Abstract
We present a case of a 60-year-old man with mild type 2 diabetes mellitus and step-wise progression of bilateral lower limb weakness, numbness and pain over a one year period. At the time of evaluation, he used a walker. He had elevated CSF protein, abnormal cooling and heat-pain thresholds on quantitative sensory testing, and NCS/EMG consistent with bilateral lumbosacral radiculoplexus neuropathies. Because it was not clear if the disease was still active, a right superficial peroneal nerve biopsy was performed and showed evidence of active axonal degeneration, ischemic injury, and microvasculitis. Based on these results, the patient was diagnosed with diabetic lumbosacral radiculoplexus neuropathy (DLRPN) and was treated with weekly intravenous methylprednisolone with marked improvement of neurological symptoms and signs. This case illustrates the typical clinical, electrophysiologic and pathological features of DLRPN and the utility of nerve biopsy to judge ongoing disease activity.
Keywords: diabetic lumbosacral radiculoplexus neuropathy, diabetic amyotrophy, diabetes mellitus, peripheral neuropathy
CASE REPORT
A 60-year-old man with a history of type II diabetes mellitus noted painless left-sided footdrop while walking across a field. This required the use of an ankle-foot orthosis, but improved significantly over the next four months. Six months after onset, his symptoms became bilateral, and he developed subacute severe burning pain and contact allodynia in both thighs, weakness of hip flexion (right greater than left), and right-sided footdrop. He then had some improvement of his pain, but worsening of his weakness, over a period of four months. At the time of evaluation, nearly one year after symptom onset, he was using a walker.
His past medical history was significant for type II diabetes mellitus, diagnosed three years earlier, for which he was taking insulin and rosiglitazone. He had no associated retinopathy or nephropathy. Two years earlier, he had developed left-sided soreness and pain in a lower thoracic dermatomal distribution, with evidence of denervation on electromyography, and had been diagnosed with a thoracic radiculopathy.
Strength examination grading (Mayo grading system: 0=normal, -1=25% weak, -2=50% weak, -3=75% weak, -3.25=antigravity, -4=100% weak) showed the following (right/left): -2/-1 iliopsoas, -1/0 gluteus maximus, -2,3/-1 quadriceps, -2,3/0,-1 hamstrings, -3.25/-3.25 anterior tibialis, -4/-3.25 toe extensors, -3.25/-1 peronei, -3/0 posterior tibialis, -2/-1 toe flexors. Strength in all other muscle groups was normal. He had absent light touch, pinprick, and temperature sensation, and reduced vibration sensation at his toes. Lower extremity reflexes were reduced to absent. Neurologic examination was otherwise normal.
Fasting glucose was normal (81 mg/dL); hemoglobin A1c was mildly elevated at 6.6. Other electrolytes, complete blood count, sedimentation rate, angiotensin converting enzyme, serum protein electrophoresis and immunofixation, antinuclear antibody, antibodies to extractible nuclear antigens, anti-neutrophil cytoplasmic antibodies, rheumatoid factor, hepatitis screen, Lyme and HIV serologies were negative or normal.
Cerebrospinal fluid analysis revealed a protein of 97 mg/dL, glucose of 84 mg/dL, a single nucleated cell, and cytology was negative. Nerve conduction studies/electromyography showed bilateral active and chronic lumbosacral plexopathies, worse on the right. There were fibrillations and long duration, polyphasic motor unit potentials with reduced recruitment in multiple lumbosacral myotomes, with marked involvement of the femoral nerve/L4-innervated muscles. There was increased insertional activity in lumbar paraspinal muscles. Quantitative sensory testing (using CASE IV1, 2) showed abnormal cooling and heat pain detection thresholds, with preserved vibratory sensation. MRI of the lumbosacral plexus was normal, without abnormal enhancement or mass lesion. Right superficial peroneal nerve biopsy was performed.
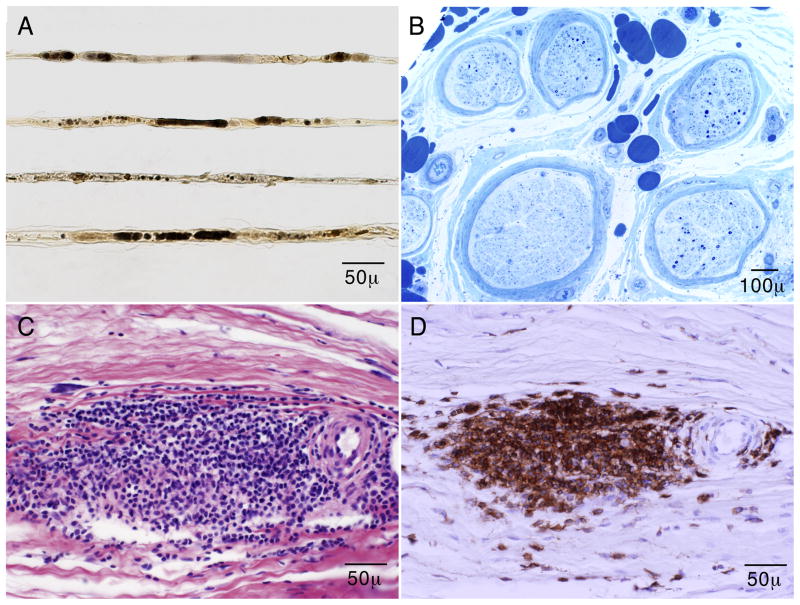
Teased fiber preparation showed a marked increased frequency of axonal degeneration (67% of the classifiable fibers) (Fig. 1A), with increased number of empty nerve strands (55). On paraffin and epoxy sections, there was a moderate to severe decrease of myelinated fiber density, in a mildly multifocal pattern (Fig. 1B). Many fibers were actively degenerating. There was focal thickening of the perineurium. Turnbull blue (iron) staining showed hemosiderin-laden macrophages in the perineurium. Neovascularization was present. Multifocal fiber loss, neovascularization, and perineurial thickening are findings characteristic of ischemic nerve injury. One epineurial blood vessel was nearly occluded. There were large (>100 cells) and moderate (50–100 cells) collections of inflammatory cells surrounding and involving the walls of epineurial blood vessels, but no fibrinoid necrosis was present (Fig. 1C).
Figure 1.
Superficial peroneal nerve biopsy: Teased fiber preparation (osmium tetrachloride) shows that most fibers are undergoing active axonal degeneration (A). Semithin epoxy sections (methylene blue) show severe loss of myelinated fibers in a multifocal distribution (some fascicles have few remaining fibers whereas others have none) (B). Serial longitudinal paraffin sections show large epineurial perivascular (sheet-like) inflammatory collections on H&E staining (C) that react to a CD45 (leukocyte common antigen) immunostain. These findings demonstrate an active inflammatory neuropathy.
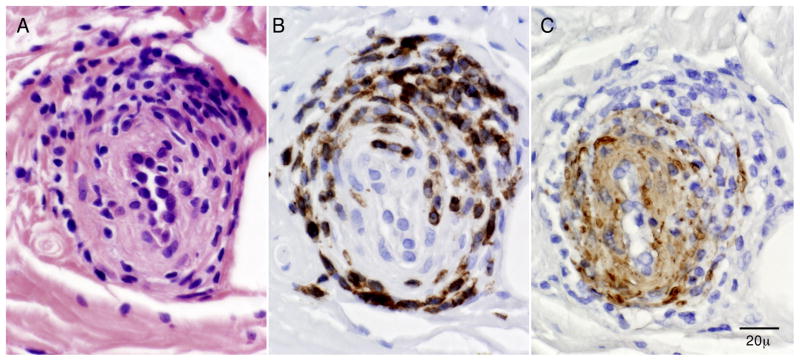
Immunohistochemical studies showed that many of the perivascular inflammatory cells were CD45 (leukocyte common antigen) positive (Fig. 1D), with greater reactivity for CD3 (T cell marker) than CD20 (B cell marker). A CD68 preparation showed that occasional macrophages were present associated with degenerating fibers in the endoneurium. Some epineurial blood vessels showed inflammation involving the muscle layers with disruption and fragmentation on smooth muscle actin preparation by CD45 positive inflammatory cells (Fig. 2). These findings were diagnostic of nerve microvasculitis.
Figure 2.
Superficial peroneal nerve biopsy (serial epineurial paraffin sections): The sections show an epineurial vessel that has inflammatory cells involving the vessel wall (on H&E) (A), that immunostain with CD45 (B) and show disruption of muscle wall elements on smooth muscle actin preparation (C). These findings are diagnostic of microvasculitis.
Answer
Bilateral diabetic lumbosacral radiculoplexus neuropathies (DLRPN)
DISCUSSION
Diabetic lumbosacral radiculoplexus neuropathy (DLRPN), also known as diabetic amyotrophy,3 diabetic polyradiculopathy,4 proximal diabetic neuropathy,5 among other names, is a disease process that affects approximately 1% of diabetic patients.6 In a large series by one of the authors (P.J.B.D.), the median age of onset was 65 years. It generally affects patients with mild diabetes mellitus; the median time from diagnosis of diabetes mellitus to onset of disease was 4.1 years. In contrast to diabetic sensorimotor polyneuropathy, which preferentially affects patients with other evidence of microvascular complications such as retinopathy or nephropathy, patients with DLRPN are actually less likely to have these complications than other patients in a community diabetic cohort (the Rochester Diabetic Neuropathy Study).7
DLRPN usually presents subacutely, and asymmetrically, with severe pain in a lower extremity, usually in the thigh (slightly more frequently than in the leg). As the pain improves, weakness is a more prominent feature, usually both proximal and distal. Associated weight loss is common. One-half of patients experience some symptoms of autonomic disturbance (orthostatic hypotension, diarrhea or constipation, change in sweating, or change in sexual function). Of 33 patients, 11 were found to have upper limb neuropathic symptoms, and 4 patients had evidence of thoracic radiculopathy. Nearly all patients eventually develop bilateral symptoms. The natural history of the disease is one of gradual, but incomplete, improvement. Pain usually is the first symptom to improve and often does to the largest degree. Though 16/33 patients were wheelchair bound at the time of their evaluation, at long-term followup, only 3 still required the use of wheelchairs.7
Cerebrospinal fluid shows elevated protein, without accompanying pleocytosis. Characteristic electrophysiologic findings include reduced motor and sensory amplitudes, which are asymmetric side to side, with evidence on needle examination of widespread (including paraspinal muscles) denervation-reinnervation changes involving root, plexus, and peripheral nerve, from multiple myotomes.7 Some authors argue that in severe cases there are two separate pathological processes occurring (a diffuse peripheral neuropathy and multiple lumbosacral radiculopathies).8 However, we believe there is usually a single underlying pathologic process (a radiculoplexus neuropathy).
Nerve biopsies from DLRPN patients have showed findings diagnostic or suggestive of ischemic injury from microvasculitis, with multifocal fiber loss, perineurial thickening, hemosiderin-laden macrophages, and/or neovascularization in many patients. Biopsies showed extensive epineurial perivascular inflammatory infiltrates, causing disruption of epineurial microvessels without fibrinoid necrosis.7 The same pathological findings have been identified in non-diabetic patients with a similar syndrome, and it is believed that both syndromes result from a microvasculitis, which may occur more frequently in diabetic patients.9, 10 More recently, a study of inflammatory markers in nerve biopsy specimens of patients with diabetic and non-diabetic lumbosacral radiculoplexus neuropathy showed significantly increased ICAM-1 positive cells in vessels, NF-kB immunoreactivity in blood vessels and the endoneurium, and (significantly in the non-diabetic patients only) increased tumor necrosis factor-alpha expression in cells in the endoneurium, compared to control specimens. These findings further support an inflammatory-immune cause for these disorders.11
Because of increasing evidence for an inflammatory-immune etiology, attempts have been made to treat these patients with immunomodulatory therapies such as corticosteroids or intravenous immunoglobulin, with some improvements in outcome.12–14 Recent prospective,double-blinded, placebo-controlled study of 75 patients with DLRPN randomized them to either a 12 week regimen of intravenous methylprednisolone or placebo; patients treated with steroids reported significantly greater improvement in symptoms (especially measures of pain and positive sensory symptoms), though the primary outcome (time to improvement of neuropathy impairment score [lower limb]) was not significantly different between the two groups.15 The lack of difference in the primary endpoint may be due to the delayed timing of initiation of treatment, and earlier intervention may have produced a better outcome.
The illustrated case also demonstrates the ongoing importance and role of nerve biopsy in the care of neuromuscular cases. Even though DLRPN was felt to be the most likely cause, it was not clear if this patient’s disease was still active as he had been symptomatic for nearly a year, and the disease is usually monophasic. Alternatively, he may have been left with the residual deficits of previous injury. The superficial peroneal nerve biopsy was very helpful in this patient’s management. It confirmed ischemic injury and active microvasculitis.
Because of the microvasculitis, this patient was treated with a 12-week course of weekly intravenous methylprednisolone infusion (1.0 grams per dose), and he had marked improvement of pain and weakness.
Acknowledgments
Supported in part by a grant obtained from the National Institutes of Neurological Disorders and Stroke (NS36797).
References
- 1.Dyck PJ, Zimmerman IR, O’Brien PC, et al. Introduction of automated systems to evaluate touch-pressure, vibration, and thermal cutaneous sensation in man. Ann Neurol. 1978;4:502–510. doi: 10.1002/ana.410040605. [DOI] [PubMed] [Google Scholar]
- 2.Dyck PJ, Zimmerman I, Gillen DA, Johnson D, Karnes JL, O’Brien PC. Cool, warm, and heat-pain detection of receptors: testing methods and inferences about anatomic distribution of receptors. Neurology. 1993;43:1500–1508. doi: 10.1212/wnl.43.8.1500. [DOI] [PubMed] [Google Scholar]
- 3.Garland H. Diabetic amyotrophy. Br Med J. 1955;2:1287–1296. doi: 10.1136/bmj.2.4951.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastron JA, Thomas JE. Diabetic polyradiculopathy: clinical and electromyographic findings in 105 patients. Mayo ClinProc. 1981;56:725–732. [PubMed] [Google Scholar]
- 5.Williams IR, Mayer RF. Subacute proximal diabetic neuropathy. Neurology. 1976;26:108–116. doi: 10.1212/wnl.26.2.108. [DOI] [PubMed] [Google Scholar]
- 6.Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 7.Dyck PJB, Norell JE, Dyck PJ. Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology. 1999;53:2113–2121. doi: 10.1212/wnl.53.9.2113. [DOI] [PubMed] [Google Scholar]
- 8.Subramony SH, Wilbourn AJ. Diabetic proximal neuropathy: clinical and electromyographic studies. JNeurolSci. 1982;53:293–304. doi: 10.1016/0022-510x(82)90014-4. [DOI] [PubMed] [Google Scholar]
- 9.Dyck PJB, Norell JE, Dyck PJ. Non-diabetic lumbosacral radiculoplexus neuropathy. Natural history, outcome and comparison with the diabetic variety. Brain. 2001;124:1197–1207. doi: 10.1093/brain/124.6.1197. [DOI] [PubMed] [Google Scholar]
- 10.Dyck PJB, Windebank AJ. Diabetic and non-diabetic lumbosacral radiculoplexus neuropathies: New insights into pathophysiology and treatment. Muscle Nerve. 2002;25(4):477–491. doi: 10.1002/mus.10080. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura N, Dyck PJ, Schmeichel AM, Engelstad JK, Low PA, Dyck PJ. Inflammatory mediators in diabetic and non-diabetic lumbosacral radiculoplexus neuropathy. Acta Neuropathol. 2008;115(2):231–239. doi: 10.1007/s00401-007-0326-2. [DOI] [PubMed] [Google Scholar]
- 12.Said G, Goulon-Goeau C, Lacroix C, Moulonguet A. Nerve biopsy findings in different patterns of proximal diabetic neuropathy. Ann Neurol. 1994;35:559–569. doi: 10.1002/ana.410350509. [DOI] [PubMed] [Google Scholar]
- 13.Krendel DA, Costigan DA, Hopkins LC. Successful treatment of neuropathies in patients with diabetes mellitus. ArchNeurol. 1995;52(11):1053–1061. doi: 10.1001/archneur.1995.00540350039015. [DOI] [PubMed] [Google Scholar]
- 14.Pascoe MK, Low PA, Windebank AJ, Litchy WJ. Subacute Diabetic Proximal Neuropathy. Mayo Clin Proc. 1997;72(12):1123–1132. doi: 10.4065/72.12.1123. [DOI] [PubMed] [Google Scholar]
- 15.Dyck PJB, O’Brien P, Bosch EP, et al. The multi-center, double-blind controlled trial of IV methylprednisolone in diabetic lumbosacral radiculoplexus neuropathy. Neurology. 2006;66(5 Suppl 2):A191. [Google Scholar]