Quervain DJF, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Rovirosa MJ, Levine S, Gordon MK, Caba M. Circadian rhythm of corticosterone secretion in the neonatal rabbit. Dev. Brain Res. 2005;158:92–96. doi: 10.1016/j.devbrainres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Schaal B, Coureaud G, Langlois D, Giniés C, Sémon E, Perrier G. Chemical and behavioural characterization of the rabbit MP. Nature. 2003;424:68–72. doi: 10.1038/nature01739. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int. J. Devl. Neurosc. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Schimchowitsch S, Moreau C, Laurent F, Stoeckel M-E. Distribution and morphometric characteristics of oxytocin-and vasopressin-immunoreactive neurons in the rabbit hypothalamus. J. Comp. Neurol. 1989;285:304–324. doi: 10.1002/cne.902850303. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a Zeitgeber. J. Biol. Rhytms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Suemaru S, Hashimoto K, Hattori T, Inoue H, Kageyama J, Ota Z. Starvation-induced changes in rat brain corticotropin-releasing factor (CRF) and pituitary-adrenocortical response. Life Sci. 1986;39:1161–1166. doi: 10.1016/0024-3205(86)90347-4. [DOI] [PubMed] [Google Scholar]
- Sugino T, Yamaura J, Yamagishi M, Ogura A, Hayashi R, Kurosa Y, Kojima M, Kangawa K, Hasegawa Y, Teraschima Y. A transient surge of ghrelin secretion before feeding is modified by different feeding regimens in sheep. Biochem. Biophys Res Commun. 2002;298:785–788. doi: 10.1016/s0006-291x(02)02572-x. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006;1088:131–140. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- Szentirmai E, Kapás L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R575–R580. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Toledo R, Aguilar-Roblero R, Canchola E, Caba M. Circadian and photic-induced expression of Fos protein in the suprachiasmatic nucleus of the Rabbit. Biol. Rhythm Res. 2005;36:47–55. [Google Scholar]
- Verbalis JG, Blackburn RE, Hoffman GE, Stricker EM. Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors. Ad. Exp. Med. Biol. 1995;395:209–225. [PubMed] [Google Scholar]
- Zarrow MX, Denenberg VH, Anderson C. Rabbit: frequency of suckling in the pup. Science. 1965;150:1835–1836. doi: 10.1126/science.150.3705.1835. [DOI] [PubMed] [Google Scholar]
- Zavaleta Y, Morgado E, Caba M. Development of the circadian gating of FOS photoinduction in the Rabbit suprachiasmatic nucleus. Soc. For Neurosci. Abs. 2007:633.14. [Google Scholar]


